Abstract
Prestorage leukoreduction of red cells is effective in reducing the incidence of HLA alloimmunization and improving the quality of stored packed red blood cells (PRBC). This study was conducted to evaluate the effectiveness of Imugard III-RC 4P in removing the leukocyte from packed red cells and the storage effects thereafter. The effects of buffy coat removal on the efficiency of leukofiltration, storage parameters of leukofiltered packed red blood cells and feasibility of prestorage leukofiltration were also assessed. Sixteen units each of buffy coat-depleted (LP) and nondepleted (NLP) PRBC were taken. Every unit was divided into two equal halves, one leukofiltered and other, non-leukofiltered. Cell counts, volume, hematocrit and hemoglobin were measured before and after filtration. Levels of K+, lactate dehydrogenase (LDH) and hemolysis were assessed in all the units weekly, post leukofiltration. Post leukofiltration, red cell and volume loss was within the specified limit in all the units. Residual leukocytes were significantly lesser in LP- PRBC compared to NLPPRBC. K+, LDH and hemolysis were significantly elevated in NLP- PRBC. Leukofiltered PRBC showed lesser elevation of K+, LDH and hemolysis towards the end of the storage period as compared to their unfiltered counterparts. Leukofilter is capable of performing ~4 log reduction. Buffy coat removal prior to filtration improves the efficiency of leukofilter and aids in improving the storage of red cells in terms of hemolysis.
Keywords: Leukofiltration, Packed red blood cells, Buffy coat, Hemolysis
Introduction
Leukocyte reduction of blood components is widely practiced to achieve a number of benefits. These include prevention of febrile reactions, avoiding leukocyte induced alloimmunization, reducing transmission of cytomegalovirus to levels comparable to those achieved through use of seronegative units [1–3]. Immunosuppression induced by allogeneic transfusion may also be abrogated by leukoreduction [4, 5]. Since 1950s, it has been clearly established that removal of buffy coat from the whole blood, removes 70–80 % of the WBCs and is sufficient in preventing many febrile non hemolytic transfusion reactions (FNHTRs) to blood but not HLA alloimmunization or cytomegalovirus transmission [6, 7].
Filtration has emerged as the most commonly used method of leukocyte reduction. Leukocyte reduction by means of filtration can be performed at three different points in time—before storage, after storage but before issue fr the blood bank, and at the bedside. Prestorage leukofiltration may offer additional benefits over leukocyte removal during or after the storage period in terms of avoiding the potential of reaction and alloimmunization, in several ways. Firstly, HLA antigens may solubilize from leukocyte membranes during storage, passing through a filter applied after storage and immunizing the recipient [8, 9]. Secondly, cytokines released from leukocytes during storage, may cause FNHTR in transfusion recipients [10, 11]. Furthermore, release of enzymes by the leukocytes during storage may be detrimental to red cell metabolism and viability [12, 13]. In addition, conducting the filtration in laboratory rather than at bedside, permits quality control checks and tighter control over the performance of the technique and leads to more uniform results [14].
This study was conducted to evaluate and document the functional properties of the Imuguard III-RC filter system and the storage effects on the leukoreduced red blood cells it produces. We have also assessed the feasibility of prestorage filtration as a routine protocol by studying the effect of buffy coat removal on the efficiency of leukofiltration and on storage parameters of packed red blood cells.
Materials and Methods
The study was reviewed and approved by the institutional ethics committee and informed consent was obtained from subjects. All subjects met standard criteria for allogeneic blood donors according to our SOP. Total 32 units of whole blood were selected by random assignment for inclusion in the study.
Plan of Study
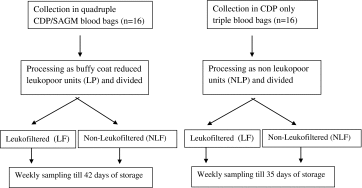
Component Preparation
Sixteen whole blood units were collected in quadruple CDP/SAGM blood bags of 450 mL (Terumo Penpol, Japan) and placed on a bench top at room temperature for 8 h. These units were processed into leuko-reduced blood components using T-ACE (M/S Terumo Penpol, Japan) automated component extractor. After separation of plasma and buffy coat, the additive solution (AS-5 Optisol, 100 ml) was added to make leukopoor packed red blood cells (LP-PRBC). Another 16 whole blood units were collected into CPD-only triple blood bags of 450 ml and manually processed into non-leukopoor packed red blood cells (NLP-PRBC). Further, each of the packed red cell units (LP-PRBC and NLP-PRBC) were weighed accurately and divided in two equal halves using sterile connecting device Composeal (M/S Fresenius, Germany). These were then stored under standard blood bank conditions at 4 °C. One semi-unit of each PRBC was leukofiltered (LF) using lab-side red cell leukofilter (Imugard III-RC 4P, Terumo Corporation, Tokyo, Japan) on the day of its processing (day 0); another semi-unit (NLF) which served as a control, was left as such, till the end of the storage period (i.e. day 35 for NLP- PRBC units and day 42 for LP- PRBC units).
