Abstract
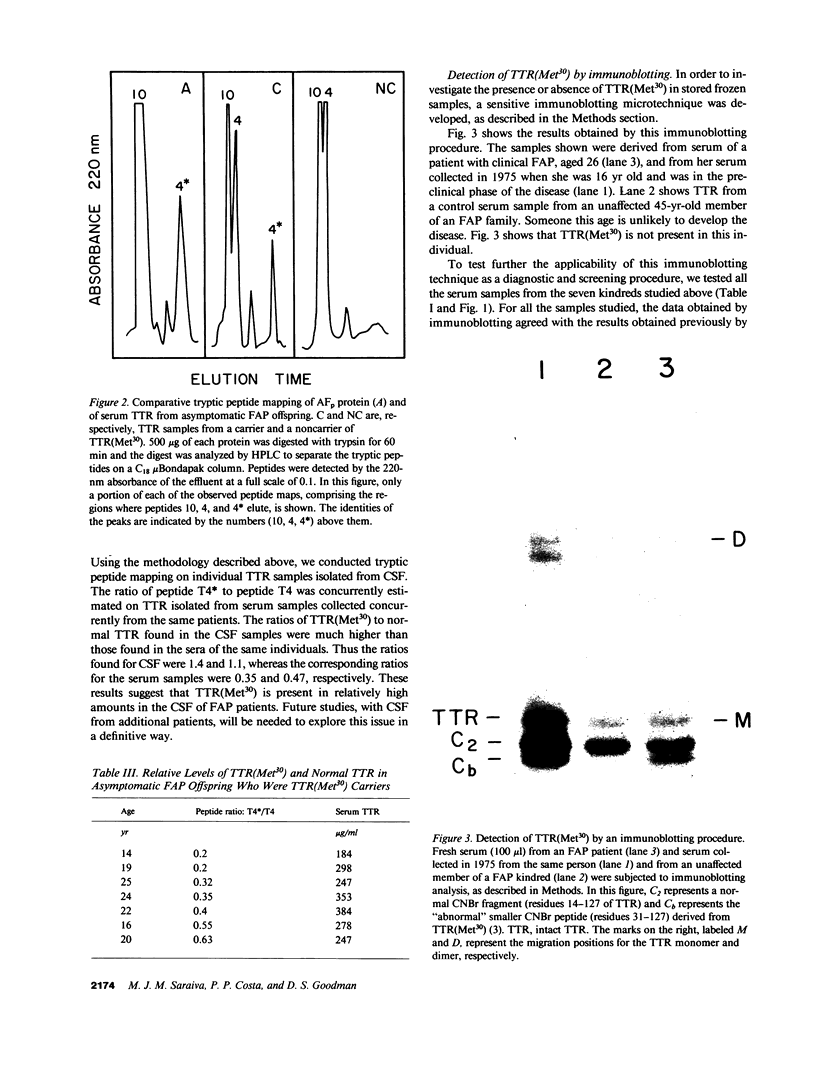
A transthyretin variant with a methionine for valine substitution at position 30 [TTR(Met30)] is found in Portuguese patients with familial amyloidotic polyneuropathy (FAP). Effective, rapid, small- and semimicro-scale (immunoblotting) procedures were developed to determine whether or not TTR(Met30) is present in the plasma of an individual subject. The immunoblotting procedure employs only 0.10 ml of serum and can serve as a reliable procedure for the screening of large numbers of persons for the presence of TTR(Met30). In family studies of seven FAP kindreds, TTR(Met30) was found in 21 out of 41 asymptomatic FAP offspring, and its presence was not related to either age or sex. Thus, the mutant TTR segregated in accordance with the known autosomal dominant mode of inheritance of FAP. Total plasma TTR levels were not reduced in asymptomatic FAP offspring who were carriers of TTR(Met30), and no difference was observed between carriers and noncarriers of the mutant TTR. The ratios of the variant to normal TTR in plasma were estimated in asymptomatic FAP offspring and were similar to those found in FAP patients. In contrast, TTR(Met30) was relatively enriched in cerebrospinal fluid samples from two FAP patients. The significance of this finding is not known, but might relate to the preferential deposition of amyloid in the nervous system in FAP. A limited study was conducted involving simultaneous analysis of both stored (collected in 1975) and fresh serum from 20 FAP offspring, all of whom had been asymptomatic in 1975. In every subject, the results obtained with the stored and the fresh serum samples were in agreement. Six of these subjects developed clinical FAP since 1975; TTR(Met30) was present in each of these subjects. These several studies strongly suggest that the presence of TTR(Met30) in plasma constitutes a predictive biochemical marker of FAP in the preclinical phase of the disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRADE C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain. 1952 Sep;75(3):408–427. doi: 10.1093/brain/75.3.408. [DOI] [PubMed] [Google Scholar]
- Aleshire S. L., Bradley C. A., Richardson L. D., Parl F. F. Localization of human prealbumin in choroid plexus epithelium. J Histochem Cytochem. 1983 May;31(5):608–612. doi: 10.1177/31.5.6341455. [DOI] [PubMed] [Google Scholar]
- Andrade C., Canijo M., Klein D., Kaelin A. The genetic aspect of the familial amyloidotic polyneuropathy. Portuguese type of paramyloidosis. Humangenetik. 1969;7(2):163–175. doi: 10.1007/BF00287080. [DOI] [PubMed] [Google Scholar]
- Benson M. D., Dwulet F. E. Identification of carriers of a variant plasma prealbumin (transthyretin) associated with familial amyloidotic polyneuropathy type I. J Clin Invest. 1985 Jan;75(1):71–75. doi: 10.1172/JCI111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carvalho J., Coimbra A., Andrade C. Peripheral nerve fibre changes in asymptomatic children of patients with familial amyloid polyneuropathy. Brain. 1976 Mar;99(1):1–10. doi: 10.1093/brain/99.1.1. [DOI] [PubMed] [Google Scholar]
- Costa P. P., Figueira A. S., Bravo F. R. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4499–4503. doi: 10.1073/pnas.75.9.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Polymorphism of human plasma thyroxine binding prealbumin. Biochem Biophys Res Commun. 1983 Jul 29;114(2):657–662. doi: 10.1016/0006-291x(83)90831-8. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Primary structure of an amyloid prealbumin and its plasma precursor in a heredofamilial polyneuropathy of Swedish origin. Proc Natl Acad Sci U S A. 1984 Feb;81(3):694–698. doi: 10.1073/pnas.81.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgenhauer K. Protein size and cerebrospinal fluid composition. Klin Wochenschr. 1974 Dec 15;52(24):1158–1164. doi: 10.1007/BF01466734. [DOI] [PubMed] [Google Scholar]
