Abstract
Genetic polymorphisms in the methylene tetrahydrofolate reductase (MTHFR) gene have been associated with the development of acute leukemias and various malignancies. The role of MTHFR polymorphism in the development of pediatric acute lymphoblastic leukemia (ALL) has been extensively studied among north Indians in various settings, yet its association with acute leukemias remains unresolved. To evaluate the relationship between functional MTHFR polymorphisms, C677T and A1298C and possible effect on risk of ALL in adults and children in North Indian population by comparing them with healthy controls. DNA was isolated from peripheral blood of 184 ALL patients (33 adults, 151 children) and 155 controls and analyzed by a PCR-restriction fragment length polymorphism assay. The frequency of MTHFR 677CT and 1298 AC genotypes were significantly lower among adult ALL cases when compared to the controls. We found a 1.74-fold reduced risk of ALL in individuals with 1298AC polymorphic variant and a 9.17-fold decreased risk of adult ALL. However, no statistically significant difference was evident between the above polymorphisms and susceptibility to ALL in children. Polymorphisms in the MTHFR gene possibly modulate risk of ALL in north Indian adults but not in children, although larger studies are needed.
Keywords: Acute lymphoblastic leukemia, Folate metabolism, MHTFR polymorphisms, North India
Introduction
Acute lymphoblastic leukemia (ALL) is a heterogeneous group of lymphoid disorders that arise from rapidly proliferating clones of hematopoietic cells having high requirements for DNA synthesis. The frequencies of the different subtypes of ALL have been related to age, ethnicity and social conditions in different countries. ALL has a bimodal age distribution: an early peak at approximately age 4–5 years followed by a second gradual increase at about 50 years. ALL, the most common childhood acute leukemia, representing about 80 % of acute leukemia, however, makes up only 20 % of adult acute leukemia. Though much progress has been made in understanding the diagnosis and therapy of this malignancy, the cause of ALL remains largely unknown. The development of ALL involves an interaction between genetic and environmental factors.
Lymphoid malignancies arise as a consequence of point mutations, chromosomal rearrangements and epigenetic alterations in hematopoeitic cells. Defects or polymorphisms in the genes of the folate dependent enzymes and deficiencies of micronutrients may influence cancer susceptibility. The methylenetetrahydrofolate reductase (MTHFR) gene is located at the end of the short arm of chromosome 1 (1p36.3) [7], and the encoded protein, MTHFR, is a key enzyme in folate metabolism. It converts 5,10-methylenetetrahydrofolate (methyleneTHF), a donor for methylating uridylate (dUMP) to thymidylate (dTMP) in DNA synthesis, to 5-methyltetrahydrofolate (methylTHF), the main circulatory form of folate that provides a methyl group for homocysteine methylation [1–5].
C677T and A1298C are two well described, commonly occurring polymorphisms in the MTHFR gene. Both variants have an impact on enzyme function: 677T affecting the catalytic and 1298C the regulatory MTHFR domain. Increased thermolability and reduced enzyme activity has been observed in 677TT and 1298CC homozygotes, and to a lesser extent in heterozygous individuals [1, 3–5]. A number of studies have reported protective effects of these common polymorphisms of the MTHFR gene in ALL, both in children and adults [2, 6–9]. One study has suggested that the effect of MTHFR polymorphisms on the risk of ALL may depend on folate status [2].
The lower MTHFR activity leads to increased plasma levels of homocysteine and reduced methylTHF formation particularly in folate-deficient states. This contributes to an increased pool of methyleneTHF, the methyl group donor for the conversion of dUMP to dTMP at the expense of a decreased pool of methylTHF. It is thought that lower MTHFR activity might favour optimal DNA synthesis by reducing uracil misincorporation rate, a potential cause of double-strand breaks and consequently chromosomal alterations during the uracil excision repairing process [1, 6, 7, 10]. This chromosomal damage may be sufficient to initiate ALL progression through the malignant transformation and clonal expansion of lymphopoietic progenitor cells [1]. The role of MTHFR variants in a number of disorders, involving disturbances in folate metabolism, such as neural tube defects, Down syndrome, venous thrombosis, eclampsia, inflammatory bowel disease, and susceptibility to colonic cancer and lymphoid malignancies, is well documented [1, 2, 11, 12].
