Abstract
Ischemia-reperfusion (I/R) injury of the kidney is a major cause of AKI. MicroRNAs (miRs) are powerful regulators of various diseases. We investigated the role of apoptosis-associated miR-24 in renal I/R injury. miR-24 was upregulated in the kidney after I/R injury of mice and in patients after kidney transplantation. Cell-sorting experiments revealed a specific miR-24 enrichment in renal endothelial and tubular epithelial cells after I/R induction. In vitro, anoxia/hypoxia induced an enrichment of miR-24 in endothelial and tubular epithelial cells. Transient overexpression of miR-24 alone induced apoptosis and altered functional parameters in these cells, whereas silencing of miR-24 ameliorated apoptotic responses and rescued functional parameters in hypoxic conditions. miR-24 effects were mediated through regulation of H2A histone family, member X, and heme oxygenase 1, which were experimentally validated as direct miR-24 targets through luciferase reporter assays. In vitro, adenoviral overexpression of miR-24 targets lacking miR-24 binding sites along with miR-24 precursors rescued various functional parameters in endothelial and tubular epithelial cells. In vivo, silencing of miR-24 in mice before I/R injury resulted in a significant improvement in survival and kidney function, a reduction of apoptosis, improved histologic tubular epithelial injury, and less infiltration of inflammatory cells. miR-24 also regulated heme oxygenase 1 and H2A histone family, member X, in vivo. Overall, these results indicate miR-24 promotes renal ischemic injury by stimulating apoptosis in endothelial and tubular epithelial cell. Therefore, miR-24 inhibition may be a promising future therapeutic option in the treatment of patients with ischemic AKI.
Keywords: acute renal failure, apoptosis, endothelium, heme oxygenase, proximal tubule, ischemia-reperfusion
Ischemia-reperfusion (I/R) injury of the kidney is one of the primary causes of AKI. It is associated with severe morbidity and mortality and thus represents a major socioeconomic health problem.1 It is a consequence of a variety of different injurious insults in native kidneys (e.g., during cardiac surgery). Moreover, it is commonly associated with the transplantation procedure and thus is an unavoidable phenomenon in transplanted kidneys.2 During ischemic AKI, a transient drop in blood flow to the kidney is followed by a reperfusion period. Reperfusion itself, although vital to restoration of kidney function, is associated with substantial additional cellular injury.3 In the kidney blood flow to the outer medulla is disproportionately reduced with respect to the reduction in total blood flow.4 Thus, epithelial cell injury is most pronounced in the S3 segment of the proximal tubule located in the outer medulla and is particularly susceptible to hypoxia.4 Capillary rarefaction during this process is associated with chronic hypoxia, potentiating tubular injury and leading to tubulointerstitial fibrosis. Currently, a targeted therapy for this important clinical disorder is not available.
MicroRNAs (miRNAs) are under intense investigation as powerful regulators of various diseases with potential critical effect on disease initiation and/or progression, including kidney disease.5 MiRNAs represent small noncoding RNA transcripts with a length of approximately 22 nucleotides, which through post-transcriptional binding of the 3′-untranslated region (UTR) of mRNA targets lead to the repression of gene/protein expression and/or translational inhibition of protein synthesis.5 Intriguingly, a single miRNA may alter the expression of many target genes, thus influencing a specific abnormality by regulating whole disease–specific pathways and signaling cascades rather than a single gene. This unique function underlines the immense importance of these small molecules. Recently, miR-24 has been shown to be critically involved in endothelial apoptosis during cardiac I/R injury as well as apoptosis of cancer and T cells.6–8 In the present study we demonstrated miR-24 to be upregulated in kidneys of renal transplant recipients with prolonged cold ischemia time (CIT) and mice subjected to experimental I/R injury. In addition, miR-24 inhibition affected kidney function and inflammation of I/R kidneys through regulation of tubular epithelial as well as endothelial cell apoptosis. Moreover, treatment with a locked nucleic acid (LNA) targeting miR-24 resulted in improved renal outcome and survival of mice subjected to bilateral I/R injury.
