Abstract
Spinal cord injury or diseases, such as amyotrophic lateral sclerosis, can cause the loss of motor neurons and therefore results in the paralysis of muscles. Stem cells may improve functional recovery by promoting endogenous regeneration, or by directly replacing neurons. Effective directional migration of grafted neural cells to reconstruct functional connections is crucial in the process. Steady direct current electric fields (EFs) play an important role in the development of the central nervous system. A strong biological effect of EFs is the induction of directional cell migration. In this study, we investigated the guided migration of embryonic stem cell (ESC) derived presumptive motor neurons in an applied EF. The dissociated mouse ESC derived presumptive motor neurons or embryoid bodies were subjected to EFs stimulation and the cell migration was studied. We found that the migration of neural precursors from embryoid bodies was toward cathode pole of applied EFs. Single motor neurons migrated to the cathode of the EFs and reversal of EFs poles reversed their migration direction. The directedness and displacement of cathodal migration became more significant when the field strength was increased from 50 mV/mm to 100 mV/mm. EFs stimulation did not influence the cell migration velocity. Our work suggests that EFs may serve as a guidance cue to direct grafted cell migration in vivo.
Keywords: Electric field, Embryonic stem cell, Motor neuron, Migration, Orientation
Introduction
Motor neurons are located in different motor neuron pools in spinal cord and brainstem nuclei. Motor neurons control skeletal muscle contraction. Spinal cord injury or diseases, such as amyotrophic lateral sclerosis, can cause the loss of motor neurons and therefore may result in muscle paralysis. It was previously reported that mouse embryonic stem cells (ESCs) can differentiate into motor neurons after they are exposedto retinoic acid (RA) and an agonist of the sonic hedgehog (Shh) signalling pathway [1]. That study raised the possibility that the pluripotent stem cell-derived motor neurons may replace the neurons lost in injury or disease and restore motor function. However, the low differentiation efficiency of mouse ESCs into motor neurons is a limitation as is the low efficiency to re-establish appropriate neural connections following transplantation. It was estimated that 20–40 % of ESCs differentiated into motor neurons after the cells were treated with RA and an activator of the Shh signalling pathway. The other 60–80 % of the cells differentiated into glutamatergic neurons and glia [2]. Wu et al. reported a two-step differentiation protocol that improved the motor neuron differentiation efficiency to 51 %. In that study, the first step was “neutralization” by adding Noggin and fibroblast growth factors (FGFs) to the cell culture medium [3]. The second step was to induce motor neuron specification.
Functional properties of the ESC-derived motor neurons have been shown in previous studies. Cultured mouse ESC-derived motor neurons expressed functionally appropriate GABA, glycine, and glutamate receptors and voltage-activated Na+, K+, and Ca2+ ion channels [2]. After embryoid bodies (EBs) were implanted into the chick embryonic spinal cord, ESC-derived motor neurons repopulated the ventral spinal cord in vivo, extended axons into the periphery, and formed synapses with muscle targets [1]. Cell motility is also an important functional property of neuronal stem cells for migration and for extension of axon growth cones towards their targets. Effective directional migration of transplanted cells to the target may promote the establishment of functional reconnection after injury or disease.
Steady direct current (DC) electric fields (EFs) play an important role in the development of the central nervous system (CNS) [4–6]. One strong biological effect of EFs is the guidance of axonal growth and induction of directional cell migration [7–10]. In vitro studies showed that EFs resembling those in the developing and regenerating nervous systems can direct spinal neuron axon growth towards the cathode [7, 8]. In vivo, EFs can enhance regrowth of damaged spinal cord axons [11]. Recent studies showed that some types of stem cells respond to EFs and display direcational migration in response to EFs. The influence of EFs on the migration speed and direction of these cells was variable [12–16]. EBs and post-mitotic motor neurons are two important stages in the ESC differentiation process. The study of the cell’s response to EFs stimulation at those stages may lead to the development of new strategies to guide the migration of grafted cells in vivo, and thus enhance recovery. For example, EFs can serve as a guidance cue to direct the migration of the cultured hippocampal neurons [17, 18]. At early cell culture stage, the bipolar hippocampal neurons with short processes were very motile and can migrate to the cathode in applied EFs [17, 18]. However EFs do not change the neuron’s migration speed. The study indicated the typical morphology of highly motile neurons and the cell migration pattern in EFs.
Here, ESCs were differentiated using a two-step procedure [19] and EFs were applied to the ESC-derived neural cells at two stages. First, we studied the migration of neural precursors from EBs in EFs. Then we investigated the EFs-directed migration of ESC-derived choline acetyltransferase (ChAT) positive cells (presumptive motor neurons) as single cells. This is the first study to characterize the migration of ESC-derived neurons in EFs.
Materials and Methods
Mouse Embryonic Stem Cell (mESC) Culture
The cell culture dishes were coated with 0.1 % gelatin ((Sigma-Aldrich, St. Louis, MO) for 30 min at room temperature. Mouse embryonic fibroblasts (MEF) (GlobalStem, Rockville, MD) were then seeded on the gelatin-coated dishes. MEF cell culture was maintained using MEF media (Dulbecco’s Modified Eagle Medium (DMEM), 10 % Fetal Bovine Serum, 1 % Penicillin-Streptomycin, 2 mM Glutamax (L-alanyl-L-glutamine dipeptide), Life Technologies, Grand Island, NY). MEFs were plated at least one day before adding mESCs. Murine ES-D3 ESC (ATCC) were seeded in cell culture dishes on top of MEFs. The mESCs were cultured with ESC medium (Dulbecco’s Modified Eagle Medium, 10 % Fetal Bovine Serum, 1 % Penicillin-Streptomycin, 2 mM Glutamax (L-alanyl-L-glutamine dipeptide), Leukemia inhibitory factor (LIF, 1,000 IU/ml, Millipore, Billerica, MA) in a 37°C incubator with 5 % CO2.
