Abstract
Tumor regression after induction chemotherapy (ICT) identifies laryngeal cancers that are responsive to chemoradiation. Patient immune parameters have recently been associated with response to chemotherapy and may identify responding patients. A retrospective analysis was performed to determine if pretreatment, circulating T lymphocyte levels, predicted ICT response in patients with advanced laryngeal cancer.
Pretreatment, circulating T lymphocyte subpopulations were correlated with response to therapy and survival. Results were compared with similar data from an identical Phase II trial involving patients with oropharyngeal cancer. An increased percentage of CD4+ cells predicted response to ICT and suggested improved survival in patients with laryngeal, but not oropharyngeal, cancer. In the combined group of patients, increased CD4 levels predicted response to ICT.
These findings demonstrate the potential importance of the immune system in chemotherapy response and clinical outcome. Differences in findings between patients with advanced laryngeal and oropharyngeal cancer may reflect different cellular immunity function in the patients with HPV-16+ oropharyngeal cancer.
Keywords: laryngeal cancer, chemotherapy, T lymphocytes, immunity, oropharyngeal cancer
INTRODUCTION
Induction chemotherapy (ICT) has proven useful for identifying patients with head and neck squamous cell carcinoma that is responsive to definitive chemoradiation.1–3 Although this approach has allowed for high rates of organ preservation and function, its shortcomings include a high cost, long duration of treatment, and potential toxicity, including a 1% to 2% mortality rate, without any demonstrable overall survival benefit from the ICT itself.4 Identification of new biomarkers that predict whether a patient’s disease will respond to organ-preserving therapy could lead to more personalized treatments and the replacement of ICT with a less costly, less time consuming, and less morbid alternative.
It is well known that both tumor biology and host immune function play important roles in a cancer’s response to therapy. Circulating and tumor infiltrating lymphocyte populations have been studied as indicators of host immune response in multiple types of cancers and have shown correlations with response to therapy and survival.5–7 In advanced oropharyngeal cancer, higher pretreatment, circulating CD8+ lymphocyte levels were predictive of response to ICT, and a low ratio of circulating %CD4+/%CD8+ lymphocytes was associated with better survival.8 In an earlier analysis of patients with advanced laryngeal cancer, we reported that pretreatment peripheral blood lymphocyte (PBL) levels were not predictive of response to ICT or survival.9 However, that report was based on preliminary data from a limited number of patients enrolled in an ongoing Phase II clinical trial that has since been completed.
A retrospective analysis of the now-completed, prospective Phase II clinical trial was undertaken to determine if there were correlations between pretreatment PBL levels and the response to ICT in patients with advanced laryngeal cancer. Pretreatment flow-cytometric analyses of CD3, CD4, CD8, NK, and B lymphocyte percentages and absolute numbers were available for nearly 80% of patients (128/163). Functional assays and serum samples for simultaneous cytokine analyses were not available. To determine if there were cancer site-specific differences of importance, we also analyzed comparative data from a separate Phase II clinical trial that investigated the same ICT approach in patients with advanced oropharyngeal cancer who received a nearly identical treatment protocol.2 To increase the statistical power and explore significant associations in a larger population with more varied disease, PBL levels were correlated with response to ICT in a cohort of patients made by combining those from the 2 separate trials.
MATERIALS AND METHODS
Patient population
Studied were 163 patients with advanced laryngeal or oropharyngeal cancer who were enrolled in 2 concurrent Phase II clinical trials that explored the utility of a single cycle of ICT for selecting patients who would benefit from subsequent organ-preserving concurrent chemoradiation. The laryngeal cancer Phase II clinical trial (UMCC 9520) included 97 patients with pathologically confirmed, resectable, previously untreated stage III or IV squamous cell carcinoma of the larynx. All patients were candidates for a total laryngectomy. The detailed patient characteristics and clinical outcomes were previously reported.1 The oropharyngeal cancer Phase II clinical trial (UMCC 9921) included 66 patients with pathologically confirmed, resectable, untreated stage III or IV squamous cell carcinoma of the oropharynx. Details of the clinical outcomes2 and immune parameter correlations8 were previously reported. The protocols were approved by the Institutional Review Board at the University of Michigan (Ann Arbor, MI), and all patients gave documented informed consent.
