Abstract
Treatment of steroid refractory autoimmune hemolytic anemia (AIHA) is challenging especially with no evidence based consensus guide lines and limited resources. The aim of this study was to evaluate the efficacy of pulse cyclophosphamide therapy in patients with severe refractory warm AIHA. The prospective study was designed to evaluate the efficacy of pulse cyclophosphamide—1 g/month for four consecutive months—in 17 patients (10 males and 7 females) with severe refractory warm AIHA [13 primary AIHA and 4 (females) secondary to SLE], all studied patients failed to respond to high dose of steroid therapy ± azathioprine ± intravenous immunoglobulin ± oral cyclophosphamide. Mean hemoglobin level, reticulocytic count and direct antiglobulin test were assessed before and after cyclophosphamide treatment every month. After the 4th cycle of cyclophosphamide (82 %, 14 patients) achieved partial response while the remaining (17 %, 3 patients) showed no response, while after 6 months follow up 47 % (8 patients) show complete response, while 53 % (9 patients) showed partial response. The mean hemoglobin levels were significantly increased after the 1st, 2nd, 3rd and 4th months of pulse cyclophosphamide therapy when compared to before treatment (P < 0.01, P < 0.001, P < 0.001 and P < 0.001) respectively, and the mean reticulocyte (%) were significantly decreased after the 2nd, 3rd and 4th months (P < 0.05, P < 0.01 and P < 0.001) respectively. We conclude that pulse cyclophosphamide therapy is well tolerated and induces good response in patients with severe refractory warm AIHA.
Keywords: Refractory, Autoimmune hemolytic anemia, Cyclophosphamide
Introduction
Autoimmune hemolytic anemia (AIHA) is a rare disease. In a recent population-based study the incidence was 0.8/1,00,000/year, but the prevalence is 17/1,00,000 [1]. Primary (idiopathic) AIHA is less frequent than secondary AIHA. Secondary cases are often challenging because not only AIHA but also the underlying disease(s) must be diagnosed and treated [2]. AIHA is essentially diagnosed in the laboratory, and considerable improvement has been made in this field. The diagnosis of AIHA is usually straightforward and made on the basis of the following laboratory findings: normocytic or macrocytic anemia, reticulocytosis, low serum haptoglobin levels, elevated lactate dehydrogenase (LDH) level, increased indirect bilirubin level, and a positive direct antiglobulin test (DAT) with a broad-spectrum antibody against immunoglobulin and complement. However, there are pitfalls, particularly in secondary cases, because not always are all of the typical laboratory findings of AIHA present [3]. For the diagnosis of secondary AIHA a careful history, including information on the onset (acute or insidious), history of infections, information on recent transfusions, exposure to drugs or vaccination, signs of immune disease (arthritis), and general clinical condition are helpful. The exclusion of a drug-induced hemolytic anemia is particularly important, because stopping the drug is the most effective therapeutic measure in this situation. A clinical examination (to rule out lymphadenopathy, splenomegaly) is obligatory. The need for additional investigations must be determined by history, clinical findings, and the type of antibody. Extended work-up relevant for treatment decisions may include abdominal examination by computed tomographic scan (to search for splenomegaly, abdominal lymphomas, ovarian dermoid cysts, renal cell carcinoma), quantitative determination of immunoglobulins, a search for a lupus anticoagulant in case of warm antibodies, or a bone marrow examination [4]. Treatment with glucocorticoids results in improvement in the majority of cases, but relapse is common. For patients whose disease becomes refractory or who do not respond to glucocorticoids, splenectomy is often employed as a second-line treatment [5]. Subsequent salvage treatments include intravenous immunoglobulin, danazol [6] and a variety of immunomodulating agents including low-dose cyclophosphamide, azathioprine, cyclosporine, and vincristine [5]. The other option for second-line treatment is the anti-CD20 antibody rituximab. The standard regimen is 375 mg/m2 on days 1, 8, 15, 22 for four doses. There is no doubt that the short-term benefit/risk ratio for rituximab is high and that rituximab is certainly the best option for patients who are not qualified for or who refuse splenectomy. The problem is the small number of selected patients, the heterogeneity of patient population, and the lack of systematic long-term data on efficacy and safety in the published reports. In ITP it has been shown that patients with a CR after rituximab can have long remission durations and that splenectomy can be avoided or postponed [7]. Such data are not available for rituximab in AIHA [4]. Azathioprine