Abstract
The ability of cells to respond and survive stressful conditions is determined, in part, by the attachment of O-linked N-acetylglucosamine (O-GlcNAc) to proteins (O-GlcNAcylation), a post-translational modification dependent on glucose and glutamine. This study investigates the role of dynamic O-GlcNAcylation of mesothelial cell proteins in cell survival during exposure to glucose-based peritoneal dialysis fluid (PDF). Immortalized human mesothelial cells and primary mesothelial cells, cultured from human omentum or clinical effluent of PD patients, were assessed for O-GlcNAcylation under normal conditions or after exposure to PDF. The dynamic status of O-GlcNAcylation and effects on cellular survival were investigated by chemical modulation with 6-diazo-5-oxo-L-norleucine (DON) to decrease or O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino N-phenyl carbamate (PUGNAc) to increase O-GlcNAc levels. Viability was decreased by reducing O-GlcNAc levels by DON, which also led to suppressed expression of the cytoprotective heat shock protein 72. In contrast, increasing O-GlcNAc levels by PUGNAc or alanyl-glutamine led to significantly improved cell survival paralleled by higher heat shock protein 72 levels during PDF treatment. Addition of alanyl-glutamine increased O-GlcNAcylation and partly counteracted its inhibition by DON, also leading to improved cell survival. Immunofluorescent analysis of clinical samples showed that the O-GlcNAc signal primarily originates from mesothelial cells. In conclusion, this study identified O-GlcNAcylation in mesothelial cells as a potentially important molecular mechanism after exposure to PDF. Modulating O-GlcNAc levels by clinically feasible interventions might evolve as a novel therapeutic target for the preservation of peritoneal membrane integrity in PD.
Peritoneal dialysis (PD) is a highly relevant RRT.1,2 Although PD is effective and convenient, long-term use of PD fluids (PDFs) leads to severe damage and chronic morphologic changes of the peritoneal membrane.3 PDFs are bioincompatible because of their composition.4–6 Whereas the acute toxicity of PDFs is mostly mediated by low pH, hyperosmolarity, and content of nonphysiologic buffer components, recent research reveals more complex glucose-mediated damage during PD.4,7–13
This glucose-related toxicity was originally regarded as an unspecific effect of hyperosmolarity caused by the at least 15-fold physiologic concentration of PDF compared with the plasma glucose level as well as additional formation of highly reactive glucose degradation products (GDPs) during heat sterilization and storage.14 Toxic GDPs are involved in oxidative stress and formation of advanced glycation end products.15–17 In addition to nonspecific toxic effects, the glucose level is increasingly regarded as the main metabolic switch for cellular responses determining cellular fate.10,18,19 One obvious mechanism is post-translational modification (PTM) of proteins.
The attachment of O-linked N-acetylglucosamine (O-GlcNAc) to proteins (O-GlcNAcylation) is a specific sugar modification of proteins.20,21 The ability of cells to respond to and survive stressful conditions is known to be influenced by glucose and glutamine-dependent O-GlcNAcylation through the hexosamine biosynthetic pathway (HBP), in which both mentioned substrates are enzymatically converted to the high-energy metabolite UDP-N-acetylglucosamine (UDP-GlcNAc), which is then covalently attached to serine, threonine, or tyrosine residues of proteins under UDP release.22–25 In contrast to other types of O- and N-linked glycosylation, O-GlcNAcylation is a primarily nuclear and cytosolic glycosylation with a single O-GlcNAc moiety and in contrast to more complex glycan structures, a dynamic process more akin to phosphorylation.20
The enzymes O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) keep the rates of glycosylation and deglycosylation in a dynamic equilibrium and ready to respond to environmental stimuli, such as the availability of glucose, combined with a hyperosmolar injurious stress.26 This dynamic interplay between glucose-related toxicity and cytoprotection, when triggered by PDF, might be potentially relevant in PD.
This study investigates the role of O-GlcNAcylation of mesothelial cell proteins for cell survival in the context of PDF toxicity as well as the influence of this abundant PTM on cellular stress responses (CSRs) and their modulation during experimental PD.
Results
Dynamic O-GlcNAc Levels of Mesothelial Cell Proteins Increase during PDF Exposure
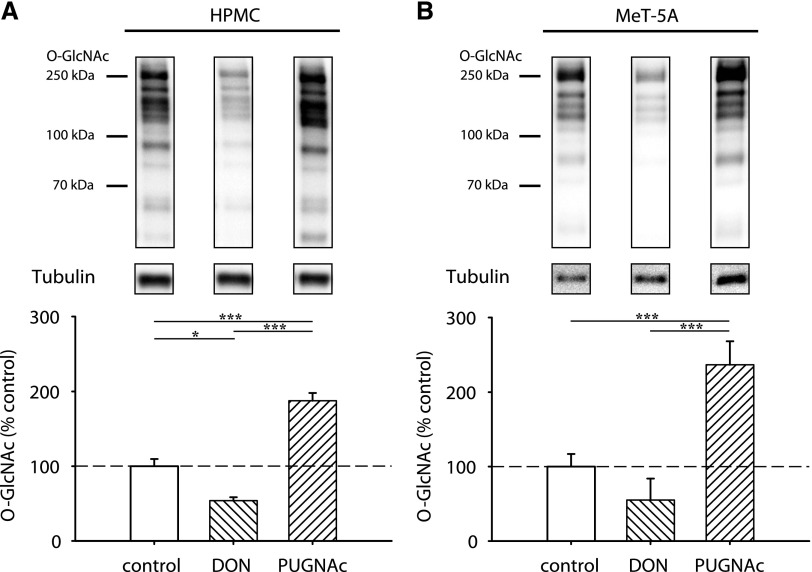
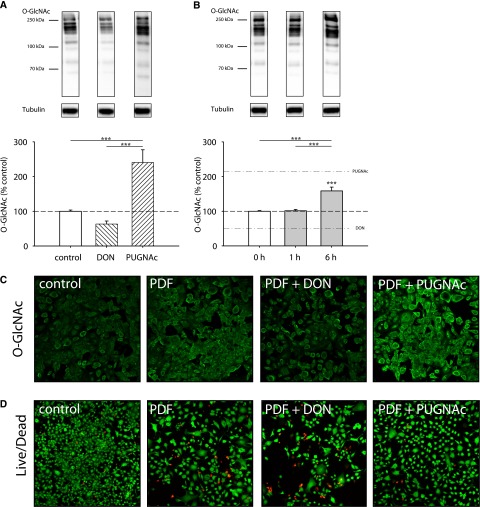
Mesothelial cells showed an intermediate O-GlcNAc abundance under control conditions in primary omentum-derived human peritoneal mesothelial cells (HPMCs) (Figure 1A) but also immortalized MeT-5A cells (Figure 1B). In both cell types, O-GlcNAc levels could be modulated by chemical inhibitors of the HBP pathway. Addition of the glutamine fructose-6-phosphate amidotransferase (GFAT) inhibitor 6-diazo-5-oxo-L-norleucine (DON) resulted in decrease (HPMC: 53.9%±4.5, P<0.05 versus control; MeT-5A: 55.1%±16.6), whereas addition of O-GlcNAcase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino N-phenyl carbamate (PUGNAc) resulted in significant increase (HPMC: 187.4%±10.6, P<0.05; MeT-5A: 263.5%±22.5, P<0.05) of O-GlcNAc abundance. As expected, DON and PUGNAc reciprocally influenced OGA and OGT abundance (Supplemental Figure 1).
