Abstract
AKI is pathologically characterized by sublethal and lethal damage of renal tubules. Under these conditions, renal tubular cell death may occur by regulated necrosis (RN) or apoptosis. In the last two decades, tubular apoptosis has been shown in preclinical models and some clinical samples from patients with AKI. Mechanistically, apoptotic cell death in AKI may result from well described extrinsic and intrinsic pathways as well as ER stress. Central converging nodes of these pathways are mitochondria, which become fragmented and sensitized to membrane permeabilization in response to cellular stress, resulting in the release of cell death–inducing factors. Whereas apoptosis is known to be regulated, tubular necrosis was thought to occur by accident until recent work unveiled several RN subroutines, most prominently receptor-interacting protein kinase–dependent necroptosis and RN induced by mitochondrial permeability transition. Additionally, other cell death pathways, like pyroptosis and ferroptosis, may also be of pathophysiologic relevance in AKI. Combination therapy targeting multiple cell-death pathways may, therefore, provide maximal therapeutic benefits.
Keywords: acute renal failure, apoptosis, renal injury
AKI is a multifactorial and multiphasic renal disease characterized by a rapid decline of renal function, resulting in the accumulation of metabolic waste and toxins and consequent complications and failure of other organs. Clinically, the causes of AKI mainly include sepsis, ischemia-reperfusion (IR) injury, and various endogenous as well as exogenous nephrotoxins. It is estimated that over 2 million people die of AKI each year around the world,1 and the prevalence of AKI has been increasing rapidly.1,2 AKI is also known for its association with unacceptably high rates of mortality. For example, in intensive care units, AKI is associated with a mortality rate of 50%–80%. Notably, even if the patients survived, the post-AKI prognosis is dismal, with 7.5% requiring long-term dialysis and 30%–70% developing complications, including CKD and ESRD. The annual cost of AKI in the United States is estimated to exceed $10 billion.3,4
Pathologically, AKI is characterized by renal tubular damage, inflammation, and vascular dysfunction. Injury and death of tubular cells are especially recognized as the precipitating factors in AKI, and as an extension, tubular repair and regeneration are considered major events in kidney recovery from AKI.5–8 Although sublethal injury is reversible, the death of tubular cells is accompanied by the inevitable loss of the function of the affected cells, and notably, it is also frequently the source of damage-associated molecular patterns (DAMPs), the stimulating and amplifying factors of inflammation in tissue damage.9 In AKI, various forms of cell death are noticeable: necrosis and apoptosis. This review summarizes the evidence for the various forms of regulated cell death in AKI, delineates their underlying mechanisms with an emphasis on the new insights, and puts forth the perspectives of targeting cell death for the prevention and therapy of kidney injury.
Apoptosis—the Classic View of Regulated Cell Death in the Kidney
Evidence for Apoptosis in AKI
In 1992, Schumer et al.10 showed DNA cleavage and nuclear condensation during renal IR injury, showing the first evidence of apoptosis in AKI. To date, the initial observation has been confirmed and extensively expanded. By morphology, apoptotic cells are identified after renal IR by electron microscopy and various nuclear staining methods.10–12 Biochemically, renal IR leads to the activation of caspases and endonucleases.13,14 In addition, regulation of apoptotic genes, including caspases and Bcl-2 family proteins, has been shown.13–16
TUNEL staining and the evaluation of in vivo cell death. TUNEL has been widely used within the last two decades to evaluate cell death in tissues. Indeed, most dead cells stain positive for TUNEL, because double-strand breaks are found in most programmed cell death. Obviously, TUNEL positivity is found in apoptotic cells, but in contrast to the widespread belief, TUNEL positivity is not limited to apoptosis, because the cells of regulated necrosis are TUNEL-positive as well.173 Therefore, detection of apoptosis requires additional staining (e.g., cleaved caspase 3 [also referred to as activated caspase 3]) either from tissue lysates in Western blot assays or immhunohistochemically. The difference between TUNEL staining and positivity for cleaved caspase 3 might even be used to quantify the amount of regulated necrosis in tissues,173 but it cannot further specify the precise pathway of regulated necrosis.174 To further distinguish necroptosis from apoptosis, one emerging marker for the direct detection of necroptosis is an mAb that detects phosphorylated MLKL.114 This antibody, however, only detects human phosphorylated MLKL.
Apoptosis is also well recognized in AKI induced by various nephrotoxins. For example, cisplatin is a widely used cancer therapy drug with a major side effect of nephrotoxicity, which limits it therapeutic efficacy.17,18 Depending on the dosage, cisplatin induces both necrosis and apoptosis in renal tubular cells in vitro as well as in vivo in animal models. Apoptosis is shown by cell morphology, caspase activation, and terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay of DNA damage. Renoprotection against cisplatin nephrotoxicity is associated by the suppression of tubular cell apoptosis,19–24 further supporting the involvement of apoptosis in cisplatin-induced renal injury. In human kidneys of sepsis-associated AKI, tubular cell apoptosis is detected by TUNEL and activated caspase 3 staining.25 Of note, in some of the previous studies, apoptosis was detected in kidney tissues by a single method, such as TUNEL assay, which may be questionable for its specificity of apoptosis (Box).
In addition, although tubular apoptosis has often been reported in various models of AKI, the upstream signaling pathways leading to apoptosis in these models can be very different. For example, distinct pathogenic mechanisms may be responsible for apoptosis in ischemic and cisplatin nephrotoxic AKI.26,27
In AKI, apoptotic cells are shown in both cortical and medullary regions. In renal tubules, apoptosis occurs in proximal tubules, distal tubules, and tubular cells of the Henle’s loop.10,12,28–36 Numerous renoprotective agents seem to ameliorate AKI, at least in part, by diminishing tubular apoptosis. For example, minocycline, a tetracycline derivative, blocks apoptosis during renal IR, which is accompanied by the amelioration of ischemic renal injury and renal failure.28,37 Remarkably, deletion of apoptotic genes specifically from proximal tubules results in marked decreases in apoptosis and protection from both ischemic and nephrotoxic AKI.38,39 Together, these studies show an important role of tubular cell apoptosis in AKI.
Main Pathways of Apoptosis in AKI
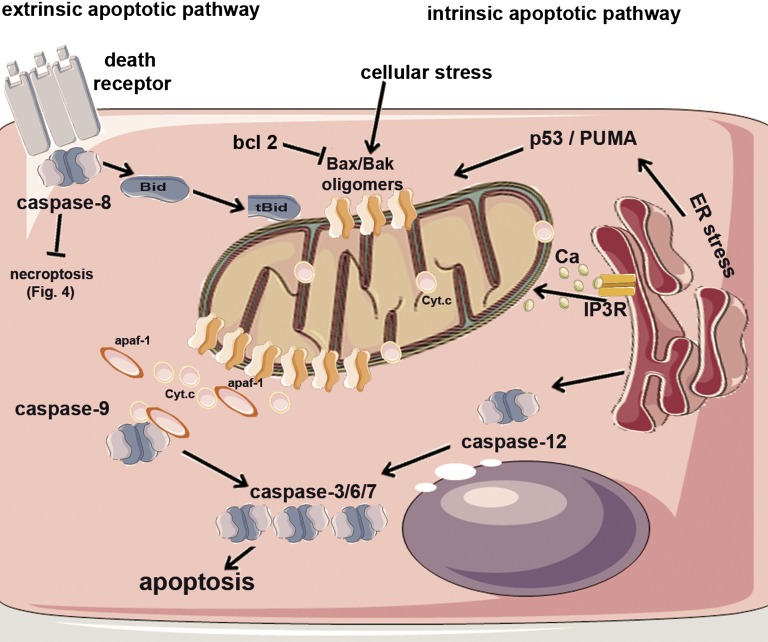
Apoptosis can be initiated through several pathways (Figure 1). In the intrinsic pathway, cell stress directly leads to mitochondrial outer membrane permeabilization (MOMP), resulting in the release of apoptogenic factors, including cytochrome c, that then bind Apaf-1 to activate caspase 9. In the extrinsic pathway, ligation of death receptors leads to the recruitment of adapter proteins and subsequent activation of caspase 8.40 Under endoplasmic reticulum (ER) stress, caspase 12 is activated,41 and more recent studies suggested that caspase 2 can be an initiator caspase for apoptosis.42
Figure 1.