Results
miR-24 in renal I/R injury
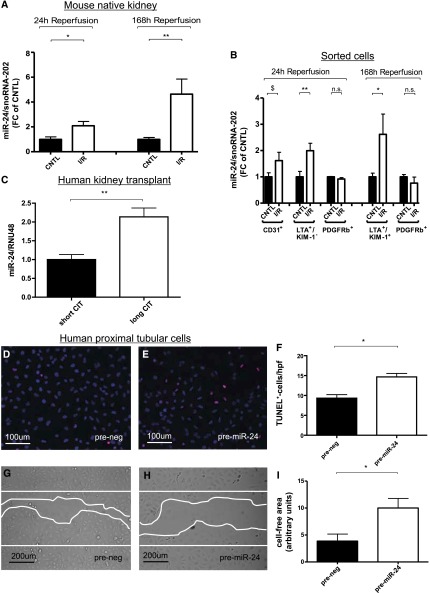
Levels of miR-24 are increased in mouse kidneys at 24 and 168 hours after induction of I/R injury compared with contralateral control kidneys (Figure 1A). Cell-sorting analysis after digestion of whole kidneys after I/R injury revealed a specific enrichment of miR-24 in tubular epithelial (LTA+/KIM-1− cells) and endothelial cells (CD31+ cells) (Figure 1B) at 24 hours of reperfusion. At a reperfusion time of 168 hours after I/R injury, miR-24 was upregulated in injured tubular epithelial cells (LTA+/KIM-1+ cells). At day 7 after reperfusion, the level of miR-24 in LTA+/KIM-1− cells (healthy tubules) changed to levels of controls. A slight, nonsignificant decrease in miR-24 expression was detected in pericytes (PDGFRb+). In kidney transplant biopsy specimens from patients with prolonged CIT (n=5 per group), miR-24 increased, indicating a distinct pathophysiologic role in this setting (Figure 1C).
Figure 1.
Expression and function of miR-24 in the kidney and distinct renal cell populations. The expression of miR-24 in mouse kidneys is depicted at 24 and 168 hours (A) after unilateral I/R injury (n=7 each). Expression of miR-24 in sorted cells after digestion of postischemic mouse kidney at reperfusion for 24 and 168 hours is shown (B). Levels of miR-24 were compared with snoRNA-202 as control. MiR-24 expression normalized to RNU-48 in biopsy specimens from kidney transplant recipients with long compared with short CIT (n=5 in each group) CD31+, endothelial cells; LTA+/KIM-1−, uninjured proximal tubular epithelial cells; LTA+/KIM-1+, injured proximal tubular epithelial cells; PDGFRb+, pericytes. (C). TUNEL staining in cultured HK-2 cells after prenegative control (D) and pre–miR-24 oligonucleotide (E) transfection and quantification of results (F) (n=6 experiments). Scratch migration analysis in normoxia in HK-2 cells after prenegative control (G) and pre–miR-24 oligonucleotide (H) transfection and quantification of results (I) (n=6 experiments). $P=0.08; **P<0.01; *P<0.05. CNTL, contralateral control kidney; hpf, high-power field.
Functional Role of miR-24 in Tubular Epithelial Cells
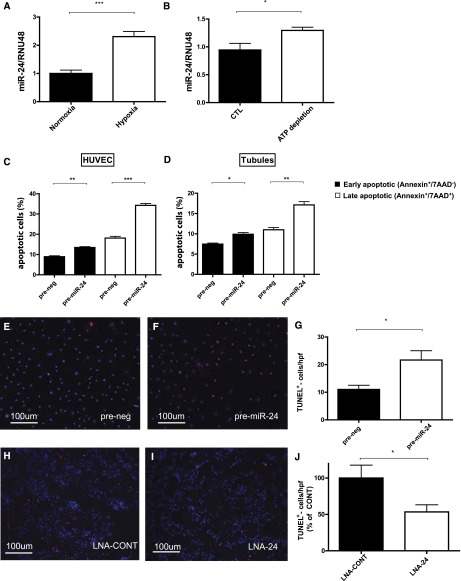
Chemical anoxia/ATP depletion for 1 hour followed by ATP repletion for 30 minutes led to an enrichment of miR-24 in HK-2 cells (Figure 2B). ATP depletion as well as treatment of cells with 0.2 μM staurosporin (to induce apoptosis) resulted in a significant increase in apoptosis, as assessed by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining (data not shown). Intriguingly, transfection of cells with miR-24 precursors without any additional cellular stressors culminated in an increase in apoptosis as assessed by TUNEL staining (Figure 1, D– F) and FACS analysis following Annexin V-Cy657/7-AAD staining of cells (Figure 2D). Analysis of 8-OHdg formation in HK-2 cells following miR-24 precursor transfection indicated a significant increase in reactive oxygen production (data not shown). Scratch migration analysis following miR-24 enrichment indicated a defect in tubular epithelial migratory capacity (Figure 1, G– I).
Figure 2.
Expression of miR-24 in distinct kidney cells and role in apoptosis regulation. Expression of miR-24 normalized to RNU-48 in hypoxic compared with normoxic control human umbilical vein endothelial cells (HUVECs) (A) and HK-2 cells exposed to ATP depletion (B) is shown. Percentage of early apoptotic (Annexin+/7AAD−) as well as late apoptotic (Annexin+/7AAD+) HUVECs (C) and proximal tubular epithelial cells (D) in FACS analysis is shown. TUNEL staining in cultured endothelial cells after prenegative control (E) and pre–miR-24 oligonucleotide (F) transfection and quantification of results (G) (n=6 experiments). TUNEL+ cells in outer medulla in mice after ischemia and reperfusion for 24 hours treated with control LNA (LNA-CONT; H) and LNA-24 (I) and quantification of results (J). ***P<0.0001; **P<0.01; *P<0.05. CTL = control.