Figure 1.
Abundance of O-GlcNAc in mesothelial cells is intermediate and can be modulated. O-GlcNAc abundance after 18 hours of incubation with 50 µM DON or 100 µM PUGNAc (hatched bars) or control cells (white bars). (A) shows results of omentum-derived HPMCs, and (B) shows results of immortalized mesothelial cells MeT-5A. The upper panels shows representative Western blots of O-GlcNAc and tubulin as loading control; densitometry represents four individual samples per bar. *P<0.05; ***P<0.001.
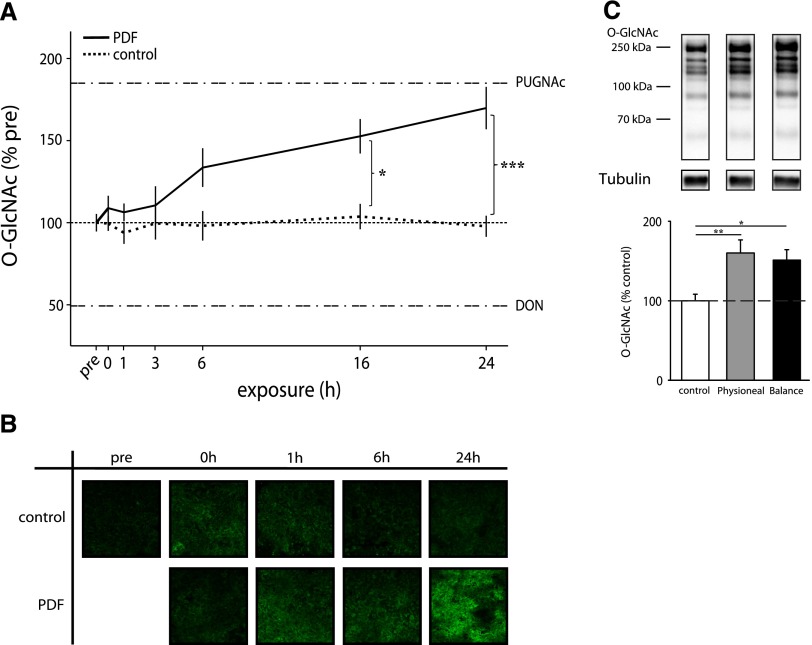
After PDF exposure, O-GlcNAc abundance in MeT-5A cells increased in a time-dependent manner up to 169.8%±12.9 (P<0.05 versus time control) after 24 hours of incubation, which is almost as high as after PUGNAc treatment (185.2%) in this setting (Figure 2A). Immunofluorescent O-GlcNAc staining (Figure 2B) confirmed the findings of the Western blot results. As shown in Figure 2C, alternative glucose-based PDF with other buffer systems and low GDP concentrations also significantly increased the O-GlcNAc abundance to 160.1%±16.5 with Physioneal and 151.2%±13.2 with Balance (both P<0.05 versus control). PDF had no significant effects on OGA and OGT abundance (Supplemental Figure 1).
Figure 2.
O-GlcNAc abundance increases during PDF exposure. O-GlcNAc abundance in MeT-5A cells after 30 minutes PDF and subsequent incubation with PDF 1:1 diluted with growth medium (0–24 hours). (A) shows significant increased levels of O-GlcNAc after 16 and 24 hours PDF (Dianeal-PD4 3.86%) exposure (black line) compared with control at corresponding time points (dashed line). The upper threshold line represents maximum O-GlcNAc levels by 100 µM PUGNAc (183%±8.8), and the lower threshold line represents minimum O-GlcNAc levels by 50 µM DON (49%±4.3). Data are shown as percentages of untreated control (pre; continuous line). Data represent n=8 in three individual experiments. (B) Immunofluorescent staining for O-GlcNAc (green) shows increased levels after PDF (Dianeal-PD4 3.86%) exposure over time compared with corresponding control cells. (C) shows significant increased levels of O-GlcNAc after 6 hours of exposure with Physioneal 40 (3.86%) and Balance (4.25%). The upper panel shows representative Western blots of O-GlcNAc and tubulin. Densitometry represents n=18 in four individual experiments. *P<0.05; **P<0.01; ***P<0.001.
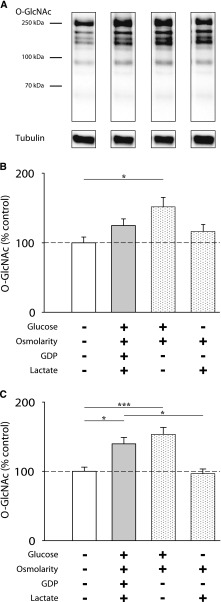
In a next step, we analyzed the influence of single properties such as high glucose and high osmolarity of the PDF on O-GlcNAcylation compared with PDF. As shown in Figure 3, growth medium containing 3.86% glucose significantly increased O-GlcNAc abundance after 6 hours to 151.8%±13.6 (P<0.05 versus control) (Figure 3B) and 153.5%±10.0 (P<0.05 versus control) after 24 hours (Figure 3C), although cell damage remained markedly lower than with PDF (data not shown). An osmolarity control (PDF containing mannitol instead of glucose) showed no significant increase (96.6%±6.8) in O-GlcNAc abundance.
Figure 3.
High glucose levels increase O-GlcNAc abundance. (A) shows representative Western blots of O-GlcNAc and tubulin after 30 minutes of PDF and subsequent 6 hours of 1:1 incubation. MeT-5A cells were treated with Dianeal-PD4 (3.86%; gray bars), growth medium with 3.86% glucose (left white spotted bars), or PD solution with mannitol (right white spotted bars) for 30 minutes and subsequently incubated with 1:1 dilutions of the fluids diluted with growth medium for (B) 6 and (C) 24 hours. Data are shown as percentages of standard growth medium (white bars). Data represent n=18 in four individual experiments. *P<0.05; ***P<0.001.