Pathways of apoptosis. In the intrinsic apoptotic pathway, cellular stress leads to the oligomerization of Bax and Bak, an event that permeabilizes the mitochondrial outer membrane, resulting in the release of apoptogenic factors, including cytochrome c (Cyt.c). In the cytosol, Cyt.c binds Apaf-1 to recruit and activate caspase 9, which further cleaves and activates executioner caspases, such as caspase 3. In the extrinsic apoptotic pathway, ligation of death receptors leads to the recruitment of adapter proteins and subsequent activation of caspase 8, which further activate executioner caspases and prevent necroptosis (Figure 4). Active caspase 8 also cleaves Bid to its truncated form tBid, which translocates to mitochondria to activate the intrinsic pathway to amplify the apoptotic cascade. In the ER stress pathway, caspase 12 mediates the activation of executioner caspases. ER stress may activate the intrinsic apoptotic pathway through Ca2+ signaling and the induction of proapoptotic Bcl-2 family proteins, such as PUMA. IP3R, inositol trisphosphate receptor.
All aforementioned apoptotic pathways have been implicated in AKI. The extrinsic pathway of apoptosis mediated by TNF-α and Fas may contribute to tubular cell loss in ischemic and septic AKI.43–45 Consistently, TNF-α receptor knockout mice are resistant to cisplatin AKI, further supporting the involvement of the TNF-α–mediated extrinsic pathway in the pathogenesis of AKI.46 In ischemic and cisplatin nephrotoxic AKI, ER stress activation has been documented, but definitive evidence for the involvement in ER stress–related apoptosis has yet to be shown.47,48 In contrast, the role played by the intrinsic pathway of apoptosis in AKI has been shown convincingly. In 1998, evidence of the activation of the intrinsic pathway in AKI was shown by using the experimental model of hypoxic incubation of renal tubular cells.49 In this model, cytochrome c is released from mitochondria followed by caspase activation and tubular cell apoptosis. Importantly, the activation of Bax and Bak, two proapoptotic Bcl-2 family proteins, was later confirmed to be key to the mitochondrial leakage or MOMP.49–51 In animals, MOMP associated by cytochrome c release was shown during ischemia and cisplatin nephrotoxic AKI.33,37,52 The critical roles of Bax and Bak in AKI have been shown recently using global and proximal tubule–specific gene knockout models.39 Notably, in humans, mitochondrial damage by Bax and Bak seems to be a key to apoptotic cell death in kidneys injured by ischemia.53,54 Upregulation of Bcl-2 either pharmacologically or by gene transfection consistently blocks Bax and Bak activation, resulting in the preservation of mitochondrial integrity and cell viability and further supporting a critical role of the intrinsic pathway of apoptosis in tubular injury in AKI.28,49–51
Mitochondria and Bcl-2 Proteins: Central Players in Apoptosis
Despite the different initiation mechanisms, most (if not all) apoptotic pathways converge on mitochondria (Figure 1). Although the extrinsic pathway of apoptosis is initiated by ligand binding of death receptors, caspase 8 (after being activated in this pathway) can activate the intrinsic pathway of apoptosis through Bcl-2 family proteins.55,56 Mitochondria also play an important role in apoptosis initiated by ER stress and caspase 2.57,58 Thus, mitochondrial damage characterized by MOMP is considered a central control point of apoptosis.59,60
At the molecular level, Bcl-2 family proteins are the key regulators of mitochondrial integrity. Defined by the presence of Bcl-2 homology (BH) domains, Bcl-2 family proteins can be proapoptotic or antiapoptotic.59,61–63 Specific functions of individual Bcl-2 proteins are dictated by the organization of the BH domains. Accordingly, antiapoptotic members, like Bcl-2 and Bcl-XL, usually contain four BH domains. Proapoptotic members can be further divided into two groups: multi-BH domain proteins, such as Bax and Bak, and BH3-only proteins, such as Bid and PUMA.59,61–63 Antiapoptotic Bcl-2 proteins protect cells by preserving the integrity of mitochondria, whereas the proapoptotic molecules kill cells by permeabilizing the organelles.59,61–63 Notably, Bax and Bak provide a requisite gateway to mitochondrial injury in various apoptotic models.64 Deletion of Bax and/or Bak consitently leads to a marked resistance to tubular apoptosis in AKI.39,65
Despite the significance of Bax and Bak in mitochondrial injury during apoptosis, the mechanism underlying their activation remains elusive. Normally, Bax is cytosolic, whereas Bak resides on the mitochondrial outer membrane. On cell stress or apoptosis, Bax translocates to mitochondria, inserts to the outer membrane, and forms oligomers. Meanwhile, Bak is also activated to oligomerize. Bax activation may involve the interaction with specific proteins, such as Bid, p53, humanin, 14-3-3 protein, ku70, and Bif-1.66–72 In renal tubular cells, Borkan and colleagues73 have recently shown the interaction of nucleophosmin with Bax, which seems to be critical to Bax activation during metabolic stress in vitro and ischemic AKI in vivo. As discussed below, the activation of Bax and Bak also involves a striking change of mitochondrial morphology and consequent alterations of the membrane property.
Mitochondrial Dynamics in Apoptosis and AKI
Mitochondrion, of the Greek mito for thread and chondro for grain, assumes distinct morphologies. Recent research has further revealed that mitochondria are a class of highly dynamic organelles.74,75 Whether mitochondria display a filamentous morphology or punctiform is determined by two opposing processes: fission and fusion. Accordingly, fusion of mitochondria promotes a filamentous network, whereas fission fragments mitochondria into short rods or spheres. In mammalian cells, mitochondrial fusion involves mitofusin-1, mitofusin-2, and OPA1, whereas fission depends on Drp-1, Fis-1, and others.
In 2001, Youle and colleagues76 reported that mitochondrial dynamics are lost on cell stress or apoptosis, leading to the fragmentation of mitochondria. Importantly, inhibition of mitochondrial fragmentation blocks cytochrome c release and apoptosis. In vivo in diseases, the role of mitochondrial fragmentation was first suggested by using experimental models of ischemic and cisplatin nephrotoxic AKI. Under these conditions, Drp1 translocates to mitochondria, which is followed by sequential events of mitochondrial fragmentation, Bax/Bak activation, cytochrome c release, caspases activation, and apoptosis. Blockade of Drp1 pharmacologically or genetically preserves the filamentous morphology of mitochondria, resulting in the suppression of tubular cell apoptosis and amelioration of AKI.77 These findings have been extended to other disease conditions, such as IR injury in the heart and brain,78,79 supporting mitochondrial fragmentation as a general pathogenic mechanism.
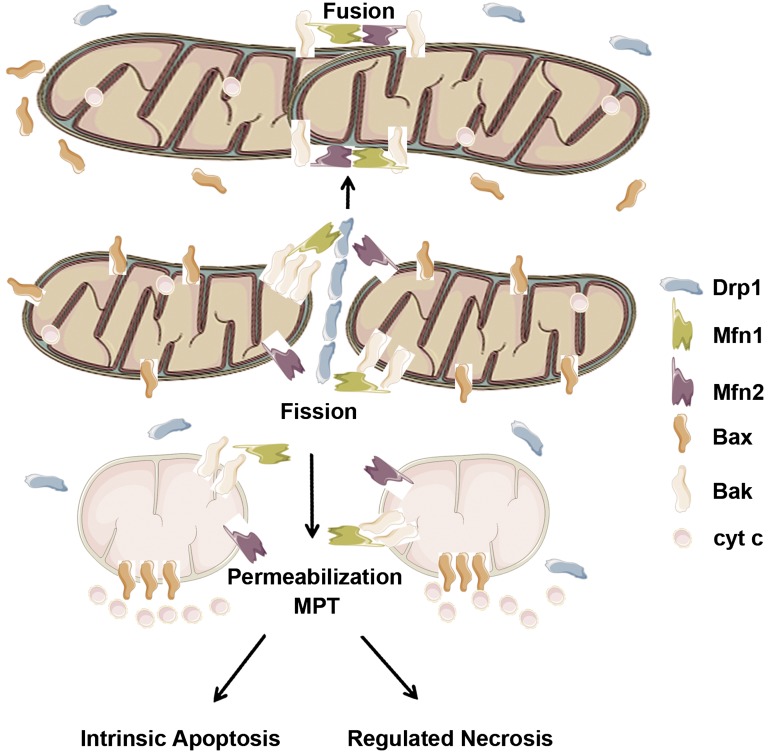
Mitochondrial fragmentation is the result of the disruption of mitochondrial dynamics. On cell stress, Drp-1 is activated (as indicated by its translocation to mitochondria) to accelerate fission. The regulation of Drp1 involves multiple post-translational modifications. In models of AKI, Drp1 is rapidly dephosphorylated in renal tubular cells, likely through calcineurin family phosphotases.80 Prevention of Drp1 dephosphorylation by calcineurin inhibitors can partially block mitochondrial fragmentation, cytochrome c release, and apoptosis, supporting a role of the dephosphorylation in Drp1 activation and mitochondrial fission.80 Interestingly, mitochondrial fragmentation not only involves hyperactivation of fission but also, depends on the arrest of fusion. Intriguingly, fusion arrest is governed by the interaction of mitofusins with Bak.81 Normally, Bak binds both mitofusin-1 and mitofusin-2. On cell stress or apoptosis, Bak dissociates from mitofusin-2 and binds mitofusin-1 at a higher affinity. Remarkably, a Bak mutant incapable of dissociating from mitofusin-2 cannot induce mitochondrial fragmentation,81 suggesting that Bak contributes to mitochondrial fragmentation by switching its binding from mitofusin-2 to mitofusin-1 (Figure 2). This key finding has been confirmed in experimental models of oxidative lung injury.82 A role of Bak in mitochondrial fragmentation has further been shown in vivo in AKI models by using gene knockout mice.39 Thus, mitochondrial fragmentation is a combined result of fission activation by Drp1 and fusion arrest mediated by Bak interaction with mitofusins.
