Abstract
A serious complication of replacement therapy in patients with bleeding disorders is the development of ‘inhibitors’, particularly FVIII inhibitors in haemophilia A patients. This leads to an increase in the management cost, morbidity and mortality, especially post-operatively. The mechanism of FVIII inhibitor development is quite complex and it is difficult to predict inhibitor development, but a prompt and accurate diagnosis is critical as early therapy can save lives. The aim of this study was to screen patients with bleeding disorders in India for inhibitors, and to analyse and compare the prevalence of inhibitors in different regions in India. Patient details were recorded and blood samples were collected in sodium citrate vacutainers from 1,505 patients with bleeding disorders, in different cities in India. Coagulation and inhibitor screening assays were performed, followed by the Bethesda assay in inhibitor positive samples to quantify the FVIII inhibitor titre. Out of the 1,505 samples analysed, 1,285 were Haemophilia A patients, out of which 78 (6.07 %) were positive for ‘FVIII Inhibitors’. The highest incidence of FVIII Inhibitors was seen in South India (13.04 %). The highest incidence of 20.99 % was observed in Chennai, followed by Hyderabad (13.33 %), Jammu (9.90 %) and Guwahati (8.51 %), respectively, with respect to the samples analysed. The other regions showed an inhibitor incidence <8 %. The incidence of inhibitors in haemophilia A patients is different in different regions of India; this may be due to the intensity of treatment, type of product or the genetic characteristics of these patients.
Keywords: Haemophilia A, Inhibitors, Epidemiology
Introduction
The development of ‘inhibitors’ or alloantibodies against the missing coagulation factor are a serious complication of factor replacement therapy in patients with bleeding disorders. Alloantibodies that neutralize the activity of the replaced deficient factor are most common in haemophilia patients, particularly in those with severe haemophilia A. The incidence of these FVIII Inhibitors differs in different regions, as reported by several groups. It has been reported to vary from 30 % in the Japanese population, to 6.2 % in the French [1–15].
An earlier report has described the overall prevalence of inhibitors to be 8.2 % in patients with inherited severe haemophilia A in India [16]. Indian haemophilia patients are still more commonly treated with blood product transfusions, and factor transfusions are usually given only on an ‘on-demand’ basis because of the prohibitive costs involved. In case of inhibitor development, the cost of management of bleeding episodes also increases significantly with the need for expensive bypassing agents in most cases. An increased tendency of FVIII inhibitor development following surgical procedures (up to 19 %) has also been reported among Indian severe haemophilia A patients [17].
The mechanism of FVIII Inhibitor development in congenital severe haemophilia A patients is thought to be the consequence of many different genetic and non-genetic risk factors, and not yet fully understood [18]. Accordingly, polymorphisms in immune-response associated genes i.e. IL1β, IL4, IL10, TNFA, CTLA4, and other genes, as well as HLA genotypes have been analysed along with FVIII mutations and/or polymorphisms, in relation to inhibitor development in several studies [19–22]. The non-genetic risk factors that could predispose to inhibitor development include age at first treatment, type of treatment product and frequency of treatment among others [23, 24].
Antibodies to FIX are more rarely encountered and typically seen in 1–3 % of haemophilia B patients, but about 60 % of patients who develop these inhibitors have FIX infusion-associated anaphylactic reactions [25, 26].
The focus of this study was to analyse and compare the prevalence of inhibitors among patients with bleeding disorders in different regions in India, with a special emphasis on the prevalence of FVIII inhibitors in Indian haemophilia A patients.
Materials and Methods
Patients and Controls
Samples from 1,505 patients with bleeding disorders included in the present study were collected and sent to the Comprehensive Haemophilia Care Centre at our institute from 2011, after written consent from the patients. The study was approved by the Institutional Ethics Committee for Research on Human Subjects. A case record form (CRF) was designed to include relevant patient information such as age, sex, ethnicity, nature & site of bleeding, parental consanguinity, family history of inhibitors, details regarding FVIII infusion including type of treatment product and frequency, etc.
Methods
Sample Collection and Processing
10 cc blood was collected in 3.2 % trisodium citrate vacutainers, and centrifuged at 4,000 rpm/15 min 4 °C to obtain platelet poor plasma (PPP) for various coagulation screening assays (PT, APTT, mixing Studies, Factor VIII:C, VWF: Ag, FVIII Inhibitor screening assays). Assays were carried out according to the latest recommendations/guidelines by the International Society on Thrombosis and Haemostasis (ISTH), and a uniform protocol was followed so as to have uniform diagnoses criteria in this large cohort. Those samples found to be inhibitor positive after the screening assay were confirmed and the inhibitor titres were quantitated by the Bethesda assay, and further by the Nijmegen modification (in case of low-titre inhibitors) [27], and depending on the results, patients were classified as having high-titre or low-titre inhibitors [28].
