Abstract
Study of the factors responsible for red cell alloimmunization can help in adopting appropriate strategy to minimize alloimmunization. However data for thalassemia patients from our region is limited. Therefore, a study was conducted to find out the frequency and the factors associated with red cell allo and autoimmunization in thalassemia patients at our center so as to enable us to take appropriate action to reduce alloimmunization. Clinical, demographic, allo and autoantibody and transfusion records of 280 thalassemia patients at our hospital were studied. Patients with and without alloantibodies were compared to find significant differences for age, gender, race, age at start of regular transfusions and splenectomy. Red cell antigen frequencies in thalassemia patients and published antigen frequencies in blood donors from the same center were compared to look antigen differences as a risk factor for alloimmunization. Twenty four thalassemia patients (8.6 %) developed 28 clinically significant alloantibodies. 18 (65 %) of the alloantibodies were of Rh system. The three most common antibodies detected was anti E (11, 39.3 %) followed by anti K (6, 21.4 %) and anti c (10.8 %). Five (1.8 %) of the 280 patients developed autoantibodies. Patient age was found to be significantly higher in alloimmunized patients than in non alloimmunized patients. Red cell antigen frequencies between blood donor and recipient populations were found to be homogenous for most of the relevant RBC antigens. The frequency of red cell alloimmunization in thalassemia patients from our center is moderate. In this setting of red cell phenotype concordant donor–recipient population requirement of extended phenotype matched transfusions may not be cost effective.
Keywords: Alloimmunization, Hemoglobinopathies, Phenotype frequency, Red cell antigens, Thalassemia, Transfusion
Introduction
The beta thalassemia is probably the commonest inherited hemoglobin disorder in Indian subcontinent; with an uneven distribution among the different endogamous populations [1]. The carrier status in Northern part of India is estimated to be around 3–4 % [2]. Beta thalassemia major usually manifests within the first year of life in 95 % of the patients and these patients are dependent on life long transfusion therapy for their survival. Transfusion therapy has been a key intervention in decreasing the thalassemia disease associated morbidity and mortality and it still remains a mainstay of treatment for majority of thalassemia patients despite the successful use of hematopoietic stem cell transplantation to cure the disease [3]. However, with multiple intermittent or chronic transfusions these patients are exposed repeatedly to risks associated with transfusion therapy i.e., infectious disease transmission, volume overload, haemolytic transfusion reactions, iron overload, increased risk of allo and autoimmunization and clinically significant delayed haemolytic transfusion reactions (DHTRs) [3, 4].
The risks of red blood cell (RBC) antigen alloimmunization in thalassemia patients are considerably greater than those in general population as evident from alloimmunization rates of 4–50 % reported in studies from various centers involved in management of these patients [5–14]. The alloimmunization can result in clinically significant DHTRs and also significantly compromise the patient care by delaying the availability of compatible blood [10]. Factors thought to be responsible for the alloimminization rates include the differences in RBC antigen frequency between blood donor and the recipient population, the immune responsiveness of the patient and the immunomodulatory effects of the allogenic transfusions on the recipient’s immune status [5, 10]. Study of the factors responsible for alloimmunization can help in adopting appropriate strategy to minimize alloimmunization frequency. However data for thalassemia patients from our region is limited [13].
Therefore, a study was conducted to find out the prevalence of allo and autoimmunization and to identify the clinical factors associated with the RBC antibody formation in thalassemia patients at our center so as to enable us to take appropriate action to reduce alloimmunization.
Materials and Methods
Patients and Transfusion Records
Clinical, demographic, allo and autoantibody and transfusion records of 280 thalassemia patients who were seen at our hospital from 1989 to 2012 and continued to be seen were reviewed. All records were available on computerized Hospital Information System since year 2001 and earlier as hard copies of patient case report files. The results of blood group antibody screening and identification tests were recorded. These patients had been receiving ABO and RhD compatible packed red blood cells (PRBCs) transfusions collected from local voluntary and replacement donors; since 2004 PRBC transfusions were by buffy coat reduced. In addition to ABO grouping and Rh D typing, pretransfusion testing consisted of major cross-matching by polyspecific Anti Human Globulin Gel cards (Diamed AG, Cressier, Sur Morat, Switzerland). Red cell antibody screening was done using three-RBC panel (Diamed) and low-ionic-strength saline (LISS) enhanced antiglobulin column gel agglutination cards (Diamed) at regular frequent intervals or as and when indicated due to inadequate response to transfusion. Antibody specificity was identified by 11 cell identification panel (Diamed) in every screening positive case. If an alloantibody was identified, the information was recorded in the blood bank records and all subsequent transfusions of RBC were selected to be relevant blood group antigen negative, crossmatch compatible. If autoantibodies were suspected further investigations were done as per the standard protocols [15]. Extended RBC antigen phenotyping was done for all new and un transfused patients since 2004 to enable or assist in identification of alloantibodies, should the patient develop them. The C, c, E and e typing was done by monoclonal saline reacting antisera (Diamed).Typing for other RBC antigens i.e. K, k, Jka, Jkb, Fya, Fyb, S, s) was done using commercially available monoclonal incomplete antisera and AHG gel cards (Diamed), M, N, Lea, Leb typing was done using IgM antisera and neutral cards (Diamed) at room temperature.