Figure 2.
Mitochondrial dynamics in apoptosis. Under normal in vivo conditions, Bax and Drp1 are located within the cytosol, whereas Bak is at the mitochondrial outer membrane, where it binds both mitofusin-1 (Mfn1) and mitofusin-2 (Mfn2) to maintain mitochondrial fusion, ensuring its filamentous morphology. On cellular stress, Drp1 translocates to mitochondria, where it forms a restriction ring to activate the cleavage of the organelles; meanwhile, Bak dissociates from Mfn2 to bind Mfn1, which leads to an arrest of fusion and mitochondrial fragmentation. Fragmented mitochondria are more sensitive to Bax oligomerization, resulting in outer membrane permeabilization followed by the release of apoptogenic factors, such as cytochrome c (cyt.c), to activate the intrinsic apoptotic pathway. In addition, mitochondrial fragmentation may also contribute to MPT, leading to necrosis.
Of note, mitochondrial fragmentation is initially reversible; in other words, fragmented mitochondria can refuse if the insult is removed from the cell in time. Then how can mitochondrial fragmentation, a seemingly morphologic change, affect mitochondrial injury? The answer may be in the changes of mitochondrial membrane properties that occur as a result of the loss of mitochondrial dynamics. It has been shown that fragmented mitochondria are sensitized to Bax insertion and oligomerization.83 Thus, mitochondrial fragmentation may participate in apoptosis by facilitating Bax insertion and oligomerization, resulting in outer membrane permeabilization and leakage of apoptogenic factors, such as cytochrome c. In addition, mitochondrial fragmentation may contribute to mitochondrial permeability transition (MPT) at the inner membrane, resulting in necrosis (Figure 2). In kidneys, mitochondrial fragmentation as a result of the disruption of mitochondrial dynamics contributes to not only AKI but also other renal diseases, including diabetic nephropathy.84,85
Regulated Necrosis
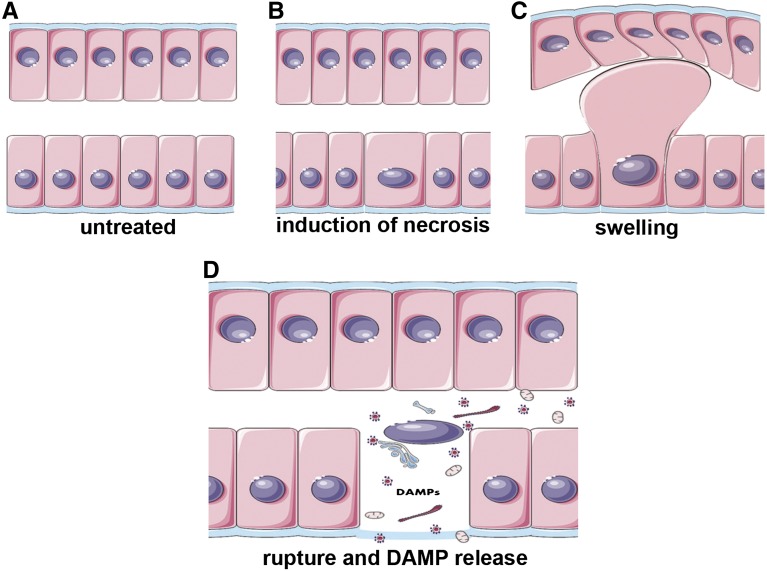
Necrosis is distinguished from apoptosis by the breakdown of the integrity of the plasma membrane. As such, necrotic cell death is accompanied by the release of unprocessed intracellular content, including cellular organelles, highly immunogenic proteins, like IL-33, F-actin, ATP, IL-1α, and HMGB1, double-stranded DNA, and RNA.86 These proimmunogenic cellular components are collectively referred to as DAMPs.86,87 The dynamics of DAMP release in the kidney have recently been shown by intravital microscopy.88 Although originally thought to be accidental, recent work has revealed several pathways of genetically determined and regulated necrosis89,90 (Figure 3), and we are beginning to understand the relative contribution of these pathways with presumably overlapping function (see below). On the molecular level, the best characterized pathway of regulated necrosis is necroptosis, an receptor-interacting protein kinase–based necrotic cell death.91
Figure 3.
Necrotic cell death in renal tubular epithelial cells. Four-phase model of necrosis-associated release of DAMPs. (A) Tubular epithelial cell layer under physiologic conditions. (B) On induction of regulated necrosis as the common mechanism of several distinct intracellular signaling pathways, individual cells begin to swell on specific genetically-determined intracellular programs that decode for regulated necrosis (like necroptosis, MPT-mediated regulated necrosis, pyroptosis, or ferroptosis). (C) Severe swelling of the luminal part of a tubular cell that undergoes regulated necrosis. (D) Plasma membrane rupture associated with DAMP release. Regulated necrosis might, therefore, trigger subsequent detrimental immune responses that cause additional organ damage beyond the primary loss of function from cell death. In transplanted organs, a classic setting for IR injury, DAMPs released from necrotic cells might trigger rejection, despite the state of immunosuppression.
Necroptosis—a Paradigm Shift
The in vivo relevance of necroptosis has been undoubtedly clarified by the rescue of caspase 8–deficient mice by ablation of receptor-interacting protein kinase 3 (RIPK3).92,93 Whereas caspase 8–deficient mice die at day 10.5 in utero and RIPK3-deficient mice have been described without phenotype,94 caspase 8/RIPK3 double-deficient mice are born at expected Mendelian frequencies and show a phenotype that was previously described as lymphoproliferation or generalized lymphoproliferative disease in mice that carry mutations in the Fas-Fas ligand pathway.92,93 From these groundbreaking experiments, it became obvious that the most important function of caspase 8 is the prevention of necroptotic cell death by a caspase 8/FLIP long heterodimer93,95 rather than the induction of either apoptosis by the caspase 8 homodimer96,97 or inflammation, possibly mediated by single caspase 8 molecules.98 In addition, from these studies, an in vivo model emerged that allows for direct investigation of mice deficient in extrinsic (receptor-mediated) apoptosis.
