Abstract
Whether urinary albumin excretion relates to higher levels of atherogenic apolipoprotein B fractions in the nondiabetic population is uncertain. Such a relationship could explain, in part, the association of elevated urinary albumin excretion with cardiovascular disease risk. We assessed the relationship of urinary albumin excretion with apolipoprotein B fractions and determined whether the association of elevated urinary albumin excretion with incident cardiovascular events is modified by high apolipoprotein B fraction levels. We performed a prospective study on 8286 nondiabetic participants (580 participants with cardiovascular disease; 4.9 years median follow-up time) with fasting lipids, apolipoprotein B, and urinary albumin excretion determined at baseline. With adjustment for sex and age, micro- and macroalbuminuria were associated with increased apolipoprotein B fractions (non-HDL cholesterol, LDL cholesterol, triglycerides, and apolipoprotein B). All four apolipoprotein B fractions modified associations of urinary albumin excretion with incident cardiovascular disease (hazard ratios for interaction terms ranged from 0.89 to 0.94 with 95% confidence intervals ranging from 0.84 to 0.99 and P values ranging from 0.001 to 0.02 by Cox proportional hazards modeling). These interactions remained present after additional adjustment for conventional risk factors, eGFR, cardiovascular history, and lipid-lowering and antihypertensive drug treatments. Such modification was also observed when urinary albumin excretion was stratified into normo-, micro-, and macroalbuminuria. We conclude that there is an association between elevated urinary albumin excretion and apolipoprotein B fraction levels and a negative interaction between these variables in their associations with incident cardiovascular events. Elevated urinary albumin excretion may share common causal pathways with high apolipoprotein B fractions in the pathogenesis of cardiovascular disease.
Keywords: albuminuria, lipids, cardiovascular disease, microalbuminuria
Robust associations of both microalbuminuria and macroalbuminuria with cardiovascular disease (CVD) are well established.1–5 Elevated plasma levels of atherogenic apolipoprotein B lipoproteins (apoB fractions; i.e., LDL cholesterol [LDL-C], very LDL-C [collectively designated non-HDL], triglycerides, and apoB) are inherent features of overt proteinuria.6–10 Interestingly, increased apoB fractions have been well established in microalbuminuric participants with types 1 and 2 diabetes mellitus11–13 as well as participants with impaired fasting glucose14 and obesity.15 Less is known on the association between moderately elevated urinary albumin excretion (UAE) and abnormalities in apoB fractions in unselected populations. Equivocal associations of elevated UAE with higher total cholesterol and LDL-C, higher triglycerides, and higher numbers of small-sized LDL particles have been observed in population-based cohorts.16–18 Thus, the extent to which elevated UAE is associated with consistent changes in various apoB fractions is still unclear.
If higher plasma apoB fraction levels were present in nondiabetic participants with elevated UAE, it would be clinically relevant to test whether the strength of the relationship of elevated UAE with cardiovascular (CV) risk is modified by concurrently high plasma levels of apoB fractions. Previous studies have already shown that the association of elevated UAE with incident CVD remains present, even when taking into account plasma total cholesterol.3,19–21 Nonetheless, it has not yet been explored whether high plasma apoB fractions modify the association of elevated UAE with incident CVD. If such associations were to be attenuated in the context of high apoB fraction levels, then it would be consistent with the hypothesis that elevated UAE and increased apoB fraction levels share pathogenic mechanisms that may contribute to the development of clinically manifest atherosclerotic CVD.22
In view of the ongoing debate as to whether a common causal pathway can be identified that underlies the relationship of microalbuminuria with atherosclerotic CVD,1 we decided to first test the associations between UAE and various apoB fractions in nondiabetic participants. Then, we examined the extent to which the relationship of incident CVD with elevated UAE is modified by high plasma apoB fraction levels. To this end, we conducted an investigation on participants of the Prevention of Renal and Vascular Endstage Disease (PREVEND) study.
Results
Table 1 gives a summary of clinical and laboratory parameters for study participants stratified according to UAE as follows: normoalbuminuria (<30 mg/24 h), microalbuminuria (30–300 mg/24 h), and macroalbuminuria (>300 mg/24 h). Unadjusted ANOVA and chi-squared testing results showed significant differences among the UAE groups for all parameters in Table 1. Most parameters traditionally associated with CVD risk tended to be increased in the higher UAE groups, namely men, age, body mass index, systolic and diastolic BPs, creatinine, glucose, C-reactive protein (CRP), total cholesterol, non-HDL cholesterol (non–HDL-C), LDL-C, triglycerides, apoB, total cholesterol:HDL-C ratio, and apoB:apoA-I ratio, whereas eGFR, HDL-C, and apoA-I were found to be lower. Results of statistical comparisons between groups revealed generally little difference in parameters for the microalbuminuric and macroalbuminuric groups, whereas these parameters in both groups differed compared with the normoalbuminuric group. With adjustment for sex and age, all lipid and lipoprotein markers remained significantly associated with the UAE groups, except for apoA-I (Table 1); with additional adjustment for eGFR, all lipid and lipoprotein markers remained significantly associated with UAE, except for apoA-I and LDL-C, although the association for LDL-C approached statistical significance (Table 1).
Table 1.