Analysis
Prevalance of Allommunization and Autoimmunization
Prevalence of alloimmunization with 95 % confidence interval was calculated by GraphPad QuickCalcs software. The cumulative incidence of RBC alloimmunization was calculated per 1,000 person years of follow up or per 100 RBC units transfused. Frequency of red cell antibody specificities was calculated.
Risk Factors for Alloimmunization
Patient related risk factors for alloantibody formation considered for analysis were age, gender, race, age at start of regular transfusions and splenectomy. Patients with and without alloantibodies were compared to find significant differences for relevant variables. t test was used for continuous variables and Chi Squares test for nominal variables.
The RBC antigen frequency among patients was calculated as per standard method [16]. The Fischer’s exact test was used for assessment of statistical significant differences between antigen frequencies between patients and published antigen frequencies in blood donors from the same center [17] to look for donor patient RBC antigen differences as a risk factor for alloimmunization. P values <0.05 were considered statistically significant.
Results
Patient Demographic Characteristics
280 beta thalassemia major patients [187 males (66.7 %) and 93 females (33.3 %); age mean 11.1 + 6.61 years (median age 10 years, range 0.7–32 years] were included in the present study. All these patients were of Asian descent and from natives of India. All patients began transfusion therapy within their first year of life. Median age at of the time of first transfusion was eight months (range 5–12 months). Total time under observation at the time of study was 2,939.6 patient years. The median number of RBC units transfused per patient was 225 (range 12–512 units). The total PRBC units transfused to all patients amounted to 70,560 RBC units in 5,292 transfusion episodes.
RBC Antibodies
Figure 1 summarizes the RBC antibodies ever detected in the study patients. As seen in Fig. 1. Twenty four thalassemia patients (8.6, 95 % confidence interval 5.5–12.8 %) developed 28 clinically significant alloantibodies. Single antibody was seen in 20 of these patients and four patients formed two alloantibodies each. The incidence of RBC alloantibody formation was 9.52 per 1,000 person years of follow up or 0.04 per 100 units transfused. Auto antibodies were also detected in five patients who included one patient who subsequently formed an alloantibody.
Fig. 1.
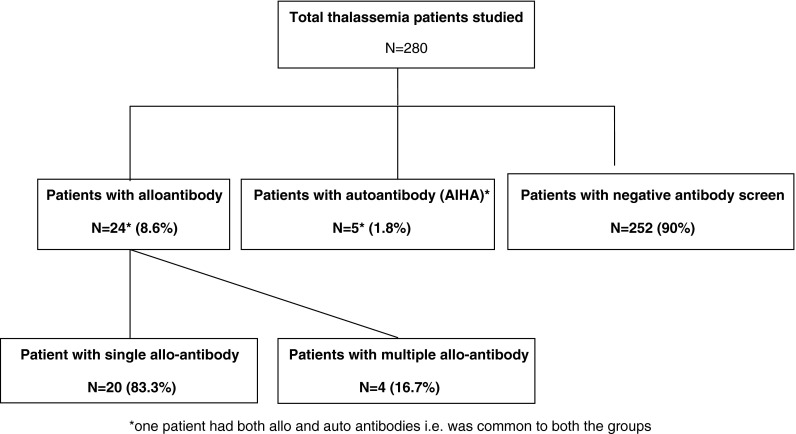
Frequency of red cell allo and autoimmunization in thalassemia patients
Table 1 shows the details of the 24 alloimmunized thalassemia patients. As seen in table 1 the alloantibodies developed 3–21 years after the initiation of transfusion therapy. These patients had received median 136.5 units (range 28–482 units; mean + SD 171.45 ± 129.87 units of RBC prior to first alloimmunization. Five out of 24 (20.8 %) alloimmunized patients had a prior splenectomy.
Table 1.