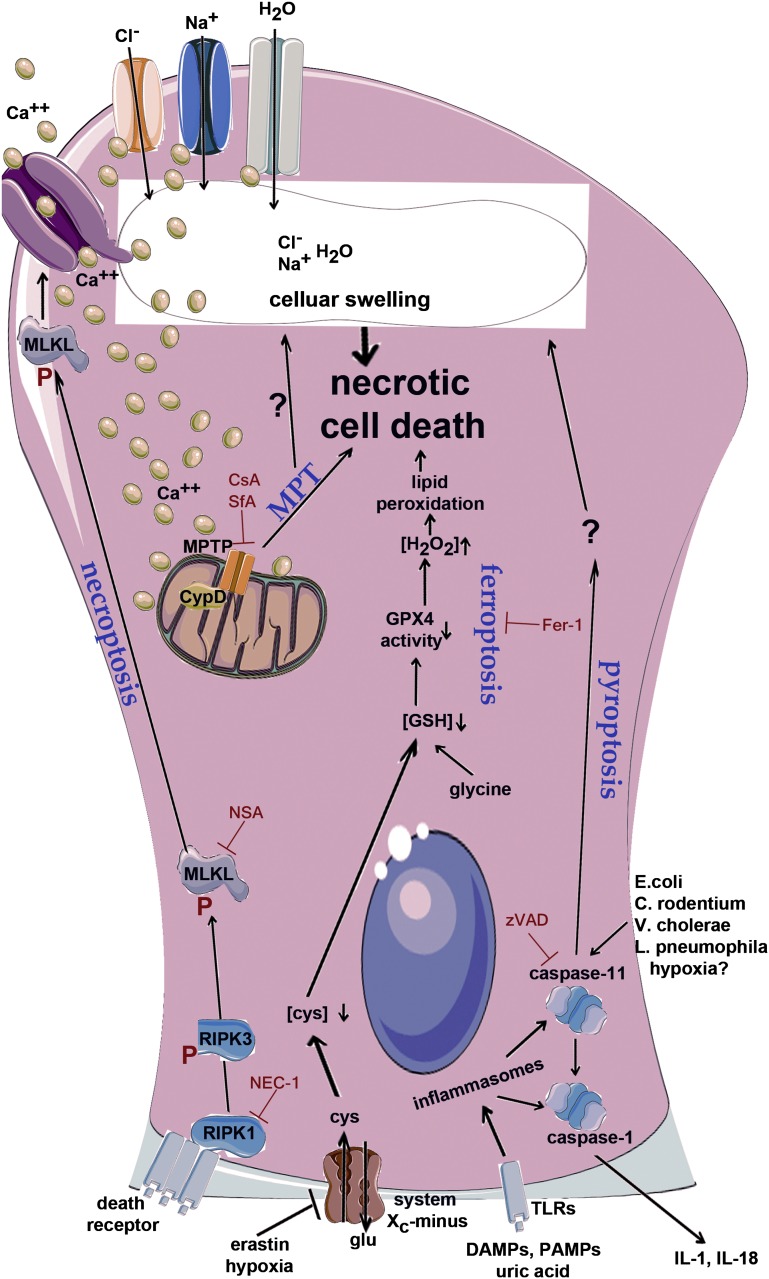
The signaling pathway of necroptosis has been reviewed in detail recently.90,99 Briefly, the main events that trigger necroptosis are engagement of death receptors in the presence of caspase inhibition,100,101 stimulation of Toll-like receptors,92,102 signaling through interferons,103,104 or recognition of intracellular viruses by the protein DAI.105,106 Any of these initial triggers uses an receptor-interacting protein–homotypic interacting motif (RHIM) domain to activate the kinase RIPK3, an essential mediator of necroptosis,107–109 which itself contains an RHIM domain and phosphorylates the downstream pseudokinase110 mixed lineage kinase domain–like (MLKL).111,112 Phosphorylation of MLKL by RIPK3 leads to a molecular switch mechanism, which exposes the N-terminal portion of MLKL113 to induce plasma membrane rupture.114
In the kidney, necroptosis was first suggested in renal ischemic AKI by showing a protective effect of necrostatin-1 (Nec-1), an inhibitor of RIPK1 (the bona fide upstream activator of RIPK3 in the necroptosis pathway) (Figure 4).115 Other groups have subsequently confirmed a role for Nec-1 in tubular cells,116–118 and the protection of RIPK3-deficient mice was shown in ischemic and cisplatin-induced AKI.27 Importantly, caspase 8/RIPK3–double knockout mice did not provide additional protection in the ischemic model but did in the cisplatin model, suggesting that, unlike previously suggested,119 extrinsic apoptosis may be of minor importance in IR injury but significantly contributes to cisplatin-induced AKI.27 This assumption is further underscored by the absence of a protective effect of the pancaspase inhibitor zVAD in the model of IR injury,115 whereas zVAD does prolong overall survival after a lethal bolus of cisplatin.27 In support of this concept, cyclosporin-mediated tubular damage120 or contrast-mediated AKI121 have been reported to be prevented by Nec-1. It should be mentioned, however, that, in these two cases, a clear detection of cell death failed and that it cannot formally be excluded that Nec-1, other than its obvious effects on necroptotic cell death, might also affect peritubular blood flow by uncharacterized means. In this sense, it is noteworthy that the highest expression of RIPK3, which is thought to indicate the cellular sensitivity of necroptosis,108 was found in glomerular endothelial cells rather than tubular cells.115
Figure 4.
Model of the integrated molecular signaling pathways of regulated necrosis in renal tubular cells. Four separate pathways of regulated necrosis may contribute to the overall organ damage in AKI. The common downstream mechanism that precedes necrotic cell death is apical swelling, which ultimately induces plasma membrane rupture as recently shown by intravital microscopy. RIPK1/RIPK3-dependent necroptosis has been extensively investigated in the kidney and is triggered by death receptors. RIPK3 is activated by phosphorylation and in turn, phosphorylates the pseudokinase MLKL, which has been suggested to be involved in the opening of plasma membrane calcium channels. Calcium-activated chloride and sodium channels may subsequently open to increase NaCl permeability, which may then cause water influx, cellular volume expansion, and plasma membrane rupture. Ca2+ from this source and others might also be involved in MPT, which directly causes regulated necrosis by unknown means. MPT and necroptosis have been clearly shown to exhibit two separate pathways in AKI. Inhibition of the cell surface cystine/glutamate antiporter system Xc-minus depletes intracellular cystine, which is reduced to cysteine on the intracellular side and together with glycine and glutamate, required for the synthesis of glutathione (GSH). Permanent hydrogen peroxide (H2O2) synthesis, especially in stressed cells, requires the GSH-dependent activity of glutathione peroxidase 4 (GPX4) to prevent H2O2-mediated lipid peroxidation followed by necrotic cell death. Finally, Toll-like receptors (TLRs) are activated by DAMPs or pathogen-associated molecular patterns (PAMPs) and crystals. TLRs activate inflammasomes, which induce caspase 1–dependent maturation of the proinflammatory cytokines IL-1β and IL-18. In parallel, inflammasomes trigger caspase 11–mediated cellular swelling and necrotic cell death, which is referred to as pyroptosis. The combination of DAMP release by necrosis and cytokine maturation renders pyroptosis an even higher immunogenic entity compared with the other pathways of regulated necrosis. Necroptosis can be prevented by the RIPK1 inhibitor Nec-1, its more stable variant, or the human-specific MLKL inhibitor necrosulfonamide (NSA). MPT can be inhibited by cyclosporin (CsA) or sanglifehrin A (SfA), ferroptosis is blocked by the small molecule ferrostatin-1 (Fer-1), and broad spectrum caspase inhibitors (e.g., zVAD-fmk) interfere with pyroptosis signaling. C. rodentium, Citrobacter rodentium; cys, Cysteine; E. coli, Escherichia coli; glu, glutamate; L pneumophila, Legionella pneumophila; V. cholerae, Vibrio cholerae.
Recently, RIPK3-deficient mice have been investigated in the model of adriamycin (ADR)-induced podocytes injury.122 As expected from previous investigations on the level of RIPK3 expression in podocyte cell lines,115 RIPK3 deficiency did not prevent ADR-induced proteinuria compared with wild-type mice but strongly prolonged overall survival in this model, unless the highest concentrations of ADR were used. Obviously, ADR-induced lethality depends on necroptosis, but it must be concluded that survival in this model is not dependent on podocyte injury, questioning the usefulness of this survival readout for podocyte damage. Taken together, necroptosis has, therefore, been shown to be critically involved in nephrotoxicity of cisplatin, cyclosporin, and ADR as well as, most notably, renal IR injury and kidney transplantation.
Necrosis by Mitochondiral Permeability Transition
Mitochondria are of outstanding interest and significance in cell death research.123,124 As outlined above, MOMP is regarded as the point of no return in the life and death decision during apoptosis.123,125 Interestingly, mitochondrial dysregulation, particularly in the form of MPT, is also capable of inducing necrotic cell death126 (Figure 2). MPT is a process that leads to the sudden exchange of solutes between the cytosol and the mitochondrial matrix through an elusive MPT pore (MPTP) that spans both the inner and outer mitochondrial membranes. The molecular composition of the MPTP remains elusive and a matter of debate.126–128 It is, however, generally accepted that the opening of the MPTP is regulated by the matrix protein cyclophilin D (CypD). In line with this finding, CypD-deficient mice have been shown to be protected from ischemic AKI.27,129,130 In addition, these mice are protected from cisplatin-induced AKI.27 Precise mechanisms about the regulation of the CypD-mediated opening of the MPTP remain unclear. In this regard, p53 has recently been suggested to be involved,131,132 but such reports are under debate.133 MPT was long known to be potentially targeted by either cyclosporin or sanglifehrin A in vitro and in vivo,27,134–136 and such effects might be of outstanding clinical importance, because trials have revealed a cyclosporin-sensitive role for MPT in myocardial infarction137; however, unfortunately, larger follow-up studies are still lacking. In addition, it is worth mentioning that there is yet another pathway of regulated necrosis called parthanatos89 because of its dependency on the nuclear protein PARP1.90 The in vivo relevance of parthanatos has been made very clear in both unilateral urethral obstruction and ischemic AKI.138,139 Future work on the potential overlay of parthanatos with MPT will be extraordinary helpful for untangling the complex web of interconnected pathways of regulated necrosis.