Results
The patients were diagnosed and classified according to the factor deficiencies. Haemophilia A (HA) was found to be most common, followed by haemophilia B (HB), von willebrand disease (VWD) and other rare factor deficiency disorders. The results have been described in Table 1. Out of the 1505 patients, 1285 (85.38 %) had haemophilia A, 160 (10.63 %) had haemophilia B, 47 (3.12 %) had VWD, and 13 (0.86 %) had rare bleeding disorders.
Table 1.
Classification of persons with bleeding disorders in the current study in India
| City/sending centre | Total no. of patients with bleeding disorders referred for inhibitor screening | No. of HA patients | No. of HB patients | No. of VWD patients | No. of patients with rare bleeding disorders |
|---|---|---|---|---|---|
| West India | |||||
| Mumbai | 288 | 246 (85.42 %) | 36 (12.50 %) | 2 (0.69 %) | 4 (1.39 %) |
| Nashik | 45 | 41 (91.11 %) | 4 (8.89 %) | 0 (0.00 %) | 0 (0.00 %) |
| Jaipur | 129 | 100 (77.52 %) | 23 (17.83 %) | 5 (3.88 %) | 1 (0.78 %) |
| Rajkot | 43 | 43 (100.00 %) | 0 (0.00 %) | 0 (0.00 %) | 0 (0.00 %) |
| Surat | 98 | 71 (72.45 %) | 10 (10.20 %) | 15 (15.31 %) | 2 (2.04 %) |
| Vadodara | 31 | 26 (83.87 %) | 1 (3.23 %) | 4 (12.90 %) | 0 (0.00 %) |
| West-total | 634 | 527 (83.12 %) | 74 (11.67 %) | 26 (4.10 %) | 7 (1.10 %) |
| South India | |||||
| Chennai | 86 | 81 (94.19 %) | 3 (3.49 %) | 2 (2.33 %) | 0 (0.00 %) |
| Hyderabad | 16 | 15 (93.75 %) | 0 (0.00 %) | 1 (6.25 %) | 0 (0.00 %) |
| Bangalore | 66 | 57 (86.36 %) | 6 (9.09 %) | 3 (4.55 %) | 0 (0.00 %) |
| Davangere | 65 | 54 (83.08 %) | 9 (13.85 %) | 1 (1.54 %) | 1 (1.54 %) |
| South-total | 233 | 207 (88.84 %) | 18 (7.73 %) | 7 (3.00 %) | 1 (0.43 %) |
| North India | |||||
| Jammu | 115 | 101 (87.83 %) | 9 (7.83 %) | 4 (3.48 %) | 1 (0.87 %) |
| Chandigarh | 29 | 26 (89.66 %) | 3 (10.34 %) | 0 (0.00 %) | 0 (0.00 %) |
| Agra | 47 | 39 (82.98 %) | 8 (17.02 %) | 0 (0.00 %) | 0 (0.00 %) |
| New Delhi | 29 | 23 (79.31 %) | 4 (13.79 %) | 1 (3.45 %) | 1 (3.45 %) |
| Lucknow | 153 | 135 (88.24 %) | 13 (8.50 %) | 5 (3.27 %) | 0 (0.00 %) |
| Varanasi | 50 | 43 (86.00 %) | 6 (12.00 %) | 0 (0.00 %) | 1 (2.00 %) |
| North-total | 423 | 367 (86.76 %) | 43 (10.17 %) | 10 (2.36 %) | 3 (0.71 %) |
| East India | |||||
| Kolkata | 138 | 117 (84.78 %) | 19 (13.77 %) | 1 (0.72 %) | 1 (0.72 %) |
| Dibrugarh | 25 | 20 (80.00 %) | 1 (4.00 %) | 3 (12.00 %) | 1 (4.00 %) |
| Guwahati | 52 | 47 (90.38 %) | 5 (9.62 %) | 0 (0.00 %) | 0 (0.00 %) |
| East-total | 215 | 184 (85.58 %) | 25 (11.63 %) | 4 (1.86 %) | 2 (0.93 %) |
| Total | 1,505 | 1,285 (85.38 %) | 160 (10.63 %) | 47 (3.12 %) | 13 (0.86 %) |
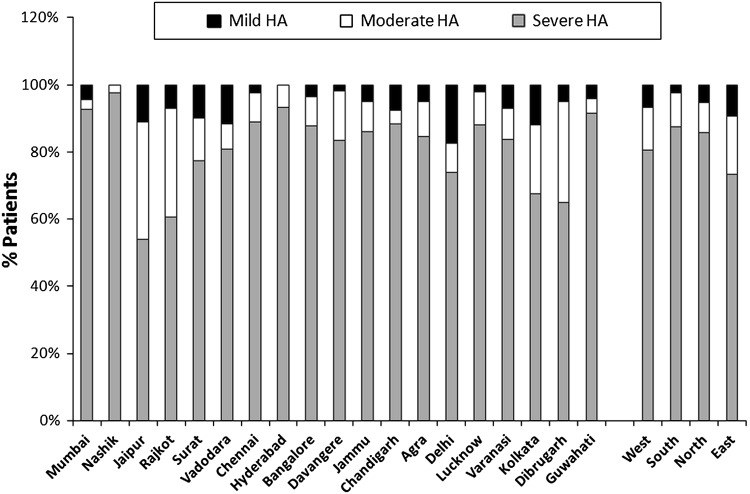
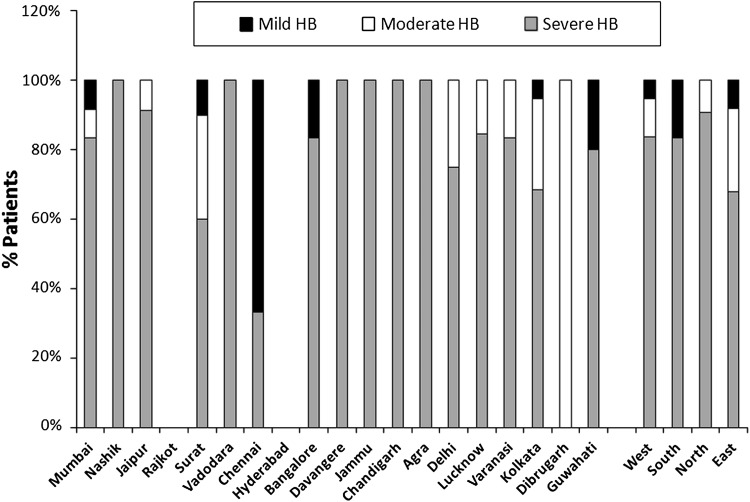