Sampling
Serial samples were taken by aseptic techniques from each halves stored at 4 °C at weekly intervals, starting on the day of processing (day 0) up to expiry for determination of characteristic storage measures. Cell counts, Hematocrit and Hemoglobin levels were assessed using automated cell analyzer (Sysmax KX-21, Cobe, Japan). Residual leukocyte counts were determined in these samples microscopically using Nageottes chamber, as per the SOP. Later, the blood samples were spun for 20 min at 2,600 rpm (Cubota, Japan) and the supernatant were analysed for following:
The level of potassium (K+) and LDH in plasma was measured by biochemistry analyser (Vitros DT60II, Ortho-Clinical diagnostics, High Wycombe, UK).
The level of plasma Hb was determined by plasma-hemoglobinometer (Hemocue, USA).
After 42 days, sterility tests were carried for all the units to rule out bacterial contamination.
Data Analyses
The data for cell counts and hemoglobin was calculated from each semi-units and then extrapolated to quantity per bag by multiplying with pre-division volume of PRBC unit of either type. Percent hemolysis was calculated using the following equation:
Percent hemolysis = [Supernatant Hb (g %)/total Hb of the bag (g %)] X [1- Hct].
Statistical analysis was performed using commercially available software for personal computers (SPSS 17.0 for Windows, SPSS GmbH, Munich, Germany). Student t test was used for parametric analyses of normally distributed data. P values <0.05 were considered significant.
Results
Filter Performance
Sixteen halves each of NLP-PRBC and LP- PRBC units were subjected to leukofilteration. Mean filtration time was 18 min for NLP-PRBC and 15 min for LP- PRBC. Mean changes in volume, hematocrit, hemoglobin, RBC count and WBC count are as shown in Table 1.
Table 1.
Filter performance for various parameters of NLP and LP packed red blood cells
| Leukofilteration | p value* | (%) Reduction | |||
|---|---|---|---|---|---|
| Pre leukofilteration (Mean ± SD) |
Post leukofilteration (Mean ± SD) |
||||
| Volume (mL) | NLP | 262.77 ± 4.72 | 212.62 ± 4.64 | >0.05 | 19.09 |
| LP | 301.11 ± 6.16 | 251.54 ± 6.36 | 16.47 | ||
| Hb conc (gm/bag) | NLP | 55.01 ± 2.78 | 50.69 ± 2.86 | >0.05 | 7.86 |
| LP | 53.06 ± 3.09 | 48.51 ± 3.62 | 8.50 | ||
| Hct (%) | NLP | 61.09 ± 2.97 | 60.27 ± 3.30 | >0.05 | 1.35 |
| LP | 56.51 ± 2.67 | 55.79 ± 2.59 | 1.18 | ||
| RBC Count (X 1011/bag) | NLP | 20.01 ± 1.73 | 17.13 ± 1.11 | >0.05 | 14.40 |
| LP | 19.46 ± 1.64 | 16.75 ± 1.16 | 13.88 | ||
| WBC Count (X 106/bag) | NLP | 2,261.56 ± 167.67 | 0.75 ± 0.171 | <0.05 | Log103.8 |
| LP | 768 ± 58.56 | 0.15 ± 0.023 | Log103.7 | ||
*p value for significance in difference between post filtration values of LP- PRBC and NLP- PRBC
Pre-filtration Values
The mean volume of NLP-PRBC units was 262.77 ± 4.72 mL whereas it was higher for LP-PRBC units (301.11 ± 6.16 mL) due to addition of SAGM in these bags. Due to buffy coat removal, the mean RBC and WBC counts per bag were lower in LP-PRBC (19.46 ± 1.64 × 1011, 768 ± 58.56 × 106) compared to NLP- PRBC units (20.01 ± 1.73 × 1011, 2,261.56 ± 167.67 × 106). The mean hematocrit and total hemoglobin content was 61.09 ± 2.97 % and 55.01 ± 2.78 g/bag for NLP-PRBC and 56.51 ± 2.67 % and 53.06 ± 3.09 g/bag for LP-PRBC.