Treatment protocol
Patients received 1 cycle of ICT consisting of cisplatin (100 mg/m2/d for 1 day) and fluorouracil (1000 mg/m2/d for 5 days). Carboplatin (area under the curve = 6) was used in place of cisplatin for patients with renal insufficiency or hearing loss. Patients were examined by direct laryngoscopy before treatment and 3 weeks after ICT to obtain bidimensional measurements of the primary tumor; the percentage change of their product was calculated to quantify tumor regression. In all patients, there was no question as to whether there was a partial response (>50% reduction in the bidimensional product) or stable disease. Among the patients with laryngeal cancer, tumor regression was ≥70% among the responders (n = 73) and ≤50% among the nonresponders (n = 22). Among the patients with oropharyngeal cancer, there were 54 responders and 22 nonresponders. Responders to ICT then underwent definitive, concurrent chemotherapy (cisplatin 100 mg/m2 on days 1, 22, and 43) and radiotherapy (70 Gy divided in daily 2-Gy fractions). Nonresponders to ICT received salvage surgery and radiotherapy. The full study designs and clinical results were previously reported.1,2
Lymphocyte subpopulations
Fresh, pretreatment peripheral blood samples were analyzed at the time of patient enrollment by routine, automated flow cytometry for WBC, CD3, CD4, CD8, the ratio of CD4 to CD8 cells, and B cells. Percentage and absolute numbers were obtained. The detailed methods have been described previously.10 In brief, counts were obtained using commercially available monoclonal antibody reagents by an indirect immunofluorescent technique and were performed in the clinical laboratories of the University of Michigan Department of Pathology. Correlations between patients’ pretreatment peripheral blood counts and their response to ICT and survival were determined. Of the 97 patients enrolled in the laryngeal cancer study, 81 patients (84%) had pretreatment peripheral blood samples analyzed. Eight of the 81 patients had incomplete sets of PBL subset counts. There were no significant differences between the subset of patients with pretreatment peripheral blood counts and those without with respect to sex, smoking status, disease stage, or response to ICT (data not shown). The median length of follow-up was 7.9 years. In the oropharynx trial, PBL levels were available for 47 patients (71%). Median length of follow-up in that trial was 6.6 years.
Statistical analysis
Statistical analysis was performed on those participants of the larynx trial with complete sets of PBL counts. Absolute counts and percentages were treated as continuous variables. The distributions of each subset were explored with histograms and quantile-quantile (Q–Q) plots to assess whether an approximately normal distribution assumption was reasonable. Initially, all counts and percentages were assumed to be approximately normal and differences in response versus nonresponse to therapy were assessed with a 2-sample t test. Because some subsets showed deviations from normality, we performed nonparametric Wilcoxon rank-sum tests as well. We found no difference in the conclusions drawn from the t test and nonparametric testing. Differences in overall survival were tested with a log-rank test and plotted using the Kaplan-Meier method based on 2 differing cutoff values: (1) the median and (2) the mean plus 2 SEM of the individual subset levels measured in a population of 40 age-matched normal subjects.10
Additional analyses were performed by combining the subset values from the larynx trial with those of the oropharynx trial. Tests for significant differences among responders and nonresponders as well as survival in the whole group were assessed in the same way as the larynx trial subjects alone. Differences in effect across trials/disease sites were then assessed using an interaction term in our regression modeling. The interaction between disease site and each peripheral blood subset was tested in logistic regression models for chemotherapy response and Cox proportional hazards models for overall survival. Data are presented as mean ± SEM.
RESULTS
Peripheral blood CD4 counts, response to ICT, and survival in patients with laryngeal cancer
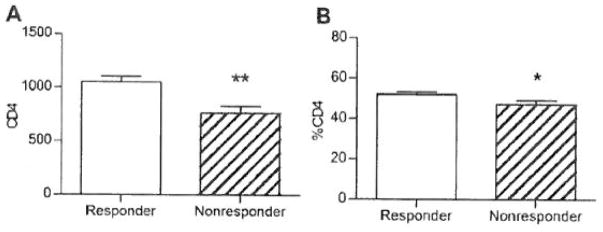
We investigated whether there was a correlation between pretreatment PBL counts and the response to ICT in patients with laryngeal cancer. We found that CD4 counts, both absolute and percentage, were significantly higher in ICT responders compared with nonresponders (see Figure 1). Decreased %CD8 (p = .11), increased CD4/CD8 ratio (p = .19), and increased %CD3 cells (p = .13) all trended toward a correlation with response to ICT. The other lymphocyte counts did not differ between ICT responders and nonresponders.