Pyroptosis—Maximal Immunogenicity of Necrosis
Pyroptosis is a necrotic-type cell death that was thought to occur exclusively in macrophages,140,141 but recent reports find comparable features in T lymphocytes,142 neurons,143 and tubular epithelial cells.144–147 The unique feature of pyroptosis compared with other pathways of regulated necrosis is the maturation of proinflammatory cytokines during the cell death process,148–150 which depends on cleavage mediated by nonapoptotic caspases. Many of the in vivo studies on pyroptosis have been performed in caspase 1–deficient mice, which have been described to carry a passenger mutation that functionally renders them caspase 1/11 double-deficient.151 It was not until the report of caspase 11–deficient mice that it was realized that caspase 11 mediates the pyroptotic cell death, whereas caspase 1 is thought to be mainly responsible for pro–IL-1β and pro–IL-18 cleavage.90 How caspase 11 mediates the downstream molecular events required for pyroptosis remains unclear, but in some similarity to necroptosis, it is speculated that plasma membrane channels are involved in the terminal cellular swelling.140 Caspase 11–deficient mice have not yet been studied in kidney diseases, including AKI; however, there are in vitro data pointing to this direction.145 Pyroptosis may be targeted by caspase inhibitors or cytokine response modifier A in the case of caspase 1.152
Ferroptosis—Iron-Dependent Necrosis
While searching for novel ways to kill tumor cells, Stockwell and colleagues153 identified a compound named erastin that induces necrotic cell death in highly resistant RAS-transformed cancer cells. Following this path, the previously unrecognized pathway of regulated necrosis turned out to be dependent on iron and was found to involve glutathione metabolism. A plasma membrane Cys/Glu exchanger (termed system Xc-minus) was identified to fuel cells with cysteine, which enables glutathione synthesis required for the reactive oxygen species–eliminating action of glutathione peroxidase 4.154 This enzyme removes H2O2 to prevent intracellular lipid peroxidation, which might directly affect, among others, lysosomal membranes and lead to lysosomal membrane permeabilization. With the detection of these molecular events, ferroptosis turned out to be a druggable pathway of regulated necrosis through the interference with the small molecule ferrostatin-1.155 With respect to kidney tubular cells, first results from kidney tubular cell lines treated with tert-butylhydroperoxide116 and freshly isolated tubules challenged with iron and hydroxyquinoline in the presence of ferrostatin-1 strongly increased cellular survival.156 Additional light has been shed on the role of iron in the pathophysiology of AKI from experiments using proximal tubular cell–specific ferritin heavy chain–deficient mice, which were shown to be sensitive to cisplatin-induced AKI and rhabdomyolysis-induced AKI.157 In addition, renal cell carcinomas were by far the most sensitive in a panel of 60 cancer cell lines from eight tissues tested with erastin.154 Therefore, ferroptosis is one of the promising therapeutic targets, especially in diseases dominated by kidney tubular necrosis,90,158 like ischemic, cisplatin nephrotoxic, and rhabdomyolysis-induced AKI.159 In some of these models, iron chelators have been investigated long before the detection of ferroptosis, but those compounds, such as desferoxamine, never made it into the clinical routine, despite considerable effects in ex vivo experiments with kidney tubules. It will, therefore, be of importance to re-evaluate renal data generated with desferoxamine in light of the molecular understanding of ferroptosis.
Relationship between Apoptosis and Regulated Necrosis
As discussed above, apoptosis and regulated necrosis are characterized by distinguished morphologic, cell biologic, and biochemical features. However, these two forms of regulated cell death are not mutually exclusive, and in many pathologic conditions, including AKI, apoptosis and necrosis coexist. It remains unclear as to what determines if a given cell will die by apoptosis or necrosis.
Apoptosis has been discussed to cause secondary necrosis. However, apoptotic cells may break down their plasma membrane during prolonged injury, displaying a necrotic morphology in vitro. In vivo, apoptotic cells and their debris are thought to be rapidly removed by phagocytic cells.160–163 However, traditional apoptotic signaling, when intercepted, may be diverted to necrosis. This result is well exemplified by necroptosis, in which death receptors are activated but caspase 8 is blocked by pharmacologic or viral inhibition,89,90 resulting in RIPK3-mediated phosphorylation of MLKL and necroptosis.
Apoptosis and regulated necrosis may also interact at various molecular and cellular levels. Clearly, as presented above, both forms of cell death involve pathologic changes in mitochondria. Moreover, Bax is known as a classic proapoptotic Bcl-2 protein that permeabilizes mitochondrial membrane to release apoptotic factors for intrinsic apoptosis. However, Bax has recently been implicated in the regulation of MPT-related necrosis by affecting mitochondrial dynamics.164 In AKI, the involvement of Bax in necrosis may depend on experimental models. Although Bax-null mice showed less tubular apoptosis and necrosis in cisplatin nephrotoxic AKI,65 Bax ablation only has significant effects on tubular apoptosis in ischemia AKI.39 Recent work also showed that RIPK3, the key player in necroptosis, is capable of promoting apoptosis when the kinase function of RIPK3 is lost in vivo165 or in complex in vitro settings, in which cellular inhibitors of apoptosis or the kinase TAK1 are inhibited.166,167 Following this thought, the effectiveness of RIPK3 kinase inhibitors seems to depend on the interaction between RIPK1 and RIPK3 through their RHIM domains.102 Although these interactions between apoptosis and necroptosis have been worked out in detail, very limited data are available on the interaction between apoptosis and other pathways of regulated necrosis, especially pyroptosis, which is hard to investigate because of the limited specificity of caspase inhibitors168 and the dependency of both of these pathways on caspases.90 It is important to understand the complex interplay of the pathways of regulated cell death, especially with the therapeutic idea to target these pathways.
In AKI, very limited information is available on functional or morphologic consequences of interference with apoptosis on other pathways of regulated cell death or vice versa.115 Moreover, the relationship between various forms of cell death in AKI remains to be examined. However, regardless the etiology, AKI is known to involve mixed forms of regulated cell death, and, as presented above, suppression of one form of cell death may have significant renoprotective effects, which nonetheless, are mostly incomplete. The contributions of different forms of cell death in AKI also depend on the nature (ischemic, nephrotoxic, or septic AKI) and severity of the injury. It is important to understand the relative contributions and the potential redundancy of the cell-death pathways to guide the therapeutic strategies for AKI therapy as well as consider the obvious immunologic consequences on the basis of the release of DAMPs from necrotic cell death.169
Targeting Renal Cell Death for AKI Therapy
To date, >1400 PubMed-listed studies on “apoptosis and kidney” or “apoptosis and renal” have been published; unfortunately, no apoptosis-targeting approach has been made into clinical routine in any field (not restricted to the prevention of AKI). Unquestionably, apoptosis is involved in pathologic conditions in kidneys, notably AKI, but whether apoptosis significantly contributes to functional organ failure was recently questioned, because caspase inhibitors (zVAD-fmk, q-VD, and zIETD-fmk) are not efficacious in blocking AKI.27,115 In addition to inhibiting caspases, these inhibitors may affect other cell-death/survival-regulatory pathways, such as autophagy.170 Moreover, at least for death receptor–independent intrinsic apoptosis, inhibition of caspases is at a downstream level of apoptosis, and without blocking upstream apoptotic events, such as those at mitochondria, the viability of renal tubular cells is ultimately lost. In this regard, intrinsic apoptosis is not completely prevented in the presence of caspase inhibitors, like q-VD,171 which raises important questions as to how to target apoptosis for the prevention and treatment of AKI. It is noteworthy that, in AKI, apoptosis does not occur immediately or at one time point; rather, it persists in kidney tissues for days to weeks after injury. For example, in ischemic AKI in mice, tubular apoptosis starts a few hours after reperfusion, reaches the maximal level at 24–48 hours, and lasts for days. Thus, apoptosis, like regulated necrosis, is a continuous process in the disease condition. Accordingly, apoptosis is detected in a relatively low percentage of tubular cells in a snapshot fashion or at any given time points. Nonetheless, the cumulative number of apoptotic cells may become remarkable. In most clinical settings of AKI, patients have passed the initial injury phase; if tubular apoptosis was still occurring at the time of diagnosis, there would be a chance for apoptosis-targeting therapy to prevent additional deterioration of tissue and renal function and time for kidney repair. Additional in vivo investigations with techniques, such as intravital microscopy, may gain insights into the dynamics of apoptosis in AKI, and, more importantly, upstream apoptotic events should be targeted for effective therapy.