Characteristics of alloimmunized β-thalassemia patients
| Patient no. | Gender | Age (years) | RBC alloantibody specificity | Age at initiation of transfusion therapy (months) | Age at detection of RBC allo-antibody (years) | Units of blood received prior to allo-antibody formation | Splenectomy |
|---|---|---|---|---|---|---|---|
| 1 | Male | 17 | Anti-JKa | 8 | 12 | 194 | No |
| 2 | Male | 8 | Anti Jka & c | 9 | 3 & 3.5 | 28 & 33 | No |
| 3 | Female | 13 | Anti K & C | 7 | 7 & 10 | 91 & 141 | No |
| 4 | Female | 22 | Anti K | 6.5 | 16 | 279 | No |
| 5 | Male | 10 | Anti K | 6 | 7.9 | 97 | No |
| 6 | Female | 16 | Anti N | 5.5 | 10.1 | 135 | No |
| 7 | Male | 13 | Anti c | 6.2 | 10.2 | 138 | No |
| 8 | Male | 25 | Anti E | 7 | 21.6 | 482 | No |
| 9 | Male | 6 | Anti D & E | 8 | 3.8 & 5.4 | 37 & 69 | No |
| 10 | Male | 13 | Anti E | 9 | 10.4 | 153 | No |
| 11 | Male | 17 | Anti K | 8.5 | 14.3 | 217 | No |
| 12 | Female | 8 | Anti E | 9.2 | 5.2 | 62 | Yes |
| 13 | Female | 7 | Anti E | 7 | 4.3 | 131 | No |
| 14 | Male | 9 | Anti K | 6 | 6.2 | 81 | No |
| 15 | Female | 23 | Anti K | 7.6 | 19.8 | 439 | No |
| 16 | Male | 16 | Anti D & E | 10.5 | 6.4 & 10.6 | 73 & 141 | No |
| 17 | Male | 19 | Anti E | 8.5 | 16.4 | 293 | No |
| 18 | Male | 22 | Anti C | 8 | 20.1 | 446 | Yes |
| 19 | Male | 10 | Anti S | 8.8 | 5.1 | 59 | Yes |
| 20 | Male | 8 | Anti E | 6 | 6.8 | 96 | No |
| 21 | Male | 15 | Anti E | 9.5 | 11.4 | 179 | No |
| 22 | Male | 13 | Anti E | 10.2 | 12.1 | 195 | Yes |
| 23 | Male | 6 | Anti c | 8 | 5.3 | 67 | No |
| 24 | Male | 14 | Anti K | 9 | 10.4 | 143 | Yes |
Table 2 summarises the specificities of alloantibodies detected. As seen 18 (65 %) of the alloantibodies were of Rh system. The three most common antibodies detected was anti E (11, 39.3 %) followed by anti K (6, 21.4 %) and anti c (10.8 %). The antibody combinations found were anti D + E in two patients, Anti Jka + c in one patient and anti K + C in one patient.
Table 2.
RBC-alloantibody specificity in alloimmunized thalassemia patients
| Blood group system | Antibody frequency Na (%) |
|---|---|
| Rh blood group | |
| Anti-E | 11 (39.3) |
| Anti-D | 2 (7.1) |
| Anti-C | 2 (7.1) |
| Anti-c | 3 (10.8) |
| Kell blood group | |
| Anti-K | 6 (21,4) |
| Kidd blood group | |
| Anti-JKa | 2 (7.1) |
| MNS blood group | |
| Anti-N | 1 (3.5) |
| Anti-S | 1 (3.5) |
aTwo patients had anti D + E, one patient each had anti Jka + c and anti K + C
Risk Factors for Alloimmunization
Table 3 summarizes the differences between the alloimmunized and non alloimmunized patients with respect to the patient related factors deemed to be associated with RBC alloantibody formation i.e., gender and age of patients, age at initiation of therapy, duration of transfusion therapy and splenectomy status. Of these only patient age was found to be significantly different (higher) in alloimmunized patients than in non alloimmunized patients.
Table 3.
Comparison of alloimmunized and non alloimmunized thalassemia patients
| Alloimmunized patients (n = 24) | Non alloimmunized patients (n = 256) | P value | |
|---|---|---|---|
| Males | 18 (75 %) | 169 (66 %) | 0.497a (NS) |
| Age (years) mean ± SD | 13.75 ± 5.62 | 10.9 ± 6.6 | 0.0419 (NS) |
| Age at initiation of transfusion therapy (months) mean ± SD | 7.9 ± 1.4 | 8.1 ± 1.6 | 0.70 (NS) |
| Duration of transfusion therapy (years) mean ± SD |
12.9 ± 5.91 | 10.3 ± 6.55 | 0.057 (NS) |
| Splenectomy | 5/24 (20.8 %) | 27/256 (10.5 %) | 0.169a (NS) |
a P value calculated by Chi Squares test, rest by t test
Table 4 shows the frequency of RBC antigens in 57 phenotyped thalassemia patients as compared to reported RBC antigen frequency in local donors for any statistically significant differences. Only Jka, Jkb, N. Lea, Leb RBC antigen frequency was found to be significantly lower in thalassemia patients than among blood donors, they however, accounted for only 3/28 (0.10 %) of alloantibodies detected. The commonest Rh phenotype among patients was DCe in 25 (43.8 %) patients followed by DCce in 14 (24.5 %), DCcEe in 11 (19.5 %), DcE in 1 (1.7 %), Dce in 2 (3.5 %), DcEe in 2 (3.5 %) and ce in 2 (3.5 %) patients.