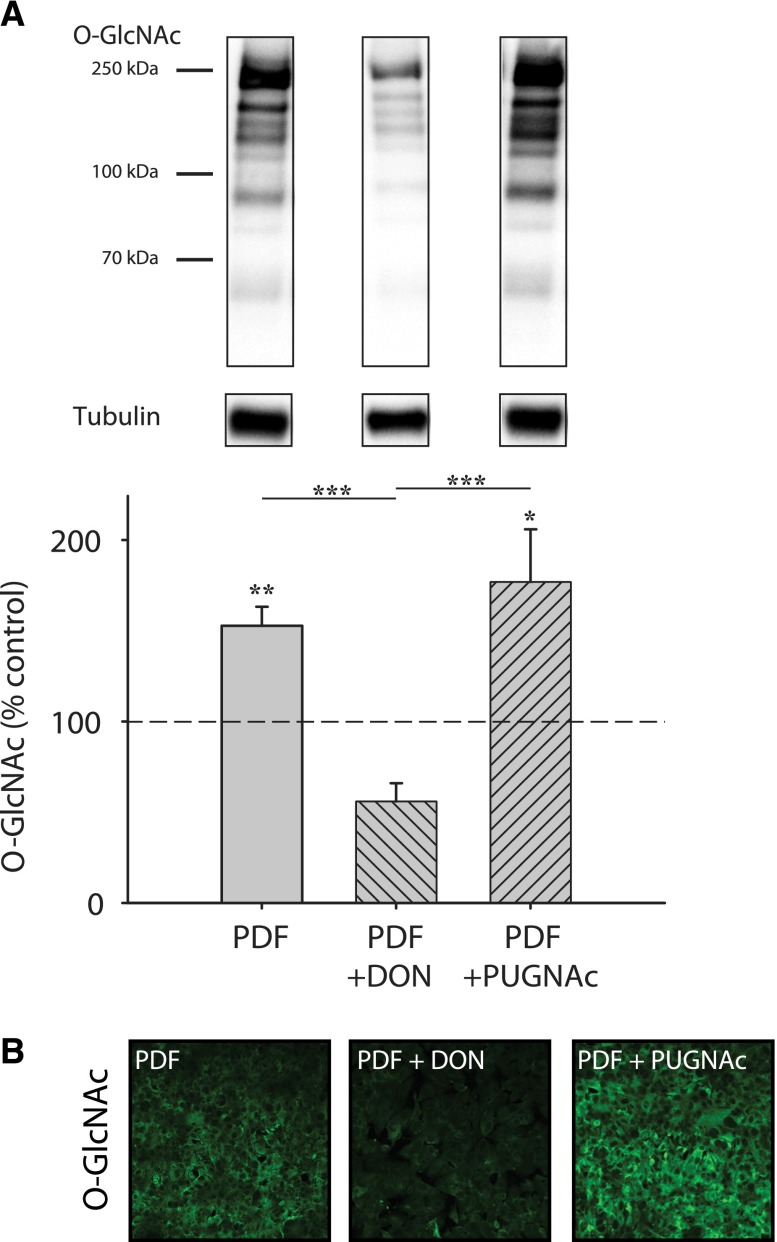
To evaluate the capacity of chemical modulation of O-GlcNAcylation during in vitro PDF exposure, DON and PUGNAc were applied before PDF treatment. As shown in Figure 4, significant modulation (DON: 56.0%±10.0; PUGNAc: 176.8%±29.1) of the O-GlcNAc abundance by chemical inhibitors is also possible in cells exposed to PDF.
Figure 4.
Modulation of O-GlcNAc abundance can be performed in combination with PDF exposure. O-GlcNAc abundance in MeT-5A cells after 30 minutes PDF and subsequent incubation with PDF 1:1 diluted with growth medium for 16 hours (gray bar). (A) shows significant changes of O-GlcNAc levels by preincubation with 50 µM DON or 100 µM PUGNAc (hatched gray bars) before PDF. The upper panel shows representative Western blots of O-GlcNAc and tubulin as loading control. Data represent three individual experiments. *P<0.05; **P<0.01; ***P<0.001. (B) Immunofluorescent staining for O-GlcNAc (green) shows altered levels after 24 hours of PDF exposure with 50 µM DON or 100 µM PUGNAc.
Modulation of O-GlcNAc Levels Significantly Alters Cell Survival
To investigate the influence of O-GlcNAc levels on cell survival, mesothelial cells were exposed to PDF with or without pretreatment with the chemical inhibitors of the HBP pathway. After exposure to PDF, cells showed significantly decreased viability and a pronounced decrease of O-GlcNAc levels through DON (Figure 5A). In contrast, elevated O-GlcNAc levels triggered by PUGNAc led to significantly improved cell survival compared with PDF treatment alone (99.8%±3.6 of control cells). Exposure to PDF resulted in a significant increase of vascular endothelial growth factor (VEGF) release (133.9%±4.2% control, P<0.05). Addition of DON resulted in a further increase (from 133.9% to 211.2%±29.1, P<0.05), whereas addition of PUGNAc had no significant effect (from 133.9% to 148.9%±4.2) (Supplemental Figure 2).
Figure 5.
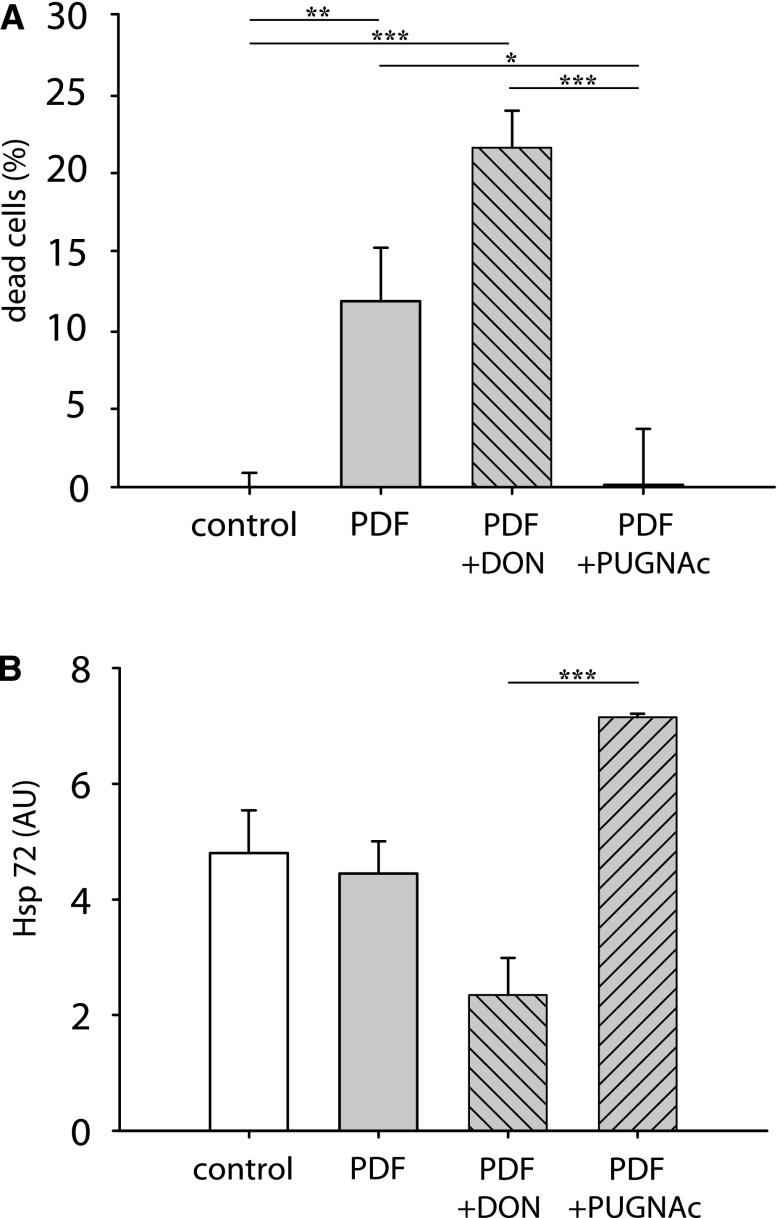
Modulation of O-GlcNAc changes viability outcome and chaperone expression after PD treatment. MeT-5A cells were treated with PDF for 30 minutes and subsequently incubated with PDF 1:1 diluted with growth medium for 16 hours (gray bars). Cells were incubated for 18 hours with 50 µM DON or 100 µM PUGNAc (hatched gray bars) before PDF exposure. (A) shows cell viability (as a percentage of dead cells) measured with neutral red uptake. Data are presented as percentages of control cells (white bars). Data represent n=42 (control/PDF) or n=20 (PDF+DON/PDF+PUGNAc) in five individual experiments. (B) shows Hsp72 expression normalized to total protein in Western blot as arbitrary units (AU). Data represent n=8 (control/PDF) or n=3/2 (PDF+DON/PDF+PUGNAc) in three individual experiments. *P<0.05; **P<0.01; ***P<0.001.