Obviously, as outlined in detail above, apoptosis is clearly involved in several pathologic conditions in the kidney, but significant prevention of the primary pathology seen in most preclinical conditions of AKI, necrosis, and functional markers of AKI has not been reproducibly reported through the addition of inhibitors of apoptosis. This finding is in line with the low levels of detection of cleaved caspase 3 in lysates taken from injured kidneys and rarely cleaved caspase 3–positive cells in immunohistochemistry. When it was realized in 2005 that regulated necrosis might serve as a therapeutic target by the identification of Nec-1,172 high hopes were raised for necroptosis-targeting strategies. It was, therefore, disappointing to realize that Nec-1 could only partially protect from ischemic AKI115 and that other pathways might be of importance other than necroptosis. Consequently, because combination therapy seems to be more effective,27 the protection is still incomplete, leaving significant histologic damage and DAMP release. Additional combinations may be useful (e.g., the addition of ferroptosis inhibitor), but it must be kept in mind that the translation of such results into clinical trials is highly problematic; control groups are required for any single- and double-therapeutic strategy, and support of such studies might become long-winded in the absence of strong, convincing preclinical evidence. In addition, for necroptosis, it is understood that plasma membrane rupture occurs as early as 20 minutes after RIPK3 dimerization,91 and application of Nec-1 30 minutes after the beginning of reperfusion has no detectable protective effect.115 Therefore, targeting regulated necrosis may be limited to such disorders in which AKI may be anticipated, like heart surgery–associated AKI, contrast-induced AKI, or kidney transplantation.
In conclusion, tubular apoptosis has been shown unequivocally in various types of AKI, including in diseased human kidneys, but despite 20 years of intensive research, an apoptosis-targeting strategy has not found its way into clinical routine. Recent work has further implicated different forms of regulated necrosis in AKI. Although significant advances have been made in the understanding of the cellular and molecular basis of cell death, targeting of signaling pathways of regulated necrosis for therapy has not yet been investigated in other than promising preclinical settings. Strategically, it is clear now that specific therapeutics have to block upstream events of cell death. In this regard, mitochondria, the converging point of cellular injury and death, may be a promising target of therapy, but mitochondria are not involved in some pathways of regulated necrosis, such as necroptosis, which are clearly relevant in AKI. Therefore, in view of the many subroutines of cell death in AKI, it is necessary to consider combination therapies that block multiple pathways of regulated cell death simultaneously or at different time points to ensure cell survival and renal function.
Disclosures
None.
Acknowledgments
The study was supported, in part, by National Natural Science Foundation of China Grant 81370791 and grants from the National Basic Research Program of China 973 Program 2012CB517601, the National Institutes of Health, and the Department of Veterans Administration. A.L. received support from Novartis, Pfizer, Fresenius, and the Else Kröner-Fresenius Stiftung.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R: Acute kidney injury: An increasing global concern. Lancet 382: 170–179, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY: Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol 24: 37–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu CY: Yes, AKI truly leads to CKD. J Am Soc Nephrol 23: 967–969, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Faubel S, Chawla LS, Chertow GM, Goldstein SL, Jaber BL, Liu KD, Acute Kidney Injury Advisory Group of the American Society of Nephrology : Ongoing clinical trials in AKI. Clin J Am Soc Nephrol 7: 861–873, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinsey GR, Sharma R, Okusa MD: Regulatory T cells in AKI. J Am Soc Nephrol 24: 1720–1726, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharfuddin AA, Molitoris BA: Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Zarjou A, Agarwal A: Sepsis and acute kidney injury. J Am Soc Nephrol 22: 999–1006, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS: Messengers without borders: Mediators of systemic inflammatory response in AKI. J Am Soc Nephrol 24: 529–536, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Schumer M, Colombel MC, Sawczuk IS, Gobé G, Connor J, O’Toole KM, Olsson CA, Wise GJ, Buttyan R: Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol 140: 831–838, 1992 [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly KJ, Plotkin Z, Vulgamott SL, Dagher PC: P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: Protective role of a p53 inhibitor. J Am Soc Nephrol 14: 128–138, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Kelly KJ, Plotkin Z, Dagher PC: Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Invest 108: 1291–1298, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basnakian AG, Ueda N, Kaushal GP, Mikhailova MV, Shah SV: DNase I-like endonuclease in rat kidney cortex that is activated during ischemia/reperfusion injury. J Am Soc Nephrol 13: 1000–1007, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Kaushal GP, Singh AB, Shah SV: Identification of gene family of caspases in rat kidney and altered expression in ischemia-reperfusion injury. Am J Physiol 274: F587–F595, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Gobé G, Zhang XJ, Willgoss DA, Schoch E, Hogg NA, Endre ZH: Relationship between expression of Bcl-2 genes and growth factors in ischemic acute renal failure in the rat. J Am Soc Nephrol 11: 454–467, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Basile DP, Liapis H, Hammerman MR: Expression of bcl-2 and bax in regenerating rat renal tubules following ischemic injury. Am J Physiol 272: F640–F647, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB: Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel) 2: 2490–2518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pabla N, Dong Z: Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Li S, Basnakian A, Bhatt R, Megyesi J, Gokden N, Shah SV, Portilla D: PPAR-alpha ligand ameliorates acute renal failure by reducing cisplatin-induced increased expression of renal endonuclease G. Am J Physiol Renal Physiol 287: F990–F998, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Baliga R: Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int 63: 1687–1696, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z: Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest 121: 2709–2722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramesh G, Reeves WB: p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol 289: F166–F174, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Sheikh-Hamad D, Cacini W, Buckley AR, Isaac J, Truong LD, Tsao CC, Kishore BK: Cellular and molecular studies on cisplatin-induced apoptotic cell death in rat kidney. Arch Toxicol 78: 147–155, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, Nick HS, Agarwal A: Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol 278: F726–F736, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Lerolle N, Nochy D, Guérot E, Bruneval P, Fagon JY, Diehl JL, Hill G: Histopathology of septic shock induced acute kidney injury: Apoptosis and leukocytic infiltration. Intensive Care Med 36: 471–478, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Havasi A, Borkan SC: Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S: Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 110: 12024–12029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z: Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279: 19948–19954, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Wei Q, Yin XM, Wang MH, Dong Z: Bid deficiency ameliorates ischemic renal failure and delays animal death in C57BL/6 mice. Am J Physiol Renal Physiol 290: F35–F42, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Wei Q, Wang MH, Dong Z: Differential gender differences in ischemic and nephrotoxic acute renal failure. Am J Nephrol 25: 491–499, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z: Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25: 4056–4066, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M: Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest 115: 3451–3459, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka T, Nangaku M, Miyata T, Inagi R, Ohse T, Ingelfinger JR, Fujita T: Blockade of calcium influx through L-type calcium channels attenuates mitochondrial injury and apoptosis in hypoxic renal tubular cells. J Am Soc Nephrol 15: 2320–2333, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery M, Thiemermann C, Yaqoob MM: Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol 15: 2115–2124, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Lee HT, Gallos G, Nasr SH, Emala CW: A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15: 102–111, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q: The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114: 795–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly KJ, Sutton TA, Weathered N, Ray N, Caldwell EJ, Plotkin Z, Dagher PC: Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 287: F760–F766, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Linkermann A, Kunzendorf U, Krautwald S: The authors reply. Kidney Int 83: 531, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z: Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int 84: 138–148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashkenazi A, Dixit VM: Death receptors: Signaling and modulation. Science 281: 1305–1308, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J: Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403: 98–103, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Lassus P, Opitz-Araya X, Lazebnik Y: Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 297: 1352–1354, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ: Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol 168: 5817–5823, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Knotek M, Rogachev B, Wang W, Ecder T, Melnikov V, Gengaro PE, Esson M, Edelstein CL, Dinarello CA, Schrier RW: Endotoxemic renal failure in mice: Role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney Int 59: 2243–2249, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Ortiz A, Lorz C, Egido J: The Fas ligand/Fas system in renal injury. Nephrol Dial Transplant 14: 1831–1834, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Ramesh G, Reeves WB: Inflammatory cytokines in acute renal failure. Kidney Int Suppl 91: S56–S61, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP: p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury. J Biol Chem 280: 31230–31239, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Baliga