FIGURE 1.
We also investigated the relationships between pretreatment PBL levels and the final tumor response, assessed 3 months after the completion of definitive chemoradiation. We found no significant associations.
When patients were grouped by T or N classification, we found no significant differences in pretreatment T cell subset levels except for slightly lower %CD8 cells in patients with Stage IV disease (p = .04).
Because pretreatment peripheral blood CD4 levels were associated with response to ICT, and induction response is usually associated with improved survival, we investigated whether CD4 levels were also associated with survival. Patients were stratified into high and low CD4 groups using the previously identified cutoff values of CD4 absolute counts and percentages that were determined by 2 methods: first, using the medians observed among all patients in the study (899.5 and 50.1%), and second, using the mean + 2SEM in 40 normal subjects (912 and 50%).10 Overall and disease-specific survival rates were compared between the patients with high and low CD4 levels using both sets of cutoff values.
We found no significant associations between CD4 levels and survival (see Figure 2). However, using the median value as a cutoff produced slightly stronger correlations than the normal subjects’ mean + 2SEM, with high CD4 counts trending toward improved overall survival. High absolute CD4 counts tended to be more strongly associated with improved survival than high CD4 percentage.
FIGURE 2.

[Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
Relationship between PBLs and response to ICT in a cohort of patients with either laryngeal or oropharyngeal cancer
Because it is generally accepted that head and neck cancer biology differs significantly by tumor site, and because the laryngeal and oropharyngeal cancer Phase II clinical trials had identical ICT protocols, we had the opportunity to determine if there were differing relationships between pretreatment PBL levels and response to ICT in a cohort of patients made by combining those from both of the separate trials (n = 81 in the larynx trial and n = 47 in the oropharynx trial). Analyses of the PBL levels and tumor response to ICT in the oropharyngeal cancer trial was previously performed.8 We also previously determined and reported the specific human papillomavirus (HPV) type and copy number in the tumor specimens from the patients with oropharyngeal trial using real-time, competitive polymerase chain reaction and matrix-assisted laser desorption time-of-flight mass spectroscopy.2
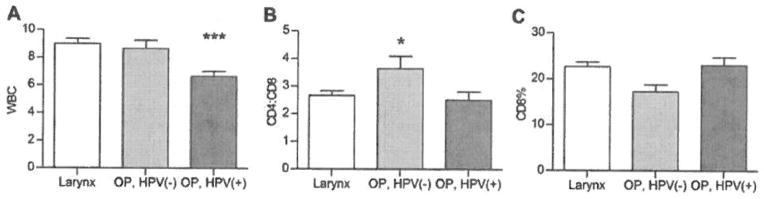
We first investigated whether there were differences in pretreatment PBL levels between the patients with laryngeal and oropharyngeal cancer. The only significant difference we observed was in the pretreatment total WBC, which was higher in the patients with laryngeal cancer (9.01 ± 0.36 in the larynx trial vs 7.58 ± 0.33 in the oropharynx trial; p = .005). When the patients with oropharyngeal cancer were separated into subgroups based on their tumor HPV-16 status, differences in CD4/CD8 ratio became significant, with lower ratios in the HPV-16+ patients, and CD8 cells trending toward a higher percentage in HPV-16+ patients with oropharyngeal cancer compared with HPV-16 patients with oropharyngeal cancer (p = .06) (see Figure 3). The CD4/CD8 ratio and CD8 levels were similar between the larynx cancer and HPV-16+ patients with oropharyngeal cancer.
FIGURE 3.
The groups of patients with laryngeal and oropharyngeal cancer were combined to determine if PBL levels correlated with response to ICT. Increased CD4 levels (% and absolute) remained significantly associated with a response to ICT (see Figure 4). A trend was also noted toward increased CD3 counts being associated with response to ICT (p = .12). Notably, CD8 levels and CD4/ CD8 ratio were not significantly associated with tumor response to ICT.