Functional Role of miR-24 in Endothelial Cells
Incubation of human umbilical vein endothelial cells in a hypoxic environment for 24 hours (0.1% O2) followed by reoxygenation for 3 hours resulted in a significant enhancement of apoptosis (TUNEL and Annexin V-Cy657/7-AAD staining of cells) (Figure 2, C–F) and upregulation of miR-24 (Figure 2A) in vitro. Transient enrichment of miR-24 suppressed tube formation capacity as well as migration capacity (Supplemental Figure 1, A–F).
Sphingosine-1-Phosphate Receptor 1; H2A Histone Family, Member X; and Heme Oxygenase-1 Are Direct Targets of miR-24 In Vitro
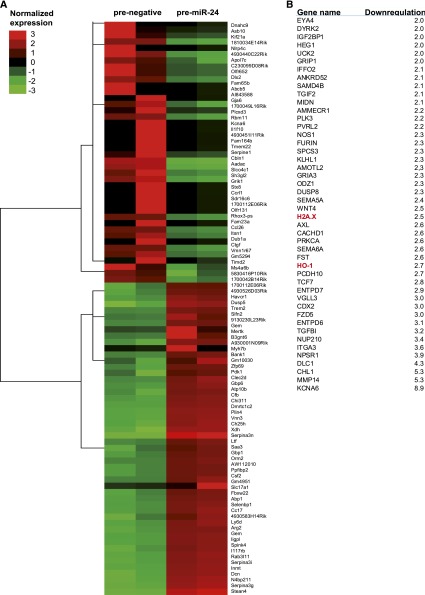
To identify miR-24 targets, which potentially induce tubular as well as endothelial cell apoptosis, we first used bioinformatic miRNA target prediction tools and observed many genes with putative 3′UTR binding sites for miR-24 that had previously been described to have important functional roles in apoptosis development. In addition, we performed a global messenger RNA expression analysis in proximal tubular epithelial cells following overexpression of miR-24 precursors (Figure 3A). In total, 1822 genes were downregulated in cells overexpressing miR-24 compared with cells transfected with a prenegative control oligonucleotide. Cluster analysis of the top 50 up- and downregulated genes identified several genes involved in apoptosis regulation (Figure 3A). Downregulated genes of the array were subsequently merged with predicted targets of miR-24 (Targetscan). These are shown in Figure 3B.
Figure 3.
Cluster analysis of miR-24 targets in tubular epithelial cells overexpressing miR-24. Affymetrix gene array and cluster analysis in tubular epithelial cells transfected with prenegative control and pre–miR-24 oligonucleotide (A) is shown. Bioinformatically predicted targets of miR-24 (as obtained from Targetscan) were cross-checked with the results of the array (B). Downregulated genes (fold regulation) of the array, subsequently merged with predicted targets of miR-24 (Targetscan), are shown in Figure 3B. Targets further analyzed (H2A.X and HO-1) are highlighted in red (B).
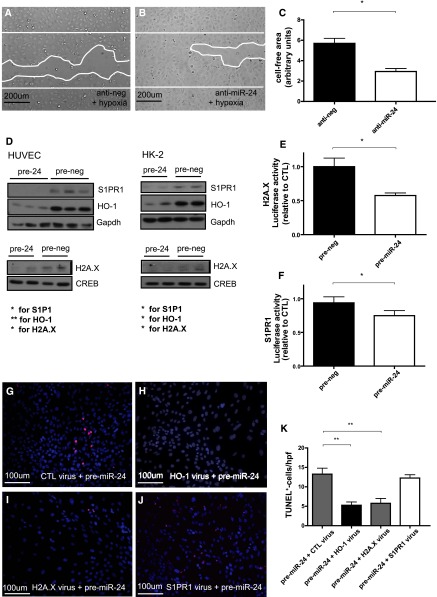
In our subsequent in vitro analyses, we focused on sphingosine-1-phosphate receptor 1 (S1PR1); H2A histone family, member X (H2A.X); and heme oxygenase-1 (HO-1). To validate these targets, proximal tubular and endothelial cells were transfected with miR-24 precursors. This resulted in the repression of H2A.X, HO-1, and S1PR1 protein expression in endothelial and tubular epithelial cells (Figure 4D).
Figure 4.