R: Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol 16: 1985–1992, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA: Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 17: 3401–3415, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA, Saikumar P: Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem 276: 18361–18374, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Mikhailov V, Mikhailova M, Degenhardt K, Venkatachalam MA, White E, Saikumar P: Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J Biol Chem 278: 5367–5376, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Qiao X, Chen X, Wu D, Ding R, Wang J, Hong Q, Shi S, Li J, Xie Y, Lu Y, Wang Z: Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci 60: 830–839, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Wolfs TG, de Vries B, Walter SJ, Peutz-Kootstra CJ, van Heurn LW, Oosterhof GO, Buurman WA: Apoptotic cell death is initiated during normothermic ischemia in human kidneys. Am J Transplant 5: 68–75, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Castaneda MP, Swiatecka-Urban A, Mitsnefes MM, Feuerstein D, Kaskel FJ, Tellis V, Devarajan P: Activation of mitochondrial apoptotic pathways in human renal allografts after ischemiareperfusion injury. Transplantation 76: 50–54, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481–490, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Li H, Zhu H, Xu CJ, Yuan J: Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Ruiz-Vela A, Opferman JT, Cheng EH, Korsmeyer SJ: Proapoptotic BAX and BAK control multiple initiator caspases. EMBO Rep 6: 379–385, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao Z, Shao Y, Jiang X: Essential roles of the Bcl-2 family of proteins in caspase-2-induced apoptosis. J Biol Chem 280: 38271–38275, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Martinou JC, Green DR: Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol 2: 63–67, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Kroemer G, Reed JC: Mitochondrial control of cell death. Nat Med 6: 513–519, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Youle RJ, Strasser A: The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Danial NN, Korsmeyer SJ: Cell death: Critical control points. Cell 116: 205–219, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Adams JM, Cory S: The Bcl-2 protein family: Arbiters of cell survival. Science 281: 1322–1326, 1998 [DOI] [PubMed] [Google Scholar]
- 64.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei Q, Dong G, Franklin J, Dong Z: The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int 72: 53–62, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Takahashi Y, Karbowski M, Yamaguchi H, Kazi A, Wu J, Sebti SM, Youle RJ, Wang HG: Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol Cell Biol 25: 9369–9382, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sawada M, Sun W, Hayes P, Leskov K, Boothman DA, Matsuyama S: Ku70 suppresses the apoptotic translocation of Bax to mitochondria. Nat Cell Biol 5: 320–329, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Yi X, Yin XM, Dong Z: Inhibition of Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J Biol Chem 278: 16992–16999, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Nomura M, Shimizu S, Sugiyama T, Narita M, Ito T, Matsuda H, Tsujimoto Y: 14-3-3 Interacts directly with and negatively regulates pro-apoptotic Bax. J Biol Chem 278: 2058–2065, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC: Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 423: 456–461, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Eskes R, Desagher S, Antonsson B, Martinou JC: Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol 20: 929–935, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR: Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303: 1010–1014, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Wang Z, Gall JM, Bonegio R, Havasi A, Illanes K, Schwartz JH, Borkan SC: Nucleophosmin, a critical Bax cofactor in ischemia-induced cell death. Mol Cell Biol 33: 1916–1924, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Youle RJ, van der Bliek AM: Mitochondrial fission, fusion, and stress. Science 337: 1062–1065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan DC: Fusion and fission: Interlinked processes critical for mitochondrial health. Annu Rev Genet 46: 265–287, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ: The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525, 2001 [DOI] [PubMed] [Google Scholar]
- 77.Brooks C, Wei Q, Cho SG, Dong Z: Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grohm J, Kim SW, Mamrak U, Tobaben S, Cassidy-Stone A, Nunnari J, Plesnila N, Culmsee C: Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ 19: 1446–1458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ: Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Cho SG, Du Q, Huang S, Dong Z: Drp1 dephosphorylation in ATP depletion-induced mitochondrial injury and tubular cell apoptosis. Am J Physiol Renal Physiol 299: F199–F206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z: Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci U S A 104: 11649–11654, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Waxman AB, Kolliputi N: IL-6 protects against hyperoxia-induced mitochondrial damage via Bcl-2-induced Bak interactions with mitofusins. Am J Respir Cell Mol Biol 41: 385–396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brooks C, Cho SG, Wang CY, Yang T, Dong Z: Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol 300: C447–C455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhan M, Brooks C, Liu F, Sun L, Dong Z: Mitochondrial dynamics: Regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int 83: 568–581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR: Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab 15: 186–200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaczmarek A, Vandenabeele P, Krysko DV: Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 38: 209–223, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Kono H, Rock KL: How dying cells alert the immune system to danger. Nat Rev Immunol 8: 279–289, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM: Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant 13: 2797–2804, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A: Molecular mechanisms of regulated necrosis [published online ahead of print February 26, 2014]. Semin Cell Dev Biol 10.1016/j.semcdb.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 90.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P: Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15: 135–147, 2014 [DOI] [PubMed] [Google Scholar]
- 91.Tait SW, Oberst A, Quarato G, Milasta S, Haller M, Wang R, Karvela M, Ichim G, Yatim N, Albert ML, Kidd G, Wakefield R, Frase S, Krautwald S, Linkermann A, Green DR: Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Reports 5: 878–885, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES: RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471: 368–372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR: Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471: 363–367, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Newton K, Sun X, Dixit VM: Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol 24: 1464–1469, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oberst A, Green DR: It cuts both ways: Reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol 12: 757–763, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Green DR: Apoptotic pathways: Ten minutes to dead. Cell 121: 671–674, 2005 [DOI] [PubMed] [Google Scholar]
- 97.Krammer PH, Arnold R, Lavrik IN: Life and death in peripheral T cells. Nat Rev Immunol 7: 532–542, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D: Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38: 27–40, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Linkermann A, Green DR: Necroptosis. N Engl J Med 370: 455–465, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J: Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1: 489–495, 2000 [DOI] [PubMed] [Google Scholar]
- 101.Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P: Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med 187: 1477–1485, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES: Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288: 31268–31279, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thapa RJ, Basagoudanavar SH, Nogusa S, Irrinki K, Mallilankaraman K, Slifker MJ, Beg AA, Madesh M, Balachandran S: NF-kappaB protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol Cell Biol 31: 2934–2946, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S: Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A 110: E3109–E3118, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Upton JW, Kaiser WJ, Mocarski ES: Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7: 302–313, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Upton JW, Kaiser WJ, Mocarski ES: DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11: 290–297, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK: Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137: 1112–1123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X: Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137: 1100–1111, 2009 [DOI] [PubMed] [Google Scholar]
- 109.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J: RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325: 332–336, 2009 [DOI] [PubMed] [Google Scholar]
- 110.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, Young SN, Varghese LN, Tannahill GM, Hatchell EC, Majewski IJ, Okamoto T, Dobson RC, Hilton DJ, Babon JJ, Nicola NA, Strasser A, Silke J, Alexander WS: The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39: 443–453, 2013 [DOI] [PubMed] [Google Scholar]
- 111.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X: Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148: 213–227, 2012 [DOI] [PubMed] [Google Scholar]
- 112.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG: Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A 109: 5322–5327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murphy JM, Lucet IS, Hildebrand JM, Tanzer MC, Young SN, Sharma P, Lessene G, Alexander WS, Babon JJ, Silke J, Czabotar PE: Insights into the evolution of divergent nucleotide-binding mechanisms among pseudokinases revealed by crystal structures of human and mouse MLKL. Biochem J 457: 369–377, 2014 [DOI] [PubMed] [Google Scholar]
- 114.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X: Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 54: 133–146, 2014 [DOI] [PubMed] [Google Scholar]
- 115.Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S: Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81: 751–761, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Nowak G, Soundararajan S, Mestril R: Protein kinase C-α interaction with iHSP70 in mitochondria promotes recovery of mitochondrial function after injury in renal proximal tubular cells. Am J Physiol Renal Physiol 305: F764–F776, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tristão VR, Gonçalves PF, Dalboni MA, Batista MC, Durão MS, Jr., Monte JC: Nec-1 protects against nonapoptotic cell death in cisplatin-induced kidney injury. Ren Fail 34: 373–377, 2012 [DOI] [PubMed] [Google Scholar]