- Gianazza E., Arnaud P. A general method for fractionation of plasma proteins. Dye-ligand affinity chromatography on immobilized Cibacron blue F3-GA. Biochem J. 1982 Jan 1;201(1):129–136. doi: 10.1042/bj2010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakazato M., Kangawa K., Minamino N., Tawara S., Matsuo H., Araki S. Identification of a prealbumin variant in the serum of a Japanese patient with familial amyloidotic polyneuropathy. Biochem Biophys Res Commun. 1984 Jul 31;122(2):712–718. doi: 10.1016/s0006-291x(84)80092-3. [DOI] [PubMed] [Google Scholar]
- Nakazato M., Kangawa K., Minamino N., Tawara S., Matsuo H., Araki S. Radioimmunoassay for detecting abnormal prealbumin in the serum for diagnosis of familial amyloidotic polyneuropathy (Japanese type). Biochem Biophys Res Commun. 1984 Jul 31;122(2):719–725. doi: 10.1016/s0006-291x(84)80093-5. [DOI] [PubMed] [Google Scholar]
- Nakazato M., Kangawa K., Minamino N., Tawara S., Matsuo H., Araki S. Revised analysis of amino acid replacement in a prealbumin variant (SKO-III) associated with familial amyloidotic polyneuropathy of Jewish origin. Biochem Biophys Res Commun. 1984 Sep 28;123(3):921–928. doi: 10.1016/s0006-291x(84)80222-3. [DOI] [PubMed] [Google Scholar]
- Nakazato M., Kurihara T., Kangawa K., Matsuo H. Childhood detection of familial amyloidotic polyneuropathy. Lancet. 1985 Jan 12;1(8420):99–99. doi: 10.1016/s0140-6736(85)91984-1. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pras M., Prelli F., Franklin E. C., Frangione B. Primary structure of an amyloid prealbumin variant in familial polyneuropathy of Jewish origin. Proc Natl Acad Sci U S A. 1983 Jan;80(2):539–542. doi: 10.1073/pnas.80.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M. J., Birken S., Costa P. P., Goodman D. S. Amyloid fibril protein in familial amyloidotic polyneuropathy, Portuguese type. Definition of molecular abnormality in transthyretin (prealbumin). J Clin Invest. 1984 Jul;74(1):104–119. doi: 10.1172/JCI111390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M. J., Birken S., Costa P. P., Goodman D. S. Family studies of the genetic abnormality in transthyretin (prealbumin) in Portuguese patients with familial amyloidotic polyneuropathy. Ann N Y Acad Sci. 1984;435:86–100. doi: 10.1111/j.1749-6632.1984.tb13742.x. [DOI] [PubMed] [Google Scholar]
- Saraiva M. J., Costa P. P., Birken S., Goodman D. S. Presence of an abnormal transthyretin (prealbumin) in Portuguese patients with familial amyloidotic polyneuropathy. Trans Assoc Am Physicians. 1983;96:261–270. [PubMed] [Google Scholar]
- Saraiva M. J., Costa P. P., Goodman D. S. Studies on plasma transthyretin (prealbumin) in familial amyloidotic polyneuropathy, Portuguese type. J Lab Clin Med. 1983 Oct;102(4):590–603. [PubMed] [Google Scholar]
- Sasaki H., Sakaki Y., Matsuo H., Goto I., Kuroiwa Y., Sahashi I., Takahashi A., Shinoda T., Isobe T., Takagi Y. Diagnosis of familial amyloidotic polyneuropathy by recombinant DNA techniques. Biochem Biophys Res Commun. 1984 Dec 14;125(2):636–642. doi: 10.1016/0006-291x(84)90586-2. [DOI] [PubMed] [Google Scholar]
- Soprano D. R., Herbert J., Soprano K. J., Schon E. A., Goodman D. S. Demonstration of transthyretin mRNA in the brain and other extrahepatic tissues in the rat. J Biol Chem. 1985 Sep 25;260(21):11793–11798. [PubMed] [Google Scholar]
- Tawara S., Nakazato M., Kangawa K., Matsuo H., Araki S. Identification of amyloid prealbumin variant in familial amyloidotic polyneuropathy (Japanese type). Biochem Biophys Res Commun. 1983 Nov 15;116(3):880–888. doi: 10.1016/s0006-291x(83)80224-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]