The distribution of haemophilia A and haemophilia B patients with regard to disease severity has been described in Figs. 1 and 2.
Fig. 1.
Distribution of Indian haemophilia A patients with regard to disease severity in the current study
Fig. 2.
Distribution of Indian haemophilia B patients with regard to disease severity in the current study
Out of the 1,285 haemophilia A patients, 1,055 (82.10 %) had severe HA with FVIII:C < 1 %, 154 (11.98 %) had moderate HA with FVIII:C between 1 and 5 %, and 76 (5.91 %) had mild HA with FVIII:C between 6 and 50 %. Out of the 160 HB patients, 133 (83.13 %) had severe HB with FIX:C < 1 %, 18 (11.25 %) had moderate HB with FIX:C between 1 and 5 %, and 9 (5.63 %) had mild HB with FIX:C between 6 and 50 %.
Also, out of the 1,285 haemophilia A patients, 78 (6.07 %) were found to be positive for inhibitors, all with FVIII:C < 1 %. The FVIII inhibitor screening results have been described in Table 2.
Table 2.
FVIII inhibitor screening results in Indian haemophilia A patients in the current study
| City/sending centre | Total no. of patients with bleeding disorders referred for inhibitor screening | No. of HA patients | No. of HA patients positive for FVIII inhibitors | High titre (>5 BU/ml) | Low titre (≤5 BU/ml) |
|---|---|---|---|---|---|
| West India | |||||
| Mumbai | 288 | 246 | 13 (5.28 %) | 9 (69.23 %) | 4 (30.77 %) |
| Nashik | 45 | 41 | 1 (2.44 %) | 1 (100.00 %) | 0 (0.00 %) |
| Jaipur | 129 | 100 | 4 (4.00 %) | 3 (75.00 %) | 1 (25.00 %) |
| Rajkot | 43 | 43 | 1 (2.33 %) | 0 (0.00 %) | 1 (100.00 %) |
| Surat | 98 | 71 | 3 (4.23 %) | 1 (33.33 %) | 2 (66.67 %) |
| Vadodara | 31 | 26 | 0 (0.00 %) | 0 (0.00 %) | 0 (0.00 %) |
| West-total | 634 | 527 | 22 (4.17 %) | 14 (63.64 %) | 8 (36.36 %) |
| South India | |||||
| Chennai | 86 | 81 | 17 (20.99 %) | 7 (41.18 %) | 10 (58.82 %) |
| Hyderabad | 16 | 15 | 2 (13.33 %) | 2 (100.00 %) | 0 (0.00 %) |
| Bangalore | 66 | 57 | 4 (7.02 %) | 2 (50.00 %) | 2 (50.00 %) |
| Davangere | 65 | 54 | 4 (7.41 %) | 3 (75.00 %) | 1 (25.00 %) |
| South-total | 233 | 207 | 27 (13.04 %) | 14 (51.85 %) | 13 (48.15 %) |
| North India | |||||
| Jammu | 115 | 101 | 10 (9.90 %) | 5 (50.00 %) | 5 (50.00 %) |
| Chandigarh | 29 | 26 | 1 (3.85 %) | 0 (0.00 %) | 1 (100.00 %) |
| Agra | 47 | 39 | 2 (5.13 %) | 0 (0.00 %) | 2 (100.00 %) |
| New Delhi | 29 | 23 | 0 (0.00 %) | 0 (0.00 %) | 0 (0.00 %) |
| Lucknow | 153 | 135 | 6 (4.44 %) | 2 (33.33 %) | 4 (66.67 %) |
| Varanasi | 50 | 43 | 1 (2.33 %) | 0 (0.00 %) | 1 (100.00 %) |
| North-total | 423 | 367 | 20 (5.45 %) | 7 (35.00 %) | 13 (65.00 %) |
| East India | |||||
| Kolkata | 138 | 117 | 4 (3.42 %) | 1 (25.00 %) | 3 (75.00 %) |
| Dibrugarh | 25 | 20 | 1 (5.00 %) | 0 (0.00 %) | 1 (100.00 %) |