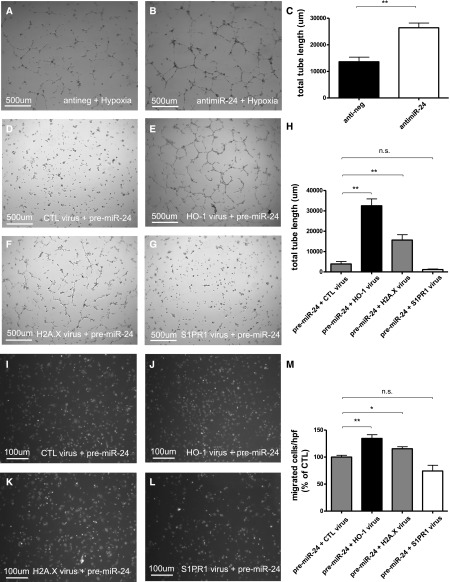
Validation of miR-24 targets and adenoviral rescue assays. Protective role of anti–miR-24 treatment in tubular epithelial cells and miR-24 targets involved in miR-24s action in renal epithelial cells and endothelial cells. Scratch migration analysis in hypoxic HK-2 cells after antinegative control (A) and anti–miR-24 oligonucleotide (B) transfection and quantification of results (C) (n=6 experiments). Western blot analysis in HUVECs and HK-2 cells of cytosolic S1PR1 and HO-1 normalized to glyceraldehyde 3-phosphate dehydrogenase (Gapdh) and nuclear H2A.× normalized to cAMP response element-binding protein after transfection with prenegative control and pre–miR-24 oligonucleotides. Results of luciferase gene reporter assays concerning H2A.X (E) and S1PR1 (F). TUNEL stainings in miR-24–overexpressing tubular epithelial cells after transduction with adenoviral constructs lacking miR-24 binding sites for control virus (CTL; G), HO-1 virus (H), H2A.X virus (I), S1PR1 virus (J), and quantification of results (K). **P<0.01; *P<0.05. hpf, high power field.
When we fused the respective 3′UTR regions to a luciferase reporter gene and determined luciferase activity in cells transfected with synthetic miR-24 precursors, miR-24 significantly repressed luciferase activity (Figure 4, E and F). We thus identified S1PR1 and H2A.X as novel direct targets of miR-24. We previously confirmed HO-1 to be a bona fide target of miR-24.9
Functionally, small interfering RNA–mediated silencing of these miR-24 targets (HO-1, H2A.X, and S1PR1) in cells subjected to hypoxia (0.1% O2 for 24 hours with subsequent reoxygenation for 3 hours) mimicked the miR-24 enrichment results and induced an impairment of endothelial tube formation (Supplemental Figure 2, A–E) and migration capacity (Supplemental Figure 2, F–J) as well as tubular (Supplemental Figure, 3A–E) and endothelial cell apoptosis (data not shown).
Protective Therapeutic Effect of miR-24 Antagonism In Vitro and Adenoviral Rescue Assays
Silencing of miR-24 in tubular epithelial cells subjected to hypoxia significantly improved migratory capacity in scratch migration assays (Figure 4, A–C). In hypoxic endothelial cells, tube formation capacity was significantly improved through silencing of miR-24 (Figure 5, A–C). To assess whether the miR-24 effects are mainly mediated by its targets H2A.X, S1PR1, and HO-1, we reconstituted those targets in miR-24–overexpressing endothelial and tubular epithelial cells by transduction with adenoviral constructs lacking miR-24 binding sites. Reconstitution of miR-24–resistant H2A.X and HO-1 rescued miR-24–mediated endothelial apoptosis (data not shown), impaired tube formation capacity (Figure 5, D–D), and migration capacity (Figure 5, I–M), as well as tubular epithelial cell apoptosis (Figure 4, G–K), while S1PR1 had no effect on any of the investigated end points. Adenoviral constructs alone (without pre-miR transfection) do not induce apoptosis.
Figure 5.
Adenoviral rescue assays in miR-24 overexpressing endothelial cells. Tube formation capacity (total tube length in micrometers) in hypoxic HUVECs transfected with antinegative control oligonucleotides (A), anti–miR-24 oligonucleotides (B), and quantification of results (C). Tube formation capacity in miR-24–overexpressing endothelial cells after transduction with adenoviral constructs lacking miR-24 binding sites for control virus (CTL; D), HO-1 virus (E), H2A.X virus (F), S1PR1 virus (G), and quantification of results (H). Migration capacity in Boyden chamber assays in miR-24–overexpressing endothelial cells after transduction with adenoviral constructs lacking miR-24 binding sites for control virus (CTL; I), HO-1 virus (J), H2A.X virus (K), S1PR1 virus (L), and quantification of results (M). **P<0.01; *P<0.05.