The role of MTHFR polymorphism in the development of childhood ALL has been extensively investigated in the past decade with conflicting results [7, 8, 13–17]. Some investigators reported that both 677T and 1298C decreased the risk of ALL in children and adults [2, 6–9] whereas others failed to confirm such an association [15, 17–20]. To date, several studies from India have attempted to correlate the association between MTHFR polymorphism and childhood ALL but has yielded discordant reports [14, 21–23]. In view of this, the present study, proposes to evaluate the possible relationship between functional MTHFR polymorphisms, C677T and A1298C and risk of ALL in adults and children in the North Indian population by comparing them with healthy controls.
Materials and Methods
Patients and Samples
The study included 184 consecutive and unrelated ALL patients.(male/female ratio-2.5:1 median age-8 years, range 0.4–55 years) All patients presented to the Department of Hematology, All India Institute of Medical Sciences, New Delhi, between June 2005 and July 2010. All patients were diagnosed according to standard methods including blood picture, bone marrow, cytochemistry for myeloperoxidase, Sudan black B and periodic acid–Schiff testing and immunophenotyping. Immunophenotyping was done for all cases by indirect immunoflorescence technique. Written consent was obtained from all patients (in case of small children consent was obtained from guardians) as well as the controls as per the institutional guidelines. Controls comprised of healthy individuals randomly selected from hospital staff, students or normal controls required by the department for coagulation tests. The controls were not necessarily North Indians.
Analysis of the MTHFR 677 and 1298 Genotypes
Genotype Analysis
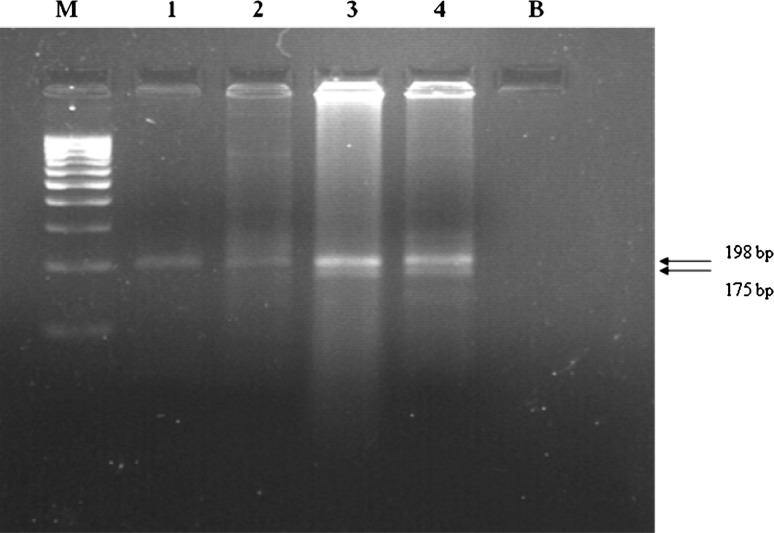
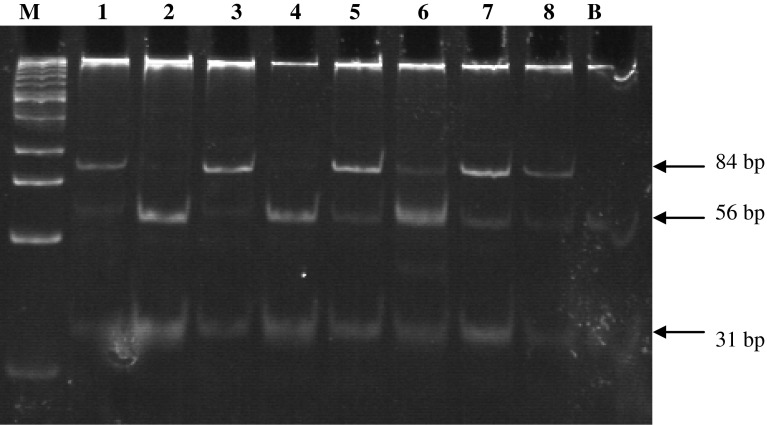
Peripheral blood sample anticoagulated by EDTA were obtained from cases and controls by vein puncture. The mononuclear cell layer was separated on Ficoll Hypaque and DNA was isolated by using a simple proteinase K treatment, followed by phenol–chloroform extraction and ethanol precipitation. Polymerase chain reaction/restriction fragment length polymorphism (PCR/RFLP) method was used for genotyping MTHFR C677T and A1298C polymorphisms, as described previously [1]. For nucleotide C677T, specific amplified PCR fragment was subjected to restriction digestion with Hinf1 and the digested PCR products were checked on 2 % agarose gel (Fig 1). For nucleotide A1298C, specific amplified PCR fragment was subjected to restriction digestion with MboII and the digested PCR products were checked on polyacrylamide gel electrophoresis (PAGE) (Fig 2).