and cyclophosphamide are both immune suppressors leading to a decrease of autoantibody production. The addition of these drugs can be considered if steroid therapy does not lead to a sufficient result, when a steroid maintenance dose of more than 20 mg/day is needed or steroid doses must be tapered due to side effects. Cyclophosphamide (100 mg/day) or azathioprine (100–150 mg/day) can be administered as monotherapy or in combination with steroids. Due to their myelosupressive effects peripheral blood cell counts must be monitored regularly and if needed dosage must be modified. From the pretreatment data in rituximab trials it appears that, in the era before rituximab, azathioprine and cyclophosphamide were popular as second-line therapy, but we have used immunosuppressants rarely because of doubts about efficacy and the fear of side effects [4].Unfortunately, many patients become refractory to multiple therapeutic approaches and develop complications of chronic high-dose steroid therapy. Because of its success in other severe autoimmune disorders, high-dose cyclophosphamide was studied in patients with severe AIHA that was refractory to standard therapies [8]. Intermittent intravenous cyclophosphamide (pulse therapy) has been reported as an effective treatment for various autoimmune diseases including nephritis associated with systemic lupus erythematosis [9].
The mechanism of action of pulse cyclophosphamide in autoimmune disorders appears to be suppression of both T and B lymphocyte numbers and function, which leads to diminished autoantibody production [10]. High-dose cyclophosphamide was initially chosen as conditioning for allogeneic bone marrow transplantation because of its potent immunosuppressive properties [11]. Lymphocytes are highly sensitive to cyclophosphamide, but primitive hematopoietic progenitors are resistant to its cytotoxic effects because they contain high levels of aldehyde dehydrogenase, an enzyme that confers resistance to cyclophosphamide [12]. High-dose cyclophosphamide without stem cell transplantation induces durable treatment-free remissions in patients with severe aplastic anemia [13]. This approach also has activity in a variety of other refractory autoimmune conditions [14], and can eliminate alloantibodies [15].
The present study was to evaluate the efficacy of pulse cyclophosphamide therapy using (1 g/month) intravenously for four consecutive months among patients with severe refractory AIHA who had failed to respond to high dose of steroid therapy.
Patients and Methods
This prospective study was designed to evaluate the efficacy of pulse cyclophosphamide (1 g/month) intravenously for four consecutive months in patients with severe refractory warm AIHA who failed to two or more treatment modalities including high dose of steroid therapy ± azathioprine ± intravenous immunoglobulin ± oral cyclophosphamide. Those patients were unable to taper the prednisone dose to less than 10 mg/day. A diagnosis of warm AIHA based on symptoms, physical finding as well as complete blood picture with reticulocytic count, a positive DAT, unconjugated hyperbilirubinemia and elevated LDH, ANF, antidouble strand DNA, and bone marrow aspirate or biopsy were done for suspected cases with secondary AIHA. Patients were invited to participate in the study after getting informed consent and the steps and aim of the research were explained to participants before taking verbal consent. The patients were recruited from clinical hematology unit, Internal Medicine department, Assuit University Hospital. Laboratory assessments including complete blood counts with reticulocytic count, DAT, bilirubin level, AST, ALT, urea and creatinine and urine analysis were performed at least monthly and up to 6 months follow up. Complete response (CR) was defined as Hb > 12, partial response (PR) was defined as Hb > 10 g/dL or at least 2 g/dL increase in Hb level and no response (NR) was defined when the response not concomitant with the definition of CR or PR.
Statistical Methods
The data obtained were calculated and statistically analyzed by using SPSS data analysis program. A value of P < 0.05 was considered to be statistically significant.
Results
This study included 17 patients (10 males and 7 females) their ages ranged from 20 to 52 years (34.52 ± 10), 13 of them had primary warm AIHA and the remaining 4 patients (females) had secondary warm AIHA due to SLE. Those patients received packed RBCs transfusion in the last year ranged from 1 to 10 units (5.6 ± 2.4), their mean hemoglobin level (g/dL) and reticulocytic count (%) were 5.6 ± 1.6 and 13.23 ± 7.28 respectively before the initiation of cyclophosphamide therapy. Follow up hemoglobin level (g/dL), DAT and reticulocyte (%) were measured every month after pulse cyclophosphamide therapy (1 g/month) intravenously for four consecutive months.