Clinical covariates and biomarker levels as mean±SD or median (interquartile range) in the total study population stratified according to 24-hour UAE as normoalbuminuria (<30 mg/24 h), microalbuminuria (between 30 and 300 mg/24 h), and macroalbuminuria (>300 mg/24 h)
| Parameter | Total Population | Normoalbuminuria (86.1% of Subjects) | Microalbuminuria | Macroalbuminuria | P Valuea | P Valueb | P Valuec |
|---|---|---|---|---|---|---|---|
| N | 8286 | 7137 | 1036 | 113 | |||
| Women, % | 50.4 | 52.6 | 36.5 | 34.5 | <0.001 (chi square) | ||
| Cardiac history, %d | 4.2 | 3.2 | 10.5 | 9.7 | <0.001 (chi square) | ||
| Statins, % | 4.6 | 3.7 | 9.5 | 12.5 | <0.001 (chi square) | ||
| Antihypertensives, % | 15.7 | 13.5 | 27.3 | 40.9 | <0.01 (chi square) | ||
| Smoking status, % | |||||||
| Never | 29.5 | 31.0 | 21.1 | 15.3 | <0.001 (chi square) | ||
| Former | 36.2 | 35.1 | 41.4 | 55.9 | |||
| Current | 34.3 | 33.9 | 37.5 | 28.8 | |||
| Age, yr | 48.9±12.6 | 47.8±12.3 | 55.5±12.6 | 56.9±12.9 | <0.001 | ||
| Body mass index, kg/m2 | 26.00±4.17 | 25.72±4.01 | 27.61±4.69 | 28.62±4.47 | <0.001 | <0.001 | <0.001 |
| Systolic BP, mmHg | 128±20 | 126±18 | 142±23 | 150±25 | <0.001 | <0.001 | <0.001 |
| Diastolic BP, mmHg | 74±10 | 73±9 | 80±11 | 82±11 | <0.001 | <0.001 | <0.001 |
| UAE, mg/24 h | 9.25 (6.27–16.9) | 8.21 (5.96–12.4) | 54.6 (38.6–90.4) | 551 (379–1077) | <0.001 | <0.001 | <0.001 |
| Creatinine, μmol/L | 83.6±14.5 | 82.6±13.5 | 89.8±26.0 | 113.4±96.2 | <0.001 | <0.001 | <0.001 |
| eGFR, ml/min per 1.73 m2 | 84.0±15.3 | 85.1±14.8 | 78.3±16.9 | 67.9±21.8 | <0.001 | <0.001 | <0.001 |
| Glucose, mmol/L | 4.74±0.66 | 4.70±0.63 | 4.99±0.75 | 4.99±0.78 | <0.001 | <0.001 | <0.001 |
| CRP, mg/L | 1.25 (0.55–2.87) | 1.13 (0.50–2.64) | 2.17 (1.00–4.43) | 2.48 (1.20–5.22) | <0.001 | <0.001 | <0.001 |
| Cholesterol, mmol/L | 5.63±1.12 | 5.60±1.12 | 5.85±1.12 | 6.13±1.35 | <0.001 | 0.003 | <0.01 |
| Non–HDL-C, mmol/L | 4.30±1.21 | 4.24±1.19 | 4.63±1.20 | 4.95±1.39 | <0.001 | <0.001 | <0.001 |
| LDL-C, mmol/L | 3.67±1.05 | 3.64±1.04 | 3.89±1.05 | 4.10±1.13 | <0.001 | 0.04 | 0.08 |
| HDL-C, mmol/L | 1.33±0.40 | 1.35±0.40 | 1.22±0.38 | 1.16±0.31 | <0.001 | <0.001 | <0.001 |
| Triglycerides, mmol/L | 1.14 (0.84–1.66) | 1.11 (0.82–1.60) | 1.38 (0.98–2.07) | 1.46 (1.08–2.17) | <0.001 | <0.001 | <0.001 |
| ApoB, g/L | 1.03±0.30 | 1.02±0.30 | 1.11±0.32 | 1.18±0.32 | <0.001 | <0.001 | <0.001 |
| ApoA-I, g/L | 1.39±0.29 | 1.40±0.29 | 1.36±0.29 | 1.35±0.28 | <0.001 | 0.32 | 0.28 |
| Cholesterol/HDL-C | 4.65±1.82 | 4.54±1.77 | 5.27±1.99 | 5.66±2.04 | <0.001 | <0.001 | <0.001 |
| ApoB/ApoA-I | 0.77±0.27 | 0.76±0.26 | 0.85±0.29 | 0.89±0.28 | <0.001 | <0.001 | <0.001 |
Chi-squared test or unadjusted ANOVA for comparisons of continuous variables in the microalbuminuric versus the normoalbuminuric group and the macroalbuminuric versus the normoalbuminuric group revealed statistically significant differences (P<0.001) for all parameters except for apoA-I in the macroalbuminuric versus normoalbuminuric comparison (P=0.09). For the macroalbuminuric versus microalbuminuric comparison, significant differences resulted for smoking score (P=0.01), systolic BP (P<0.001), diastolic BP (P<0.01), UAE (P<0.001), creatinine (P<0.001), eGFR (P<0.001), cholesterol (P=0.01), and non–HDL-C (P<0.01).
ANOVA adjusted for sex and age.
ANOVA adjusted for sex, age, and eGFR.
Cardiac history included hospitalization for myocardial infarction, revascularization procedures, or obstructive coronary artery disease.
Risk Associations and Interactions of UAE with Lipids and Lipoproteins
There were 580 participants with CV events in the study population (n=8286) identified with a median follow-up time of 4.9 (interquartile range=2.2–7.5) years. Of these events, 417, 143, and 20 events occurred in the normo-, micro-, and macroalbuminuric groups (corresponding to event rates of 5.84%, 13.80%, and 17.70%, respectively). To examine potential associations of UAE and lipids with CVD risk, Cox multivariable proportional hazards models adjusted for sex and age were formulated with risk as a function of UAE and the individual lipid and lipoprotein parameters shown in Table 1. Results (Table 2) showed significant risk associations for all lipid and lipoprotein parameters (direct associations for cholesterol, non–HDL-C, LDL-C, triglycerides, apoB, cholesterol:HDL-C ratio, and apoB:apoA-I ratio and inverse association for HDL-C and apoA-I).
Table 2.
Interaction of UAE with lipid parameters as assessed by Cox proportional hazards models adjusted for sex and age
| Parameters | Model without Interaction | Model with interaction | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| UAE | 1.19 | 1.12 to 1.28 | <0.001 | 1.25 | 1.17 to 1.35 | <0.001 |
| Cholesterol | 1.27 | 1.17 to 1.37 | <0.001 | 1.33 | 1.23 to 1.44 | <0.001 |
| Interaction | 0.92 | 0.87 to 0.97 | 0.002 | |||
| UAE | 1.17 | 1.10 to 1.25 | <0.001 | 1.25 | 1.16 to 1.35 | <0.001 |
| Non–HDL-C | 1.37 | 1.27 to 1.48 | <0.001 | 1.43 | 1.32 to 1.54 | <0.001 |
| Interaction | 0.92 | 0.87 to 0.97 | 0.001 | |||
| UAE | 1.20 | 1.12 to 1.28 | <0.001 | 1.25 | 1.16 to 1.35 | <0.001 |
| LDL-C | 1.30 | 1.20 to 1.41 | <0.001 | 1.36 | 1.25 to 1.49 | <0.001 |
| Interaction | 0.94 | 0.87 to 0.99 | 0.02 | |||
| UAE | 1.17 | 1.10 to 1.25 | <0.001 | 1.25 | 1.16 to 1.34 | <0.001 |
| Triglycerides | 1.28 | 1.17 to 1.39 | <0.001 | 1.37 | 1.25 to 1.49 | <0.001 |
| Interaction | 0.89 | 0.84 to 0.95 | 0.001 | |||
| UAE | 1.20 | 1.12 to 1.28 | <0.001 | 1.25 | 1.16 to 1.35 | <0.001 |
| ApoB | 1.27 | 1.18 to 1.37 | <0.001 | 1.30 | 1.21 to 1.40 | <0.001 |
| Interaction | 0.93 | 0.88 to 0.98 | 0.01 | |||
| UAE | 1.18 | 1.10 to 1.26 | <0.001 | 1.22 | 1.13 to 1.32 | <0.001 |
| HDL-C | 0.71 | 0.63 to 0.79 | <0.001 | 0.69 | 0.61 to 0.77 | <0.001 |
| Interaction | 1.07 | 0.98 to 1.16 | 0.12 | |||
| UAE | 1.21 | 1.13 to 1.29 | <0.001 | 1.21 | 1.12 to 1.30 | <0.001 |
| ApoA-I | 0.76 | 0.70 to 0.84 | <0.001 | 0.76 | 0.69 to 0.84 | <0.001 |
| Interaction | 1.00 | 0.94 to 1.07 | 0.92 | |||
| UAE | 1.19 | 1.11 to 1.26 | <0.001 | 1.17 | 1.08 to 1.26 | <0.001 |
| Cholesterol/HDL-C | 1.27 | 1.22 to 1.32 | <0.001 | 1.28 | 1.22 to 1.34 | <0.001 |
| Interaction | 1.01 | 0.98 to 1.05 | 0.46 | |||
| UAE | 1.19 | 1.11 to 1.28 | <0.001 | 1.21 | 1.12 to 1.31 | <0.001 |
| ApoB/ApoA-I | 1.40 | 1.31 to 1.50 | <0.001 | 1.40 | 1.31 to 1.50 | <0.001 |
| Interaction | 0.98 | 0.93 to 1.03 | 0.41 | |||
Interactions between UAE and the lipid and lipoprotein parameters were also examined by inclusion of corresponding interactions terms into the sex- and age-adjusted models. Results showed significant negative interactions (hazard ratio [HR], <1.00) for the apoB-associated fractions (cholesterol, non–HDL-C, LDL-C, triglycerides, and apoB), which is also shown in Table 2. Interactions were nonsignificant for HDL-C and apoA-I as well as the cholesterol:HDL-C ratio and the apoB:apoA-I ratio.