Post Filtration Values
The approximate volume loss was 50 mL for both type of PRBC. The mean decrease in hematocrit per bag was 1.35 % for NLP- PRBC and 1.18 % for LP- PRBC. The mean difference in hemoglobin (about 2 g) between two groups observed before filtration is still found after filtration, showing that hemoglobin loss due to filtration is similar for both groups. For LP- PRBC, decrease in hemoglobin per bag was 4.55 g/dL (8.5 %) and the mean decrease in RBC count per bag was 2.71 × 1011 (13.88 %). The mean leukocyte count per bag reduced to 0.15 ± 0.023 × 106 after leukofilteration. For NLP- PRBC, the mean decrease in hemoglobin per bag was 4.32 g/dL (7.86 %) and the mean decrease in RBC count per bag was 2.88 × 1011(14.40 %). The mean leukocyte count per bag was reduced to 0.75 ± 0.171 × 106 after leukofiltration.
As shown in Table 2, initially in the LF subunits, the levels of K+ (2.93 ± .35 mmol/L), LDH (226.88 ± 43.26U/L) and hemolysis (0.0412 ± 0.0083 %) were higher compared to NLF units (K+2.73 ± .46 mmol/L, LDH 197.38 ± 51.75 U/L, hemolysis 0.0363 ± 0.0096 %). This difference was however not significant statistically (p > 0.05). The mean levels of these biochemical parameters remained higher in LF subunits as compared to their NLF counterparts, till 4 weeks of storage. Afterwards, the levels of K+, LDH and hemolysis increased in NLF subunits as compared to LF subunits and this increase was progressive. At the end of the storage period, the levels of these biochemical parameters were higher in NLF subunits compared to LF units but the difference was not statistically significant (p < 0.05).
Table 2.
Alterations in various storage parameters with leukofiltration in LP and NLP- PRBC (Mean ± SD)
| Week 0 | 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LF | NLF | LF | NLF | LF | NLF | LF | NLF | ||
| K+ (mmol/L) | LP | 2.93 ± 0.35 | 2.73 ± 0.46 | 8.30 ± 0.87 | 7.92 ± 0.91 | 16.55 ± 1.32 | 15.63 ± 1.93 | 24.56 ± 1.57 | 24.38* ± 2.04 |
| NLP | 3.16 ± 0.37 | 2.93 ± 0.33 | 8.74 ± 0.79 | 8.31 ± 0.74 | 17.14 ± 1.03 | 16.46 ± 1.49 | 25.35 ± 1.62 | 26.53 ± 1.14 | |
| LDH (U/L) | LP | 226.88 ± 43.26 | 197.38 ± 51.75 | 365.75 ± 58.14 | 349.13 ± 55.07 | 576.50 ± 95.28 | 547.88 ± 115.33 | 718.75 ± 118.02 | 679.94* ± 117.18 |
| NLP | 229 ± 34.60 | 199.75 ± 31.69 | 391.56 ± 37.91 | 371.38 ± 32.24 | 610.50 ± 68.19 | 585.38 ± 62.38 | 772.25 ± 78.64 | 793.69 ± 70.64 | |
| Hemolysis (%) | LP | 0.0412 ± 0.0096 | 0.0363 ± 0.0096 | 0.1081 ± 0.0207 | 0.0938 ± 0.0244 | 0.1600 ± 0.0346 | 0.1506 ± 0.0306 | 0.2475 ± 0.0580 | 0.2413 ± 0.0427 |
| NLP | 0.0419 ± 0.0083 | 0.0369 ± 0.0094 | 0.1169 ± 0.0153 | 0.1088 ± 0.014 | 0.1738 ± 0.0252 | 0.1675 ± 0.0201 | 0.2669 ± 0.0199 | 0.2550 ± 0.0309 | |
| 4 | 5 | 6 | |||||
|---|---|---|---|---|---|---|---|
| LF | NLF | LF | NLF | LF | NLF | ||
| K+ (mmol/L) | LP | 33.50* ± 2.10 | 34.36* ± 2.30 | 43.31* ± 1.47 | 44.93* ± 1.52 | 55.98 ± 1.95 | 58.16 ± 1.33 |
| NLP | 35.63 ± 1.28 | 37.33 ± 1.60 | 47.35 ± 1.31 | 48.62 ± 1.21 | |||