Modulation of O-GlcNAc levels resulted in concordant changes of the cellular heat shock response represented by heat shock protein 72 (Hsp72) counteracting PDF toxicity. Reducing the O-GlcNAc levels by DON was reflected by concordant reduction of viability and Hsp72 expression.
Increased O-GlcNAc levels under addition of PUGNAc correspondingly resulted in improved survival and significantly increased Hsp72.
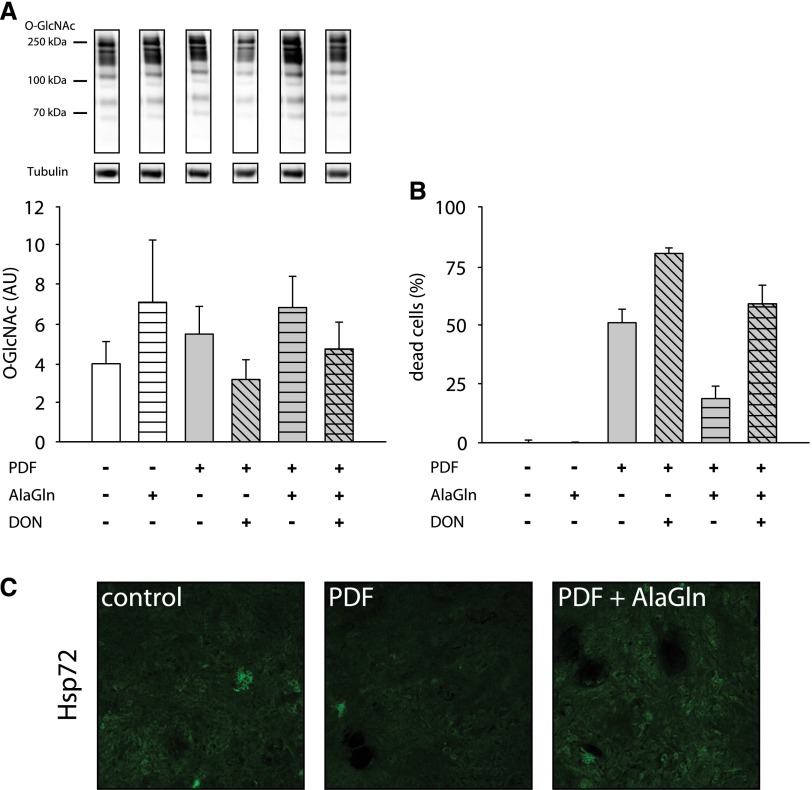
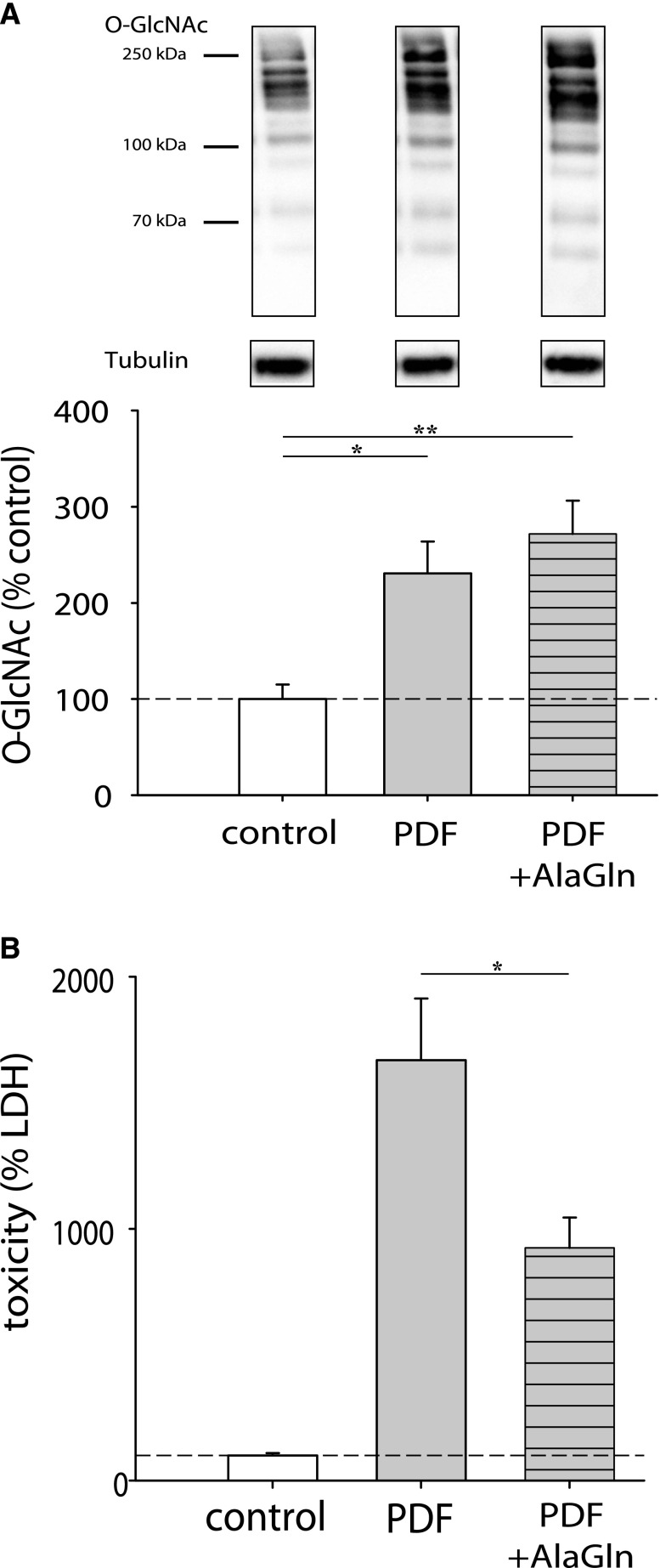
In the next step, we evaluated the influence of the clinically feasible HBP modulator glutamine (administrated as the stable dipeptide alanyl-glutamine [AlaGln]) in this exposure system. Addition of 8 mM AlaGln during PDF exposure increased the O-GlcNAc abundance and also improved cellular survival during PDF treatment. Cells coincubated with AlaGln and DON showed a slight decrease of O-GlcNAcylation (Figure 6A) and cell viability (Figure 6B). Immunofluorescent staining in Figure 6C shows that addition of AlaGln during PDF exposure also increased Hsp72 expression in mesothelial cells.
Figure 6.
Addition of AlaGln causes cytoprotective effects. (A) shows O-GlcNAc abundance of HPMCs after 16 hours of incubation with PDF 1:1 diluted with growth medium (gray bars). Cells were incubated for 18 hours with 50 µM DON (hatched gray bars) before PDF exposure; 8 mM AlaGln was added at PDF treatment start (striped bars). The upper panel shows representative Western blots of O-GlcNAc and tubulin as loading control. Densitometry represents results of two independent HPMC cultures. (B) shows cell viability (percentage of dead cells) measured as neutral red uptake after 30 minutes incubation with PDF and subsequent incubated with PDF 1:1 diluted with growth medium for 24 hours (gray bars) in MeT-5A cells. Data are presented as percentages of untreated control (precontrol is not shown). Cells were incubated for 18 hours with 50 µM DON (hatched gray bars) before PDF exposure. Addition of AlaGln (8 mM) was carried out at the start of the PDF exposure (striped bars). Data represent n=6 per treatment. (C) shows immunofluorescent staining of Hsp72 (green) after 30 minutes incubation with PDF and subsequent incubation with PDF 1:1 diluted with growth medium for 24 hours±8 mM AlaGln.