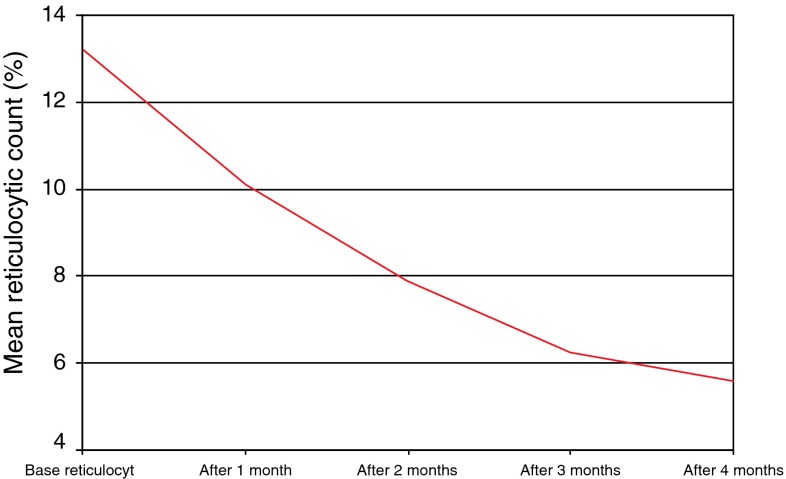
In this study after the 4th cycle of cyclophosphamide (82 %, 14 patients) achieved PR while the remaining (17 %, 3 patients) showed NR, the hemoglobin level of 7 patients (41 %) raised up to 10 g/dL or greater without blood transfusion while taking less than or equal to 10 mg/day prednisone, while 8 patients (47 %) their hemoglobin levels were between 9 and 10 g/dL taking less than or equal to 10 mg/day prednisone and transfusion independence and the 2 (11.7 %) remaining patients their hemoglobin levels were 8.9 and 7.4 g/dL. After follow up for 6 months after stoppage of cyclophosphamide (47 %, 8 patients) showed CR, while (53 %, 9 patients) show PR on less than or equal to 10 mg/day prednisone and transfusion independence (Table 1). There was a significant increase of the mean hemoglobin level after (1st, 2nd, 3rd and 4th) months of initiation of cyclophosphamide therapy when compared to that before cyclophosphamide therapy (P < 0.01, P < 0.001, P < 0.001 and P < 0.001) respectively, while the mean reticulocyte (%) were significantly decreased after the 2nd, 3rd and 4th months (P < 0.05, P < 0.01 and P < 0.001) respectively. The mean hemoglobin levels were significantly gradually increased after every cyclophosphamide cycle to reach its maximum level after the 4th cycle (Table 2), while the mean reticulocyte (%) were significantly gradually decreased after every cyclophosphamide cycle to reach its minimum level after the 4th cycle (Fig. 1). No abnormalities were detected with regard to WBCs, platelet count and renal chemistry during the study.
Table 1.