| LDH (U/L) | LP | 843* ± 123.84 | 855.19* ± 99.16 | 1013.06* ± 130.31 | 1060.88* ± 128.94 | 1304 ± 123.40 | 1372.56 ± 129.94 |
| NLP | 1002.75 ± 68.63 | 1049.06 ± 70.10 | 1241.19 ± 80.31 | 1298.75 ± 91.119 | |||
| Hemolysis (%) | LP | 0.2950 ± 0.0668 | 0.3075* ± 0.0558 | 0.3550* ± 0.0666 | 0.3788* ± 0.0637 | 0.4306 ± 0.0668 | 0.4581 ± 0.0660 |
| NLP | 0.3250 ± 0.0203 | 0.3481 ± 0.0258 | 0.3963 ± 0.0212 | 0.4256 ± 0.0199 | |||
*p value <0.05 between LP and NLP- PRBC
Effect of Buffy Coat Removal
As evident from Table 1, there is no significant difference (p > 0.05) between postfiltration values of volume, RBC count, hemoglobin content and hematocrit of NLP- PRBC and LP- PRBC units. However, the leukofiltration achieved in LP- PRBC is significantly greater compared to NLP- PRBC units (p < 0.05).
The weekly estimates of mean K+, LDH and hemolysis in NLF and LF halves of both NLP and LP-PRBC units are as shown in Table II. Pre-leukofiltration, the mean levels of biochemical parameters in LP-PRBC units (K+2.73 ± 0.46 mmol/L, LDH 197.38 ± 51.75 U/L, hemolysis 0.0363 ± .0096 %) were almost similar to those of NLP-PRBC units (K+ 2.93 ± .033 mmol/L, LDH 199.75 ± 31.69 U/L, hemolysis 0.0369 ± .0094 %). After leukofilteration, there was a rise in their levels, in both types of units which was not significantly different (p > 0.05).
In the first and second week, the levels of these markers were higher in NLP- PRBC units compared to LP-PRBC units in both LF and NLF groups, but the difference was not statistically significant.
In the third week, in NLF subunits of NLP- PRBC units, the mean levels of K+ (26.53 ± 1.14 mmol/L) and LDH (793.69 ± 70.64U/L) were significantly higher (p < 0.05) compared to LP- PRBC units (K+ 24.38 ± 2.04 mmol/L, LDH 679.94 ± 117.18 U/L). The increase in hemolysis in NLP-PRBC units (0.2550 ± .0309 vs. 0.2413 ± .0427 %) was not statistically significant (p > 0.05). Neither of the parameters showed a statistically significant difference (p > 0.05) in the LF subunits of both type of PRBC.
In the fourth week, in NLF subunits of NLP- PRBC units, the mean levels of K+ (37.33 ± 1.60 mmol/L), LDH (1049.06 ± 70.10U/L) and hemolysis (0.3481 ± .0258 %) were all significantly higher statistically (p < 0.05) compared to LP- PRBC (K+ 34.36 ± 2.30 mmol/L, LDH 855.19 ± 99.16 U/L, hemolysis 0.3075 ± .0558 %). In LF subunits, the differences were statistically significant for the mean levels of K (35.63 ± 1.28 vs. 33.50 ± 2.10 mmol/L) and LDH (1002.75 ± 68.63 vs. 843 ± 123.84 U/L) of NLP- PRBC and LP- PRBC units (p < 0.05).
At the end of the storage period i.e. fifth week, the mean levels of K, LDH and hemolysis were significantly higher (p < 0.05) in both NLF subunits (K+ 48.62 ± 1.21 vs. 44.93 ± 1.52 mmol/L, LDH 1298.75 ± 91.119U/L vs. 1060.88 ± 128.94 U/L, hemolysis 0.4256 ± 0.0199 vs. 0.3788 ± 0.0637 %) and LF subunits (K+ 47.35 ± 1.31 vs. 43.31 ± 1.47 mmol/L, LDH 1241.19 ± 80.31 U/L vs 1013.06 ± 130.31 U/L, hemolysis 0.3963 ± 0.0212 vs. 0.3550 ± 0.0666 %) of NLP-PRBC units compared to LP- PRBC units.