Interaction of UAE with ApoB Fractions
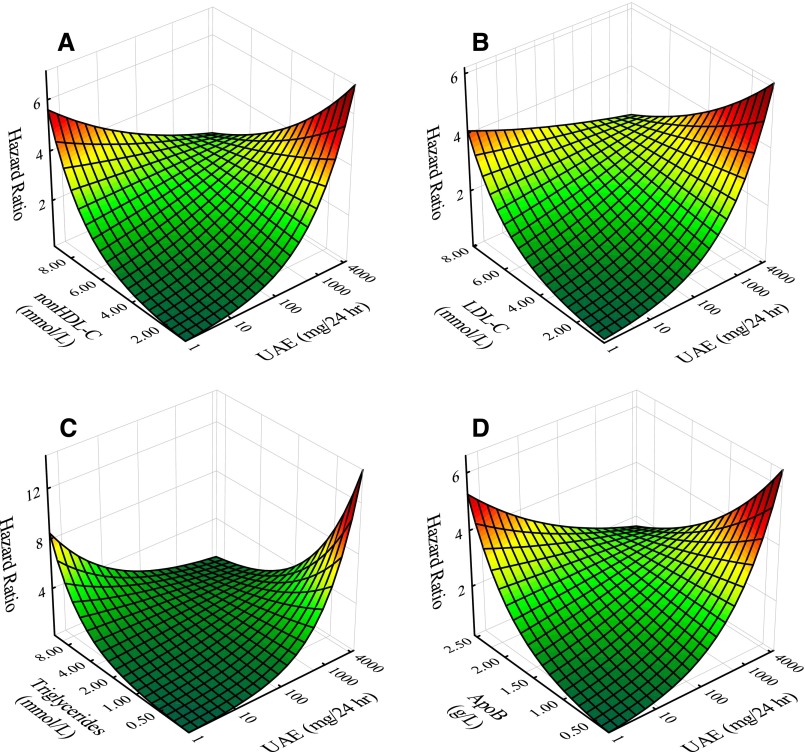
To further investigate resultant negative interactions of UAE and apoB-associated fractions on risk, individual Cox models as functions of cholesterol, non–HDL-C, LDL-C, triglyceride, and apoB levels as continuous variables were developed with the addition of adjustments beyond sex and age, including CV history, statin use, anti-hypertensive use, smoking, systolic BP, eGFR, and glucose. As a representation of apoA-I–containing particles, corresponding apoA-I modeling was also generated for comparison with the apoB parameters. For each of the apoB fractions (Table 3), significant negative interaction with UAE was maintained. Again, for apoA-I, the interaction term was nonsignificant. Negative interactions between UAE and representative apoB fractions (non–HDL-C, LDL-C, triglycerides, and apoB) are illustrated graphically in Figure 1. Figure 1 presents HR surfaces generated analytically from the corresponding Cox regression equations including interaction terms.
Table 3.
Interaction of UAE with apoB-associated parameters and apoA-I as assessed by Cox proportional hazards models adjusted for sex, age, CV history, statin use, anti-HTN use, smoking, systolic BP, eGFR, and glucose
| Parameters | Model with Interaction | ||
|---|---|---|---|
| HR | 95% CI | P Value | |
| UAE | 1.10 | 1.00 to 1.19 | 0.05 |
| Cholesterol | 1.40 | 1.28 to 1.54 | <0.001 |
| Interaction | 0.92 | 0.86 to 0.98 | <0.01 |
| UAE | 1.10 | 1.00 to 1.20 | 0.05 |
| Non–HDL-C | 1.49 | 1.35 to 1.63 | <0.001 |
| Interaction | 0.91 | 0.86 to 0.97 | <0.01 |
| UAE | 1.07 | 0.98 to 1.17 | 0.13 |
| LDL-C | 1.40 | 1.27 to 1.54 | <0.001 |
| Interaction | 0.96 | 0.89 to 1.01 | 0.10 |
| UAE | 1.11 | 1.01 to 1.21 | 0.03 |
| Triglycerides | 1.25 | 1.13 to 1.39 | <0.001 |
| Interaction | 0.91 | 0.84 to 0.98 | 0.01 |
| UAE | 1.07 | 0.98 to 1.17 | 0.14 |
| ApoB | 1.28 | 1.18 to 1.39 | <0.001 |
| Interaction | 0.94 | 0.88 to 1.00 | 0.06 |
| UAE | 1.02 | 0.94 to 1.12 | 0.61 |
| ApoA-I | 0.80 | 0.72 to 0.90 | <0.001 |
| Interaction | 0.96 | 0.88 to 1.04 | 0.30 |
HTN, hypertensive.
Figure 1.
Plots illustrating negative interaction effects in terms of continuous variables (Table 2) of UAE with apoB fractions (non–HDL-C, LDL-C, triglycerides, and apoB). Plots give HRs for CV events adjusted for sex and age as a function of UAE and apoB fractions as follows: (A) non–HDL-C, (B) LDL-C, (C) triglycerides, and (D) apoB. HRs were derived analytically using Cox modeling results including UAE, apoB fraction, and the respective interaction term.