Table 4.
RBC antigens frequency in thalassemia patients compared with local blood donor population
| RBC antigen | AF% in thalassemia patients in present study (%) (n = 57) | AF% reported in blood donors from same center [17] | P calculated by Fischers exact test two tailed |
|---|---|---|---|
| D | 55 (96.4) | 96 | NS |
| C | 50 (87.7) | 95.19 | NS |
| C | 32 (56.1) | 69.23 | NS |
| E | 14 (24.5) | 15.38 | NS |
| E | 56 (98.2) | 98.07 | NS |
| K | 0 (0) | 1.92 | NS |
| k | 57 (100) | 100 | NS |
| Jka* | 34 (59.6) | 75.96 | 0.02 |
| Jkb | 28 (49.12) | 68.26 | 0.0008 |
| Fya | 49 (85.9) | 73.07 | 0.034 |
| Fyb | 31 (54.3) | 53.84 | NS |
| M | 49 (85.9) | 77.88 | NS |
| N | 30 (52.6) | 73.07 | 0.005 |
| S | 40 (70) | 63.46 | NS |
| s | 50 (87.7) | 45.19 | 0.001 |
| Lea | 19 (13.3) | 36.53 | 0.001 |
| Leb | 15 (26.3 %) | 69.23 | 0.001 |
RBC Autoimmunization
As seen in Fig. 1, five (1.8 %) of the 280 patients developed autoantibodies as determined by a persistent or transient positive Direct antiglobulin test (DAT) that ranged from 1+ to 4+. In one (20 %) of these patients autoimmunization occurred subsequent to alloimmunization. Three (60 %) of these patients had a prior splenectomy. In all of these patients there were warm IgG alloantibodies with panaggulination reaction and positive autocontrol. All these patients developed clinically significant Autoimmune hemolytic anemia (AIHA) which had to be treated with steroids.
Discussion
Only a few studies have objectively studied the causes and risk factors for alloimmunization specifically the phenotype differences between the patients and blood donors. In the present study we studied these elements to look for the best solutions to reduce alloimmunization in our thalassemia patients.
Frequency of Alloimmunization and Autoimmunization
Table 5 summarises the alloimmunization and autoimmunization rates in thalassemia patients reported from various populations for comparison. As seen the reported alloimmunisation rates in thalassemics from other parts of India vary from 3.79–9.48 % [8, 13, 19]. In our study frequency of alloimmunization and autoimmunization was 8.6 and 1.8 % respectively which is similar to that reported from other centers in our country. These alloimmunization rates can be termed as moderate. In contrast, low immunization rates are reported in blood donors (0.8 %) or other hospital base patients (1–2 %) [24, 25]; or high alloimmunization rates of more than 20 % reported in thalassemics from China [7], USA and UK [6], USA [5], Taiwan [14], Arab[19] and in Sickle Cell Disease (SCD) patients [10]. The alloimmunization rates in other frequently transfused patients from our country have also been reported to be moderate i.e. 3.4 % in multiply transfused patients [26] and 9.8 % in renal failure patients [27].
Table 5.
Red cell immunization in patients with thalassemia
| Country [Reference number] | No. of patients | Frequency of autoimmunization (%) | Frequency of alloimmunization (%) | Comments |
|---|---|---|---|---|
| India [13] | 211 | 0.47 | 3.8 | Anti E, anti K, anti D |
| India [18] | 116 | – | 9.48 | Anti E followed by anti K |
| India [8] | 32 | – | 18.8 | Anti E followed by anti c |
| Taiwan [14] | 30 | – | 37 | Anti E, anti c, anti Mia, anti D, anti S |
| Iran [9] | 711 | 1.7 | 5.3 | Anti K, anti D, anti E |
| Arab [19] | 190 | 11 | 30 | – |
| Pakistan [20] | 97 | – | 9.2 | Anti K, anti D, anti E |
| Pakistan [21] | 161 | 1.87 | 5 | Anti K, anti Jka, anti Jsb |
| Malaysia [22] | 58 | 1.7 | 8.6 | Anti E, anti c, anti K, anti Jka, anti N, anti S |
| China [7] | 382 | 4.7 | 18.3 | Anti E followed by anti Mi/Mur and anti c |
| Taiwan Chinese [23] | 64 | – | 9.4 | 66 % anti E, followed by anti Mia. Anti C |
| Asians in UK and US [6] | 335 | 4.9 % | 13.3 | Anti C, anti E, anti K |
| Caucasians in UK and US [6] | 328 | 21.2 | ||
| Italy [11] | 1,435 | – | 5.2 | Anti C, anti E, anti K |
| Spanos [12] | 973 | – | 22.6 | Anti C, anti E, anti K |
| USA Asians [5] | 48 | 25 | 20.8 | Anti K followed by anti E |
| Present study | 280 | 1.8 | 8.6 | Most common alloab anti E followed by anti K and then anti c |
In our study frequency of autoimmunization was found to be 1.8 %. In a study by Ahmed et al. the incidence of autoantibodies in thalassemics was 28.8 % [28]. Their data was similar to an Asian study which reported autoantibodies in 25 % of their thalassaemia patients [5]. Also a study from Kuwait observed autoantibodies in 11 % of their patients [19]. However, a study in Iran found autoantibodies in 1.4 % of their thalassemia patients [9]. Another study from Malaysia reported that only one of their patient developed autoantibodies [22]. We observed a great variation in reported autoimmunization rates however why there is so much variation and whether these were also clinically significant autoantibodies need to be studied.