Discussion
Transfusion of leukoreduced blood components is assuming greater significance with increase in patient population requiring multiple transfusions such as onco-hematology and thalassemia patients. Prestorage leukofiltration of blood components has been emphasized in studies conducted in recent past to prevent cytokine accumulation leading to FNHTRs, HLA alloimmunization and transfusion related immunomodulation. This study was designed to evaluate the properties of the Imugard III-RC filter system, assess the effect of buffy coat removal on the efficiency of leukofiltration and on RBC hemolysis parameters in packed red blood cells. To avoid the introduction of other variables (inter-donor variability of Hb, hematocrit, WBC count and RBC count), we used a design in which one PRBC unit was divided into two equal subunits and prestorage leukofilteration of one subunit allowed us to specifically assess the effect of post storage leukoreduction on identical RBCs.
The Imugard III-RC prestorage leukofiltration system provides a means to remove ~4 log10 leukocytes from a Red Blood Cell unit. Leukocyte content below 5 × 106/unit is necessary for a unit to be considered ‘leukoreduced’ as per the AABB standards (this value is medically appropriate and practically achievable as per AABB Comments to FDA on Draft Guidance relating to Leukoreduction on 5/1/01) and leukocyte reduction by filtration of RBCs must not result in a red cell loss of >15 % [15]. Residual leukocytes must be below 1 × 106/unit according to the Council of Europe’s standards and a minimum of 40 g of hemoglobin must be present in each unit after leukoreduction [16]. All units prepared with the Imugard III-RC filter in this study met all these criteria. Other parameters such as volume, hematocrit etc. were also within the range specified by British committee for standards in hematology [17]. Prestorage leukoreduction is said to have certain potential drawbacks that have limited its application. One of these is retention of red cells in the filter and tubing. To combat this, Imugard III-RC prestorage leukoreduction system incorporates an air valve that automatically allows filtered (sterile) air into the system at the conclusion of filtration to drain the red cells from the filter housing and tubing. A similar study was conducted in Thailand to evaluate the effectiveness of Imugard III-RC in removing leukocyte from packed red cells. The authors have reported approximately 15 % red cell loss and 99.99 % WBC reduction, similar to the findings of this study [18].
A comparison of filtered and unfiltered units over a period of 42 days showed that hemolysis rates were higher in the filtered PRBC units initially as compared to unfiltered PRBC units in both groups. It has been assumed that during leukoreduction damaged or fragile cells may be removed selectively because undeformable cells are easily trapped in capillary beds or filter matrices [19]. This selective depletion of damaged cells accounts for increased hemolysis immediately after filtration as seen in our study as well as in other studies also [20]. Towards the end of the storage period, the picture was reversed i.e. the unfiltered units were showing a greater degree of hemolysis, compared to filtered ones, yet the level of hemolysis in all the units was still well below the limit of 0.8 % suggested by the international guidelines [21]. The quantitative data arising from this study did not support concerns raised by receiving clinicians of increased hemolysis in the leukofiltered units, consistent with other studies in the literature [22–25]. Most studies ascribe this improvement of RBC survival of filtered units to the lack of hydrolytic enzymes derived from WBCs dying in the storage container [26, 27]. Small but statistically significant decreases in hemolysis, increases in ATP levels and improvements in poststorage in vivo recovery have been reported in association with prestorage leukofiltration [12, 13].
In terms of residual leukocytes, the results confirm that buffy-coat removal obviously has improved the filtration performances similar to that reported by a previous study [28]. This difference is obviously ascribed to lower influent leukocyte count in LP-PRBC units, but all units were WBC reduced to the level, which is presumed to be sufficient for the avoidance of alloimmunization against HLA antigens and for significantly reducing the risk of cytomegalovirus transmission [3].
Although hemolysis of all the units increased with time during standard storage conditions, the hemolysis present in prestorage LP-PRBC units was consistently lower than hemolysis in the corresponding NLP-PRBC units (Table 2). The differences between the units increased with storage time although significance (p < 0.05) between the hemolysis parameters of LP-PRBC and NLP-PRBC was seen only after 3 weeks of storage onward. These findings are in accordance with early studies of Hoegman who showed that increased hemolysis was induced by leucocytes if red cell concentrates were stored in protein free additive solution [26]. No unit, however, in either arm contained a level of hemolysis above the normally accepted level of 1 percent. In our study, the differences in K+, LDH and hemolysis values in LF and NLF units were more distinct for the NLP- PRBC than for the LP- PRBC units. Our results are in line with those of previous studies and indicate that the beneficial effects of prestorage leukofiltration on storage measures are greater for conventionally prepared PRBCs with a larger WBC load, whereas improvements in filtered RBCs are less in buffy coat-depleted units. [23, 29].