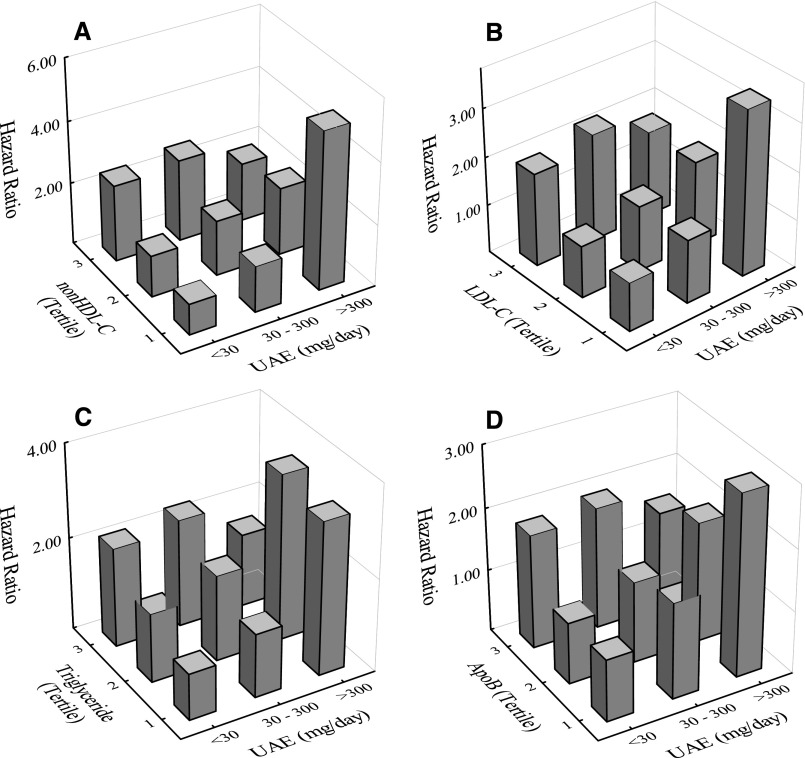
We next examined whether negative interactions of UAE with apoB fractions on risk would also be manifested in Cox models using stratified variables: UAE (<30, 30–300, and >300 mg/d) and apoB fractions (tertiles). In each case, participants with UAE<30 mg/d and lowest tertile of the respective representative apoB fraction served as reference groups. Table 4 gives corresponding HRs, 95% confidence intervals (95% CIs), P values, and numbers of participants in each UAE category. Results again showed relatively lower sex- and age-adjusted risk for participants with the highest levels of UAE and apoB fraction. Negative interactions of UAE with the apoB fractions for the stratified variable models are illustrated in the bar plots in Figure 2. For comparison, corresponding results for apoA-I are given in Supplemental Figure 1 and Supplemental Table 1.
Table 4.
HRs, 95% CIs, P values, and N values from Cox proportional hazards modeling of time to event as a function of stratified UAE (<30, 30–300, and >300 mg/d) and tertiles of apoB fractions (non–HDL-C, LDL-C, triglycerides, and apoB) adjusted for sex and age
| Model | UAE (<30 mg/d) | UAE (30–300 mg/d) | UAE (>300 mg/d) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | N | HR | 95% CI | P Value | N | HR | 95% CI | P Value | N | |
| Non–HDL-C | ||||||||||||
| Tertile 1 | 1.00 | — | — | 2429 | 1.48 | 0.86 to 2.52 | 0.15 | 235 | 5.09 | 1.82 to 14.25 | 0.002 | 18 |
| Tertile 2 | 1.34 | 0.96 to 1.86 | 0.08 | 2300 | 1.79 | 1.15 to 2.79 | 0.01 | 344 | 2.19 | 0.97 to 4.95 | 0.06 | 39 |
| Tertile 3 | 2.47 | 1.82 to 3.37 | <0.001 | 2191 | 2.65 | 1.81 to 3.89 | <0.001 | 437 | 1.90 | 0.91 to 3.97 | 0.09 | 54 |
| LDL-C | ||||||||||||
| Tertile 1 | 1.00 | — | — | 2376 | 1.32 | 0.81 to 2.17 | 0.27 | 253 | 3.43 | 1.24 to 9.50 | 0.02 | 20 |
| Tertile 2 | 1.08 | 0.79 to 1.49 | 0.62 | 2277 | 1.36 | 0.88 to 2.09 | 0.16 | 336 | 1.76 | 0.78 to 3.94 | 0.17 | 36 |
| Tertile 3 | 1.93 | 1.44 to 2.59 | <0.001 | 2199 | 2.22 | 1.53 to 3.22 | <0.001 | 402 | 1.77 | 0.86 to 3.66 | 0.12 | 50 |
| Triglycerides | ||||||||||||
| Tertile 1 | 1.00 | — | — | 2447 | 1.37 | 0.81 to 2.31 | 0.23 | 228 | 3.28 | 1.19 to 9.06 | 0.02 | 16 |
| Tertile 2 | 1.47 | 1.10 to 1.96 | 0.01 | 2323 | 1.84 | 1.24 to 2.75 | 0.003 | 316 | 3.56 | 1.63 to 7.78 | 0.001 | 39 |
| Tertile 3 | 2.12 | 1.61 to 2.78 | <0.001 | 2156 | 2.31 | 1.64 to 3.25 | <0.001 | 472 | 1.58 | 0.77 to 3.22 | 0.21 | 56 |
| ApoB | ||||||||||||
| Tertile 1 | 1.00 | — | — | 2349 | 1.54 | 0.94 to 2.52 | 0.09 | 226 | 2.92 | 1.04 to 8.15 | 0.04 | 18 |
| Tertile 2 | 0.99 | 0.73 to 1.36 | 0.97 | 2217 | 1.29 | 0.85 to 1.98 | 0.23 | 337 | 1.93 | 0.83 to 4.51 | 0.13 | 34 |
| Tertile 3 | 1.83 | 1.37 to 2.43 | <0.001 | 2125 | 1.95 | 1.34 to 2.83 | <0.001 | 411 | 1.57 | 0.78 to 3.15 | 0.20 | 57 |
Reference group taken as UAE<30 mg/d and lowest tertile of respective apoB fraction.
Figure 2.
Plots showing negative interaction effects in terms of stratified variables (Table 3) of UAE with apoB fractions (non–HDL-C, LDL-C, triglycerides, and apoB). Plots give HRs for CV events adjusted for sex and age as a function of UAE (<30, 30–300, and >300 mg/d) and tertiles of apoB fractions as follows: (A) non–HDL-C, (B) LDL-C, (C) triglyceride, and (D) apoB. The reference group in each case was taken as UAE<30 mg/d and the lowest tertile of respective apoB fraction.
Mediation Analyses
Mediation analyses were performed to elucidate potential mechanistic effects of apoB fractions on the association of UAE with CVD outcomes. Thus, in Cox models of incident CVD as a function of UAE, changes in the coefficient of UAE (continuous) were estimated for models adjusted for apoB fractions separately. In a sex-, age-, and eGFR-adjusted model, adjustments for non–HDL-C, LDL-C, triglycerides, and apoB resulted (Table 5) in significant reductions in the coefficient of the UAE-incident CVD association of 18% (P=0.002), 9% (P=0.02), 17% (P<0.01), and 8% (P=0.001), respectively.
Table 5.