Alloantibody Specificity
Table 5 also shows the specificity of alloantibodies reported in various studies. As seen, antibodies are reported mainly against Rh and K antigens. In our study anti c was the third most common antibody (10.8 %, 3/28) following anti E (39.3, 11/28) and anti K (21.4 %, 6/28). Various studies have reported high prevalence of anti E and anti c in Asian population [5, 21, 22, 26]. Based on this Chaudhari et al. [8] proposed that there is heterogeneous distribution of Rh E and c Antigen in Indian population. Papiha [29] had reported R1R1 phenotype frequency to be 50 % in North India to 70 % in Mongoloid population. However in our study we did not find significant differences in E and c antigen frequency between thalassemia patients and local donor population. In our study frequency of R1R1 phenotype in thalassemia patients was 43.8 %. Another explanation put forward to explain the antibody specificities observed is that Rh c and E are known to be potent antigens with relative potency of 0.041–0.0338 respectively, next to only potency of RhD and K (Kell) and thus exposure leads to immunization in antigen negative subjects [30]. The concurrent antibodies in our study were anti D + E (two patients), anti K + C (one patient) and anti Jka + c (one patient). However we did not find anti c + E as reported by others. It has been reported that R1R1 subjects who develop anti E are prone to develop anti c subsequently and delayed hemolytic transfusion reaction [31]. Anti c/E pair has been reported to be the third commonest concurrent antibodies after anti K/E and anti D/C [32].
In most reports from Western countries, RBC antibodies most commonly associated with alloimmunization in thalassaemia are directed against C, E and Kell antigens [6, 11, 12]. Anti-D alloantibodies are also frequently reported even though most patients receive RBC products crossmatched for ABO and RhD blood groups worldwide. Unlike SCD, it is not known if variant RH genotypes that could predispose patients to alloimmunization are more prevalent in thalassaemia patients. Kell antibodies are identified less often in Chinese thalassaemia patients compared to Caucasians [7], however, antibodies against Mia and Mur antigens occur more frequently in Chinese and Southeast Asians [7, 14]. Mia and Mur antigens are part of the Miltenberger subsystem in the MNS blood group and can be associated with severe haemolytic disease of the newborn as well as haemolytic transfusion reactions.
Risk Factors for Alloimmunization
RBC Antigenic Differences Between Blood Donor and Recipient
Differences in alloimmunization rates are often attributed to the ethnic or racial disparity of the donor recipient population. The relative homogeneity of donor and recipient populations in Greece and Italy has been shown to contribute to lower alloimmunization rates for transfused patients with thalassaemia [11, 12] compared to more heterogeneous populations in other countries, such as Kuwait [19] and Taiwan [14]. One study from USA reported an alloimmunization rate of 20.8 % in Asians patients with thalassaemia where only 5 % of local blood donors were Asian [5]. In a larger geographically diverse report, 13.3 % of Asians with thalassaemia in North America and the United Kingdom were alloimmunized, which was not significantly different from other races or ethnic groups; the ethnicities of donors were not noted [6]. Our findings demonstrated objectively that blood donor and recipient populations are homogenous in our region for most of the relevant RBC antigens. In this setting of red cell phenotype concordant donor-recipient population requirement of extended phenotype matched transfusions may not be cost effective.
Other Factors
Of the patient related factors deemed to be associated with RBC alloantibody formation i.e., gender and age of patients, age at initiation of therapy, duration of transfusion therapy and splenectomy status only patient age was found to be significantly different (higher/more) in alloimmunized patients than in non alloimmunized patients. Duration of transfusion support and older age have been reported to be associated with increased rates of alloimmunization in thalassaemia patients. In our study alloantibodies formed 3–21 years after transfusion or after transfusion of median 136 units of red cells. Although gender has been identified a risk factor for alloimmunization in the general population [33], studies of thalassaemia have not found higher rates in women [6, 19, 34]. Splenectomy, which is typically performed in thalassaemia patients to alleviate hypersplenism or stabilize transfusion requirements, has been reported to be a significant risk factor for alloimmunization [5, 6, 34]. The mechanism by which removal of the spleen increases alloantibody formation is not clear. As reported, when the spleen is absent or removed, immune responses are altered [35]. It has been postulated that post-splenectomy conformational changes in RBC membranes enhance immunomodulation that result in allosensitization. While splenectomy is performed less frequently in thalassaemia [6, 36], the significantly increased risk of alloimmunization in splenectomized younger patients is concerning and deserves further study.