Demographic characters of the patients before and after cyclophosphamide therapy
| Age (years) and sex of the patients | Types of AIHAa | Therapy prior to pulse cyclophosphamide | Patients status | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before pulse cyclophosphamide | After 4th cycle | After 6 months follow up | |||||||||
| HB (g/dL) |
RC % |
DAT | HB (g/dL) |
RC % |
DAT | Type of response | HB (g/dL) |
Type of response | |||
| 20/M | Primary | Steroids +azathioprine | 3 | 33 | + | 9.9 | 7 | _ | PR | 11 | PR |
| 30/F | Primary | Steroids + azathioprine | 4 | 23 | + | 10.3 | 6 | _ | PR | 12.3 | CR |
| 40/F | Secondary (SLE) | Steroids + azathioprine + Intravenous immunoglobulin + oral cyclophosphamide | 5 | 12 | + | 11.2 | 4 | _ | PR | 12.7 | CR |
| 50/F | Primary | Steroids + azathioprine + oral cyclophosphamide | 3.5 | 14 | + | 10.7 | 6 | _ | PR | 13 | CR |
| 30/M | Primary | Steroids + azathioprine | 6.3 | 12 | + | 9.7 | 6 | + | PR | 10.8 | PR |
| 33/M | Primary | Steroids + oral cyclophosphamide | 5.3 | 23 | + | 10.2 | 7 | PR | 11.5 | PR | |
| 23/F | Primary | Steroids + azathioprine + Intravenous immunoglobulin + oral cyclophosphamide | 4.7 | 18 | + | 9.4 | 5 | + | PR | 12.3 | CR |
| 35/M | Primary | Steroids + azathioprine | 6.8 | 14 | + | 9 | 4 | + | PR | 10.5 | PR |
| 22/F | Secondary (SLE) | Steroids + azathioprine + oral cyclophosphamide | 4.5 | 7 | + | 10.2 | 4 | PR | 12.3 | CR | |
| 46/M | Primary | Steroids + azathioprine | 4.6 | 8 | + | 8.9 | 5 | + | PR | 9.2 | PR |
| 52/M | Primary | Steroids + azathioprine | 7.3 | 7 | + | 9.3 | 4 | _ | NR | 11.2 | PR |
| 43/F | Secondary (SLE) | Steroids + azathioprine + oral cyclophosphamide | 6.4 | 7 | + | 10.6 | 6 | _ | PR | 12.6 | CR |
| 44/M | Primary | Steroids + azathioprine + oral cyclophosphamide | 8.3 | 12 | + | 9.4 | 7 | _ | NR | 13.3 | CR |
| 27/F | Secondary (SLE) | Steroids + azathioprine + oral cyclophosphamide | 5.7 | 8 | + | 9.3 | 6 | _ | PR | 12 | PR |
| 35/M | Primary | Steroids + azathioprine | 6.2 | 12 | + | 9 | 6 | + | PR | 11.1 | PR |
| 34/M | Primary | Steroids + azathioprine | 8.3 | 8 | + | 9 | 6 | + | NR | 12.9 | CR |
| 33/M | Primary | Steroids + azathioprine | 4.5 | 7 | + | 7.4 | 6 | + | PR | 9 | PR |
M male, F female, CR complete response defined as Hb > 12, PR partial response, defined as Hb > 10 g/dL or at least 2 g/dL increase in Hb, NR No response(not concomitant with the definition of CR or PR), RC reticulocyte
aAll included patients are warm AIHI
Table 2.
Comparison of hemoglobin level before and 1, 2, 3 and 4 months after cyclophosphamide therapy
| Hemoglobin level (g/dL) | P value |
|---|---|
| Before initiation of cyclophosphamide therapy (5.6 ± 1.6) vs. | |
| After 1 month | ** |
| After 2 months | *** |
| After 3 months | *** |
| After 4 months | *** |
| After 1 month (7.1 ± 1.2) vs. | |
| After 2 months | ** |
| After 3 months | *** |
| After 4 months | *** |
| After 2 months (8.1 ± 0.8) vs. | |
| After 3 months | ** |
| After 4 months | *** |
| After 3 months (8.8 ± 0.8) vs. | |
| After 4 months (9.6 ± 0.9) | ** |
The values were represented by mean ± standard deviation
** P < 0.01, *** P < 0.001
Fig. 1.
Reticulocytic count before and after cyclophosphamide therapy
Discussion
Treatment of steroid refractory AIHA is challenging especially in patients who failed to respond to maximum dose of steroids ± azathioprine ± intravenous immunoglobulin ± oral cyclophosphamide, also their preference to avoid surgery (splenectomy), restrictions imposed by health funding authorities to provide rituximab and the unavailability of compatible blood transfusion even washed RBCs superadded more difficulties and put the patients in a critical situation, so our trial using pulse cyclophosphamide therapy monthly showed good response with no detectable hazardous effects in our patients.