Mediation analysis showing changes in the coefficient relating the association of UAE with incident CVD risk in a Cox proportional hazards model adjusted for sex, age, and eGFR after separate entry of apoB fractions into the models
| Mediation Modeling | Coefficient | P Value | Percent Coefficient Reduction | P Value |
|---|---|---|---|---|
| Model 1a | 0.18 (0.11–0.25) | <0.001 | NA | |
| Model 1+non–HDL-C | 0.15 (0.08–0.21) | <0.001 | 18 (7–30) | 0.002 |
| Model 1+LDL-C | 0.16 (0.10–0.23) | <0.001 | 9 (2–16) | 0.02 |
| Model 1+triglycerides | 0.15 (0.08–0.22) | <0.001 | 17 (5–29) | <0.01 |
| Model 1+apoB | 0.16 (0.10–0.23) | <0.001 | 8 (2–15) | 0.01 |
NA, not applicable.
Model 1 is a base model consisting of Cox proportional hazards regression with time to incident CVD event as a function of UAE (continuous) with adjustment for sex, age, and eGFR.
Sensitivity Analyses
Sensitivity analyses were performed to assess the robustness of our findings. We addressed whether the enrichment of the PREVEND study with participants with higher UAE affected the findings of negative interactions of UAE with apoB fractions. Similar Cox analyses were, therefore, performed on a subsample (n=3338) of the PREVEND cohort representative of the general population23 that was generated by removal of the microalbuminuric participants who were initially added for enrichment to the PREVEND cohort of this study. Essentially similar results were obtained with models including UAE and apoB fractions adjusted for sex and age, showing continued significance of HRs: UAE (P=0.002) with non–HDL-C (P<0.001), UAE (P=0.002) with LDL-C (P<0.001), UAE (P=0.002) with triglycerides (P<0.001), and UAE (P<0.01) with apoB (P<0.001). Inclusion of respective interaction terms between UAE and apoB fractions to base models showed comparable negative interaction terms with the respective apoB fractions, giving results for the interaction term with UAE as follows: non–HDL-C (HR, 0.89; 95% CI, 0.81 to 0.98; P=0.02), LDL-C (HR, 0.83; 95% CI, 0.73 to 0.95; P<0.01), triglycerides (HR, 0.90; 95% CI, 0.79 to 1.02; P=0.11), and apoB (HR, 0.79; 95% CI, 0.70 to 0.90; P<0.001).
Discussion
This study shows that elevated UAE is associated with four measures of plasma apoB fractions (non–HDL-C, LDL-C, triglycerides, and apoB) in analyses in which sex and age were taken into account. Although both UAE and each of these apoB fractions predicted CV outcome in sex- and age-adjusted analysis, we were also able to show that these apoB fractions modified the extent to which elevated UAE predicts incident CVD. In particular, there were negative interactions of each of these apoB fractions with elevated UAE on CV outcome, irrespective of elevated UAE being expressed as a continuous variable or stratified into normo-, micro-, and macroalbuminuria. Such effect modification by these apoB fractions on incident CVD remained present after additional adjustment for individual conventional CVD risk factors (smoking, systolic BP, and glucose), kidney function (eGFR and serum creatinine), a positive CVD history, and lipid-lowering and antihypertensive drug treatment. If elevated UAE and apoB fractions were totally unrelated factors that affect incident CVD, then it would be anticipated that there were no interactions on CV outcome.22 Additionally, mediation analysis also indicated that part of the association of elevated UAE with incident CVD was attributable to apoB fractions as mediating factors. We interpret these results to be consistent with our hypothesis that elevated UAE and increased apoB fraction levels share, to some degree, causal pathways that are involved in the pathogenesis of atherosclerotic CVD.
This population-based study revealed that elevated UAE confers consistent proatherogenic changes in plasma lipids and (apo)lipoproteins, including higher total cholesterol, LDL-C, non–HDL-C, triglycerides, and apoB. Remarkably, higher apoB fraction levels were already evident in conjunction with microalbuminuria, with only minor additional changes being observed between micro- and macroalbuminuria. Previous population-based studies variably showed slightly higher total cholesterol, LDL-C, and triglycerides.16–18 We believe that the currently observed increases in the various apoB fractions with elevated UAE in nondiabetic participants emphasize the pathophysiologic relevance of elevated UAE per se for alterations in the metabolism of apoB lipoproteins. Our observations, therefore, raise the possibility that abnormalities in the metabolism of apoB lipoproteins, such as increased LDL production, impaired LDL clearance, or decreased very LDL catabolism, which are all well documented in overt proteinuria,7–9,24–27 may already be operative in conjunction with moderately elevated UAE.
Of potential interest, evidence has accumulated in support of the possibility that elevated UAE does not only represent an early sign of renal disease but also that it should be regarded as a marker of generalized endothelial damage.1,28,29 Thus, it is plausible to propose that apoB-containing lipoproteins are more readily retained in the arterial wall in the context of a defective endothelial barrier,11,29 which was more recently evidenced by a diminished glycocalyx barrier.30,31 It is, furthermore, likely that atherosclerotic vascular diseases are also featured by enhanced tissue accumulation of advanced glycation end products (AGEs),32–34 a process that includes glycation of apoB lipoproteins.35 AGE accumulation in tissue may further aggravate vascular leakiness and precipitate tissue damage.36 Of additional note, enhanced tissue accumulation of AGE, at least in diabetic subjects, is dependent on the degree of albuminuria.37 Elevated UAE and increased apoB fractions could, therefore, act in concert in promoting the development of atherosclerosis.
Our choice for selecting non–HDL-C, LDL-C, triglycerides, and apoB as representative apoB fractions related to elevated UAE was driven by the statistically significant differences in these apoB fractions according to UAE groups after adjustment for sex and age. The relationship of elevated UAE with non–HDL-C, triglycerides, and apoB remained significant after additional adjustment for eGFR, the latter being an important covariate, because elevated UAE coincides with changes in kidney function3,21 and mildly impaired kidney function is associated with atherogenic changes in apoB lipoproteins.38 Although the association of LDL-C with elevated UAE did not reach formal statistical significance after additional adjustment for eGFR, all four fractions as well as total cholesterol were found to interact negatively with elevated UAE on incident CVD. From a clinical perspective, it is relevant that non–HDL-C and apoB levels are considered to be at least as good as LDL-C in CV risk prediction.19,39–43
Several other methodological aspects and limitations of our study need to be considered. First, the PREVEND cohort is enriched with participants with microalbuminuria.44 However, relevant bias caused by overrepresentation of participants with higher albuminuria is very unlikely, because a sensitivity analysis on a subsample of participants representative of the general population yielded similar point estimates of the negative interactions between UAE and the four apoB fractions. Second, reliable information regarding the use of lipid-lowering and antihypertensive medication was available at baseline but not during follow-up. Because changes in the relationship of UAE with apoB fractions in response to antihypertensive treatment cannot be excluded, they may have resulted in some underestimation in the effect modifying of apoB fractions on UAE-related CV risk.45 Additionally, it can be envisaged that statin treatment initiated during follow-up would weaken the relationship of incident CVD with LDL-C, because statin administration results in a shift in the relationship of LDL-C with apoB to lower LDL-C for a given apoB concentration.46,47 Third, although our study was focused on the association of apoB lipoproteins with UAE, additional analyses showed that neither HDL-C nor apoA-I were found to modify the relationship of incident CVD with elevated UAE.