Allogeneic white blood cells within RBC products have been implicated in the past with allergic and febrile transfusion reactions, as well as alloimmunization, [5, 37–39] and transfusion of leucoreduced blood has been offered as a solution to reduce alloimmunization. However, the precise mechanism and the host factors that mediate allosensitization are not clear. CD4+ regulatory T cells, which are prime regulators of immune responses, had reduced activity in alloimmunized thalassaemia patients compared to other chronically transfused patients who remain non-alloimmunized [40]. This suggests that identification of molecular markers of hosts that predict a propensity to form alloantibodies could be useful in designing strategies to mitigate this risk.
The pathogenesis of erythrocyte autoantibody formation following transfusion is also not well understood, though, clinical evidence of autoimmune hemolytic anaemia has been seen with high amounts of RBC-associated IgG [41]. It was also suggested that alloantibody binding to the RBCs could lead to conformational changes of the antigenic epitope that ultimately stimulates production of autoantibodies [42]. It is possible that certain people are genetic responders who have an increased tendency to develop RBC autoantibodies and the tendency toward autoantibody formation could reflect an overall dysfunction of the immune system [43].In study by Ahmed there was a significant association of splenectomy with alloimmunization and auto antibodies (P = 0.03, 0.001 respectively).Wiener et al. [44], demonstrated significant elevations in RBC bound IgG in thalassaemia patients which was more abundant in splenectomized than nonsplenectomized subjects. They found that the absence of a spleen may further enhance the immune response to the infused foreign antigens which are not effectively filtered. In contrast, in Malaysia despite a higher rate of patients with splenectomy only one patient had been reported to have autoantibodies [22, 41].
In summary, our data shows that frequency of alloimmunization to minor RBC antigens is moderate in our transfused thalassemia patients; this finding is commensurate with the homogeneity of our blood donor and recipient population for clinically significant RBC antigens. To further reduce the alloimmunization the feasibility and usefulness of prophylactic extended/limited phenotype matched and leucopoor PRBC transfusions should be studied. In patients with poor transfusion outcomes probability of autoimmunization and AIHA should be considered.
Conflict of interest
None.
References
- 1.Sukumaran PK. Abnormal hemoglobin in India. In: Sen NN, Basu AK, editors. Trends in hematology. Calcutta: Sree Saraswaty Press Ltd.; 1975. [Google Scholar]
- 2.Bichele SK. Symposium: thalassemias (part-one) Indian J Haematol Blood Transfus. 1992;10:1–20. [Google Scholar]
- 3.Prati D. Benefits and complications of regular blood transfusion in patients with beta thalassemia major. Vox Sang. 2000;79:129–137. doi: 10.1046/j.1423-0410.2000.7930129.x. [DOI] [PubMed] [Google Scholar]
- 4.Rebulla P, Modell B. Transfusion requirements and effects in patients with beta thalassemia major. Lancet. 1991;337:227–280. doi: 10.1016/0140-6736(91)90881-O. [DOI] [PubMed] [Google Scholar]
- 5.Singer ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP. Alloimmunization and erythrocyte autoimmunization in transfusion dependent thalassemia patients of predominantly Asian descent. Blood. 2000;96:3369–3373. [PubMed] [Google Scholar]
- 6.Thompson AA, Cunningham MJ, Singer ST, Neufeld EJ, Vichinsky E, Yamashita R, Thalassemia Clinical Research Network Investigators et al. Red cell alloimmunization in a diverse population of transfused patients with thalassaemia. Br J Haematol. 2011;153:121–128. doi: 10.1111/j.1365-2141.2011.08576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng CK, lee CK, Lin CK. Clinically significant red blood cell antibodies in chronically transfused patients: a survey of Chinese thalassemia major patients and literature review. Transfusion. 2012;52:2220–2224. doi: 10.1111/j.1537-2995.2012.03570.x. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhari CN. Red cell antibodies in multiple transfused thalassemia patients. MJAFI. 2011;67:34–37. doi: 10.1016/S0377-1237(11)80008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karimi M, Nikrooz P, Kashef S, Jamalian N, Davatolhagh Z. RBC alloimmunization in blood transfusion-dependent beta-thalassemia patients in southern Iran. Int J Lab Hematol. 2007;29:321–326. doi: 10.1111/j.1365-2257.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 10.Chou ST, Liem RL, Thompson AA. Challenges of alloimmunization in patients with hemoglobinopathies. Br J Haematol. 2012;158:394–404. doi: 10.1111/bjh.12061. [DOI] [PubMed] [Google Scholar]