FIGURE 4.

Predictive potential of PBLs and response to ICT by statistical modeling
We used a logistic regression interaction model to explore the potential use of PBL counts to predict response to ICT in patients with either laryngeal and oro-phayngeal cancer. PBL counts were log-transformed to improve normality. Patients from the 2 trials were pooled to develop the model. The model was then applied to the same patients, taking into account their disease site, to examine whether PBL counts had different predictive patterns between sites.
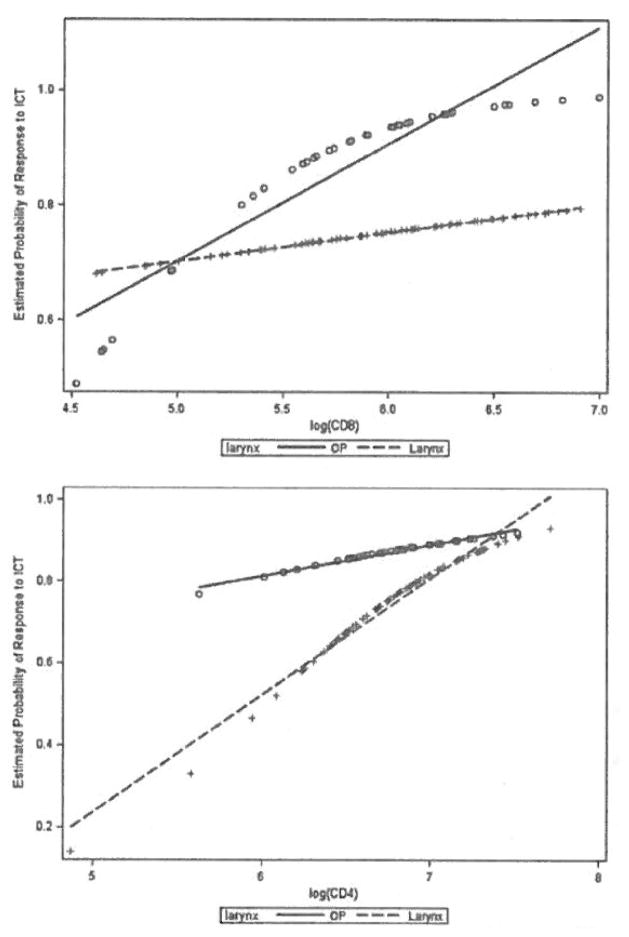
There were interesting differences in the predictive potential for CD4 and CD8 counts between patients with laryngeal and oropharyngeal cancer (see Figure 5). In Figure 5, the predictive association of a given PBL level with response to ICT is represented by the slope of a line, with a steeper slope indicating a stronger predictive relationship. CD4 levels in the patients with laryngeal cancer showed a strong, positive predictive potential (meaning higher CD4 values predicted a higher probability of response to ICT), consistent with the significant correlation between CD4 levels and the response to ICT we observed. In the patients with oropharyngeal cancer, a significant correlation between CD4 levels and the response to ICT was not observed using this model; however, a positive predictive potential was evident, although not as strong as in the larynx. These data suggest that if the oropharynx trial included more patients, a correlation between high CD4 counts and response to ICT may have reached significance. For CD8 levels, the model shows a clear difference in the predictive potential between the patients with laryngeal and oropharyngeal cancer. In the oropharynx, where HPV-16 status is very important, there is a strong, positive predictive potential for CD8 counts, whereas in the larynx there is almost no predictive potential (indicated by a line slope of nearly 0). Finally, CD3 counts showed a strong positive predictive potential in both sites (data not shown).
FIGURE 5.
[Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
DISCUSSION
A major finding of this study was that high pretreatment peripheral blood CD4 levels (both % and absolute) predicted a clinical tumor response to a single cycle of ICT, and trended toward predicting improved survival in patients with advanced laryngeal cancer. Furthermore, low CD8, high CD4/CD8 ratio, and high CD3 levels showed trends toward an association with tumor response to ICT. These preliminary findings, in a small retrospective cohort, provide additional evidence that systemic host immune reactivity is involved in the clinical response to cytotoxic chemotherapy. These results are in contrast to our previous, work-in-progress report that found no associations between pretreatment circulating lymphocyte levels and the response to ICT in patients with laryngeal cancer.9 The difference in findings between these reports was likely due to the increased number of patients that were included in the current analysis, which was needed to reach sufficient power to detect the observed difference in CD4 levels between the ICT responders and nonresponders. These results are important because they suggest that PBL levels could be useful in identifying patients with laryngeal cancers that would be responsive to organ-preserving therapy. Furthermore, PBL counts reflect one aspect of cellular immune reactivity, which may be important in a patient’s overall prognosis.
CD4+ lymphocytes are known to exhibit variable roles in tumor immunity. CD4+ Th cells act beneficially to facilitate tumor eradication, whereas regulatory T cells (Tregs), which are largely CD4+CD25+, are potent inhibitors of the antitumor response.11 This study extends the evidence for the predictive utility of pretreatment, circulating CD4+ cell levels to potentially identify patients for organ-preserving treatment. It remains to be determined if the increased levels of CD4 cells in responders are due to the expansion of Th or Treg cells. The mechanism by which circulating CD4+ lymphocytes are associated with tumor response to chemotherapy is not clear. At the time that this study was conducted, the role of Tregs in cancer was not known, so we did not analyze for the different subsets of CD4+ lymphocytes. Presumably, it is a greater number of Th cells, and not Treg cells, in the pretreatment peripheral blood that is responsible for the correlation with response to therapy. However, further prospective investigation into the types and activities of pretreatment, circulating CD4+ cells, and tumor infiltrating lymphocytes in laryngeal cancer is needed.
Tumor biology also plays a critical role in response to therapy. The results of this study show striking differences in response to therapy between the tumor sites. The difference in findings between the laryngeal and oropharyngeal cancer trials suggests that, immunologically, these tumors likely differ by biological and genetic factors, in addition to site, and that these may be related to differing immune responses. A closely related confirmation of this is seen in patients with oropharyngeal cancer, where tumor HPV-16 status was significantly associated with altered T lymphocyte levels and response to therapy.8 The current results in laryngeal cancer also differ from a similar study that investigated the predictive utility of pretreatment, circulating lymphocytes in patients with HPV-16-related oropharyngeal cancer.8 In that study, a higher %CD8 cells and a low CD4/CD8 ratio were found to predict response to ICT and improved survival; in contrast to our findings in patients with laryngeal cancer, where mean CD8 percentages were similar to those in HPV-16+ patients with oropharyngeal cancer but were not related to chemotherapy response.
Pretreatment WBC and CD4/CD8 ratios differed among the patients grouped and analyzed by larynx, HPV-16+ oropharynx, and HPV-16- oropharynx cancer types. The total WBC counts were lower in patients with HPV-16+ oropharyngeal cancer, which may be due in part to lower rates of smoking in this group, in that many of these patients were nonsmokers and smoking is known to increase WBC counts.12 The CD4/CD8 ratio was higher and the CD8 counts trended lower in patients with HPV-16 oropharyngeal cancer, which had the worst response to therapy among the groups studied. The statistical prediction model was consistent with the previously demonstrated correlation between high CD8 levels and a response to ICT in the oropharynx, and additionally, it showed no such pattern in the larynx, further illustrating the potentially important differences in host-tumor interactions between patients with tumors arising at these sites.
Another interesting finding was that high CD4 cell levels were predictive of a response to ICT in the combined group of larynx and oropharynx trial patients. The correlation between high CD4 levels and response to therapy was less strong in the combined group than in the larynx group alone, suggesting that this association may be driven largely by the larynx group, although the prediction model showed that this association might also be evident in the oropharynx if a greater number of patients were studied. High CD3 levels became more strongly correlated with a response to therapy in the combined group than in the larynx patient group alone, but it did not reach statistical significance. The prediction model strongly supported this finding.