In conclusion, this study shows that, in nondiabetic participants, elevated UAE is consistently associated with higher levels of non–HDL-C, LDL-C, triglycerides, and apoB taking into account sex and age. Furthermore, we showed that the increased CVD risk attributable to elevated UAE is attenuated in the context of concurrently high apoB fraction levels. It is, therefore, conceivable that elevated UAE and increased apoB fractions share, at some level, common pathophysiologic mechanisms that contribute to the pathogenesis of atherosclerotic CVD.
Concise Methods
Study Population
PREVEND is a large, general population-based prospective cohort study that was started in 1997 with the goal of investigating relationships of increased albuminuria with CV and renal disease. A complete description of the PREVEND study protocol has been presented previously.44 In summary, a one-page questionnaire was sent by mail to all inhabitants of the city of Groningen, The Netherlands, ages 28–75 years old, of whom 48% responded (n=40,856). Exclusions included pregnant women and type 1 diabetic individuals. Enrichment of the cohort with microalbuminuric participants was achieved by inviting participation of all individuals having urinary albumin concentration≥10 mg/L (6000 of 7768 participated). In addition, a random sample of participants with urinary albumin concentration<10 mg/L was also invited (2592 of 3395 participated), resulting in a total of 8592 participants from the PREVEND cohort. The study population of this work (n=8286) was derived by exclusion of type 2 diabetic participants (defined by use of glucose-lowering drugs according to questionnaires or pharmacy data or an increased glucose concentration). The PREVEND study was approved by the Medical Ethics Committee of the University of Groningen. Informed written consent was obtained from all participants. The study adhered to the ethical principles set by the Declaration of Helsinki.
Measurements and Definitions
UAE was determined at baseline on entry into the study as the mean value of two 24-hour urine collections taken over 2 consecutive days. Urinary albumin concentration was measured by nephelometry (BNII; Dade Behring, Marburg, Germany), with lower limit of sensitivity of 2.3 mg/L and intra- and interassay coefficients of variation of 2.2% and 2.6%, respectively. UAE blood biomarker analyses were performed on plasma and serum venous samples from participants fasted overnight, with collection after 15 minutes of rest. Levels of creatinine, glucose, CRP, total cholesterol, HDL-C, triglycerides, apoB, and apoA-I were determined by standard techniques as described previously.48,49 Interassay coefficients of variation for apoB and apoA-I analyses were <5% in each case. eGFR was determined using the serum creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation.50 Non–HDL-C was calculated as the difference between total cholesterol and HDL-C. LDL-C levels were estimated using the Friedewald equation.
Smoking status was assessed using a trilevel variable (never been a smoker, past smoker, and current smoker). Body mass index was calculated as weight divided by height squared (kilograms per meter2). BP was measured for 10 minutes at 1-minute intervals in the supine position using automatic instrumentation (Dinamap XL Model 9300; Johnson-Johnson Medical, Tampa, FL); reported values were the means of the last two recordings.51 Initial exclusion of type 2 diabetic participants was on the basis of subject medical history, use of glucose-lowering drugs, and/or fasting plasma glucose level>7.0 mmol/L (according to the 1997 American Diabetes Association criteria). Information on medication use was checked by the use of pharmacy-dispensing data from all community pharmacies in the city of Groningen, which covers complete information on drug use in 95% of PREVEND participants.19
Outcome
History of previous CVD (hospitalization for myocardial infarction, revascularization procedures, or obstructive coronary artery disease) was acquired from questionnaires. Incident outcome events included CV-related mortality and hospitalization. Outcome events (International Classification of Diseases [ICD], ninth revision and classification of interventions) were followed over time and included acute myocardial infarction (ICD-410), acute and subacute ischemic heart disease (ICD-411), coronary artery bypass grafting, and percutaneous transluminal coronary angioplasty. Hospitalizations for heart failure and stroke were not counted as outcome events, because it is known that, in general, these events show no or only weak associations with lipid parameters.41,52–56 Survival time was defined as date of initial urine collection (1997–1998) to date of first CV event or December 31, 2008. If a person was lost to follow-up (396 of the overall cohort of 8592 participants), censoring date was date of removal from the municipal registry. Mortality data and causes of death were acquired by record linkage with the Dutch Central Bureau of Statistics. CV morbidity data were obtained from PRISMANT, the Dutch national registry of hospital discharge diagnoses.
Data Analyses
Statistica 10.0 (StatSoft, Inc., Tulsa, OK) was used for statistical and graphical analyses. Results are reported as means±SDs for normally distributed variables and medians (interquartile ranges) for non-normal variables (UAE, triglycerides, and CRP). ANOVA was used to assess differences among groups with Bonferroni correction for multiple comparisons. Non-normal variables were conditioned for analysis by logarithmic transformation. Chi-squared testing was used to assess differences among groups for categorical variables. Multivariable Cox–proportional hazards modeling was used to follow CV outcomes over time, with continuous independent variables standardized by transformation to distributions with means=0 and SDs=1. HRs are per 1 SD unit. Interactions between risk variables were assessed by inclusion of multiplicative interaction terms into base models. The proportional hazard assumption was verified by evaluation of correlation between survival time and scaled Schoenfeld residuals.
Illustration of interactions of UAE with apoB fractions (non–HDL-C, LDL-C, triglycerides, and apoB) for continuous variable models was performed by analytically graphing Cox regression equations (including interaction terms) to give plots of HR as a function of UAE and apoB fraction. Using apoB as an example, it would involve evaluation of the expression exp(βUAE×UAE+βapoB×apoB+βinteract×UAE×apoB). Results are presented as three-dimensional plots, showing HR as a surface extending over clinically relevant domains of UAE and apoB fraction. Interactions were also illustrated for stratified variable models. Risk variables were stratified into three levels of UAE as 30, 30–300, and >300 mg/d and apoB fractions as tertiles. Interactions were shown by calculating HRs of the resulting nine cells using as reference the cell with the lowest UAE and the lowest tertile of apoB fraction (HR, 1.00). HRs as a function of UAE and apoB fractions were plotted as a three-dimensional bar graph.
Mediation analysis was performed to estimate the change in the coefficient of the UAE versus incident CVD association when adjusted for apoB fractions (i.e., what percentage of the association between UAE and CV events could be explained by apoB fractions as mediators).22,57 Sensitivity analyses were performed on a subsample of participants representative of the general population to assess the robustness of our findings regarding whether the enrichment of the PREVEND study with participants with higher UAE values affected findings of interactions between UAE and apoB fractions.
Two-sided P values <0.05 were considered statistically significant except for interaction terms, where a more conservative P value <0.10 was taken to indicate statistical significance as proposed by Selvin58 and advocated by the Food and Drug Administration.59
Disclosures
None.