Fig. 1.
Agarose gel electrophoresis of the PCR–RFLP; Lane M is the 100 bp DNA marker lane1and 2 show 198 bp fragment (wild type) lane 3 and 4 show 198 and 175 bp fragments (heterozygous for C677T mutation)
Fig. 2.
Polyacrylamide gel electrophoresis of the PCR–RFLP for the detection of A1298C mutation; Lane M, 25 bp DNA marker; Lane 1 and 3 show 84 bp and 31 bp fragments (homozygous mutant) Lane 5–8 show 84 bp, 56 bp, 31 bp fragments (heterozygous mutant) Lane 2 and 4 show 56 bp, 31 bp fragments (wild type)
Statistical Analysis
All analyses were performed by using STATA. Independent T test and Wilcoxon rank-sum (Mann–Whitney) test were applied to find out if there was any difference in age between cases and controls. Pearson Chi square and Fisher’s exact test were applied to find if there was any difference in sex between cases and controls (when cell frequencies were <5, exact methods were used to compute the risk estimates). Odds ratios and 95 % confidence intervals were computed by using conditional logistic regression (univariate and multiple regression analysis as applied).
Results
Of the 184 cases of ALL, 33 were adults (>15 years) and 151 were children (<15 years). Among the adults, 67 % were male, with a mean age of 29.4 years (SD = 15.5, median = 24 years), among children 73 % were male, with a mean age of 6.9 years (SD = 3.8, median = 6 years). Based on immunophenotyping 89 % patients were categorized as B-lineage and 11 % as T-lineage ALL. No significant differences in the MTHFR 677 and 1298 mutant frequencies were observed between these groups. Therefore, B-lineage and T-lineage ALL were considered as a single group for this analysis.
Among the 184 patients diagnosed with ALL, the MTHFR 677 polymorphic allele frequency was 20.38 % compared with 21.29 % among the 155 control subjects. For MTHFR 1298, we observed a polymorphic allele frequency of 35.87 % in the cases and 40.00 % in the controls. The observed frequencies in the controls and patients for MTHFR 677 and 1298 were in accordance with Hardy–Weinberg laws of equilibrium.
MTHFR 1298AC polymorphic variant was underrepresented among the patients when compared with controls (42.39 % vs. 55.48 %), conferring a 1.74-fold decreased risk of ALL among subjects with the 1298AC variant of MTHFR relative to 1298AA (OR = 0.574, adjusted OR = 0.540, 95 % CI = 0.359–0.916, adjusted 95 % CI = 0.334–0.873, P = 0.020 adjusted P = 0.012). No difference was observed in the distribution of MTHFR 677 between cases and controls and hence no such protective effect was seen for this polymorphism.
We next investigated the joint effects of the two polymorphisms and found that individuals with the 677CC/1298AC variant had a 1.96-fold decreased risk of developing ALL (OR = 0.847, adjusted OR = 0.449, 95 % CI = 0.394–1.82, adjusted 95 % CI = 0.236–0.855, P = 0.034, adjusted P = 0.015) and those with the 677CT/1298AC variant had a 2.18-fold reduced risk (OR = 0.459, adjusted OR = 0.402, 95 % CI = 0.226–0.930, adjusted 95 % CI = 0.194–0.832, P = 0.031, adjusted P = 0.014) when using 677CC/1298AA as the reference group. Although there was no evidence of statistical interaction between MTHFR 677 and MTHFR 1298 (χ2 = 9.133, P = 0.237), we did observe linkage between the two. There was total absence of the double- homozygous mutants.
Prevalence of the MTHFR 677 and 1298 Genotypes in Adult ALL Cases and Controls
Among the 33 adult patients diagnosed with ALL, the MTHFR 677 polymorphic allele frequency was 15.15 % compared with 24.14 % among the 58 control subjects. For MTHFR 1298, we observed a polymorphic allele frequency of 24.24 % in the cases and 45.69 % in the controls. The genotype frequencies of MTHFR 677CC, 677CT, and 677TT and MTHFR 1298AA, 1298AC, and 1298CC are listed in Table 1.
Table 1.