Confirmation in the Ex Vivo System Using HPMCs Derived from PD Effluent
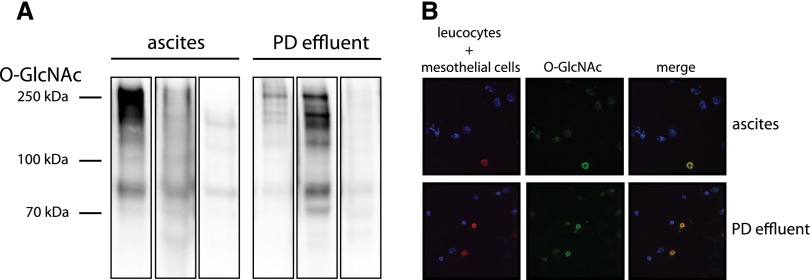
O-GlcNAc levels in clinical PD effluents and ascites fluids of six patients were heterogeneous. When crude peritoneal cell extracts were assessed, cytospin staining showed that O-GlcNAc was selectively expressed in detached HPMCs (Figure 7). As the next step, we validated our findings by cultivating HPMCs derived from peritoneal effluents from uremic donors on PD, resulting in a biologically relevant ex vivo system. Effluent-derived HPMCs also showed intermediate levels of O-GlcNAc, which could be decreased by incubation with DON (63.1%±9.0) or increased with PUGNAc (240.3%±36.9) (Figure 8A). Cells exposed to PDF showed significant increase of O-GlcNAc levels (158.6%±11.0) (Figure 8B) with increased intensity in immunofluorescent O-GlcNAc staining (Figure 8C). Confirming the findings of immortalized cells, reduced O-GlcNAc levels (by DON) resulted in concordant reduction of viability, whereas increased O-GlcNAc levels (by PUGNAc) resulted in improved survival (Figure 8D). Addition of 8 mM AlaGln to the PDF resulted in increased O-GlcNAc levels as well as decreased cell injury (Figure 9).
Figure 7.
O-GlcNAc signals in PD effluents and ascites samples mainly originate from mesothelial cells. (A) shows Western blots for O-GlcNAc in cells of ascites fluid or PD effluent (three samples each); 5 µg total protein was loaded per sample. (B) shows immunofluorescent staining of cytospin slides with 20,000 cells derived from ascites (upper row) or PD effluent bags (lower row). Leukocytes: anti–CD-45 (blue); mesothelial cells: anti–Pan-Keratin (red); O-GlcNAc: anti–O-GlcNAc (green).
Figure 8.
PDF exposure and modulation of O-GlcNAc levels influence cell survival in the ex vivo system of PD effluent derived HPMCs. (A) shows modulated O-GlcNAc abundance after incubation with 50 µM DON or 100 µM PUGNAc (hatched bars) compared with control (white bars). The upper panel shows representative Western blots of O-GlcNAc and tubulin as loading control. (B) shows O-GlcNAc abundance after 30 minutes PDF and subsequent incubation with PDF 1:1 diluted with growth medium (1 or 6 hours; gray bars). Upper dashed line represents mean of maximum O-GlcNAc levels by 100 µM PUGNAc (215%), and lower dashed line represents mean of minimum O-GlcNAc levels by 50 µM DON (55.5%) for 24 hours. Data are shown as percentages of untreated control. Data represent n=10 in cultures of six different PD patients. ***P<0.001. (C) Immunofluorescent staining for O-GlcNAc (green) after 30 minutes PDF and subsequent incubation with PDF 1:1 diluted with growth medium for 6 hours. DON: 24 hours at 50 µM; PUGNAc: 24 hours at 100 µM. (D) shows representative images of live/dead stain (green: living cells; red: dead cells) after 30 minutes PDF and subsequent incubation with PDF 1:1 diluted with growth medium for 6 hours. DON: 24 hours at 50 µM; PUGNAc: 24 hours at 100 µM. n=4.
Figure 9.
Addition of AlaGln causes cytoprotetive effects in the ex vivo system of PD effluent derived HPMCs. Effluent-derived HPMCs were incubated for 30 minutes with PDF followed by 6 hours of incubation with PDF 1:1 diluted with growth medium. (A) shows O-GlcNAc levels after incubation with PDF±8 mM AlaGln. Data represent four cultures of two different PD patients. (B) shows toxicity as a percentage of control cell lactate dehydrogenase (LDH) release after incubation with PDF±8 mM AlaGln. Data represent 24 cultures of three different PD patients. *P<0.05; **P<0.01.
Discussion
This study shows dynamic changes of O-GlcNAc levels in mesothelial cells during PDF exposure. These changes were amenable to pharmacologic interventions and resulted in concordant effects on cellular survival, which were at least, in part, mediated by modulating cytoprotective CSRs.
Most proteins undergo PTMs to attain their full molecular functionality.27 Their activity often is strongly affected by PTM like protein phosphorylation, which can rapidly switch the status of activity.27,28 The attachment of a single-sugar N-acetylglucosamine is another common PTM. Whereas classical glycosylation is static and mostly occurs on secreted or membrane-associated proteins, O-GlcNAcylation is a highly dynamic modification occurring on nuclear, cytoplasmatic, and mitochondrial proteins.20,23,29,30 O-GlcNAcylation is thought to act similar to phosphorylations as a dynamic regulator of signal transduction: O-GlcNAcylation on serine and threonine residues of amino acids occurs reciprocally as well as combined with phosphorylation but also independently.21,22,30,31 In contrast to hundreds of enzymes with kinase or phosphatase activity, the complete turnover of O-GlcNAcylation is catalyzed by only two enzymes, OGT and OGA,32 underlining the versatile role of this PTM in multiple relevant cellular processes.20 Until now, the effect of glucose-based PDF on O-GlcNAc abundance and the potential relevance of this PTM for the stress response have not been investigated; nothing is known about its effect and clinical relevance in mesothelial cells during PD.
Our study in experimental PD is the first to show that exposure to glucose-based PDF leads to significant increase of O-GlcNAcylation in mesothelial cells. This finding is not unexpected, because O-GlcNAcylation has been shown to be influenced by transient changes in glucose concentration.22 O-GlcNAcylation has been reported in other models to mediate effects of glucose stress, a major determinant in peritoneal membrane function and longevity during PD.21,33 With conventional PDF, at least 15-fold physiologic concentrations of glucose are applied to the mesothelial cells. On physiologic glucose exposure, 2%–5% of the glucose influx is known to be converted inside the cell to UDP-GlcNAc through the HBP.20,34 UDP-GlcNAc serves as direct donor for modification of proteins with O-GlcNAc.20 Mesothelial cells are not able to downregulate their glucose transporters in high ambient glucose concentrations.35 PDF exposure will likely directly translate into high intracellular glucose levels and then increased O-GlcNAcylation in mesothelial cells. Our data show that primary and immortalized mesothelial cells have an intermediate status of O-GlcNAcylation that was clearly amenable to modulation with chemical inhibitors of the HBP. In this system, comparison of the effects of PDF, glucose, and hyperosmolarity showed that the increase of O-GlcNAc was driven by glucose. DON as inhibitor of the GFAT significantly decreased O-GlcNAc abundance, whereas PUGNAc as inhibitor of the O-GlcNAcase prevents O-GlcNAc removal and therefore, leads to significantly increased O-GlcNAc levels—with and without concomitant exposure to PDF.