In conclusion, the filter evaluated in the study is capable of reducing WBC content by ~4 log. The total numbers of WBCs in the filtered units were within acceptable limits, regardless of the nature of the PRBC preparation procedure. The buffy coat removal of PRBC units offers additional benefit in terms of lesser residual leukocytes and lesser elevation of hemolysis markers during storage.
Conflict of Interest
None.
Contributor Information
Atul Sonker, Email: dratulsonkar@yahoo.com.
Anju Dubey, Phone: +918004904431, FAX: 0522-2668017, Email: dranjudubey@gmail.com.
Rajendra Chaudhary, Email: rkcsgpgi@gmail.com.
References
- 1.Meryman HT. Transfusion-induced alloimmunization and immunosuppression and the effects of leukocyte depletion. Transfus Med Rev. 1989;3:180–193. doi: 10.1016/S0887-7963(89)70078-X. [DOI] [PubMed] [Google Scholar]
- 2.Sniecinski I, O’Donnell MR, Nowicki B, Hill LR. Prevention of refractoriness and HLA-alloimmunization using filtered blood products. Blood. 1988;71:1402–1407. [PubMed] [Google Scholar]
- 3.Bowden RA, Slichter SJ, Sayers M, et al. A comparison of leukocyte-reduced and cytomegalovirus (CMV) seronegative blood products for the prevention of transfusion-associated CMV infection after marrow transplant. Blood. 1995;86:3598–3603. [PubMed] [Google Scholar]
- 4.Busch MP, Lee TH, Heitman J. Allogeneic leukocytes but not therapeutic blood elements induce reactivation and dissemination of latent human immunodeficiency virus type 1 infection: implications for transfusion support of infected patients. Blood. 1992;80:2128–2135. [PubMed] [Google Scholar]
- 5.Blajchman MA, Bardossy L, Carmen R, et al. Allogeneic blood transfusion-induced enhancement of tumor growth: two animal models showing amelioration by leukodepletion and passive transfer using spleen cells. Blood. 1993;81:1880–1882. [PubMed] [Google Scholar]
- 6.Payne R. The association of febrile transfusion reactions with leuko-agglutinins. Vox Sang. 1957;2:233–241. doi: 10.1111/j.1423-0410.1957.tb03698.x. [DOI] [PubMed] [Google Scholar]
- 7.Perkins HA, Payne R, Ferguson J, Wood M. Nonhaemolytic febrile transfusion reactions. Quantitative effects of blood components with emphasis on isoantigenic incompatibility of leukocytes. Vox Sang. 1966;11:578–600. doi: 10.1111/j.1423-0410.1966.tb04256.x. [DOI] [PubMed] [Google Scholar]
- 8.Blajchman MA, Bardossy L, Carmen RA, et al. An animal model of allogeneic donor platelet refractoriness: the effect of the time of leukodepletion. Blood. 1992;79:1371–1375. [PubMed] [Google Scholar]
- 9.Ramos RR, Curtis BR, Duffy BF, Chaplin H. Low retention of white cell fragments by polyester fiber white cell-reduction platelet filters. Transfusion. 1994;34:31–34. doi: 10.1046/j.1537-2995.1994.34194098599.x. [DOI] [PubMed] [Google Scholar]
- 10.Stack G, Snyder EL. Cytokine generation in stored platelet concentrates. Transfusion. 1994;34:20–25. doi: 10.1046/j.1537-2995.1994.34194098597.x. [DOI] [PubMed] [Google Scholar]
- 11.Heddle NM, Klama L, Singer J, et al. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med. 1994;331:625–628. doi: 10.1056/NEJM199409083311001. [DOI] [PubMed] [Google Scholar]
- 12.Brecher ME, Pineda AA, Torloni AS. Prestorage leukocyte depletion: effect on leukocyte and platelet metabolites, erythrocyte lysis, metabolism and in vivo survival. Semin Hematol. 1991;28(suppl 5):3–9. [PubMed] [Google Scholar]
- 13.Davey RJ, Carmen RA, Simon TL. Preparation of white cell-depleted red cells for 42 day storage using an integral in-line filter. Transfusion. 1989;29:496–499. doi: 10.1046/j.1537-2995.1989.29689318446.x. [DOI] [PubMed] [Google Scholar]