Distribution of MTHFR C677T and A1298C genotypes in adult cases of ALL and controls
| Genotype | Cases (%) | Controls (%) | OR (95 % CI) | P value |
|---|---|---|---|---|
| 677CC | 25 (75.76) | 33 (56.90) | 1 | |
| 677CT | 6 (18.18) | 22 (37.93) | 0.36 (0.127–1.020) | 0.055 |
| 677TT | 2 (6.06) | 3 (5.17) | 0.88 (0.136–5.670) | 0.893 |
| 1298AA | 22 (66.67) | 14 (24.14) | 1 | |
| 1298AC | 6 (18.18) | 35 (60.34) | 0.109 (0.036–0.326) | 0.000 |
| 1298CC | 5 (15.15) | 9 (15.52) | 0.353 (0.098–1.274) | 0.112 |
OR crude odds ratios, CI confidence interval with 95 % of probability, NA not applicable
MTHFR 1298AC heterozygotes were underrepresented among the patients when compared with controls (18.18 % vs. 60.34 %), as was the MTHFR 677 CT variant (18.18 % vs. 37.93 %) suggesting a protective effect of these variants. We observed a 9.17-fold decreased risk of adult ALL among subjects with the 1298AC variant of MTHFR relative to 1298AA (OR = 0.109, adjusted OR = 0.104, 95 % CI = 0.036–0.326, adjusted 95 % CI = 0.034–0.318, P = 0.000) and a 2.88-fold reduced risk in individuals with 677CT variant of MTHFR relative to 677CC when adjusted for sex (adjusted OR = 0.347, 95 % CI = 0.121–0.996, P = 0.05).
On investigating the joint effects of the two polymorphisms as shown in Table 2, we found that a 17.86-fold decreased risk of adult ALL in individuals with the 677CT/1298AA variant (OR = 0.056, 95 % CI = 0.012–0.241, P = 0.02), a 5.95-fold reduced risk in those with the 677CC/1298CC combination (OR = 0.19, 95 % CI = 0.02–1.52, P = 0.027), a 6.80-fold decreased risk in those with the 677CC/1298AC polymorphism (OR = 0.147, 95 % CI = 0.026–0.810, P = 0.00), and a 23.81-fold reduced risk in those with the 677CT/1298AC variant (OR = 0.042, 95 % CI = 0.007–0.250, P = 0.001) when using 677CC/1298AA as the reference group. There was evidence of statistical interaction between MTHFR 677 and MTHFR 1298 (χ2 = 24.432, P = 0.000) and we did observe linkage between the two; namely, all cases and controls with the 677TT genotype had the 1298AA genotype, and very few with the 1298CC genotype had 677CT genotype. There was total absence of the double-homozygous mutants.
Table 2.
Joint effects of the two polymorphisms of MTHFR C677T and A1298C in adult cases and controls and their association with ALL cases and controls
| Genotype | Cases (%) | Controls (%) | OR (95 % CI) | P value |
|---|---|---|---|---|
| 677CC/1298AA | 17 (51.52) | 5 (8.62) | 1 | |
| 677CT/1298AA | 3 (9.09) | 6 (10.34) | 0.056 (0.012–0.241) | 0.028 |
| 677TT/1298AA | 2 (6.06) | 3 (5.17) | 0.168 (0.034–0.817) | 0.119 |
| 677CC/1298AC | 4 (12.12) | 21 (36.21) | 0.147 (0.026–0.810) | 0.000 |
| 677CT/1298AC | 2 (6.06) | 14 (24.14) | 0.042 (0.007–0.250) | 0.001 |
| 677TT/1298AC | 0 (0.00) | 0 (0.00) | 0.147 (0.010–1.978) | |
| 677CC/1298CC | 4 (12.12) | 7 (12.07) | 0.196 (0.025–1.520) | 0.027 |
| 677CT/1298CC | 1 (3.03) | 2 (3.45) | 0.148 | |
| 677TT/1298CC | 0 (0.00) | 0 (0.00) |
OR crude odds ratios, CI confidence interval with 95 % of probability, NA not applicable
Test for interaction: χ 2 = 24.432 (P = 0.000)
Prevalence of the MTHFR 677 and 1298 Genotypes in Children with ALL and Controls
Among the 151 children diagnosed with ALL, the MTHFR 677 polymorphic allele frequency was 21.52 % compared to 19.59 % among the 97 control subjects. For MTHFR 1298, we observed a polymorphic allele frequency of 38.41 % in the cases and 36.60 % in the controls. The genotype frequencies of MTHFR 677CC, 677CT, and 677TT and MTHFR 1298AA, 1298AC, and 1298CC are listed in Table 3.
Table 3.