Recent research in other systems showed that O-GlcNAc represents a relevant component of cytoprotective CSRs.23,36–39 PDFs contain different stressors with the potential to induce the CSR, such as hyperosmolar concentrations of glucose, GDP, and acidic lactate. Whereas GDP and acidic lactate are associated with acute adverse effects of PDF, only high glucose was necessary to induce O-GlcNAc. These data, therefore, discriminate cytotoxic factors from factors driving potentially cytoprotective O-GlcNAcylation of cellular proteins in mesothelial cells exposed to PDF. In other models, elevating O-GlcNAc before cellular insults has been shown to improve survival, whereas lowering O-GlcNAc was followed by decreased survival of cells and tissues.23,26,40 In our study, the viability and function of mesothelial cells after PDF exposure were also clearly affected by modulation of O-GlcNAcylation. Increased O-GlcNAc levels in mesothelial cells with PUGNAc treatment led to significantly improved viability, whereas cells with decreased O-GlcNAc with DON treatment showed decreased survival and increased VEGF secretion after PDF exposure. These data clearly support that O-GlcNAc levels influence biologically relevant processes and pathways that are involved in resistance against PDF toxicity.
One particularly attractive mechanism of how O-GlcNAc may influence resistance against PDF is by regulating the expression of HSPs.22,26 Our group has previously shown that HSP expression represents an essential part of the cytoprotective CSR of mesothelial cells exposed to PDF.41–44 After inhibition or enhancement of expression of Hsp72, cellular survival clearly correlated with abundance of this major marker and effector protein.41,43–47 In this study, elevation of O-GlcNAc by PUGNAc increased the expression of Hsp72, whereas reduction of O-GlcNAc by DON resulted in suppression of Hsp72 expression in mesothelial cells exposed to PDF. These results show O-GlcNAc–dependent regulation of Hsp72 expression in this system. However, PDF exposure itself did not result in increased Hsp72 expression in mesothelial cells, despite the PDF-induced increase of O-GlcNAc. Although the molecular mechanism of this apparent paradox has to be described in future studies, we hypothesize that this disconnect between Hsp72 expression and O-GlcNAcylation is likely caused by complex effects of PDF exposure on upstream regulation of heat shock factor 1 (HSF-1). The activation of HSF-1, the key transcription factor of Hsp72, is a multistep process involving multiple regulators that might be independently regulated by factors prevalent in PDF.48,49 O-GlcNAc-mediated regulation of HSF-1 is also supported by the recent work by Kazemi et al.,23 which reported a link between Hsp72, HSF-1, glycogen synthase kinase 3β, and O-GlcNAcylation.
Recently, we have shown that the addition of glutamine to PDF protects mesothelial cells in in vitro and in vivo models of PD by enhancing CSRs after PDF exposure.45,46 AlaGln is a stable dipeptide in clinical use as an additive to parenteral nutrition.
Glutamine is a critical component in the generation of UDP-GlcNAc by GFAT, the rate-limiting enzyme of the HBP. Glutamine and DON have similar chemical structures, but whereas DON inhibits GFAT by covalent binding, glutamine is channeled into the pathway.50 Indeed, AlaGln increased levels of O-GlcNAc in mesothelial cells and added to the effects of PDF. Addition of AlaGln counteracted the decrease of O-GlcNAc levels by DON treatment and attenuated the cytotoxicity of DON during PDF exposure. The data suggest that glutamine mediated its effects through the HBP also during PDF exposure, similar to the suggestion by Wischmeyer and colleagues.25 Confirming our previous in vitro and in vivo data, addition of glutamine resulted in increased expression of Hsp72 in mesothelial cells during PDF exposure.45,46 These findings are also in agreement with studies in cell systems of cardiac dysfunction and sepsis, which showed that glutamine enhanced HSP expression through the upregulation of O-GlcNAcylation.23–25 The concordance of O-GlcNAc levels, HSP expression, and improved survival of mesothelial cells proposes a possible mechanistic explanation for the cytoprotection observed with the addition of AlaGln to PDF in experimental PD.
In clinical PD effluents, O-GlcNAc abundance in cellular extracts did not differ from that of ascites. More detailed analysis of PD effluents by staining of cytospins showed that O-GlcNAcylation was primarily detected in the mesothelial cells. Different O-GlcNAc levels in PD effluents and ascites thus rather reflected the heterogeneity of the peritoneal cell populations than actual expression levels in mesothelial cells. Primary cultures of these effluent-derived mesothelial cells from patients on PD currently represent the most accepted system to bridge the gap between experimental in vitro models and clinical studies. In the final part of this study, we, therefore, set out to confirm the role of O-GlcNAc after exposure to PDF in this biologically relevant experimental model of PD. Our results confirmed increased O-GlcNAc levels after PDF exposure. Supporting the data of PDF exposure obtained in the highly reproducible cell line experiments, the addition of DON to PDF also resulted in increased cell death, whereas the addition of PUGNAc and glutamine led to reduced cellular damage in peritoneal effluent–derived mesothelial cells exposed to PDF. Although ex vivo exposure to diluted PDF still represents an artificial system, this model likely reflects the conditions prevalent during an intraperitoneal PD dwell and has previously been shown to be suitable for assessing the balance of mesothelial cell injury and repair processes.46,49
In conclusion, this study identified O-GlcNAcylation in mesothelial cells as a potentially important PTM after exposure to PDF. Dynamic changes of O-GlcNAc levels on PDF exposure were shown in immortalized mesothelial cells and primary cultures of HPMCs obtained from patients on PD. O-GlcNAc levels were amenable to pharmacologic interventions that resulted in concordant effects on cellular survival, which were at least, in part, mediated by modulating expression of HSP. Enhancing O-GlcNAc levels by clinically feasible interventions, such as by adding AlaGln to PDF, might thus evolve as a novel therapeutic target for the preservation of peritoneal membrane integrity in PD. Additional studies will be needed to assess the complex interplay of O-GlcNAc with specific target proteins involved in modulation of HSF-1 activation to better understand the role of O-GlcNAc in HSP-mediated CSRs during experimental PD and further define the specific pattern of individual mesothelial proteins that are O-GlcNAcylated in response to PDF exposure.
Concise Methods
Mesothelial Cells
HPMCs were isolated from specimens of omentum obtained from consenting nonuremic patients undergoing elective abdominal surgery. Cells were enzymatically isolated and characterized as previously described.51 All experiments were performed using cells from the second passage.
HPMCs from PD effluents (effluent HPMCs) were grown from randomly collected peritoneal effluents of consenting patients undergoing continuous ambulatory PD at the Vienna General Hospital. The collected peritoneal effluent fluid was surplus to diagnostic requirements. The ethics committee of the Medical University of Vienna has approved the study in which the cells were obtained (EK867/2010). PD effluents were collected from dwells with at least 4 hours of 1.5% glucose-based PDF in stable PD patients.
Cells were collected and propagated as described before.46 Only cobblestone-like epithelial cultures were used for incubation experiments, showing stable morphology during two cell passages.
All HPMCs, including immortalized human mesothelial cells (MeT-5A [ATCC-CRL-9994]; LGC Standards GmbH, Wesel, Germany), were propagated in M199 culture medium supplemented with 0.1 g/L l-glutamine, 50 units/ml penicillin, 50 µg/ml streptomycin, 2.2 g/L sodium bicarbonate, 5.96 g/L HEPES, and 10% FCS and cultured at 5% CO2 and 37°C in a humidified atmosphere.