- 14.Freedman JJ, Blajchman MA, McCombie N. Canadian red cross society symposium on leukodepletion: report of proceedings. Transfus Med Rev. 1994;8:1–14. doi: 10.1016/S0887-7963(94)70093-6. [DOI] [PubMed] [Google Scholar]
- 15.Roback JD, Combs MR, Grossman BJ, Hillyer CD. American Association of Blood Banks: Technical Manual, 16th ed Bethesda; 2008
- 16.Council of Europe . Guide to the preparation, use and quality assurance of blood components. 12. France: Council of Europe Publishing Strasbourg; 2006. [Google Scholar]
- 17.British Committee for Standards in Haematology Guidelines on the clinical use of leukocyte- depleted blood components. Transfusions Med. 1998;8:59–71. doi: 10.1046/j.1365-3148.1998.00129.x. [DOI] [PubMed] [Google Scholar]
- 18.Kijkornphan S, Moungkote T, Somboonvit P, Noparat K, Chiewsilp P. Effectiveness of leukocyte removal by Imugard III. J Med Assoc Thai. 1997;80(Suppl 1):S9–S12. [PubMed] [Google Scholar]
- 19.Baerlocher GM, Meiselman HJ, Reinhart WH. Gel-filtration of sickle erythrocytes: separation based on cell deformability. Clin Hemorheol Microcirc. 2001;24:11–18. [PubMed] [Google Scholar]
- 20.Muller-Steinhardt M, Janetzko K, Kirchner H, Kluter H. Effect of whole blood preparation and leukocyte filtration on storage of erythrocyte concentrates over 42 days. Beitr Infusionsther Transfusionsmed. 1997;34:53–57. [PubMed] [Google Scholar]
- 21.Council of Europe . Guide to the preparation, use, and quality assurance of blood components. France: Council of Europe Press Strasbourg; 1995. [Google Scholar]
- 22.Brecher ME, Pineda AA, Torloni AS, et al. Prestorage leukocyte depletion: effect on leukocyte and platelet metabolites, erythrocyte lysis, metabolism and in vivo survival. Semin Hematol. 1991;28:3–9. [PubMed] [Google Scholar]
- 23.Heaton WA, Holme S, Smith K, et al. Effects of 3–5 log10 prestorage leukocyte depletion on red cell storage and metabolism. Br J Haematol. 1994;87:363–368. doi: 10.1111/j.1365-2141.1994.tb04923.x. [DOI] [PubMed] [Google Scholar]
- 24.AuBuchon JP, Elfath MD, Popovsky MA, et al. Evaluation of a new prestorage leukoreduction filter for red blood cell units. Vox Sang. 1997;72:101–106. doi: 10.1046/j.1423-0410.1997.7220101.x. [DOI] [PubMed] [Google Scholar]
- 25.Williamson LM, Rider JR, Swann ID, et al. Evaluation of plasma and red cells obtained after leucocyte depletion of whole blood. Transfus Med. 1999;9:51–61. doi: 10.1046/j.1365-3148.1999.009001051.x. [DOI] [PubMed] [Google Scholar]
- 26.Hogman CF, Hedlund K, Akerblom O, Venge P. Red blood cell preservation in protein-poor media. I. Leukocyte enzymes as a cause of hemolysis. Transfusion. 1978;18:233–241. doi: 10.1046/j.1537-2995.1978.18278160591.x. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama T, Hanaoka K. Hemolysis in stored red blood cell concentrates: modulation by haptoglobin or ulinastatin, a protease inhibitor. Crit Care Med. 2001;29:1979–1982. doi: 10.1097/00003246-200110000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Masse M, Andreu G, Angue M, et al. A multicenter study on the efficiency of white cell reduction by filtration of red cells. Transfusion. 1991;31:792–797. doi: 10.1046/j.1537-2995.1991.31992094664.x. [DOI] [PubMed] [Google Scholar]
- 29.Muller-Steinhardt M, Janetzko K, Kandler R, et al. Impact of various red cell concentrate preparation methods on the efficiency of prestorage white cell filtration and on red cells during storage for 42 days. Transfusion. 1997;37:1137–1142. doi: 10.1046/j.1537-2995.1997.37111298088042.x. [DOI] [PubMed] [Google Scholar]