Distribution of MTHFR C677T and A1298C genotypes in all childhood cases of ALL and controls
| Genotype | Cases (%) | Controls (%) | OR (95 % CI) | P value |
|---|---|---|---|---|
| 677CC | 94 (62.25) | 63 (64.95) | 1 | |
| 677CT | 49 (32.45) | 30 (30.93) | 1.094 (0.628–1.907) | 0.749 |
| 677TT | 8 (5.30) | 4 (4.12) | 1.340 (0.387–4.640) | 0.644 |
| 1298AA | 57 (37.75) | 36 (37.11) | 1 | |
| 1298AC | 72 (47.68) | 51 (52.58) | 0.891 (0.514–1.545) | 0.683 |
| 1298CC | 22 (14.57) | 10 (10.31) | 1.389 (0.590–3.270) | 0.451 |
OR crude odds ratios, CI confidence interval with 95 % of probability, NA not applicable
No difference was observed in the distribution of MTHFR 677 and 1298 between cases and controls; hence no such protective effect was seen for these polymorphisms.
On investigating the joint effects of the two polymorphisms as shown in Table 4, no protective effect was seen for either polymorphism. Although there was no evidence of statistical interaction between MTHFR 677 and MTHFR 1298 (χ2 = 3.275, P = 0.908), we did observe linkage between the two as there were very few cases and controls with the 677CT/1298CC, 677TT/1298AA, and 677TT/1298AC genotypes.
Table 4.
Joint effects of the two polymorphisms of MTHFR C677T and A1298C in childhood cases and controls and their association with ALL cases and controls
| Genotype | Cases (%) | Controls (%) | OR (95 % CI) | P value |
|---|---|---|---|---|
| 677CC/1298AA | 29 (19.21) | 21 (21.65) | 1 | |
| 677CT/1298AA | 24 (15.89) | 12 (12.37) | 1.448 (0.593–0.533) | 0.416 |
| 677TT/1298AA | 4 (2.65) | 3 (3.09) | 0.965 (0.195–0.776) | 0.966 |
| 677CC/1298AC | 44 (29.14) | 32 (32.99) | 0.995 (0.483–0.051) | 0.991 |
| 677CT/1298AC | 24 (15.89) | 18 (18.56) | 0.965 (0.421–2.214) | 0.934 |
| 677TT/1298AC | 4 (2.65) | 1 (1.03) | 2.896 (0.301–27.816) | 0.357 |
| 677CC/1298CC | 21 (13.91) | 10 (10.31) | 1.520 (0.594–3.890) | 0.382 |
| 677CT/1298CC | 1 (0.66) | 0 (0.00) | ||
| 677TT/1298CC | 0 (0.00) | 0 (0.00) |
OR crude odds ratios, CI confidence interval with 95 % of probability, NA not applicable
Test for interaction: χ 2 = 3.275 (P = 0.908)
Discussion
MTHFR, methylenetetrahydrofolate reductase, uses 5,10MTHF (methylenetetrahydro-folate) as substrate to form 5-MTHF (methylhydrofolate)[2].Common polymorphisms in MTHFR are 677C > T and 1298A > C. These polymorphisms were associated with a reduced enzymatic activity of MTHFR in a number of studies [3, 4, 6, 8]. The mechanism proposed to explain these associations was the shunt of folate metabolism verses thymidine and purine synthesis, which would slow the incorporation of uracil into DNA and protect against carcinogenesis.
Epidemiological data on polymorphism-disease association in ALL is limited among Asians, unlike the large cohort studies in Western population. The differences among different groups, including our data, reflect the expected ethnic variability between different populations. The frequency of MTHFR C677T allele among our population is similar to those of other Asian studies however; it was lower than those reported by others [2, 6, 8, 15].
As regards the frequencies of MTHFR 1298 allele, our results are in concordance with those of other populations [1, 14]. However, Filipino population had a lower frequency of A1298C. The differences among different groups, including our data, reflect the expected ethnic variability between different populations.
