Abstract
Present management of β thalassemia major by regular packed red blood cell (PRBC) transfusions poses risk of alloimmunization not only to red blood cell antigens, but also to human platelet antigens (HPA) and Human leucocyte antigens class I (HLA I). However data in this context is very limited in Indian population. The aim of the study was to determine the prevalence of alloimmunization to HPA and HLA I in β thalassemia major patients who have received multiple PRBC transfusions over the years. A cross sectional study was performed at our tertiary care blood bank. β thalassemia major patients of more than 6 years of age were included who were receiving fresh, leucoreduced and irradiated PRBC units regularly with annual requirement of more than ten PRBC transfusions. A total of 9 out of 80 (11.25 %) patients were found to be alloimmunized for HPA antigens of various specificity and 24 out of 80 (30 %) developed antibodies to HLA I. The awareness of development of alloimmunization to HPA and HLA antigens in multi PRBC transfused thalassemics, despite use of leucofilters will prompt us, to look for improvement in our current PRBC preparations to minimise platelet alloimmunisation. Further studies are required to validate the findings and build the base line data in this regard. This is of importance, especially in view of providing suitable cross-matched platelets when required in future especially when considering future haematopoietic stem cell transplantation (HSCT).
Keywords: Anti-human platelet antigens, Anti-human leucocyte antigens, Haematopoietic stem cell transplantation, Packed red blood cell concentration
Introduction
The most effective management in a patient of β Thalassemia major includes lifelong transfusion at 2–4 weeks interval to maintain pre transfusion hemoglobin concentration of 9–10.5 g/dl [1]. Lifelong transfusion therapy poses multiple problems which complicate their management. The most widely studied complication is RBC alloimmunization and autoimmunization which causes delay in getting compatible blood [2, 3]. Alloimmunization is not limited to RBC antigens alone but also to platelet antigens and HLA antigens. We practice universal leucoreduction for PRBC in β thalassemia patients in a bid to prevent febrile non haemolytic transfusion reactions caused by cytokines produced from leucocytes, to prevent HLA alloimmunization, and to prevent transfusion of viruses like cytomegalovirus (CMV). However there are scattered reports of alloimmunization to the human platelets antigens (HPA) and Human leucocytes antigen I (HLA I) in this group as well. Hematopoietic stem cell transplantation (HSCT) is considered as the definitive treatment for β thalassemia major patients. Immune-mediated refractoriness to platelet transfusion is a major problem in successful engraftment in patients undergoing HSCT. Marktel et al. found a high incidence of refractoriness because of anti-HLA antibodies during post-Hematopoietic stem cell transplantation (HSCT) aplasia in freshly transplanted β Thalassemia patients. The risk factors predicting a negative platelet transfusion outcome were presence of spleen and the number of anti-HLA antibodies [4], but the author has not commented on the role of anti HPA antibodies. Hence considering that few studies have been done in this setting [5, 6], and considering the high incidence of platelet alloimmunization in other multiply transfused populations such as hemato oncology patients [7], we screened the sera of multi PRBC transfused β Thalassemia major patients. The aim of this study was to find out the prevalence of platelet alloimmunization; in multi PRBC transfused β Thalassemia major patients who were receiving regular leucoreduced product, and its implication in the definitive treatment of β Thalassemia major.
Materials and Methods
This cross sectional study was done in the Department of Immunohematology and Blood Transfusion at a tertiary care centre of western India. The study was approved by the institutional ethical committee.
Inclusion Criteria
We considered 80 β Thalassemia major patients who had undergone more than 50 transfusions. Age of patient in our study varied from 6–21 years (mean 11.6 yrs). There were 46 female and 34 male patients. Informed consent for participation in the study was obtained from guardian or from adult patient themselves. The patients were on regular transfusions every 15–45 days and their yearly requirement was more than ten PRBCs. These patients, hence, had more than 50 transfusions overall, and therefore, were ideal candidates to develop alloantibodies. All patients received leucoreduced, irradiated fresh blood.
Exclusion Criteria
Unwilling patients and those who had history of platelet transfusions were excluded. Patients of age less than 6 years, and those who were receiving immunosuppressive medication such as corticosteroids, were excluded. If the patient was febrile or suspected of having an infection, collection of blood for the study was deferred until the patient was well.
Phlebotomy and Sample Collection
Three ml blood was collected in EDTA vacutainer for pretransfusion compatibility testing. No extra phlebotomy was performed. The samples were centrifuged and cross matching was performed. The left over sera was used in the study. This sera was immediately alliquoted in small volumes and kept frozen below −30° C, until we collected all 80 patient samples and thawed them only when we performed platelets antibody assay in order to minimize inter-operator variability. Haemolysed, lipemic, and icteric samples were exluded as per the manufacturer instructions.