AIHA frequently has an acute onset, but in most cases it must be considered as a chronic disease with few exceptions. In primary WAIHA, there is only a low chance of spontaneous or drug induced long-term remission or cure. Thus, the primary goal of treatment is to keep the patient clinically comfortable and to prevent “hemolytic crises” with the use of medical interventions with the lowest possible short- and long-term side effects [4]. It is surprising and regrettable that treatment of AIHA is still not evidence-based, but essentially experience-based. There are no randomized studies and only a few prospective phase 2 trials, otherwise, only retrospective studies. There is no formal consensus on the definition of complete (CR) or partial (PR) hematologic remission and refractoriness [4]. There is little consensus on how to manage AIHA when corticosteroid therapy fails and when splenectomy is ineffective or is not an option [5]. Treatments for these patients include low-dose cytotoxic therapy [16] danazol and intravenous immunoglobulin [17]. Most of these treatments are only partially successful, with many patients becoming dependent on glucocorticoid maintenance therapy, and eventually suffering the consequences of chronic steroid administration [18]. However, progress in treatment has been much slower [19]. Therapy has been reviewed by several investigators, but no treatment guidelines have yet been published [20].
For patients with AIHA in whom glucocorticoid treatment fails, splenectomy is frequently offered as second-line treatment [16]. However, this approach is limited because splenectomy is less effective and have a higher complication rate in secondary AIHA [21]. There is lack of systematic long-term data on efficacy and safety in the published reports for rituximab in AIHA, also rituximab therapy has to be repeated every 1–3 years, and this may increase the risk of infections, including progressive multifocal leukoencephalopathy PML [4]. In practice the choice of the sequence of second line treatments in patients with WAIHA mainly depends on the personal experience of the physician, patient factors such as age and co morbidity, the availability and cost of drugs, and the preference of the patient. The main factor for the selection of any drug should be safety, because the curative potential of all these drugs is low, and treatment may be more dangerous for the patient than the disease to be treated. Decisions are always made on an individual basis after discussion of experienced hematologists and then with the patient [4].
The opinion that cyclophosphamide is highly effective appears to be based on data from two earlier articles [22, 23]. Those studies provided overall results but no specific patient details
Our results were in concord with the study by Moyo et al. [8] which stated that, the use of high-dose cyclophosphamide (50 mg/kg ideal body weight per day) intravenously in combination with mesna and G-CSF for 4 consecutive days is well tolerated and effective in patients with refractory AIHA and they added that further study of this approach as treatment for refractory AIHA is warranted. In our study a nearly similar results to that [8] were obtained with relatively small dose (1 g/month for four consecutive months) without use of mesna and G-CSF. The hazards of the high-dose cyclophosphamide they used was nausea, vomiting, transient alopecia, and neutropenic fever were not observed in our patients. More studies with large number of patients are recommended to compare between the two regimens.
Conclusion
Pulse cyclophosphamide therapy with relatively small dose (1 g/month for four consecutive months) induces remission in patients with severe refractory AIHA and provides reasonable option for AIHA away from the risks of splenectomy and toxicity and the higher costs of rituximab and needed to be studied in large scale of patients.
Contributor Information
Ahmad F. Thabet, Phone: 01061461306, Email: drahmedfarag2005@yahoo.com
Mostafa Faisal, Phone: 01003071038, Email: mostafafaisal82@yahoo.com.
References
- 1.Klein NP, Ray P, Carpenter D, et al. Rates of autoimmune diseases in Kaiser Permanente for use in vaccine adverse event safety studies. Vaccine. 2010;28(4):1062–1068. doi: 10.1016/j.vaccine.2009.10.115. [DOI] [PubMed] [Google Scholar]