A major strength of this study was the prospective clinical trial design with uniform treatment regimens, a relatively large number of patients, and long follow-up. Additionally, the flow-cytometric analyses were performed by experts in a clinical pathology laboratory on fresh peripheral blood samples. Unfortunately, only retrospective correlations with immune markers were possible, and although we assume that T lymphocyte numbers reflect cellular immune system homeostasis, they do not provide information on immunological function. They also do not allow for conclusions about the potential mechanisms responsible for the correlation between PBLs and response to therapy. Levels of PBLs also do not necessarily reflect the immune response in the tumor microenvironment. However, they do begin to provide additional evidence that the balance among T cell subsets, which are influenced by cytokines and regulatory T cells, is important in patients with head and neck cancer. Consistent with this notion, a functioning immune system has recently been related to effective chemotherapy.13,14 Future investigations should focus on the functional interaction between tumor genetics, tumor biology, and the immune system, including both PBL and tumor infiltrating lymphocytes, prior to and over the course of treatment.
CONCLUSIONS
In this study, we found that, in patients with advanced laryngeal cancer, increased pretreatment peripheral blood CD4 levels were highly predictive of a response to ICT and demonstrated a trend toward improved overall survival. In a combined group of patients with laryngeal or oropharyngeal cancer, response to ICT was also predicted by increased CD4 levels, but statistical modeling indicated that the predictive relationship of lymphocyte subsets and tumor response differed significantly by the site of tumor origin. These differences may be explained in part by the differing tumor biology of HPV-16-related cancer.
In an age of multidisciplinary, multimodal therapy, these observations add to previous evidence that immune parmeters may be useful for identifying subsets of patients with disease that will respond to organ-preserving therapy. A better understanding of host immune function and tumor biology should allow for the development of less morbid, more personalized, less costly, and less time-consuming methods of selecting the optimal therapy for each individual patient.
Acknowledgments
Contract grant sponsor: Institute of Cancer/National Institute of Health; contract grant number: P50 CA097248; contract grant sponsor: Sinabaldo and Diane Tozzi Research Fund.
Footnotes
Presented at the 2012 Multidisciplinary Head and Neck Cancer Symposium, Phoenix, Arizona, January 26–28, 2012.
References
- 1.Urba S, Wolf G, Eisbruch A, et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol. 2006;24:593–598. doi: 10.1200/JCO.2005.01.2047. [DOI] [PubMed] [Google Scholar]
- 2.Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26:3138–3146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 4.Pignon JP, Bourhis J, Domenge C, Designé L, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 5.Wahlin BE, Sundstrom C, Holte H, et al. T cells in tumors and blood predict outcome in follicular lymphoma treated with rituximab. Clin Cancer Res. 2011;17:4136–4144. doi: 10.1158/1078-0432.CCR-11-0264. [DOI] [PubMed] [Google Scholar]
- 6.Hernberg MM, Hahka-Kemppinen MH, Pyrhönen SO. The prognostic role of CD4+ and CD8+ lymphocytes during chemoimmunotherapy in metastatic melanoma. Melanoma Res. 2004;14:493–500. doi: 10.1097/00008390-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Molling JW, Langius JAE, Langendijk JA, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–868. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 8.Wansom D, Light E, Worden F, et al. Correlation of cellular immunity with human papillomavirus 16 status and outcome in patients with advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2010;136:1267–1273. doi: 10.1001/archoto.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf GT, Bradford CR, Urba S, et al. Immune reactivity does not predict chemotherapy response, organ preservation, or survival in advanced laryngeal cancer. Laryngoscope. 2002;112:1351–1356. doi: 10.1097/00005537-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Wolf GT, Schmaltz S, Hudson J, et al. Alterations in T-lymphocyte subpopulations in patients with head and neck cancer. Correlations with prognosis. Arch Otolaryngol Head Neck Surg. 1987;113:1200–1206. doi: 10.1001/archotol.1987.01860110066010. [DOI] [PubMed] [Google Scholar]
- 11.Kosmaczewska A, Ciszak L, Potoczek S, Frydecka I. The significance of Treg cells in defective tumor immunity. Arch Immunol Ther Exp. 2008;56:181–191. doi: 10.1007/s00005-008-0018-1. [DOI] [PubMed] [Google Scholar]
- 12.Fröhlich M, Sund M, Löwel H, Imhof A, Hoffmeister A, Koenig W. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95) Eur Heart J. 2003;24:1365–1372. doi: 10.1016/s0195-668x(03)00260-4. [DOI] [PubMed] [Google Scholar]
- 13.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 201O;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 14.Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]