Markers of Kidney Damage, Endothelial Activation, and Inflammation in Unilateral I/R Injury after miR-24 Silencing
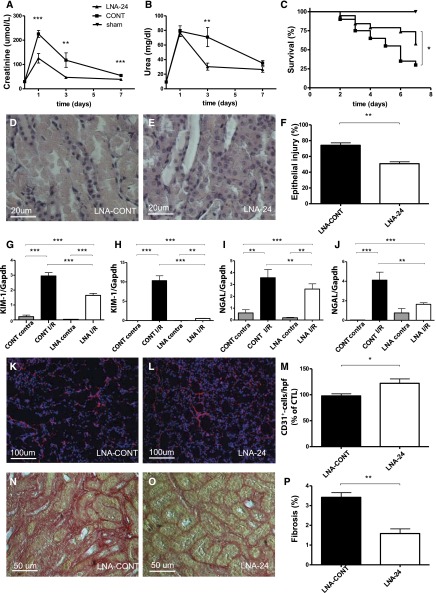
Treatment of mice with an LNA-modified anti-miR targeting miR-24 before unilateral I/R injury (Figure 6, G–J) resulted in a marked reduction of kidney injury marker gene expression (NGAL and KIM-1). Capillary rarefaction on day 1 after I/R injury was significantly improved in animals treated with an LNA-modified anti-miR targeting miR-24 (Figure 6, K–M). In addition, markers of endothelial activation (vascular cellular adhesion molecule-1) decreased significantly (data not shown).
Figure 6.
In vivo effect of miR-24 antagonism in murine I/R injury. Protective rescue of renal I/R injury following anti–miR-24 therapy. Renal function parameters (serum creatinine [A] and urea [B]) as well as Kaplan–Meier curve survival analysis (C) in mice treated with an LNA-modified anti-miR targeting miR-24 (LNA-24) and a mismatch control LNA (CONT) 24 hours before induction of I/R injury, as well as sham-operated animals. Bilateral renal I/R injury was performed for 27 minutes. Observation period from days 0 to 7; n=20 per treatment group, n=4 in the sham group. Differences in urea levels at day 7 are underestimated because of loss of uremic mice in the control group. Histologic degree of epithelial injury after ischemia and reperfusion for 24 hours in mice receiving LNA-MM (D) and LNA-24 (E) and unilateral clamping of renal pedicles, as well as quantification of results (F, n=7 each). KIM-1 as well as NGAL mRNA levels in postischemic unilaterally clamped kidneys after reperfusion for 24 hours (G and I) and 168 hours (H and J); CONT contra, contralateral kidney of mice with LNA-CONT; CONT IR, clamped kidney of mice with LNA-CONT; LNA contra, contralateral kidney of mice with LNA-24; LNA IR, clamped kidney of mice with LNA-24. n=7 mice in each group and time point. Capillary rarefaction (CD31-staining) analysis in mice treated with control LNA (LNA-CONT) (K) and LNA-24 (L) and quantification of results (M) (n=7 per group) at reperfusion for 24 hours after clamping. Fibrosis development (sirius red staining) analysis in mice treated with control LNA (LNA-CONT) (N) and LNA-24 (O) and quantification of results at reperfusion for 168 hours (P) (n=7 each). *P<0.05; **P<0.01; ***P<0.0001.
Kidney Morphology, Infiltration of Immune Cells, and Level of Apoptosis after miR-24 Silencing
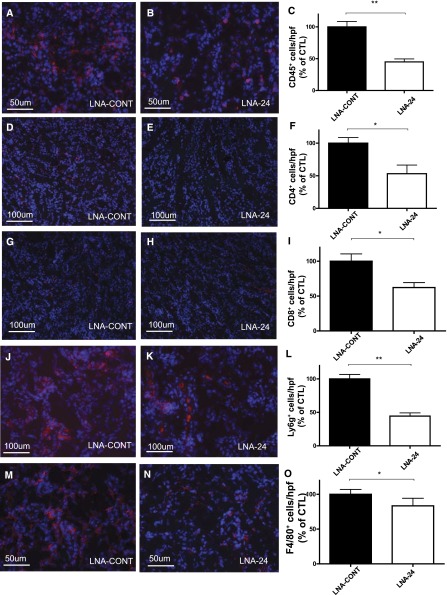
LNA-24 treatment resulted in a significant improvement of kidney morphology on day 1 after I/R injury in unilateral (Figure 6, D and E) as well as bilateral I/R injury (Supplemental Figure 4, D and E) and a reduction in epithelial injury in mice (see Figure 6F for unilateral, Supplemental Figure 4F for bilateral I/R injury). Infiltration of CD45+, F4/80+ macrophages, CD4+ and CD8+ T cells, and Ly6g+ neutrophils significantly decreased after LNA-24 treatment as assessed by immunofluorescence at all investigated time points (Figure 7A–O, data for reperfusion of 168 hours in unilateral I/R are shown). Tubular cell apoptosis as assessed by TUNEL staining was significantly lower in LNA-24–treated animals at 24 hours of reperfusion (Figure 2, G–I).
Figure 7.