| Guwahati | 52 | 47 | 4 (8.51 %) | 1 (25.00 %) | 3 (75.00 %) |
| East-total | 215 | 184 | 9 (4.89 %) | 2 (22.22 %) | 7 (77.78 %) |
| Total | 1,505 | 1,285 | 78 (6.07 %) | 37 (47.44 %) | 41 (52.56 %) |
The highest incidence of FVIII Inhibitors i.e. 20.99 % was seen in South India i.e. Chennai, followed by Hyderabad, with an incidence of 13.33 %, and subsequently Davangere and Bangalore with incidences of 7.41 and 7.02 % respectively. Jammu showed the highest incidence of FVIII Inhibitors in North India (9.90 %), followed by Agra which showed an incidence of 5.13 %. In East India, Guwahati had an incidence of 8.51 % FVIII Inhibitors, and in West India, Mumbai had an incidence of 5.28 %, with respect to the samples analysed. The other regions showed an Inhibitor incidence <5 %.
Among the haemophilia A patients, the mean age of the inhibitor positive patients was 19.31 years (range 3–58 years) and the mean age of the inhibitor negative patients was 17.76 years (7 months–68 years). FVIII inhibitor levels ranged between 1.4 to 256 BU/ml. 37 (47.44 %) patients were found to have high-titre inhibitors >5 BU/ml, and 41 (52.56 %) patients were found to have low-titre inhibitors ≤5 BU/ml. Thus, the number of inhibitor positive patients with high-titre and low-titre inhibitors was nearly equal in this study. The overall FVIII Inhibitor incidence in the haemophilia A samples studied was 6.07 %, not much different from the earlier described incidence of 8.2 %. Haemarthrosis still remains the most clinically challenging bleeding manifestation in both groups of patients.
A comparative analysis of the treatment products, (either only factor concentrates/only blood products/both factor concentrates and blood products) the most important treatment-related risk factor, in the inhibitor positive and inhibitor negative patients has been described in Table 3. With the exception of Guwahati, the difference was not statistically significant in the other regions. Among the Guwahati haemophilia A patients, those being treated with factor concentrates were inhibitor positive already (P value <0.0001****; OR 783.00; 95 % CI 13.789–44464, using the approximation of Woolf), and those being treated with a combination of both factor concentrates and blood products are inhibitor negative so far (P value <0.0001****; OR 0.001277; 95 % CI 2.249E-05-0.07252). The zone-wise comparison remained significant in the East India inhibitor positive group treated with only factor concentrates (P value 0.0022**; OR 13.200; 95 % CI 3.060–56.947). Other information collected in the clinical case record form was not significantly different between the two groups and has not been described in detail.
Table 3.