Several case control studies have been conducted to investigate the presence of a relationship between gene variants and the impact of MTHFR polymorphism as a risk modifier in susceptibility of ALL. Most of the studies showed a protective effect of the enzyme variants [1, 2, 4, 6–9, 20] while others failed to detect such results [13, 15, 18]. Some studies have reported significant increased risk of childhood ALL with MTHFR gene variants [16, 17, 19]. Studies carried out among the Indian pediatric population also reveal conflicting results. Sood et al. [21] and Reddy et al. [14] reported the genotype 677CT to be significantly associated with an elevated risk of ALL whereas, Sadananda et al. [22] and Nikbakht et al. [23] found no significant difference in susceptibility to ALL. Our results for the genotype 677CT are in line with the finding of Sadananda et al. and Nikbakht et al. For MTHFR 1298AC Reddy et al. found an elevated risk of ALL, however, Sood et al. and Sadananda et al. did not find any significant difference in susceptibility to ALL. In the present study, we found a statistically significant reduced risk of ALL in a small group of 33 adult patients with the 1298AC genotype (P = 0.020) although, no such association was observed in a much larger group of childhood ALL. We observed a statistically significant reduced risk in patients with 1298AC polymorphism in the unadjusted category (OR = 0.109, 95 % CI = 0.036–0.326, P = 0.00) and 677 CT polymorphic variant when adjusted for sex (OR = 0.347, 95 % CI = 0.121–0.996, P = 0.05). On investigating the joint effects of the two polymorphisms, we found that a 17.86-fold decreased risk of adult ALL in individuals with the 677CT/1298ACA variant (OR = 0.056, 95 % CI = 0.012–0.241, P = 0.02), a 5.95-fold reduced risk in those with the 677CC/1298CC combination (OR = 0.19, 95 % CI = 0.02–1.52, P = 0.027), a 6.80-fold decreased risk in those with the 677CC/1298AC polymorphism (OR = 0.147, 95 % CI = 0.026–0.810, P = 0.00) and a 23.81-fold reduced risk in those with the 677CT/1298AC variant (OR = 0.042, 95 % CI = 0.007–0.250, P = 0.001) when using 677CC/1298AA as the reference group. There was evidence of statistical interaction between MTHFR 677 and MTHFR 1298 (χ2 = 24.432, P = 0.000). Thus, analysis of the combined genotype frequencies of both polymorphisms showed that MTHFR variants 677CC/1298AC, 677CC/1298CC, 677CT/1298AA and 677CT/1298AC were significantly higher in controls as compared to adult ALL patients suggestive of a protective role. This is in agreement with the results of Skibola et al. [1], who found an OR of 0.23 (95 %CI = 0.06–0.81) for MTHFR 677 TT homozygous state and the OR for heterozygous and homozygous MTHFR A1298C were 0.33 (95 % CI = 0.15–0.73) and 0.07 (95 % CI = 0.00–1.77) respectively. In their study the joint effect of the two polymorphisms was found in cis form and the total absence of the double-homozygous mutants. This is in concordance to our study and as reported by skibola et al. [1].
We also observed the protective effect of A1298C variant higher than that of C677T. This may be partly explained by the fact that T677 and C1298 exert their action by different mechanisms. The Ala222Val replacement is located in the region encoding the N-terminal catalytic domain, whereas Glu429Ala substitution resides within C-terminal SAM regulatory domain of the enzyme. It has been shown that SAM inhibits MTHFR and that this feedback loop is essential for methyl group biogenesis and prevention of methyleneTHF depletion. SAM-insensitive MTHFR, on the other hand, is expected to direct more one-carbon units to methylTHF and hence to SAM synthesis. It is thus possible that C1298 variant might affect this regulation and, as suggested by Wiemels et al. [7], act through a different pathway than T677, leading to a prevention of aberrant methylation pattern.
In contrast to our data, there are studies who have not found any association between the two variants of MTHFR and risk of ALL in adults [13, 18]. Similarly, Schnakenberg et al. [15] reported the same in children. Several studies reported that the MTHFR variants 677 and 1,298 are associated with an increased risk of childhood ALL [16, 17]. However, one study by Kim et al. reported the increased susceptibility of ALL in adults with only 677 MTHFR variant. In our study, we observed an association between MTHFR variants and ALL in adults but not in children. A plausible cause for this may be that mothers of the children with ALL took folate supplementation which is part of the prenatal services during pregnancy in India, and, adequate folate levels negates the effect of MTHFR polymorphic variants in children with leukemia [2]. More conceivable would be the relation between the folate status and genotype in adult ALL, where folate status might be much more conditional to the subject’s own genotype and folate intake.
The above reported case–control studies exhibit similarities as well as discrepancies. Various reasons have been cited for these apparently conflicting results. The controversy in the literature has been attributed to many factors including sample size, age group and ethnic variability. This necessitates that such studies have to be performed locally in each community in order to detect population at risk and potential environmental hazards. Furthermore, different MTHFR polymorphisms may exhibit differential impact on the risk of leukemia in different populations due to different genotype frequencies of polymorphisms, differences in genetic background, gene-environment interactions such as diet, nutritional intake of folate, gene–gene interactions, and differences in the etiology of adult as opposed to childhood leukemia.