Infiltration of inflammatory cells following miR-24 antagonism in vivo. Immunofluorescence stainings and quantification of representative cryosections (4 μm) in outer medulla of mice treated with control LNA (LNA-CONT) and LNA-24 concerning CD45+ (A–C), CD4+ (D–F), CD8+ (G–I), Ly6g+- neutrophils (J–L), and F4/80+ macrophages (M–O) at reperfusion time of 168 hours. Specific immunofluorescence stainings in red and DAPI in blue. **P<0.01; *P<0.05.
Regulation of miR-24, Survival, Kidney Function, and Markers of Kidney Damage and Inflammation in Bilateral I/R Injury In Vivo
Treatment of mice with an LNA-modified anti-miR targeting miR-24 before induction of I/R injury (24 hours) resulted in significant improvement of survival compared with mismatch control-treated animals (Figure 6C). This was accompanied by preserved kidney function (lower levels of serum creatinine and urea levels; see Figure 6, A and B). On day 7 after induction of bilateral I/R injury, levels of renal function decreased further and normalized to levels in sham-operated animals on day 14 (data not shown). Moreover, treatment of mice with an LNA-modified anti-miR targeting miR-24 in bilateral renal I/R injury (Supplemental Figure 4, B and C) resulted in a marked reduction of kidney injury marker gene expression (NGAL and KIM-1). In addition, expression of inflammatory gene expression (IL-1β, IL-6, monocyte chemoattractant protein 1, macrophage inflammatory protein-2α, TNF-α) in bilateral I/R injury was significantly lower in animals treated with LNA-modified anti-miR-24 after I/R injury (Supplemental Figure 5, D–H). In vivo, in a model of bilateral I/R injury, we found HO-1 to be significantly upregulated after miR-24 antagonism compared with LNA-mismatch treated animals in the outer medulla (P<0.01). H2A.X was also upregulated by miR-24 inhibition, although not to a statistically significant level (P=0.08). S1PR1 was not regulated in vivo (Supplemental Figure 4, G–I, for H2A.X; Supplemental Figure 4, J–L, for S1PR1; Supplemental Figure 4, M–O, for HO-1).
MiR-24 in the Progression from AKI to CKD
At a reperfusion time of 168 hours, the level of developing fibrosis was highly attenuated in mice treated with an LNA-modified anti-miR targeting miR-24 (Figure 6, N–P) compared with mismatch treated animals subjected to unilateral I/R injury. In addition, the expression of fibrosis-associated genes, including those for collagen I α2, collagen III, and α–smooth muscle actin, was blunted in LNA-24 treated mice at all three investigated time points (see Supplemental Figure 6, A–I, for unilateral I/R; Supplemental Figure 5, A–C, for bilateral I/R).
Discussion
In the present study, we found that miR-24 affected endothelial and tubular epithelial cell apoptosis in murine renal I/R injury as well human kidney transplant–associated renal I/R injury. A global messenger RNA expression analysis in proximal tubular epithelial cells revealed many apoptosis-associated genes to be deregulated after miR-24 modulation. In particular, miR-24 was found to target prominent antiapoptotic proteins, including S1PR1, H2A.X, and HO-1 in vitro. Additionally, HO-1 and H2A.X were prominent targets of miR-24 in vivo. Viral reconstitution of these targets in miR-24–overexpressing endothelial and tubular epithelial cells ameliorated various functional parameters in vitro. Finally, silencing of miR-24 in vivo ameliorated renal I/R injury, infiltration of various immune cells and survival as well as kidney function in mice. Silencing of miR-24 in vivo culminated in a repression of renal fibrosis following I/R injury.
Several studies have recently examined the role of specific miRNAs in the pathogenesis of renal I/R injury. For instance, Godwin and coworkers elegantly identified miR-21 as one of the most highly induced miRNAs during renal I/R injury.10 MiR-127 contributed to the severity of renal I/R injury through a modulation of cell trafficking.11 However, none of the studies presented so far evaluated the protective potential of specific pharmacologic miRNA inhibition during renal I/R injury. We provide evidence of the in vivo relevance of miR-24 in human and murine renal I/R injury. Moreover, we identify the underlying molecular mechanisms by analyzing specific apoptosis-associated targets of miR-24. Most important, we demonstrate the feasibility and efficacy of in vivo miR-24 inhibition as a protective therapeutic option during renal I/R injury.