Supplementary Material
Acknowledgments
The contributions of R.T. Gansevoort, S.J.L. Bakker, and R.P.F. Dullaart were made on behalf of the Prevention of Renal and Vascular Endstage Disease (PREVEND) study group, and we are indebted to all PREVEND collaborators. Dade Behring (Marburg, Germany) is acknowledged for supplying equipment (Behring Nephelometer II) to determine apolipoproteins and urinary albumin.
This work is supported by The Netherlands Heart Foundation Grant 2001.005.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013121256/-/DCSupplemental.
References
- 1.Stehouwer CDA, Smulders YM: Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J Am Soc Nephrol 17: 2106–2111, 2006 [DOI] [PubMed] [Google Scholar]
- 2.de Jong PE, Gansevoort RT, Bakker SJL: Macroalbuminuria and microalbuminuria: Do both predict renal and cardiovascular events with similar strength? J Nephrol 20: 375–380, 2007 [PubMed] [Google Scholar]
- 3.Smink PA, Lambers Heerspink HJ, Gansevoort RT, de Jong PE, Hillege HL, Bakker SJL, de Zeeuw D: Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: The PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 60: 804–811, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Hillege HL, Janssen WM, Bak AA, Diercks GF, Grobbee DE, Crijns HJ, Van Gilst WH, De Zeeuw D, De Jong PE, Prevend Study Group : Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 249: 519–526, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Ordoñez JD, Hiatt RA, Killebrew EJ, Fireman BH: The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int 44: 638–642, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Joven J, Villabona C, Vilella E, Masana L, Albertí R, Vallés M: Abnormalities of lipoprotein metabolism in patients with the nephrotic syndrome. N Engl J Med 323: 579–584, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Warwick GL, Caslake MJ, Boulton-Jones JM, Dagen M, Packard CJ, Shepherd J: Low-density lipoprotein metabolism in the nephrotic syndrome. Metabolism 39: 187–192, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Warwick GL, Packard CJ, Demant T, Bedford DK, Boulton-Jones JM, Shepherd J: Metabolism of apolipoprotein B-containing lipoproteins in subjects with nephrotic-range proteinuria. Kidney Int 40: 129–138, 1991 [DOI] [PubMed] [Google Scholar]
- 9.de Sain-van der Velden MG, Kaysen GA, Barrett HA, Stellaard F, Gadellaa MM, Voorbij HA, Reijngoud DJ, Rabelink TJ: Increased VLDL in nephrotic patients results from a decreased catabolism while increased LDL results from increased synthesis. Kidney Int 53: 994–1001, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Krikken JA, Waanders F, Dallinga-Thie GM, Dikkeschei LD, Vogt L, Navis GJ, Dullaart RP: Antiproteinuric therapy decreases LDL-cholesterol as well as HDL-cholesterol in non-diabetic proteinuric patients: Relationships with cholesteryl ester transfer protein mass and adiponectin. Expert Opin Ther Targets 13: 497–504, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Dullaart RP, Dikkeschei LD, Doorenbos H: Alterations in serum lipids and apolipoproteins in male type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia 32: 685–689, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Jones SL, Close CF, Mattock MB, Jarrett RJ, Keen H, Viberti GC: Plasma lipid and coagulation factor concentrations in insulin dependent diabetics with microalbuminuria. BMJ 298: 487–490, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegele RA, Harris SB, Zinman B, Hanley AJG, Connelly PW: Increased plasma apolipoprotein B-containing lipoproteins associated with increased urinary albumin within the microalbuminuria range in type 2 diabetes. Clin Biochem 32: 143–148, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Sung KC, Rhee EJ, Kim H, Park JB, Kim YK, Woo S, Wilson AM: An elevated apolipoprotein B/AI ratio is independently associated with microalbuminuria in male subjects with impaired fasting glucose. Nutr Metab Cardiovasc Dis 21: 610–616, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Basdevant A, Cassuto D, Gibault T, Raison J, Guy-Grand B: Microalbuminuria and body fat distribution in obese subjects. Int J Obes Relat Metab Disord 18: 806–811, 1994 [PubMed] [Google Scholar]
- 16.Shankar A, Klein R, Moss SE, Klein BEK, Wong TY: The relationship between albuminuria and hypercholesterolemia. J Nephrol 17: 658–665, 2004 [PubMed] [Google Scholar]
- 17.de Boer IH, Astor BC, Kramer H, Palmas W, Rudser K, Seliger SL, Shlipak MG, Siscovick DS, Tsai MY, Kestenbaum B: Mild elevations of urine albumin excretion are associated with atherogenic lipoprotein abnormalities in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 197: 407–414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung KC, Kim BJ, Ryu S: An association of a variety of cardiovascular risk factors with low grade albuminuria in Korean men. Atherosclerosis 196: 320–326, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Kappelle PJWH, Gansevoort RT, Hillege JL, Wolffenbuttel BHR, Dullaart RPF, PREVEND study group : Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein. J Intern Med 269: 232–242, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Fox CS, Matsushita K, Woodward M, Bilo HJG, Chalmers J, Heerspink HJL, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG, Chronic Kidney Disease Prognosis Consortium : Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 380: 1662–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR, Chronic Kidney Disease Prognosis Consortium : Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: A meta-analysis. BMJ 346: f324, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JS, Kaye J, Wright LK: Moderating and mediating effects in causal models. Issues Ment Health Nurs 22: 63–75, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Lambers Heerspink HJ, Brantsma AH, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort RT, PREVEND Study Group : Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol 168: 897–905, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Vaziri ND: Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int 63: 1964–1976, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Kronenberg F: Dyslipidemia and nephrotic syndrome: Recent advances. J Ren Nutr 15: 195–203, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Trevisan R, Dodesini AR, Lepore G: Lipids and renal disease. J Am Soc Nephrol 17[Suppl 2]: S145–S147, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kwakernaak AJ, Lambert G, Slagman MCJ, Waanders F, Laverman GD, Petrides F, Dikkeschei BD, Navis G, Dullaart RPF: Proprotein convertase subtilisin-kexin type 9 is elevated in proteinuric subjects: Relationship with lipoprotein response to antiproteinuric treatment. Atherosclerosis 226: 459–465, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A: Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 32: 219–226, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Tabas I, Williams KJ, Borén J: Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 116: 1832–1844, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JAE, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JBL, Kastelein JJP, Stroes ES, Vink H: Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 55: 1127–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Garsen M, Rops ALWMM, Rabelink TJ, Berden JHM, van der Vlag J: The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrol Dial Transplant 29: 49–55, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Lutgers HL, Graaff R, de Vries R, Smit AJ, Dullaart RP: Carotid artery intima media thickness associates with skin autofluoresence in non-diabetic subjects without clinically manifest cardiovascular disease. Eur J Clin Invest 40: 812–817, 2010 [DOI] [PubMed] [Google Scholar]