In conclusion, we report similarities and variability in the relative frequencies of MTHFR. Our study represents another attempt at unraveling the putative mechanisms of leukemogenesis in acute leukemia. We found a decreased risk of ALL in adult patients with MTHFR polymorphic variant C677T, although the number studied was only thirty-three. No significant association was found between the genetic polymorphisms in susceptibility to childhood ALL. Thus, we observed that MTHFR variants may not be the markers for the progression or the development of childhood leukemia and could not be used as treatment strategies for childhood ALL. Our results need confirmation by other larger studies; further well designed studies including folate level measurements as well as larger sample size are required to evaluate the interactive effects of folate intake and genotype in the etiology of ALL. Role of gene–gene and other gene-environment interactions in leukemogenesis also need to be examined.
Contributor Information
Sudha Sazawal, Email: sudha_sazawal@hotmail.com.
Rekha Chaubey, Email: rekha79_aiims@yahoo.co.in.
Pawandeep Kaur, Email: pmroke@yahoo.com.
Sunita Chikkara, Email: sunita1409@gmail.com.
Bijender Kumar, Email: bbohra@gmail.com.
Sameer Bakshi, Email: sambakh@hotmail.com.
L. S. Arya, Email: lsarya@rediffmail.com
Vinod Raina, Email: vinodraina@hotmail.com.
Alakananda Das Gupta, Email: alakanandadasgupta@gmail.com.
Renu Saxena, Phone: +91-11-6594797, FAX: +91-11-6862663, Email: renusax@hotmail.com.
References
- 1.Skibola CF, Smith MT, Kane E, Roman E, Rollinson S, Cartwright RA, et al. Polymorphisms in the methylenetetrahydrofolate reductase gene are associated with susceptibility to acute leukemia in adults. Proc Natl Acad Sci USA. 1999;96:12810–12815. doi: 10.1073/pnas.96.22.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krajinovic M, Lamothe S, Labuda D, Lemieux-Blanchard E, Theoret Y, Moghrabi A, et al. Role of MTHFR genetic polymorphisms in the susceptibility to childhood acute lymphoblastic leukemia. Blood. 2004;103:252–257. doi: 10.1182/blood-2003-06-1794. [DOI] [PubMed] [Google Scholar]
- 3.Robien K, Ulrich CM. 5,10-Methylenetetrahydrofolate reductase polymorphisms and leukemia risk: a HuGE minireview. Am J Epidemiol. 2003;157:571–582. doi: 10.1093/aje/kwg024. [DOI] [PubMed] [Google Scholar]
- 4.de Jonge R, Tissing WJE, Hooijberg JH, Jansen G, Kaspers GJL, Lindemans J, et al. Polymorphisms in folate-related genes and risk of pediatric acute lymphoblastic leukemia. Blood. 2009;113:2284–2289. doi: 10.1182/blood-2008-07-165928. [DOI] [PubMed] [Google Scholar]
- 5.Wiesberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169–172. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 6.Franco RF, Simoes BP, Tone LG, Gabellini SM, Zago MA, Falcao RP. The methylenetetrahydrofolate reductase C677T gene polymorphism decreases the risk of childhood acute lymphocytic leukaemia. Br J Haematol. 2001;115:616–618. doi: 10.1046/j.1365-2141.2001.03140.x. [DOI] [PubMed] [Google Scholar]
- 7.Wiemels JL, Smith RN, Taylor GM, Eden OB, Alexander FE, Greaves MF. Methylenetetrahydrofolate reductase (MTHFR) polymorphisms and risk of molecularly defined subtypes of childhood acute leukemia. Proc Natl Acad Sci USA. 2001;98:4004–4009. doi: 10.1073/pnas.061408298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gemmati D, Ongaro A, Scapoli GL, et al. Common gene polymorphisms in the metabolic folate and methylation pathway and the risk of acute lymphoblastic leukemia and non-Hodgin’s lymphoma in adults. Cancer Epidemiol Biomarkers Prev. 2004;13:787–974. [PubMed] [Google Scholar]
- 9.Kamel AM, Moussa HS, Ebid GT, Bu RR, Bhatia KG. Synergistic effect of methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms as risk modifiers of pediatric acute lymphoblastic leukemia. J Egypt Natl Canc Inst. 2007;19:96–105. [PubMed] [Google Scholar]