- 11.Sirchia G, Zanella A, Parravicini A, Morelati F, Rebulla P, Masera G. Red cell alloantibodies in thalassemia major. Results of an Italian cooperative study. Transfusion. 1985;25:110–112. doi: 10.1046/j.1537-2995.1985.25285169198.x. [DOI] [PubMed] [Google Scholar]
- 12.Spanos T, Karageorga M, Ladis V, Peristari J, Hatzihami A, Kattamis C. Red cell alloantibodies in patients with thalassemia. Vox Sang. 1990;58:50–55. doi: 10.1111/j.1423-0410.1990.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 13.Pahuja S, Pujani M, Gupta SK, Chandra I, Jain M. Alloimmunization and red cell autoimmunization in multitransfused thalassemics of Indian origin. Hematology. 2010;15:174–177. doi: 10.1179/102453309X12583347114013. [DOI] [PubMed] [Google Scholar]
- 14.Wang LY, Liang DC, Liu HC, Chang EC, Wang CL, Chan YS, et al. Alloimmunization among patients with transfusion-dependent thalassemia in Taiwan. Transfus Med. 2006;16:200–203. doi: 10.1111/j.1365-3148.2006.00656.x. [DOI] [PubMed] [Google Scholar]
- 15.Brecher ME. Technical manual. 15. Bethesda: American Association of Blood Banks; 2005. [Google Scholar]
- 16.Thakral B, Saluja K, Sharma RR, Marwaha N. Phenotype frequencies of blood group systems (Rh, Kell, Kidd, Duffy, MNS, P, Lewis and Lutheran) in north Indian blood donors. Transfus Apher Sci. 2010;43:17–22. doi: 10.1016/j.transci.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhary Rk, Shukla JS, Ray V. Minor red cell antigens in north Indian blood donor population. Indian J Haematol Blood Transfus. 2003;21:34–36. [Google Scholar]
- 18.Gupta R, Singh DK, Singh B, Rusia U. Alloimmunisation to red cells in thalassemia: emerging problems and future strategies. Transfus Apher Sci. 2011;45:167–170. doi: 10.1016/j.transci.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Ameen R, Al-Shemmari S, Al-Humood S, Chowdhury RJ, Al Eyaadi O, Al Basir A. RBC alloimmunization and autoimmunization among transfusion-dependent Arab thalassemia patients. Transfusion. 2003;43:1604–1610. doi: 10.1046/j.1537-2995.2003.00549.x. [DOI] [PubMed] [Google Scholar]
- 20.Bilwani F, Kakepoto GN, Adil SN, Usman M, Hassan F, Khursid M. Frequency of irregular red cell alloantibodies in patients with thalassemia major: a bicenter study. J Pak Med Assoc. 2005;55:563–565. [PubMed] [Google Scholar]
- 21.Bhatti FA, Salamat N, Nadeem A, Shabbir N. Red cell immunization in beta thalassaemia major. J Coll Phys Surg Pak. 2004;14:657–660. [PubMed] [Google Scholar]
- 22.Noor Haslina MN, Ariffin N, Illuni Hayati I, Rosline H. Red cell immunization in multiply transfused Malay thalassemic patients. Southeast Asian J Trop Med Public Health. 2006;37:1015–1020. [PubMed] [Google Scholar]
- 23.Chao YH, Wu KH, Lu JJ, Shih MC, Peng CT, Chang CW. Red blood cell alloimmunisation among Chinese patients with beta thalassemia major in Taiwan. Blood Transfus. 2013;11:71–74. doi: 10.2450/2012.0153-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tormey CA, Fisk I, Stack G. Red blood cell alloantibody frequency, specificity and properties in a population of male military veterans. Transfusion. 2008;48:2069–2076. doi: 10.1111/j.1537-2995.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 25.Heddle NM, Soutar RL, O’Hoski P, Singer J, McBride JA, Ali MA, et al. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol. 1995;91:1000–1005. doi: 10.1111/j.1365-2141.1995.tb05425.x. [DOI] [PubMed] [Google Scholar]
- 26.ThakraI B, Saluja K, Sharma RR, Marwaha N. Red cell alloimmuttization in a transfused patients population: a study from a tettiary care hospital in north India. Hematology. 2008;13:313–318. doi: 10.1179/102453308X343419. [DOI] [PubMed] [Google Scholar]