Analysis of the treatment products given to the Indian haemophilia A patients in the study
| Region | Inhibitor status | Factor concentrates | Blood products | Factor concentrates + blood products | Total |
|---|---|---|---|---|---|
| Mumbai | Inhibitor positive | 1 (7.69 %) | 1 (7.69 %) | 11 (84.62 %) | 13 |
| Inhibitor negative | 42 (18.03 %) | 7 (3.00 %) | 184 (78.97 %) | 233 | |
| P value | 0.4757 | 0.3565 | 1.000 | ||
| Nashik | Inhibitor positive | 0 (0.00 %) | 0 (0.00 %) | 1 (100.00 %) | 1 |
| Inhibitor negative | 0 (0.00 %) | 8 (20.00 %) | 32 (80.00 %) | 40 | |
| P value | 0.000 | 1.000 | 1.000 | ||
| Jaipur | Inhibitor positive | 0 (0.00 %) | 0 (0.00 %) | 4 (100.00 %) | 4 |
| Inhibitor negative | 0 (0.00 %) | 7 (7.29 %) | 89 (92.71 %) | 96 | |
| P value | 0.000 | 1.000 | 1.000 | ||
| Rajkot | Inhibitor positive | 0 (0.00 %) | 0 (0.00 %) | 1 (100.00 %) | 1 |
| Inhibitor negative | 5 (11.91 %) | 2 (4.76 %) | 35(83.33 %) | 42 | |
| P value | 1.000 | 1.000 | 1.000 | ||
| Surat | Inhibitor positive | 0 (0.00 %) | 1 (33.33 %) | 2 (66.67 %) | 3 |
| Inhibitor negative | 3 (4.41 %) | 21(30.88 %) | 44(64.71 %) | 68 | |
| P value | 1.000 | 1.000 | 1.000 | ||
| Vadodara | Inhibitor positive | 0 (0.00 %) | 0 (0.00 %) | 0 (0.00 %) | 0 |
| Inhibitor negative | 5 (19.23 %) | 2 (7.69 %) | 19 (73.08 %) | 26 | |
| P value | NA | NA | NA | ||
| West-total | Inhibitor positive | 1 (4.55 %) | 2 (9.09 %) | 19 (86.36) | 22 |
| Inhibitor negative | 55 (10.89 %) | 47 (9.31 %) | 403 (79.80 %) | 505 | |
| P value | 0.4959 | 1.0000 | 0.5915 | ||
| Chennai | Inhibitor positive | 5 (29.41 %) | 2 (11.77 %) | 10 (58.82 %) | 17 |
| Inhibitor negative | 35 (54.69 %) | 1(1.56 %) | 28 (43.75 %) | 64 | |
| P value | 0.1004 | 0.1100 | 0.2893 | ||
| Hyderabad# | - | - | - | - | 16 |
| Bangalore# | - | - | - | - | 66 |
| Davangere# | - | - | - | - | 65 |
| South-total$ | Inhibitor positive | 5 (29.41 %) | 2 (11.77 %) | 10 (58.82 %) | 17 |
| Inhibitor negative | 35 (54.69 %) | 1 (1.56 %) | 28 (43.75 %) | ||
| 64 | |||||
| P value | 0.1004 | 0.1100 | 0.2893 | ||
| Jammu | Inhibitor positive | 0 (0.00 %) | 0 (0.00 %) | 10 (100.00 %) | 10 |
| 91 | |||||
| Inhibitor negative | 0 (0.00 %) | 2 (2.20 %) | 89 (97.80 %) | ||
| P value | 0.000 | 1.000 | 1.000 | ||
| Chandigarh | Inhibitor positive | 0 (0.00 %) | 0 (0.00 %) | 1 (100.00 %) | 1 |
| Inhibitor negative | 4 (16.00 %) | 4 (16.00 %) | 17 (68.00 %) | ||
| 25 | |||||
| P value | 1.000 | 1.000 | 1.000 | ||
| Agra | Inhibitor positive | 2 (100.00 %) | 0 (0.00 %) | 0 (0.00 %) | 2 |
| Inhibitor negative | 31 (83.78 %) | 0 (0.00 %) | 6 (16.22 %) | ||
| 37 | |||||
| P value | 1.000 | 0.000 | 1.000 | ||
| New Delhi | Inhibitor positive | 0 (0.00 %) | 0 (0.00 %) | 0 (0.00 %) | 0 |
| Inhibitor negative | 0 (0.00 %) | 0 (0.00 %) | 23(100.00 %) | ||
| 23 | |||||
| P value | NA | NA | NA | ||
| Lucknow | Inhibitor positive | 3 (50.00 %) | 0 (0.00 %) | 3 (50.00 %) | 6 |
| Inhibitor negative | 62 (48.06 %) | 0 (0.00 %) | 67 (51.94 %) | ||
| 129 | |||||
| P value | 1.000 | 0.000 | 1.000 | ||
| Varanasi | Inhibitor positive | 0 (0.00 %) | 0 (0.00 %) | 1 (100.00 %) | 1 |
| Inhibitor negative | 4 (9,52 %) | 2 (4.76 %) | 36 (85.72 %) | ||
| 42 | |||||
| P value | 1.000 | 1.000 | 1.000 | ||
| North-total | Inhibitor positive | 5 (25.00 %) | 0 (0.00 %) | 15 (75.00 %) | 20 |
| Inhibitor negative | 101 (29.11 %) | 8 (2.30 %) | 238 (68.59 %) | ||
| 347 | |||||
| P value | 0.8040 | 1.000 | 0.6279 | ||
| Kolkata | Inhibitor positive | 0 (0.00 %) | 0 (0.00 %) | 4 (100.00 %) | 4 |
| Inhibitor negative | 8 (7.08 %) | 12(10.62 %) | 93 (82.30 %) | ||
| 113 | |||||
| P value | 1.000 | 1.000 | 1.000 | ||
| Dibrugarh | Inhibitor positive | 0 (0.00 %) | 0 (0.00 %) | 1 (100.00 %) | 1 |
| 19 | |||||
| Inhibitor negative | 2 (10.53 %) | 10 (52.63 %) | 7 (36.84 %) | ||
| P value | 1.000 | 1.000 | 0.4000 | ||
| Guwahati | Inhibitor positive | 4 (100.00 %) | 0 (0.00 %) | 0 (0.00 %) | 4 |
| Inhibitor negative | 0 (0.00 %) | 0 (0.00 %) | 43 (100.00 %) | ||
| 43 | |||||
| P value | <0.0001**** | 0.000 | <0.0001**** | ||
| East-total | Inhibitor positive | 4 (44.44 %) | 0 (0.00 %) | 5 (55.56 %) | 9 |
| Inhibitor negative | 10 (5.71 %) | 22 (12.57 %) | 143 (81.72 %) | ||
| 175 | |||||