- 10.Ames BN. Cancer prevention and diet: help from single nucleotide polymorphisms. Proc Natl Acad Sci USA. 1999;96:12216–12218. doi: 10.1073/pnas.96.22.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000;151:862–877. doi: 10.1093/oxfordjournals.aje.a010290. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo K, Hamajima N, Suzuki R, et al. Methylenetetrahydrofolate reductase gene (MTHFR) polymorphisms and reduced risk of malignant lymphoma. Am J Hematol. 2004;77:351–357. doi: 10.1002/ajh.20215. [DOI] [PubMed] [Google Scholar]
- 13.Chiusolo P, Reddiconto G, Cimino G, Sica S, Fiorini A, Farina G, et al. Methylenetetrahydrofolate reductase genotypes do not play a role in acute lymphoblastic leukemia pathogenesis in the Italian population. Haematologica. 2004;89:139–144. [PubMed] [Google Scholar]
- 14.Reddy H, Jamil K. Polymorphisms in the MTHFR gene and their possible association with susceptibility to childhood acute lymphocytic leukemia in an Indian population. Leuk Lymphoma. 2006;47:1333–1339. doi: 10.1080/10428190600562773. [DOI] [PubMed] [Google Scholar]
- 15.Schnakenberg E, Mehles A, Cario G, Rehe K, Seidemann K, Schlegelberger B, et al. Polymorphism of methylenetetrahydrofolate reductase (MTHFR) and susceptibility to pediatric acute lymphoblastic leukemia in a German study population. BMC Med Genet. 2005;6:23. doi: 10.1186/1471-2350-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alcasabas P, Ravindranath Y, Goyette G, Haller A, Del Rosario L, Lesaca-Medina MY, et al. 5,10-Methylenetetrahydrofolate reductase (MTHFR) polymorphisms and the risk of acute lymphoblastic leukemia (ALL) in Filipino children. Pediatr Blood Cancer. 2008;51:178–812. doi: 10.1002/pbc.21511. [DOI] [PubMed] [Google Scholar]
- 17.Milne E, de Klerk NH, van Bockxmeer F, Kees UR, Thompson JR, Baker D, et al. Is there a folate-related gene-environment interaction in the etiology of childhood acute lymphoblastic leukemia? Int J Cancer. 2006;119:229–232. doi: 10.1002/ijc.21803. [DOI] [PubMed] [Google Scholar]
- 18.Oh D, Kim NK, Jang MJ, Kim HC, Lee JH, Lee JA, et al. Association of the 5,10-Methylenetetrahydrofolate reductase (MTHFR C677T and A1298C) polymorphisms in Korean patients with adult acute lymphoblastic leukemia. Anticancer Res. 2007;27:3419–3424. [PubMed] [Google Scholar]
- 19.Kim HN, Kim YK, Lee IK, Yang DH, Lee JJ, Shin MH, et al. Association between polymorphisms of folate-metabolizing enzymes and hematological malignancies. Leuk Res. 2009;33:82–87. doi: 10.1016/j.leukres.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Pereira TV, Rudnicki M, Pereira AC, Pombo-de-Oliveira MS, Franco RF. 5,10-Methylenetetrahydrofolate reductase polymorphisms and acute lymphoblastic leukemia risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1956–1963. doi: 10.1158/1055-9965.EPI-06-0334. [DOI] [PubMed] [Google Scholar]
- 21.Sood S, Das R, Trehan A, Ahluwalia J, Sachdeva MU, Varma N, et al. Methylenetetrahydrofolate reductase gene polymorphisms: association with risk for pediatric acute lymphoblastic leukemia in north Indians. Leuk Lymphoma. 2010;51:928–932. doi: 10.3109/10428191003719023. [DOI] [PubMed] [Google Scholar]
- 22.Sadananda MN, Chandy S, Ramachandra N, Appaji L, Aruna BS, Ramaswamy G, et al. Methylenetetrahydrofolate reductase gene polymorphisms and risk of acute lymphoblastic leukemia in children. Ind J Cancer. 2010;47:40–45. doi: 10.4103/0019-509X.58858. [DOI] [PubMed] [Google Scholar]
- 23.Nikbakht M, MalekZadeh K, Jha AK, Askari M, Marwaha RK, Kaul D, et al. Polymorphisms of MTHFR and MTR genes are not related to susceptibility to childhood ALL in North India. Exp Oncol. 2012;34:43–48. [PubMed] [Google Scholar]