In Vitro PDF Treatment
Mesothelial cells were exposed for 30 minutes to commercially available 3.86% glucose-based PD4-Dianeal (Baxter AG, Vienna, Austria) followed by incubation with PDF diluted 1:1 with normal growth medium for the indicated times. Parallel incubation with medium served as a control. FCS was added to all final mixtures to yield a final concentration of 10%.
As chemical inhibitors of the O-GlcNAc pathway, 50 µM DON to decrease O-GlcNAc abundance or 100 µM PUGNAc to inhibit removal of O-GlcNAc and therefore, increase O-GlcNAc abundance was added to the cultures, starting 18 hours before PDF incubation.
In separate experiments, MeT-5A cells were exposed for 30 minutes to commercially available 3.86% glucose-based PD4-Dianeal, Physioneal 40 3.86% (Baxter AG), or Balance (Fresenius Medical Care AG, Vienna, Austria) followed by incubation with PDF diluted 1:1 with normal growth medium for 6 hours. Cells were also incubated with growth medium containing 3.86% glucose or (laboratory-made) PD4 with mannitol as osmolarity control.
Preparation of Peritoneal Cells
Human peritoneal cells from either ascites or PD effluents were obtained from six patients at the Vienna General Hospital (three of each). PD effluents were collected from dwells in stable PD patients as described above. Ascites samples were collected from patients with congestive heart failure and refractory ascites. All samples were taken from surplus material and promptly anonymized. The fluid of the whole bag was centrifuged, cells were counted, and cytospin slides (20,000 cells per slide) or cell lysates (1×106 cells per 100 µl lysis buffer46) were produced.
Western Blot and ELISA
Cell extracts were prepared as described46; equal amounts of total protein were separated by SDS-PAGE and electroblotted onto polyvinylidene fluoride (PVDF) membranes. The membranes were incubated with antibodies against O-GlcNAc (RL2; Abcam, Inc., Cambridge, United Kingdom), Hsp72 (SPA-810; Enzo Life Sciences, Farmingdale, NY), OGA (Abcam, Inc.), OGT (SAB2101676; Sigma-Aldrich, St. Louis, MO), or β-tubulin (691261; MP Biochemicals, Solon, OH).
VEGF in cell culture supernatants was measured with ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Viability Assays
After in vitro exposure, neutral red uptake was measured using a standard reagent (50 µg/ml) according to the manufacturer’s protocol (Sigma-Aldrich).
Using the LIVE/DEAD viability/cytotoxicity kit (Invitrogen, Paisley, UK), intracellular esterase activity (staining of living cells) and plasma membrane integrity (staining of dead cells) were simultaneously stained and analyzed using a laser-scanning microscope (LSM-700; Carl Zeiss MicroImaging GmbH, Jena, Germany).
Lactate dehydrogenase release in cell culture supernatants was assessed using the TOX-7 LDH Kit (Sigma-Aldrich) according to the manufacturer’s instructions.
Immunofluorescent Staining
Cells cultured on chamber slides (BD Falcon, Millville, NJ) were incubated with anti–O-GlcNAc antibody (RL-2; Abcam, Inc.) followed by an FITC-labeled secondary antibody (Abcam, Inc.). Cytospin slides were incubated with anti–CD-45 (APC; Miltenyi Biotec, Bergisch Gladbach, Germany), anti–Pan-Keratin (PE; Cell Signaling Technologies, Danvers, MA), and anti–O-GlcNAc antibody (RL-2; Abcam, Inc.) detected by FITC-labeled secondary antibody (Abcam, Inc.). Slides were analyzed using an LSM-700.
Statistical Analyses
All statistical analyses were performed using Statistical Package for the Social Sciences 17 (SPSS Inc., Chicago, IL) and Sigmaplot 11.0 (Systat Software GmbH, Erkrath, Germany). Values from different groups were compared using t tests or ANOVAs where appropriate. In the case of ANOVA, Tukey's honest significant difference test was used as a post hoc test. P values lower than 0.05 were considered significant. The results are presented as means±SEMs.
Disclosures
R.H., K.B., and K.K. are employees of Zytoprotec GmbH. C.A. is a cofounder of Zytoprotec GmbH, a spinoff of the Medical University Vienna that holds the patent “Carbohydrate-based peritoneal dialysis fluid comprising glutamine residue” (International Publication Number WO 2008/106702 A1).
Supplementary Material
Acknowledgments
The authors thank the Imaging Core Facility of the Medical University of Vienna (M. Gröger and S. Rauscher) for the opportunity to use facility equipment and their support. We also thank A. Hellman for helping with the submission of the manuscript and K. Rusai for critically reading the manuscript.
This study was funded by ZIT-Technology Agency of the City of Vienna (ID 648625). K.B. and K.K. were supported by the European Training and Research in Peritoneal Dialysis (EuTRiPD) Programme, a project funded by the European Union within the Marie Curie scheme (287813).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101128/-/DCSupplemental.
References
- 1.Li S, Zhou Y, Fan J, Cao S, Cao T, Huang F, Zhuang S, Wang Y, Yu X, Mao H: Heat shock protein 72 enhances autophagy as a protective mechanism in lipopolysaccharide-induced peritonitis in rats. Am J Pathol 179: 2822–2834, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson AR, Hayes WN, Vondrak K, Ariceta G, Schmitt CP, Ekim M, Fischbach M, Edefonti A, Shroff R, Holta T, Zurowska A, Klaus G, Bakkaloglu S, Stefanidos C, Van de Walle J, European Paediatric Dialysis Working Group : Factors influencing choice of renal replacement therapy in European paediatric nephrology units. Pediatr Nephrol 28: 2361–2368, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Davies SJ, Phillips L, Griffiths AM, Russell LH, Naish PF, Russell GI: What really happens to people on long-term peritoneal dialysis? Kidney Int 54: 2207–2217, 1998 [DOI] [PubMed] [Google Scholar]
- 4.McIntyre CW: Update on peritoneal dialysis solutions. Kidney Int 71: 486–490, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Breborowicz A, Oreopoulos DG: Biocompatibility of peritoneal dialysis solutions. Am J Kidney Dis 27: 738–743, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Jörres A, Topley N, Gahl GM: Biocompatibility of peritoneal dialysis fluids. Int J Artif Organs 15: 79–83, 1992 [PubMed] [Google Scholar]
- 7.Topley N, Kaur D, Petersen MM, Jörres A, Passlick-Deetjen J, Coles GA, Williams JD: Biocompatibility of bicarbonate buffered peritoneal dialysis fluids: Influence on mesothelial cell and neutrophil function. Kidney Int 49: 1447–1456, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Holmes CJ: Biocompatibility of peritoneal dialysis solutions. Perit Dial Int 13: 88–94, 1993 [PubMed] [Google Scholar]
- 9.Breborowicz A, Rodela H, Oreopoulos DG: Toxicity of osmotic solutes on human mesothelial cells in vitro. Kidney Int 41: 1280–1285, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Witowski J, Bender TO, Wisniewska-Elnur J, Ksiazek K, Passlick-Deetjen J, Breborowicz A, Jörres A: Mesothelial toxicity of peritoneal dialysis fluids is related primarily to glucose degradation products, not to glucose per se. Perit Dial Int 23: 381–390, 2003 [PubMed] [Google Scholar]