Antibody Assay
PAKPLUS® is a qualitative solid phase enzyme linked immunosorbent assay (ELISA) in which microwell provides monoclonal captured platelet glycoprotein IIb/IIIa and Ia/IIa obtained from group O donors of known platelet types. HLA class I and platelet glycoprotein Ib/IX and IV are provided as affinity purified glycoprotein. The test is designed to detect and differentiate between antibodies to HLA class I and platelet specific antigens like HPA-1a, HPA-1b, HPA-3a. HPA-3b, HPA-4a, HPA-5a, and HPA-5b. Patient plasma is added to micro well coated with platelet and HLA protein allowing antibody, if present, to bind. Unbound antibodies are than washed away. An alkaline phosphatase labelled antihuman globulin reagent (anti IgG/M) is added to the wells and incubated. The unbound anti IgG/M is washed away and the substrate p-nitrophenyl phosphate (PNPP) is added. After a 30 mins incubation period the reaction is stopped with Stopping Solution. The optical density of the colour that develops is measured in a spectrophotometer. All the additions of reagents were done according to the manufacturer’s instructions.
Results
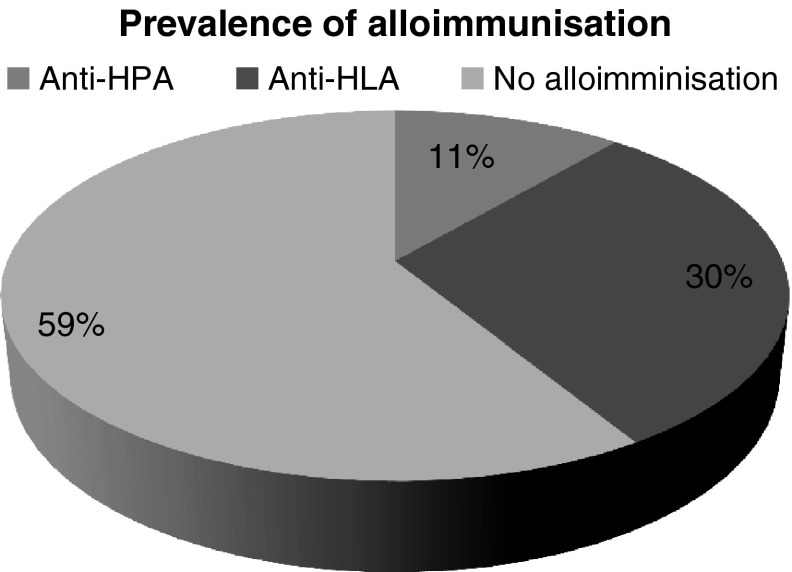
We found that 9 out of 80(11.25 %) patient developed anti-HPA antibodies and 24 developed antibodies to HLA antigens. HPA specificities found in 80 serum samples were HPA1a, 2(2.5 %); HPA1b, 3(3.75 %); and HPA5b, 4(5 %); respectively. Antibodies to HLA antigens were 24 out of 80(30 %) (Fig. 1). Our results highlight that HPA and HLA I alloimmunization in PRBC multitransfused β thalassemia patients is a real possibility even when leucoreduced and irradiated blood is used.
Fig. 1.
Prevalence of alloimmunization against human platelet antigen (HPA) and Human leucocyte antigen class I (HLAI) in β Thalassemia major patients
Discussion
Platelets are the major source of HLA Class I antigens in blood. HLA Class I antigens are intrinsic transmembrane proteins synthesized and acquired at the megakaryocyte stage before platelets become cytoplasmic fragments [8]. There have been limited studies of HPA alloimmunization in patients who received multiple transfusions of packed red blood cell. Interest in sensitization rates in such patients has increased, particularly with the advent of HSCT as a definitive therapy for patients of β thalassemia major. Sensitization to HLA I and HPA can inhibit platelet transfusion responses increasing the risk of thrombocytopenic hemorrhage in patients undergoing HSCT who require multiple platelet transfusions. One study of chronically transfused sickle cell disease patients determined that 85 % of those with at least 50 past red cell transfusions had evidence of platelet-reactive antibodies, predominately anti-HLA, whereas 48 % of those with fewer than 50 past exposures were sensitized [9]. Another study of 60 β thalassemia major patients examined the development of HLA antibodies and found that 32 (53 %) were positive for HLA antibodies at baseline and seven more became sensitized in the follow-up period of 1 year, accounting for a total HLA sensitization rate of 65 % [5] Neither study used RBC units that were leukocyte reduced to the extent that HLA sensitization would be lessened. Our study analysed the β Thalassemia major patients receiving only fresh, leucoreduced and irradiated blood. We used leucocytes reduction technology by filtration which has emerged as the most commonly used method. The current high-performance leukocyte removal filters can reduce the residual white cell (WBC) content by at least 3 logs and typically achieve 4 log or greater removal. The technology represents an important advance in the preparation of blood components. However, leukocyte reduction represents the largest incremental increase in the cost of producing blood components in history. Bipin et al. [9] reported the prevalence of HPA antigen as 79.45 % homozygous for HPA1-a, 19.75 % heterozygous for HPA1a-HPA1b and 0.99 % homozygous for HPA1b. If antibodies were to develop against high prevalent antigens, then finding the suitably matched platelets would be a difficult proposition. In PRBC multitranfused patient’s alloimmunization is usually regarded as strictly related to the presence of HLA I alloantibodies, since these are responsible for most of the FNHTR. However HPA may also be implicated [6]. The possibility of alloimmunization to platelets due to multiple PRBC transfusions may interfere with the outcome of hematopoietic stem cell transplant (HSCT) treatment. The immune responses of β thalassemia major patients may differ from those of other groups of multiply transfused patients and BMT candidates because of the development of hypersplenism that develops during the course of illness. Hence refractoriness might develop earlier in the course of transplant for β Thalassemia major than for malignant diseases. β thalassemia major patients receive almost exclusively RBC products and there is a difference in the persistence of platelet alloimmunization that may be related to differences in underlying disease, in immunologic function, or in the lack of immunosuppressive therapy in β thalassemia major patients compared with cancer or other HSCT patients [8].