- 27.Shukla JS, Chaudhary RK. Red cell alloimmunization in multitransfused chronic renal failure patients undergoing hemodialysis. Indian J Pathol Microbiol. 1999;42:299–302. [PubMed] [Google Scholar]
- 28.Ahmed AM, Hasan NS, Ragab SH, Habib SA, Emara NA, Aly AA. Red cell alloimmunization and autoantibodies in Egyptian transfusion-dependent thalassemia patients. Arch Med Sci. 2010;4:592–598. doi: 10.5114/aoms.2010.14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papiha SS. Genetic variation in India. Hum Biol. 1996;68:607–628. [PubMed] [Google Scholar]
- 30.Eder AF, Chambers LA. Noninfectious complications of blood transfusion. Arch Pathol Lab Med. 2007;131:708–718. doi: 10.5858/2007-131-708-NCOBT. [DOI] [PubMed] [Google Scholar]
- 31.Judd WJ, Dake LR, Davenport RD. On a much higher than reported incidence of anti-c in R1R1 patients with anti-E. Immunohematology. 2005;21:94–96. [PubMed] [Google Scholar]
- 32.Tormey CA, Stack G. The characterization and classification of concurrent blood group antibodies. Transfusion. 2009;49:2709–2718. doi: 10.1111/j.1537-2995.2009.02337.x. [DOI] [PubMed] [Google Scholar]
- 33.Bauer MP, Wiersum-Osselton J, Schipperus M, Vandenbroucke JP, Briet E. Clinical predictors of alloimmunization after red blood cell transfusion. Transfusion. 2007;47:2066–2071. doi: 10.1111/j.1537-2995.2007.01433.x. [DOI] [PubMed] [Google Scholar]
- 34.el-Danasoury AS, Eissa DG, Abdo RM, Elalfy MS. Red blood cell alloimmunization in transfusion-dependent Egyptian patients with thalassemia in a limited donor exposure program. Transfusion. 2012;52:43–47. doi: 10.1111/j.1537-2995.2011.03234.x. [DOI] [PubMed] [Google Scholar]
- 35.Shih-Ching K, Choudhry MA, Matsutani T, Schwacha MG, Rue LW, Bland KI, et al. Splenectomy differentially influences immune responses in various tissue compartments of the body. Cytokine. 2004;28:101–108. doi: 10.1016/j.cyto.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Thruet I, Pondarre C, Loundou A, Steschenko D, Girot R, Bachir, et al. Complications and treatment of patients with beta thalassemia in France: results of the National Registry. Haematologica. 2010;95:724–729. doi: 10.3324/haematol.2009.018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blumberg N, Heal IM, Gettings KF. WBC reductions of RBC transfusions is associated with a decreased incidence of RBC alloimmunization. Transfusion. 2003;43:945–952. doi: 10.1046/j.1537-2995.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- 38.Blajchman MA. The clinical benefits of the leucoreduction of blood products. J Trauma. 2006;60:583–590. doi: 10.1097/01.ta.0000199537.09201.7b. [DOI] [PubMed] [Google Scholar]
- 39.Schonewille H, van de Watering LM, Brand A. Additional red blood cell alloantibodies after blood transfusion in a non hematologic alloimmunized patient cohort: is it time to take precautionary measures? Transfusion. 2006;46:630–635. doi: 10.1111/j.1537-2995.2006.00764.x. [DOI] [PubMed] [Google Scholar]
- 40.Bao W, Zhong H, Li X, Lee MT, Schwartz J, Sheth S, et al. Immuneregulation in chronically transfused allo-antibody responder and non responder patients with sickle cell disease and beta-thalassemia major. Am J Hematol. 2011;86:1001–1006. doi: 10.1002/ajh.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noor Haslina MN, Ariffin N, Illuni Hayati I, Rosline H. Red cell autoantibodies among thalassemia patients in Hospital University Sains Malaysia. Singap Med J. 2007;48:922–925. [PubMed] [Google Scholar]
- 42.Adani R, Sorenson S, Shinar E, Lande W, Rachmilewitz E, Schrier SL. Characterization and comparison of the red blood cell membrane damage in severe human alpha and beta thalassemia. Blood. 1992;79:1058–1063. [PubMed] [Google Scholar]
- 43.Castellino SM, Combs MR, Zimmerman SA, Issitt PD, Ware RE. Erythrocyte autoantibodies in pediatric with sickle cell disease receiving transfusion therapy: frequency, characteristics and significance. Br J Haematol. 1999;104:189–194. doi: 10.1046/j.1365-2141.1999.01127.x. [DOI] [PubMed] [Google Scholar]
- 44.Wiener E, Wanachiwanawin W, Kotipan K, Fucharoen S, Wasi P, Wickramasinghe S. Erythroblast and erythrocyte bound antibodies in alpha and beta thalassemia syndromes. Transfus Med. 1991;1:229–238. doi: 10.1111/j.1365-3148.1991.tb00038.x. [DOI] [PubMed] [Google Scholar]