| P value | 0.0022** | 0.6024 | 0.0749 |
**P≤0.01; ****P≤0.0001
#Treatment related details were not entirely provided in the CRFs for Hyderabad, Bangalore & Davangere
$Excluding Hyderabad, Bangalore, Davangere
There were no FIX Inhibitors detected in any of the haemophilia B patients. Among those with rare bleeding disorders (13 cases), Factor X (FX) deficiency was most commonly diagnosed (5 cases), followed by afibrinogenemia (2 cases), combined Factor V and FVIII deficiency (2 cases), factor II (FII) deficiency (1 case), factor V (FV) deficiency(1 case), Factor VII (FVII) deficiency (1 case), and factor XI (FXI) deficiency (1 case). There were no inhibitors detected in any of these patients with rare bleeding disorders who were screened. Many haemophilia patients were diagnosed at our centre to be actually VWD patients and were thus excluded from the FVIII/FIX inhibitor prevalence analysis.
Discussion
This is the first study from India on a large series of patients with bleeding disorders from different regions of India. There is a distinct variation in the prevalence of inhibitors in different regions; Chennai (in South India), showing the highest prevalence i.e., 20.99 % and Vadodara (in West India) as well as New Delhi (in North India) showing the least prevalence i.e. 0.0 %. Overall, the prevalence of inhibitors in Western India has been found to be low ranging from 0 % in Vadodara to 5.3 % in Mumbai. Altogether among the 527 haemophilia A patients screened, only 22 were positive for inhibitors (4.17 %). Similar is the finding in East India, wherein among the 184 HA patients screened 9 were positive i.e. 4.89 %. In North India, except Jammu which shows an incidence of 9.90 %, the prevalence of inhibitors has been found to be low in the other centres. The centres in South India have shown an increased average incidence of inhibitors compared to the other zones (East, West and North India), with Chennai having the highest incidence of nearly 21 %. It is important to note that the sample size from certain regions was too small; for instance 23 and 26 HA patients from New Delhi and Vadodara each. The 0 % prevalence in these small series may not represent the actual prevalence of inhibitors in these regions.
An earlier report, which largely included patients from Western India, has shown an inhibitor incidence of 8.2 % among 292 severe haemophilia A patients analysed [16], while another report from south India describes an incidence of 13 % among 200 haemophilia patients [29]. The number of patients described in this series is much higher, but the prevalence is not significantly different from these earlier reports. A study of the FVIII haplotypes in Indian haemophilia A patients [30] has suggested that the distribution of these haplotypes (which possibly explains the higher inhibitor incidence in African-Americans because of mismatched transfusions), are not a predisposing risk factor in Indians. This could partially explain the low overall inhibitor prevalence in Indian haemophiliacs.
The results described with regard to the treatment products need to be interpreted with caution. Even though statistically significant results were observed in Guwahati, the number of inhibitor positive patients was very low i.e. only 4 patients. Similarly this is also applicable to the other regions as the number of inhibitor positive patients in each region is very low, and may not provide statistically conclusive results as yet.
When further zone-wise analysis was carried out, the difference remained significant only in the East India inhibitor positive group who were treated solely with factor concentrates. Again, the low number of patients is not enough yet to conclude that factor concentrates alone could predispose to inhibitors. Also, the South India patients who showed the highest incidence of inhibitors did not show any significant difference with regard to treatment products yet.
As the treatment-related factors can be modified if necessary, further analysis in a larger cohort of patients along with other variables may provide more information on this complex process of inhibitor development that would help in the clinical management of these patients.