Our findings may be predictive of an increased risk of platelet refractoriness and perhaps an increased risk of significant haemorrhage in HSCT-aplasia phase. In our study, fortunately, antibodies against the low frequency antigens HPA-1b, and HPA-5b and HPA-2b appear to be the most common. We routinely perform and advocate extended phenotype of patients RBCs before the start of transfusions, but role of platelet phenotyping and antibody panel for better management is not well established and further studies are required. To overcome platelet refractoriness, HLA matched platelets are most sought after, but studies have proved that up to 40 % of HLA-matched platelet transfusions remain unsuccessful. HLA typing of patients as well as platelet donors is expensive and the long turnaround time decreases its utility in some clinical situations. Also the role of splenomegaly as a non-immunological cause of platelet refrectoriness in β thalassemia major patients has to be kept in mind. To overcome the problem related to HLA matching, flow-cytometry has been advocated as a platelet cross matching method thereby providing crossmatched platelets [11]. In addition platelet antigen and antibody data may serve as handy and time saving. Computerized platelet serology for antigens and antibodies from committed regular donors may enrich our blood banks with the choice of finding appropriate platelet cross match. Platelet cross-matching may provide a potentially more rapid first-line alternative to HLA-matched platelets transfusions for refractory patients who do not yet have HLA typing performed [12]. The high prevalence and possibly the high persistence of platelet alloimmunization in heavily transfused β Thalassemia major patients suggest that as a group they may be more immunocompetent than heavily transfused cancer or other HSCT patients. For this reason, leukodepletion of blood products might be less efficacious in these patients than in cancer and HSCT patients for prevention of platelet alloimmunization and hence total reliability on filter may not be a good idea. Rather, it will be prudent to anticipate the development of anti HPA and respond accordingly. However, for the same reason, prevention of platelet and HLA alloimmunization may be especially important for patients who are candidates of HSCT. Thus, it may be appropriate to consider extending the indication for leucofilteration for the prevention of platelet and HLA alloimmunization in β thalassemia major patients as they are potential HSCT recipients. Leucofilters although very effective, seem insufficient to completely remove leucocytes, platelets, their fragments and cytokines already produced by them. To make leucofilteration more effective we have to improve our procedures. In this context the best policy would be prestorage leucofilteration. Differences in the viscoelastic properties of red cells and leukocytes are particularly pronounced at low temperatures. This account for the better performance of red cell leukocyte reduction filters at refrigerated temperatures and more studies may be called for its efficacy and implementations thereafter. Poststorage leukocyte filtration that is filtration of components before issue from the blood bank, allows better inventory management. Prestorage leukocyte leucofilteration would have additional advantages by preventing transfusion of leukocyte fragments that would otherwise develop during storage. Alloimmunization to platelets can be explained due to residual platelets in plasma which is left in PRBC units as suspending medium. The additive solutions like SAGM, when used, enable us to separate maximum plasma out and hence help in minimizing residual content of platelet in red cell units. We assume that using dedicated units of SAGM- PRBC for β thalassemia major may have an impact on minimizing platelet alloimmunization. There is a great importance of awareness of the possibility of alloimmunization development even after present leucofiltration methods and existing packed RBC storage methods. The knowledge of platelet antigens and platelet antibodies will help us to prepare committed platelet donor pools who are negative to respective antigens. This will enable us to tide over refractoriness after platelet infusions due to immunological causes. Together with sophisticated molecular HPA genotyping methods, this knowledge will help us to find better matched platelet units during HSCT in β thalassemia major cases. HLA and HPA immunization is provoked by residual leucocytes and platelet fragments in residual plasma in PRBC units which can be minimised if SAGM bags are used which replace plasma maximally and when leucoreduction is performed at the earliest possible time during component preparation preferably using inline leucofilter blood bags at low temperatures.