- 2.Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. J Autoimmun. 2007;29(1):1–9. doi: 10.1016/j.jaut.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valent P, Lechner K. Diagnosis and treatment of autoimmune haemolyticanaemias in adults: a clinical review. Wien KlinWochenschr. 2008;120(5–6):136–151. doi: 10.1007/s00508-008-0945-1. [DOI] [PubMed] [Google Scholar]
- 4.Lechner K, Jäger U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831–1838. doi: 10.1182/blood-2010-03-259325. [DOI] [PubMed] [Google Scholar]
- 5.Petz LD, Garratty G. Management of autoimmune hemolytic anemias. In: Petz LD, Garratty G, editors. Acquired immune hemolytic anemias. New York: Churchill Livingstone; 1980. pp. 392–440. [Google Scholar]
- 6.Ahn YS, Harrington WJ, Mylvaganam R, Ayub J, Pall LM. Danazol therapy for autoimmune hemolytic anemia. Ann Intern Med. 1985;102:298–301. doi: 10.7326/0003-4819-102-3-298. [DOI] [PubMed] [Google Scholar]
- 7.Cooper N, Stasi R, Cunningham-Rundles S, et al. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125(2):232–239. doi: 10.1111/j.1365-2141.2004.04889.x. [DOI] [PubMed] [Google Scholar]
- 8.Moyo VM, Smith D, Brodsky I, Crilley P, Jones RJ, Brodsky RA. High-dose cyclophosphamide for refractory autoimmune hemolytic anemia. Blood. 2002;100(2):704–706. doi: 10.1182/blood-2002-01-0087. [DOI] [PubMed] [Google Scholar]
- 9.McCune WJ, Bolbus J, Zeldes W, Dunne R, Bohlke P, Fox DA. Clinical and immunologic effects of monthly administration of intravenous cyclophosphamide in severe systemic lupus erythematosus. N Engl J Med. 1988;318:1423. doi: 10.1056/NEJM198806023182203. [DOI] [PubMed] [Google Scholar]
- 10.Verlin M, Laros RK, Penner JA. Treatment of refractory thrombocytopenic purpura with cyclophosphamide. Am J Hematol. 1996;1:97. doi: 10.1002/ajh.2830010111. [DOI] [PubMed] [Google Scholar]
- 11.Thomas ED, Storb R, Fefer A, et al. Aplastic anaemia treated by marrow transplantation. Lancet. 1972;1:284–289. doi: 10.1016/S0140-6736(72)90292-9. [DOI] [PubMed] [Google Scholar]
- 12.Jones RJ, Barber JP, Vala MS, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85:2742–2746. [PubMed] [Google Scholar]
- 13.Jaime-Perez JC, Gonzalez-Llano O, Gomez-Almaguer D. High-dose cyclophosphamide in the treatment of severe aplastic anemia in children. Am J Hematol. 2001;66:71. doi: 10.1002/1096-8652(200101)66:1<71::AID-AJH1019>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 14.Hayag MV, Cohen JA, Kerdel FA. Immunoablative high-dose cyclophosphamide without stem cell rescue in a patient with pemphigus vulgaris. J Am Acad Dermatol. 2000;43:1065–1069. doi: 10.1067/mjd.2000.110397. [DOI] [PubMed] [Google Scholar]
- 15.Brodsky RA, Fuller AK, Ratner LE, Jones RJ, Leffell MS. Elimination of alloantibodies by immunoablative high-dose cyclophosphamide. Transplantation. 2001;71:482–484. doi: 10.1097/00007890-200102150-00025. [DOI] [PubMed] [Google Scholar]
- 16.Rosse W, Bussel J, Ortel T (1997) Challenges in managing autoimmune disease. American Society of Hematology Education Book 92–101
- 17.Flores G, Cunningham-Rundles C, Newland AC, Bussel JB. Efficacy of intravenous immunoglobulin in the treatment of autoimmune hemolytic anemia: results in 73 patients. Am J Hematol. 1993;44:237–242. doi: 10.1002/ajh.2830440404. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz RS, Berkman EM, LE Silberstein . Autoimmune hemolyticanemias. In: Hoffman RH, Benz EJ Jr, Shattil SJ, editors. Hematology: basic principles and practice. 3. Philadelphia: Churchill Livingstone; 2000. pp. 611–630. [Google Scholar]
- 19.Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev. 2008;22(1):1–15. doi: 10.1016/j.blre.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Packman CH. Hemolytic anemia due to warm autoantibodies. Blood Rev. 2008;22(1):17–31. doi: 10.1016/j.blre.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Akpek G, McAneny D, Weintraub L. Comparative response to splenectomy in Coombs-positive autoimmune hemolytic anemia with or without associated disease. Am J Hematol. 1999;61:98–102. doi: 10.1002/(SICI)1096-8652(199906)61:2<98::AID-AJH4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Zupańska B, Sylwestrowicz T, Pawelski S. The results of prolonged treatment of autoimmune haemolytic anaemia. Haematologia (Budap) 1981;14(4):425–433. [PubMed] [Google Scholar]
- 23.Sakalová A, Hrubisko M. Cyclophosphamide in the treatment of immune hemocytopenias. FoliaHaematolInt Mag KlinMorpholBlutforsch. 1975;102(5):559–564. [PubMed] [Google Scholar]