- 33.de Vos LC, Noordzij MJ, Mulder DJ, Smit AJ, Lutgers HL, Dullaart RPF, Kamphuisen PW, Zeebregts CJ, Lefrandt JD: Skin autofluorescence as a measure of advanced glycation end products deposition is elevated in peripheral artery disease. Arterioscler Thromb Vasc Biol 33: 131–138, 2013 [DOI] [PubMed] [Google Scholar]
- 34.de Vos LC, Mulder DJ, Smit AJ, Dullaart RPF, Kleefstra N, Lijfering WM, Kamphuisen PW, Zeebregts CJ, Lefrandt JD: Skin autofluorescence is associated with 5-year mortality and cardiovascular events in patients with peripheral artery disease. Arterioscler Thromb Vasc Biol 34: 933–938, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Soran H, Durrington PN: Susceptibility of LDL and its subfractions to glycation. Curr Opin Lipidol 22: 254–261, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Schmidt AM: Skin autofluorescence, 5-year mortality, and cardiovascular events in peripheral arterial disease: All that glitters is surely not gold. Arterioscler Thromb Vasc Biol 34: 697–699, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, Smit AJ: Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care 30: 107–112, 2007 [DOI] [PubMed] [Google Scholar]
- 38.de Boer IH, Astor BC, Kramer H, Palmas W, Seliger SL, Shlipak MG, Siscovick DS, Tsai MY, Kestenbaum B: Lipoprotein abnormalities associated with mild impairment of kidney function in the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol 3: 125–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL: Lipoprotein management in patients with cardiometabolic risk: Consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol 51: 1512–1524, 2008 [DOI] [PubMed] [Google Scholar]
- 40.van der Steeg WA, Boekholdt SM, Stein EA, El-Harchaoui K, Stroes ES, Sandhu MS, Wareham NJ, Jukema JW, Luben R, Zwinderman AH, Kastelein JJ, Khaw KT: Role of the apolipoprotein B-apolipoprotein A-I ratio in cardiovascular risk assessment: A case-control analysis in EPIC-Norfolk. Ann Intern Med 146: 640–648, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J, Emerging Risk Factors Collaboration : Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302: 1993–2000, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Angelantonio E, Gao P, Pennells L, Kaptoge S, Caslake M, Thompson A, Butterworth AS, Sarwar N, Wormser D, Saleheen D, Ballantyne CM, Psaty BM, Sundström J, Ridker PM, Nagel D, Gillum RF, Ford I, Ducimetiere P, Kiechl S, Koenig W, Dullaart RP, Assmann G, D’Agostino RB, Sr., Dagenais GR, Cooper JA, Kromhout D, Onat A, Tipping RW, Gómez-de-la-Cámara A, Rosengren A, Sutherland SE, Gallacher J, Fowkes FG, Casiglia E, Hofman A, Salomaa V, Barrett-Connor E, Clarke R, Brunner E, Jukema JW, Simons LA, Sandhu M, Wareham NJ, Khaw KT, Kauhanen J, Salonen JT, Howard WJ, Nordestgaard BG, Wood AM, Thompson SG, Boekholdt SM, Sattar N, Packard C, Gudnason V, Danesh J, Emerging Risk Factors Collaboration : Lipid-related markers and cardiovascular disease prediction. JAMA 307: 2499–2506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kappelle PJ, Gansevoort RT, Hillege HJ, Wolffenbuttel BH, Dullaart RP, PREVEND Study Group : Common variation in cholesteryl ester transfer protein: Relationship of first major adverse cardiovascular events with the apolipoprotein B/apolipoprotein A-I ratio and the total cholesterol/high-density lipoprotein cholesterol ratio. J Clin Lipidol 7: 56–64, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Pinto-Sietsma SJ, Janssen WM, Hillege HL, Navis G, De Zeeuw D, De Jong PE: Urinary albumin excretion is associated with renal functional abnormalities in a nondiabetic population. J Am Soc Nephrol 11: 1882–1888, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Schnack C, Hoffmann W, Hopmeier P, Schernthaner G: Renal and metabolic effects of 1-year treatment with ramipril or atenolol in NIDDM patients with microalbuminuria. Diabetologia 39: 1611–1616, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Kappelle PJ, Zwang L, Huisman MV, Banga JD, Sluiter WJ, Dallinga-Thie GM, Dullaart RP: Atorvastatin affects low density lipoprotein and non-high density lipoprotein cholesterol relations with apolipoprotein B in type 2 diabetes mellitus: Modification by triglycerides and cholesteryl ester transfer protein. Expert Opin Ther Targets 13: 743–751, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Kappelle PJ, Dallinga-Thie GM, Dullaart RP, Diabetes Atorvastatin Lipid Intervention (DALI) study group : Atorvastatin treatment lowers fasting remnant-like particle cholesterol and LDL subfraction cholesterol without affecting LDL size in type 2 diabetes mellitus: Relevance for non-HDL cholesterol and apolipoprotein B guideline targets. Biochim Biophys Acta 1801: 89–94, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Borggreve SE, Hillege HL, Wolffenbuttel BHR, de Jong PE, Zuurman MW, van der Steege G, van Tol A, Dullaart RPF, PREVEND Study Group : An increased coronary risk is paradoxically associated with common cholesteryl ester transfer protein gene variations that relate to higher high-density lipoprotein cholesterol: A population-based study. J Clin Endocrinol Metab 91: 3382–3388, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Corsetti JP, Gansevoort RT, Sparks CE, Dullaart RPF: Inflammation reduces HDL protection against primary cardiac risk. Eur J Clin Invest 40: 483–489, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Greeff A, Reggiori F, Shennan AH: Clinical assessment of the DINAMAP ProCare monitor in an adult population according to the British Hypertension Society Protocol. Blood Press Monit 12: 51–55, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Shahar E, Chambless LE, Rosamond WD, Boland LL, Ballantyne CM, McGovern PG, Sharrett AR, Atherosclerosis Risk in Communities Study : Plasma lipid profile and incident ischemic stroke: The Atherosclerosis Risk in Communities (ARIC) study. Stroke 34: 623–631, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Bhatia M, Rothwell PM: A systematic comparison of the quality and volume of published data available on novel risk factors for stroke versus coronary heart disease. Cerebrovasc Dis 20: 180–186, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Everett BM, Kurth T, Buring JE, Ridker PM: The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol 48: 2235–2242, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canouï-Poitrine F, Luc G, Bard JM, Ferrieres J, Yarnell J, Arveiler D, Morange P, Kee F, Evans A, Amouyel P, Ducimetiere P, Empana JP, PRIME Study Group : Relative contribution of lipids and apolipoproteins to incident coronary heart disease and ischemic stroke: The PRIME Study. Cerebrovasc Dis 30: 252–259, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, Armitage J, Collins R, Heart Protection Study Collaborative Group : Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation 125: 2469–2478, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Baron RM, Kenny DA: The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51: 1173–1182, 1986 [DOI] [PubMed] [Google Scholar]
- 58.Selvin S: Statistical Analysis of Epidemiological Data, New York, Oxford University Press, 1996 [Google Scholar]
- 59.Lu M, Lyden PD, Brott TG, Hamilton S, Broderick JP, Grotta JC: Beyond subgroup analysis: Improving the clinical interpretation of treatment effects in stroke research. J Neurosci Methods 143: 209–216, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.