- 11.Schwenger V, Morath C, Salava A, Amann K, Seregin Y, Deppisch R, Ritz E, Bierhaus A, Nawroth PP, Zeier M: Damage to the peritoneal membrane by glucose degradation products is mediated by the receptor for advanced glycation end-products. J Am Soc Nephrol 17: 199–207, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Holmes CJ: Reducing cardiometabolic risk in peritoneal dialysis patients: Role of the dialysis solution. J Diabetes Sci Tech 3: 1472–1480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes CJ: Glucotoxicity in peritoneal dialysis—solutions for the solution! Adv Chronic Kidney Dis 14: 269–278, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS: Long-term exposure to new peritoneal dialysis solutions: Effects on the peritoneal membrane. Kidney Int 66: 1257–1265, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Müller-Krebs S, Kihm LP, Zeier B, Gross ML, Wieslander A, Haug U, Zeier M, Schwenger V: Glucose degradation products result in cardiovascular toxicity in a rat model of renal failure. Perit Dial Int 30: 35–40, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Zeier M, Schwenger V, Deppisch R, Haug U, Weigel K, Bahner U, Wanner C, Schneider H, Henle T, Ritz E: Glucose degradation products in PD fluids: Do they disappear from the peritoneal cavity and enter the systemic circulation? Kidney Int 63: 298–305, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Kim YL, Cho JH, Choi JY, Kim CD, Park SH: Systemic and local impact of glucose and glucose degradation products in peritoneal dialysis solution. J Ren Nutr 23: 218–222, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Brownlee M: The pathobiology of diabetic complications: A unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Sitter T, Sauter M: Impact of glucose in peritoneal dialysis: Saint or sinner? Perit Dial Int 25: 415–425, 2005 [PubMed] [Google Scholar]
- 20.Hart GW, Housley MP, Slawson C: Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446: 1017–1022, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Ruan HB, Singh JP, Li MD, Wu J, Yang X: Cracking the O-GlcNAc code in metabolism. Trends Endocrinol Metab 24: 301–309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW: Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 279: 30133–30142, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kazemi Z, Chang H, Haserodt S, McKen C, Zachara NE: O-linked beta-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3beta-dependent manner. J Biol Chem 285: 39096–39107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singleton KD, Wischmeyer PE: Glutamine induces heat shock protein expression via O-glycosylation and phosphorylation of HSF-1 and Sp1. JPEN J Parenter Enteral Nutr 32: 371–376, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Hamiel CR, Pinto S, Hau A, Wischmeyer PE: Glutamine enhances heat shock protein 70 expression via increased hexosamine biosynthetic pathway activity. Am J Physiol Cell Physiol 297: C1509–C1519, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groves JA, Lee A, Yildirir G, Zachara NE: Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones 18: 535–558, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S: Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4: 1633–1649, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Bohnert KA, Gould KL: On the cutting edge: Post-translational modifications in cytokinesis. Trends Cell Biol 21: 283–292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells L, Whelan SA, Hart GW: O-GlcNAc: A regulatory post-translational modification. Biochem Biophys Res Commun 302: 435–441, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Slawson C, Housley MP, Hart GW: O-GlcNAc cycling: How a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem 97: 71–83, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Snow DM, Hart GW: Nuclear and cytoplasmic glycosylation. Int Rev Cytol 181: 43–74, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Butkinaree C, Park K, Hart GW: O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta 1800: 96–106, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouché C, Serdy S, Kahn CR, Goldfine AB: The cellular fate of glucose and its relevance in type 2 diabetes. Endocr Rev 25: 807–830, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marbán E: Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117: 1172–1182, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Fischereder M, Schröppel B, Wiese P, Fink M, Banas B, Schmidbauer S, Schlöndorff D: Regulation of glucose transporters in human peritoneal mesothelial cells. J Nephrol 16: 103–109, 2003 [PubMed] [Google Scholar]
- 36.Champattanachai V, Marchase RB, Chatham JC: Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol 294: C1509–C1520, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darley-Usmar VM, Ball LE, Chatham JC: Protein O-linked β-N-acetylglucosamine: A novel effector of cardiomyocyte metabolism and function. J Mol Cell Cardiol 52: 538–549, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ku NO, Toivola DM, Strnad P, Omary MB: Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat Cell Biol 12: 876–885, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nöt LG, Brocks CA, Vámhidy L, Marchase RB, Chatham JC: Increased O-linked beta-N-acetylglucosamine levels on proteins improves survival, reduces inflammation and organ damage 24 hours after trauma-hemorrhage in rats. Crit Care Med 38: 562–571, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zachara NE, Molina H, Wong KY, Pandey A, Hart GW: The dynamic stress-induced “O-GlcNAc-ome” highlights functions for O-GlcNAc in regulating DNA damage/repair and other cellular pathways. Amino Acids 40: 793–808, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehm M, Bergmeister H, Kratochwill K, Vargha R, Lederhuber H, Aufricht C: Cellular stress-response modulators in the acute rat model of peritoneal dialysis. Pediatr Nephrol 25: 169–172, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Kratochwill K, Lechner M, Siehs C, Lederhuber HC, Rehulka P, Endemann M, Kasper DC, Herkner KR, Mayer B, Rizzi A, Aufricht C: Stress responses and conditioning effects in mesothelial cells exposed to peritoneal dialysis fluid. J Proteome Res 8: 1731–1747, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Bidmon B, Endemann M, Arbeiter K, Ruffingshofer D, Regele H, Herkner K, Eickelberg O, Aufricht C: Overexpression of HSP-72 confers cytoprotection in experimental peritoneal dialysis. Kidney Int 66: 2300–2307, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Bender TO, Böhm M, Kratochwill K, Vargha R, Riesenhuber A, Witowski J, Jörres A, Wieslander A, Aufricht C: Peritoneal dialysis fluids can alter HSP expression in human peritoneal mesothelial cells. Nephrol Dial Transplant 26: 1046–1052, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Bender TO, Böhm M, Kratochwill K, Lederhuber H, Endemann M, Bidmon B, Aufricht C: HSP-mediated cytoprotection of mesothelial cells in experimental acute peritoneal dialysis. Perit Dial Int 30: 294–299, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Kratochwill K, Boehm M, Herzog R, Lichtenauer AM, Salzer E, Lechner M, Kuster L, Bergmeister K, Rizzi A, Mayer B, Aufricht C: Alanyl-glutamine dipeptide restores the cytoprotective stress proteome of mesothelial cells exposed to peritoneal dialysis fluids. Nephrol Dial Transplant 27: 937–946, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Endemann M, Bergmeister H, Bidmon B, Boehm M, Csaicsich D, Malaga-Dieguez L, Arbeiter K, Regele H, Herkner K, Aufricht C: Evidence for HSP-mediated cytoskeletal stabilization in mesothelial cells during acute experimental peritoneal dialysis. Am J Physiol Renal Physiol 292: F47–F56, 2007 [DOI] [PubMed] [Google Scholar]
- 48.van Oosten-Hawle P, Morimoto RI: Transcellular chaperone signaling: An organismal strategy for integrated cell stress responses. J Exp Biol 217: 129–136, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rusai K, Herzog R, Kuster L, Kratochwill K, Aufricht C: GSK-3β inhibition protects mesothelial cells during experimental peritoneal dialysis through upregulation of the heat shock response. Cell Stress Chaperones 18: 569–579, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu F, Lukinius A, Bergström M, Eriksson B, Watanabe Y, Långström B: A mechanism behind the antitumour effect of 6-diazo-5-oxo-L-norleucine (DON): Disruption of mitochondria. Eur J Cancer 35: 1155–1161, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Stylianou E, Jenner LA, Davies M, Coles GA, Williams JD: Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int 37: 1563–1570, 1990 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.