- 118.Zhang L, Jiang F, Chen Y, Luo J, Liu S, Zhang B, Ye Z, Wang W, Liang X, Shi W: Necrostatin-1 attenuates ischemia injury induced cell death in rat tubular cell line NRK-52E through decreased Drp1 expression. Int J Mol Sci 14: 24742–24754, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Daemen MA, van ’t Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, Buurman WA: Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest 104: 541–549, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ouyang Z, Zhu S, Jin J, Li J, Qiu Y, Huang M, Huang Z: Necroptosis contributes to the cyclosporin A-induced cytotoxicity in NRK-52E cells. Pharmazie 67: 725–732, 2012 [PubMed] [Google Scholar]
- 121.Linkermann A, Heller JO, Prókai A, Weinberg JM, De Zen F, Himmerkus N, Szabó AJ, Bräsen JH, Kunzendorf U, Krautwald S: The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice. J Am Soc Nephrol 24: 1545–1557, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hakroush S, Cebulla A, Schaldecker T, Behr D, Mundel P, Weins A: Extensive podocyte loss triggers a rapid parietal epithelial cell response [published online ahead of print December 12, 2013]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Green DR, Ferguson T, Zitvogel L, Kroemer G: Immunogenic and tolerogenic cell death. Nat Rev Immunol 9: 353–363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tait SW, Green DR: Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11: 621–632, 2010 [DOI] [PubMed] [Google Scholar]
- 125.Kroemer G, Galluzzi L, Brenner C: Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Kim JS, He L, Lemasters JJ: Mitochondrial permeability transition: A common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304: 463–470, 2003 [DOI] [PubMed] [Google Scholar]
- 127.Bernardi P: The mitochondrial permeability transition pore: A mystery solved? Front Physiol 4: 95, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabó I, Lippe G, Bernardi P: Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A 110: 5887–5892, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Devalaraja-Narashimha K, Diener AM, Padanilam BJ: Cyclophilin D gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol 297: F749–F759, 2009 [DOI] [PubMed] [Google Scholar]
- 130.Park JS, Pasupulati R, Feldkamp T, Roeser NF, Weinberg JM: Cyclophilin D and the mitochondrial permeability transition in kidney proximal tubules after hypoxic and ischemic injury. Am J Physiol Renal Physiol 301: F134–F150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baumann K: Cell death: Multitasking p53 promotes necrosis. Nat Rev Mol Cell Biol 13: 480–481, 2012 [DOI] [PubMed] [Google Scholar]
- 132.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM: p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149: 1536–1548, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Karch J, Molkentin JD: Is p53 the long-sought molecular trigger for cyclophilin D-regulated mitochondrial permeability transition pore formation and necrosis? Circ Res 111: 1258–1260, 2012 [DOI] [PubMed] [Google Scholar]
- 134.Clarke SJ, McStay GP, Halestrap AP: Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem 277: 34793–34799, 2002 [DOI] [PubMed] [Google Scholar]
- 135.Weinberg JM, Roeser NF, Davis JA, Venkatachalam MA: Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int 52: 140–151, 1997 [DOI] [PubMed] [Google Scholar]
- 136.Feldkamp T, Park JS, Pasupulati R, Amora D, Roeser NF, Venkatachalam MA, Weinberg JM: Regulation of the mitochondrial permeability transition in kidney proximal tubules and its alteration during hypoxia-reoxygenation. Am J Physiol Renal Physiol 297: F1632–F1646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, André-Fouët X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M: Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 359: 473–481, 2008 [DOI] [PubMed] [Google Scholar]
- 138.Kim J, Padanilam BJ: Loss of poly(ADP-ribose) polymerase 1 attenuates renal fibrosis and inflammation during unilateral ureteral obstruction. Am J Physiol Renal Physiol 301: F450–F459, 2011 [DOI] [PubMed] [Google Scholar]
- 139.Kim J, Long KE, Tang K, Padanilam BJ: Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int 82: 193–203, 2012 [DOI] [PubMed] [Google Scholar]
- 140.Bergsbaken T, Fink SL, Cookson BT: Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol 7: 99–109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bergsbaken T, Fink SL, den Hartigh AB, Loomis WP, Cookson BT: Coordinated host responses during pyroptosis: Caspase-1-dependent lysosome exocytosis and inflammatory cytokine maturation. J Immunol 187: 2748–2754, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Muñoz-Arias I, Greene WC: Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505: 509–514, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Adamczak SE, de Rivero Vaccari JP, Dale G, Brand FJ, 3rd, Nonner D, Bullock MR, Dahl GP, Dietrich WD, Keane RW: Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab 34: 621–629, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chung SD, Lai TY, Chien CT, Yu HJ: Activating Nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS ONE 7: e47299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang JR, Yao FH, Zhang JG, Ji ZY, Li KL, Zhan J, Tong YN, Lin LR, He YN: Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J Physiol Renal Physiol 306: F75–F84, 2014 [DOI] [PubMed] [Google Scholar]
- 146.Lorenz G, Darisipudi MN, Anders HJ: Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant 29: 41–48, 2014 [DOI] [PubMed] [Google Scholar]
- 147.Krautwald S, Linkermann A: The fire within: Pyroptosis in the kidney. Am J Physiol Renal Physiol 306: F168–F169, 2014 [DOI] [PubMed] [Google Scholar]
- 148.Case CL, Shin S, Roy CR: Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun 77: 1981–1991, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sauer JD, Pereyre S, Archer KA, Burke TP, Hanson B, Lauer P, Portnoy DA: Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proc Natl Acad Sci U S A 108: 12419–12424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A: Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11: 1136–1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM: Non-canonical inflammasome activation targets caspase-11. Nature 479: 117–121, 2011 [DOI] [PubMed] [Google Scholar]
- 152.Krautwald S, Ziegler E, Rölver L, Linkermann A, Keyser KA, Steen P, Wollert KC, Korf-Klingebiel M, Kunzendorf U: Effective blockage of both the extrinsic and intrinsic pathways of apoptosis in mice by TAT-crmA. J Biol Chem 285: 19997–20005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR: RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447: 864–868, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR: Regulation of ferroptotic cancer cell death by GPX4. Cell 156: 317–331, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR: Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR: Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136: 4551–4556, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, Balla J, Verlander J, Darshan D, Kuhn LC, Agarwal A: Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123: 4423–4434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dixon SJ, Stockwell BR: The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10: 9–17, 2014 [DOI] [PubMed] [Google Scholar]
- 159.Fähling M, Mathia S, Paliege A, Koesters R, Mrowka R, Peters H, Persson PB, Neumayer HH, Bachmann S, Rosenberger C: Tubular von Hippel-Lindau knockout protects against rhabdomyolysis-induced AKI. J Am Soc Nephrol 24: 1806–1819, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Poon IK, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, Ravichandran KS: Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 507: 329–334, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, Ravichandran KS: Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 493: 547–551, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS: Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461: 282–286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vanden Berghe T, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N, Guerin CJ, Brunk UT, Declercq W, Vandenabeele P: Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ 17: 922–930, 2010 [DOI] [PubMed] [Google Scholar]
- 164.Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, Crow MT, Gavathiotis E, Dorn GW. 2ndO’Rourke B, Kitsis RN: Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A 109: 6566–6571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, Roose-Girma M, Warming S, Dixit VM: Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343: 1357–1360, 2014 [DOI] [PubMed] [Google Scholar]
- 166.Dondelinger Y, Aguileta MA, Goossens V, Dubuisson C, Grootjans S, Dejardin E, Vandenabeele P, Bertrand MJ: RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ 20: 1381–1392, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Remijsen Q, Goossens V, Grootjans S, Van den Haute C, Vanlangenakker N, Dondelinger Y, Roelandt R, Bruggeman I, Goncalves A, Bertrand MJ, Baekelandt V, Takahashi N, Berghe TV, Vandenabeele P: Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis. Cell Death Dis 5: e1004, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA: Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem 273: 32608–32613, 1998 [DOI] [PubMed] [Google Scholar]
- 169.Anders HJ, Schaefer L: Beyond tissue injury—damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis [published online ahead of print April 24, 2014]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Herzog C, Yang C, Holmes A, Kaushal GP: zVAD-fmk prevents cisplatin-induced cleavage of autophagy proteins but impairs autophagic flux and worsens renal function. Am J Physiol Renal Physiol 303: F1239–F1250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Josefsson EC, Burnett DL, Lebois M, Debrincat MA, White MJ, Henley KJ, Lane RM, Moujalled D, Preston SP, O’Reilly LA, Pellegrini M, Metcalf D, Strasser A, Kile BT: Platelet production proceeds independently of the intrinsic and extrinsic apoptosis pathways. Nat Commun 5: 3455, 2014 [DOI] [PubMed] [Google Scholar]
- 172.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J: Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1: 112–119, 2005 [DOI] [PubMed] [Google Scholar]
- 173.Weinlich R, Oberst A, Dillon CP, Janke LJ, Milasta S, Lukens JR, Rodriguez DA, Gurung P, Savage C, Kanneganti TD, Green DR: Protective roles for caspase-8 and cFLIP in adult homeostasis. Cell Reports 5: 340–348, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Linkermann A, De Zen F, Weinberg J, Kunzendorf U, Krautwald S: Programmed necrosis in acute kidney injury. Nephrol Dial Transplant 27: 3412–3419, 2012 [DOI] [PubMed] [Google Scholar]