A recent study showed that targeted deletion of DICER from the proximal tubular epithelium protects against I/R-induced renal injury through expression changes of various miRNAs.12 Interestingly, DICER knockdown was associated with reduced expression of miR-27a. This miRNA belongs to an miRNA cluster, which is simultaneously transcribed. The miR-23⁄27⁄24 gene cluster is composed of two separate gene clusters located at different genomic loci.13 The intergenic miR-24–2 cluster is encoded on human chromosome 19p13.13, expressing miR-23a, miR-27a, and miR-24–2.13,14 This cluster is involved in angiogenesis and endothelial apoptosis in cardiac ischemia and retinal vascular development.13 Moreover, miR-24 regulates apoptosis of cancer and T cells.7,8 Overexpression of the miR-23a, miR-27a, and miR-24–2 cluster induced apoptosis of human embryonic kidney cells.15 The initial finding of our study, that miR-24 is highly enriched in kidneys of transplant recipients with prolonged CIT, prompted us to identify the underlying mechanisms in a mouse model of renal I/R injury. Most strikingly, miR-24 inhibition ameliorated kidney injury and function as well as overall survival of mice. We demonstrated specific enrichment of miR-24 in endothelial (CD31+ cells) and tubular epithelial cells (LTA+/KIM-1− cells) at 24 hours of reperfusion through sorting of cells based on distinct surface receptors. Interestingly, at a reperfusion time of 168 hours after I/R injury, miR-24 was also upregulated in injured tubular epithelial cells (LTA+/KIM-1+ cells). We believe that miR-24 is elevated soon after induction of I/R injury in tubular epithelial cells and drives the subsequent injurious events in these cells. In line with this hypothesis, we saw robust upregulation of miR-24 in injured tubular epithelial cells at day 7 (LTA+/KIM-1+ cells).
To identify the downstream mechanism of miR-24–regulated protection, we used a global mRNA array analysis that revealed many deregulated apoptosis-associated genes. We focused on H2A.X, HO-1, and S1PR1, all of which have established roles in recovery of renal I/R injury and/or apoptosis.16–18 H2A.X and HO-1 are protective concerning DNA-damage and oxidative stress and were among the strongest down-regulated targets in our profiling approach.17,19 S1PR1 is a predicted target of miR-24 and has previously been described as an important factor in the resolution of renal I/R injury.16 Interestingly, reconstitution of H2A.X and HO-1 in miR-24 overexpressing endothelial and tubular epithelial cells underlined their critical importance in miR-24–mediated effects on apoptosis regulation. In vivo, HO-1 was significantly upregulated by miR-24 antagonism, underscoring its in vivo significance as a downstream effector of miR-24. H2A.X showed a trend for regulation, while S1PR1 was not regulated. We thus propose HO-1 as the major factor in miR-24–mediated ischemic AKI.
MiR-24 inhibition in renal I/R injury primarily resulted in protection against endothelial and tubular epithelial apoptosis. Strikingly, postischemic fibrosis development was also highly attenuated in mice treated with an LNA-modified anti-miR targeting miR-24. These effects can be attributed to enhanced capillary density and tubular epithelial cell survival after miR-24 inhibition.
In conclusion, we show that miR-24 contributes to renal I/R injury by influencing endothelial and tubular epithelial apoptosis through regulation of antiapoptotic HO-1 and H2A.X. MiR-24 has also previously been shown to have an antiapoptotic function in cancer cells and cardiomyocytes.20,21 However, here we provide clear evidence of the proapoptotic role of miR-24 in renal I/R injury. Silencing of miR-24 ameliorates the apoptotic response in vivo, leading to suppressed tubular epithelial and endothelial apoptosis; this, in turn, is associated with enhanced capillary density and reduced tubulointerstitial fibrosis and potentially blunting of the AKI-to-CKD continuum. Of note, this is the first report showing that pharmacologic miRNA inhibition might be a viable therapeutic option in the treatment of patients with this life-threatening clinical disorder. Intriguingly, miR-24 is also enriched in transplanted kidneys of patients with prolonged CIT, indicating its potential role in human renal I/R- injury This study highlights that miR-24 modulation might ultimately lead to the first targeted clinically applicable therapy for AKI.
Concise Methods
Patients
In kidney transplant recipients, who presented with prolonged (707±73 minutes, n=5) and short (208±56 minutes, n=5) CIT, renal biopsy specimens and clinical and demographic data were collected. RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. For detection of miRNAs in samples, TaqMan miR-24 primer assays (Applied Biosystems) were applied. The small RNA molecule RNU-48 was amplified as a control. The Ethics Committee of the Hannover Medical School approved the study, and all patients gave their written informed consent (approval number 2765). Patient characteristics are shown in Table 1.
Table 1.
Demographic characteristics of transplant recipients with long and short CIT
| Characteristic | Short CIT | Long CIT |
|---|---|---|
| Men/women (n/n) | 1/4 | 2/3 |
| Recipient age (yr) | 46.2±3.9 | 47.0±4.7 |
| Cause of ESRD (n) | ||
| GN | 3 | 2 |
| Hypertensive/diabetic nephropathy | 1 | 2 |
| Unknown | 1 | 1 |
| CIT (min) | 208±56 | 707±73 |
Values expressed with a plus/minus sign are the mean±SD.
Animal Studies
Male C57BL/6 mice were purchased from Charles River (Sulzfeld, Germany) and were housed under standard conditions. Mice 10–12 weeks old weighing between 20 and 30 g were used for all experiments.