Our study concludes that alloimmunization is a real possibility for HPA and HLA antibodies even after practicing universal leucoreduction. However larger studies are recommended. There is still scope for better methods to minimize alloimmunization. This is of significant importance in the context of possible platelet refractoriness in future HSCT as many of them are potential candidate for HSCT provided they have HLA matched donors. Our study also highlights that the most common anti HPA found in PRBC multitransfused β thalassemia patients are anti HPA 1a, HPA 2b and HPA5b. This could be of prime importance when searching for proper cross match for platelet transfusion. More studies from different parts of the country will help to form a data-base and in developing better strategies to prevent platelet alloimmunization, in this group, and also be helpful in formulating an efficient donor pool, thereby preparing us to provide for suitable platelet cross match when required in future, especially when considering future HSCTs.
Conflict of Interest
The authors have no competing interest.
Contributor Information
Joseph Philip, Phone: +020-26026267, Email: eoj_in@yahoo.com.
Sudeep Kumar, Email: sudeepkumar80.33@gmail.com.
T. Chatterjee, Email: ctathagat@hotmail.com
R. S. Mallhi, Email: pragraj@yahoo.com
References
- 1.Androulla Eleftheriou (2003) About thalassemia. In: Thalassemia International Federation, pp 29
- 2.Karimi M, Nikrooz P, Kashef S, Jamalian N, Davatolhagh Z. RBC alloimmunization in blood transfusion-dependent beta-thalassemia patients in southern Iran. Int J Lab Hematol. 2007;29(5):321. doi: 10.1111/j.1365-2257.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 3.Saied DA, Kaddah AM, Badr Eldin RM, Mohaseb SS. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent Egyptian thalassemic patients. J Pediatr Hematol Oncol. 2011;33(6):409–414. doi: 10.1097/MPH.0b013e3182208154. [DOI] [PubMed] [Google Scholar]
- 4.Marktel S, Napolitano S, Zino E, Cappelli B, et al. Platelet transfusion refractoriness in highly immunized beta β thalassemia assemia children undergoing stem cell transplantation. Pediatr Transplant. 2010;14(3):393–401. doi: 10.1111/j.1399-3046.2009.01282.x. [DOI] [PubMed] [Google Scholar]
- 5.Lo SC, Lin DT, Lin SW, Chang JS. Platelet alloimmunization after longterm red cell transfusion in transfusion dependent thalassemia patient. Transfusion. 2005;45:761–765. doi: 10.1111/j.1537-2995.2005.04246.x. [DOI] [PubMed] [Google Scholar]
- 6.Tazzari PL, Ricci F, Tassi C, Bontadini A, Fruet F, Conte R. Alloimmunization against human platelet antigen 2 (HPA2) in a series of multitransfused β-thalassemia patient. Haematologica. 1998;83(8):765–766. [PubMed] [Google Scholar]
- 7.Meenu Bajpai, babita Kaura, neelam marwaha, et al. Platelet alloimmunization in multitransfused patients with haemato-oncological disorders. Natl Med J India. 2005;18:134–136. [PubMed] [Google Scholar]
- 8.Curtis Brian R, McFarland Janice G. Rossi’s Principle of transfusion medicine. 4. Bethesda: AABB press; 2009. Platelet immunology and alloimmunization; p. 168. [Google Scholar]
- 9.Friedman DF, Lukas MB, Jawad A, et al. Alloimmunization to platelets in heavily transfused patients with sickle cell disease. Blood. 1996;88:3216–3222. [PubMed] [Google Scholar]
- 10.Bipin K, Dipika Mohanty K Ghosh. Frequency distribution of antigen in the human platelet antigen system in the western Indian population. Transfusion. 2002;42:317–320. doi: 10.1046/j.1537-2995.2002.00048.x. [DOI] [PubMed] [Google Scholar]
- 11.Lin Jeong-Shi, Ying-Ju Jau-Yi Lyou, et al. Screening for platelet antibodies in adultidiopathic thrombocytopenic purpura: a comparative study using solid phase red cell adherence assay and flow cytometry. J Chin Med Assoc. 2006;69:569–574. doi: 10.1016/S1726-4901(09)70331-4. [DOI] [PubMed] [Google Scholar]
- 12.Wiita Arun P, Nambiar Ashok. Longitudinal management with crossmatch-compatible platelets for refractory patients: alloimmunization, response to transfusion, and clinical outcomes. Transfusion. 2012;52:2146–2154. doi: 10.1111/j.1537-2995.2012.03593.x. [DOI] [PubMed] [Google Scholar]