Acknowledgments
This work was supported by a grant from Novo Nordisk India Pvt. Ltd. to the National Institute of Immunohaematology (ICMR), Mumbai, India. We would also like to acknowledge the following physicians: Dr R Dayal (HFI Agra), Dr A K Arya (HFI Kanpur), Dr Shubha Phadke (HFI Lucknow), Dr K K Gupta (HFI Varanasi), Dr Thilagavathy, Dr Vardarajan (HFI Chennai), Dr Usha (HFI Coimbatore), Dr Vardarajan (HFI Trichy), Dr Anburajan (HFI Tiruneveli), Dr Raveendran (HFI Salem), Dr Nalini & Dr TK Dutta (HFI Pondicherry), Dr Cecil Ross, Dr Sathish Kumar (HFI Bangalore), Dr Suresh Hanganvadi (HFI Davangere), Dr P K Gogoi (HFI Guwahati), Dr Ruby Reshy & Dr Sarawat (HFI Srinagar), Dr K K Kaul, Dr Rekha Harish, Dr JP Singh (HFI Jammu), Dr Maganthi Prasad (HFI Vijayawada), Dr Mallikarjuna, Dr AMVR Narendra (HFI Hyderabad), Dr Shantanu Basu (HFI Kolkata), Dr Purushotam, Dr Mary (HFI Angamaly/Kunnamkulam), Dr Ajith Kumar, Dr PK Sashidaran (HFI Calicut), Dr Ashok Kumar, Dr VK Srikala, Dr Shankar VH (HFI Trivandrum), Dr Sudhakaran (HFI Kannur), Dr David, Dr Vijaykumar, Dr Jaykumar (HFI Angamally), Dr Kiran Shah, Dr Vijay Shah (Hemophilia Society Surat Chapter), Dr Seema Bhatwadekar (Hemophilia Society Vadodara Chapter), Dr Jagdish Singh, Dr Sudheer Mehta (HFI Jaipur).
References
- 1.Shirahata A, Fukutake K, Higasa S, Mimaya J, Oka T, Shima M, et al. Study Group on Factors involved in Formation of Inhibitors to Factor VIII and IX Preparations An analysis of factors affecting the incidence of inhibitor formation in patients with congenital haemophilia in Japan. Haemophilia. 2011;17:771–776. doi: 10.1111/j.1365-2516.2011.02599.x. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter SL, Michael Soucie J, Sterner S, Presley R, Treatment Center Network (HTCN) Investigators Increased prevalence of inhibitors in Hispanic patients with severe haemophilia A enrolled in the universal data collection database. Haemophilia. 2012;18:e260–e265. doi: 10.1111/j.1365-2516.2011.02739.x. [DOI] [PubMed] [Google Scholar]
- 3.Webert KE, Rivard GE, Teitel J, Carcao M, Lillicrap D, St-Louis J, Walker IR. Low prevalence of inhibitor antibodies in the Canadian haemophilia population. Haemophilia. 2012;18:e254–e259. doi: 10.1111/j.1365-2516.2011.02694.x. [DOI] [PubMed] [Google Scholar]
- 4.Au WY, Lee V, Kho B, Ling AS, Chan D, Chan EY, et al. A synopsis of current haemophilia care in Hong Kong. Hong Kong Med J. 2011;17:189–194. [PubMed] [Google Scholar]
- 5.Wang XF, Zhao YQ, Yang RC, Wu JS, Sun J, Zhang XS, et al. The prevalence of factor VIII inhibitors and genetic aspects of inhibitor development in Chinese patients with haemophilia A. Haemophilia. 2010;16:632–639. doi: 10.1111/j.1365-2516.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- 6.Iorio A, Oliovecchio E, Morfini M, Mannucci PM, on behalf of the Association of Italian Hemophilia Centres Directors Italian registry of haemophilia and allied disorders. objectives, methodology and data analysis. Haemophilia. 2008;14:444–453. doi: 10.1111/j.1365-2516.2008.01679.x. [DOI] [PubMed] [Google Scholar]
- 7.Sharifian R, Hosseini M, Safai R, Tugeh Gh, Ehsani AH, Lak M, Jazebi M. Prevalence of inhibitors in a population of 1280 hemophilia A patients in Iran. Acta Medica Iranica. 2003;41:66–68. [Google Scholar]
- 8.Borhany M, Kumari M, Shamsi T, Naz A, Farzana T. Frequency of factor VIII (FVIII) inhibitor in haemophilia A. J Coll Physician Surg Pak. 2012;22:289–293. [PubMed] [Google Scholar]
- 9.Darby SC, Keeling DM, Spooner RJ, Wan Kan S, Giangrande PL, Collins PWUK, UK Haemophilia Centre Doctors’ Organisation et al. The incidence of factor VIII and factor IX inhibitors in the haemophilia population of the UK and their effect on subsequent mortality, 1977–99. J Thromb Haemost. 2004;2:1047–1054. doi: 10.1046/j.1538-7836.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 10.Hay CR, Palmer B, Chalmers E, Liesner R, Maclean R, Rangarajan S, et al, United Kingdom Haemophilia Centre Doctors’ Organisation (UKHCDO) Incidence of factor VIII inhibitors throughout life in severe haemophilia A in the United Kingdom. Blood. 2011;117:6367–6370. doi: 10.1182/blood-2010-09-308668. [DOI] [PubMed] [Google Scholar]
- 11.Aledort LM, Dimichele DM. Inhibitors occur more frequently in African-American and Latino haemophiliacs. Haemophilia. 1998;4:68. doi: 10.1046/j.1365-2516.1998.0146c.x. [DOI] [PubMed] [Google Scholar]