All animal experimental procedures agreed with institutional and legislative regulations and were approved by the local authorities (approval number 08/1434).
Other methodologic details are given in the Supplemental Material.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to acknowledge the support of the Else-Kröner-Fresenius Foundation awarded to J.L. and T.T. and the assistance of the Cell Sorting Core Facility of the Hannover Medical School, supported in part by Braukmann-Wittenberg-Herz-Stiftung and Deutsche Forschungsgemeinschaft. The technical assistance of Annette Just is greatly appreciated.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013121329/-/DCSupplemental.
References
- 1.Kelly KJ: Acute renal failure: Much more than a kidney disease. Semin Nephrol 26: 105–113, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bon D, Chatauret N, Giraud S, Thuillier R, Favreau F, Hauet T: New strategies to optimize kidney recovery and preservation in transplantation. Nat Rev Nephrol 8: 339–347, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Weight SC, Bell PR, Nicholson ML: Renal ischaemia—reperfusion injury. Br J Surg 83: 162–170, 1996 [PubMed] [Google Scholar]
- 4.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenzen JM, Haller H, Thum T: MicroRNAs as mediators and therapeutic targets in chronic kidney disease. Nat Rev Nephrol 7: 286–294, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, Galuppo P, Kneitz S, Pena JT, Sohn-Lee C, Loyer X, Soutschek J, Brand T, Tuschl T, Heineke J, Martin U, Schulte-Merker S, Ertl G, Engelhardt S, Bauersachs J, Thum T: MicroRNA-24 regulates vascularity after myocardial infarction. Circulation 124: 720–730, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Xie Y, Tobin LA, Camps J, Wangsa D, Yang J, Rao M, Witasp E, Awad KS, Yoo N, Ried T, Kwong KF: MicroRNA-24 regulates XIAP to reduce the apoptosis threshold in cancer cells. Oncogene 9; 32(19): 2442–2451, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunner S, Herndler-Brandstetter D, Arnold CR, Wiegers GJ, Villunger A, Hackl M, Grillari J, Moreno-Villanueva M, Bürkle A, Grubeck-Loebenstein B: Upregulation of miR-24 is associated with a decreased DNA damage response upon etoposide treatment in highly differentiated CD8(+) T cells sensitizing them to apoptotic cell death. Aging Cell 11: 579–587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiedler J, Stöhr A, Gupta SK, Hartmann D, Holzmann A, Just A, Hansen A, Hilfiker-Kleiner D, Eschenhagen T, Thum T: Functional microRNA library screening identifies the hypoxaMiR miR-24 as a potent regulator of smooth muscle cell proliferation and vascularization [published online ahead of print Nov 19, 2013]. Antioxid Redox Signal 10.1089/ars.2013.5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J: Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci U S A 107: 14339–14344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguado-Fraile E, Ramos E, Sáenz-Morales D, Conde E, Blanco-Sánchez I, Stamatakis K, del Peso L, Cuppen E, Brüne B, Bermejo ML: miR-127 protects proximal tubule cells against ischemia/reperfusion: Identification of kinesin family member 3B as miR-127 target. PLoS ONE 7: e44305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei Q, Bhatt K, He HZ, Mi QS, Haase VH, Dong Z: Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 21: 756–761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bang C, Fiedler J, Thum T: Cardiovascular importance of the microRNA-23/27/24 family. Microcirculation 19: 208–214, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Chan MC, Hilyard AC, Wu C, Davis BN, Hill NS, Lal A, Lieberman J, Lagna G, Hata A: Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. EMBO J 29: 559–573, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhabra R, Adlakha YK, Hariharan M, Scaria V, Saini N: Upregulation of miR-23a-27a-24-2 cluster induces caspase-dependent and -independent apoptosis in human embryonic kidney cells. PLoS ONE 4: e5848, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, Haase VH, Macdonald TL, Lynch KR, Okusa MD: Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG: Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature 458: 591–596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blydt-Hansen TD, Katori M, Lassman C, Ke B, Coito AJ, Iyer S, Buelow R, Ettenger R, Busuttil RW, Kupiec-Weglinski JW: Gene transfer-induced local heme oxygenase-1 overexpression protects rat kidney transplants from ischemia/reperfusion injury. J Am Soc Nephrol 14: 745–754, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Olszanecki R, Rezzani R, Omura S, Stec DE, Rodella L, Botros FT, Goodman AI, Drummond G, Abraham NG: Genetic suppression of HO-1 exacerbates renal damage: Reversed by an increase in the antiapoptotic signaling pathway. Am J Physiol Renal Physiol 292: F148–F157, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Qin W, Shi Y, Zhao B, Yao C, Jin L, Ma J, Jin Y. miR-24 regulates apoptosis by targeting the open reading frame (ORF) region of FAF1 in cancer cells. PLoS One 25; 5(2):e9429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med 14; 208(3):549–560, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.