- 12.Knobe KE, Sjörin E, Tengborn LI, Petrini P, Ljung RC. Inhibitors in the Swedish population with severe haemophilia A and B: a 20-year survey. Acta Paediatr. 2002;91:910–914. doi: 10.1111/j.1651-2227.2002.tb02854.x. [DOI] [PubMed] [Google Scholar]
- 13.Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9:418–435. doi: 10.1046/j.1365-2516.2003.00780.x. [DOI] [PubMed] [Google Scholar]
- 14.Windyga J, Lopaciuk S, Stefanska E, Juszynski A, Wozniak D, Strzelecki O, Szczepanik AB. Haemophilia in poland. Haemophilia. 2006;12:52–57. doi: 10.1111/j.1365-2516.2006.01188.x. [DOI] [PubMed] [Google Scholar]
- 15.Sultan Y. Prevalence of inhibitors in a population of 3435 haemophilia patients in France. French Hemophilia Study Group. Thromb Haemost. 1992;67:600–602. [PubMed] [Google Scholar]
- 16.Ghosh K, Shetty S, Kulkarni B, Nair S, Pawar A, Khare A, et al. Development of inhibitors in patients with haemophilia from India. Haemophilia. 2001;7:273–278. doi: 10.1046/j.1365-2516.2001.00505.x. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh K, Jijina F, Shetty S, Madkaikar M, Mohanty D. First-time development of FVIII inhibitor in haemophilia patients during the postoperative period. Haemophilia. 2002;8:776–780. doi: 10.1046/j.1365-2516.2002.00687.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh K, Shetty S. Immune response to FVIII in haemophilia A: an overview of risk factors. Clinic Rev Allergy Immunol. 2009;37:58–66. doi: 10.1007/s12016-009-8118-1. [DOI] [PubMed] [Google Scholar]
- 19.Astermark J, Berntorp E, White GC, Kroner BL. The Malmö International Brother Study (MIBS): further support for genetic predisposition to inhibitor development in hemophilia patients. Haemophilia. 2001;7:267–272. doi: 10.1046/j.1365-2516.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- 20.Oldenburg J, Pavlova A. Genetic risk factors for inhibitors to factors VIII and IX. Haemophilia. 2006;12(Suppl. 6):15–22. doi: 10.1111/j.1365-2516.2006.01361.x. [DOI] [PubMed] [Google Scholar]
- 21.Reding MT. Immunological aspects of inhibitor development. Haemophilia. 2006;12(Suppl. 6):30–35. doi: 10.1111/j.1365-2516.2006.01363.x. [DOI] [PubMed] [Google Scholar]
- 22.Pinto P, Ghosh K, Shetty S. Immune regulatory gene polymorphisms as predisposing risk factors for the development of factor VIII inhibitors in Indian severe haemophilia A patients. Haemophilia. 2012;18:794–797. doi: 10.1111/j.1365-2516.2012.02845.x. [DOI] [PubMed] [Google Scholar]
- 23.Astermark J. Basic aspects of inhibitors to factors VIII and IX and the influence of non-genetic risk factors. Haemophilia. 2006;12(Suppl. 6):8–14. doi: 10.1111/j.1365-2516.2006.01360.x. [DOI] [PubMed] [Google Scholar]
- 24.Leissinger C, Cooper DL, Solem CT, HTRS Investigators Assessing the impact of age, race, ethnicity and inhibitor status on functional limitations of patients with severe and moderately severe haemophilia A. Haemophilia. 2011;17:884–889. doi: 10.1111/j.1365-2516.2011.02509.x. [DOI] [PubMed] [Google Scholar]
- 25.Chitlur M, Warrier I, Rajpurkar M, Lusher JM. Inhibitors in factor IX deficiency a report of the ISTH-SSC international FIX inhibitor registry (1997–2006) Haemophilia. 2009;15:1027–1031. doi: 10.1111/j.1365-2516.2009.02039.x. [DOI] [PubMed] [Google Scholar]
- 26.Coppola A, Capua MD, Dario MN, Minno D, Palo MD, Marrone E, et al. Treatment of haemophilia: a review of current advances and ongoing issues. J Blood Med. 2010;1:183–195. doi: 10.2147/JBM.S6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg HM, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII: C inhibitor. Improved specificity and reliability. Thromb Haemost. 1995;73:247–251. [PubMed] [Google Scholar]
- 28.White GC II, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J, Factor VIII and Factor IX Subcommittee (2001) Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost 85: 560 [PubMed]
- 29.Mathews V, Nair SC, David S, Viswabandya A, Srivastava A. Management of haemophilia in patients with inhibitors: the perspective from developing countries. Semin Thromb Hemost. 2009;36:820–826. doi: 10.1055/s-0029-1245115. [DOI] [PubMed] [Google Scholar]
- 30.Pinto P, Ghosh K, Shetty S (2013) Factor VIII haplotypes in severe haemophilia A patients in India. Ann Hematol 92:999–1000 [DOI] [PubMed]