Neural Induction and Presumptive Motor Neuron Specification
The cultured mESCs and MEF cells were trypsinized with 0.25 % trypsin/EDTA (Life Technologies, Grand Island, NY) and the trypsin was inactivated by adding fresh ESC medium. The collected cells were centrifuged, resuspended in medium and the cell pellet was triturated for a few times and then were seeded in the 0.1 % gelatin-coated cell culture plates and incubated at 37°C for 30 min. Most MEF cells adhered to the cell culture plates within an hour. The floating cells, which were enriched for mESCs, were collected, centrifuged, and resuspended. The presumptive motor neuron differentiation was then performed as reported previously [3]. In brief, the cell pellets were re-suspended in DMEM supplemented with ESC culture grade FBS, monothioglycerol (Sigma-Aldrich, St. Louis, MO), non-essential amino acids (Life Technologies, Grand Island, NY), Noggin, and FGF-8 (PEPROTECH, Rocky Hill, NJ). The cells were then cultured in suspension culture dishes (Fisher Scientific, Pittsburgh, PA). The ESCs formed embryoid bodies (EBs) in the suspension cell culture plate. After 1 day of suspension culture, the cell culture medium was changed with fresh neural differentiation medium and the EBs were cultured for another 24 h. Then the cells were re-suspended and cultured in motor neuron differentiation medium (ES-Cult basal medium-A supplemented with knockout serum replacement (Life Technologies, Grand Island, NY), retinoic acid (Sigma-Aldrich, St. Louis, MO), smoothened agonist (SAG, Millipore, Billerica, MA)) in the suspension culture plate for 6 days. Then the EBs were either cultured for additional 24 h or used for the investigation of cell migration in EFs.
For the EBs cultured for 7 days in suspension culture plate, the EBs were dissociated using Accumax (Millipore, Billerica, MA) or mechanically by pipetting. Then the cells were cultured with ES-Cult basal medium-A (STEMCELL Technologies Inc.) supplemented with knockout serum replacement, brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), glial cell-derived neurotrophic factor (GDNF) and neurotrophin-3 (NT-3) (PEPROTECH, Rocky Hill, NJ). After the cells were cultured for 3 to 4 days, they were trypsinized and the dissociated cells were seeded in the glass chamber for 2 days. Then the cells were subjected to the direct current EFs stimulation as described below.
Embryonic Stem Cell Derived Presumptive Motor Neuron Migration in EFs
To investigate cell migration in EFs, an electric field stimulation system was established as reported previously [17, 20]. In brief, cells were grown in a glass chamber for migration experiments. The chamber was made from coverslips and its final dimensions were 30 mm×0.8 mm×0.15 mm. The plastic surface of the chamber was coated with poly-DL-ornithine (100 ug/ml, Sigma-Aldrich, St. Louis, MO) at 4°C overnight and then the coating solution was aspirated and the chamber was coated with laminin (20 mg/ml, Sigma-Aldrich, St. Louis, MO) at 4°C overnight. To apply the EFs to the cultured cells in the chamber, agar-salt bridges (filled with Steinberg’s solution (58 mM NaCl, 0.67 mM KCl, 0.44 mM Ca(NO3)2·4H20, 1.3 mM MgSO4·7H20 and 4.6 mM Trizma base) gelled with 1 % agar) were used to connect silver–silver chloride electrodes in beakers of Steinberg’s solution to pools of excess culture medium at either side of the chamber. Culture conditions in control were identical except no EFs were applied.
To study the cell migration from EBs with EFs stimulation, the EBs were placed in the chamber and cultured with motor neuron differentiation medium. After 24 h culture, the EBs attached to the plastic surface of the chamber and some cells migrated out of the EBs. Then the steady DC EFs of 50–100 mV/mm were applied to the cultured EBs for 20 h in a CO2 incubator (37°C). The voltage was supplied by Bio-Rad 200/2.0 Power Supply (Bio-Rad, Hercules, CA). The EFs were measured with an electric meter (DT830B, JAMECO BENCHPRO, Belmont, CA). At the beginning, during the stimulation and at the end of the stimulation the voltage was checked to confirm the applied electric fields..
To study the cell migration of the dissociated presumptive motor neurons, the dissociated cells were seeded in the glass chamber with a cell seeding density of 2–3×104 per chamber. Steady DC EFs of 50 mV/mm or 100 mV/mm were applied to the cultured cells in culture chambers and migration was recorded by a time-lapse microscopy. The microscope was placed in a plastic incubator with 37°C and 5 % CO2. Sterile conditions will be maintained throughout. To study migration of the cells in an applied EF, an area with cells was selected after the cells were cultured for 24 h. Cell migration was recorded by capturing images every 3 min during one hour control period. Then the EF was switched on and cell migration was recorded every 3 min for one hour. To confirm cell migration direction in EFs, the EF polarity was reversed without changing the field strength and cell migration will be recorded for one more hour.
Time-lapse Imaging and Quantification of Cell Behavior
Cell migration was recorded with a Zeiss Axio Observer microscope. Time-lapse image recording was performed using a ZEN 2011 imaging microscope software and the images were taken by digital camera (AxioCam MRm Rev.3 with FireWire). The images were analysed by NIH ImageJ software (National Institutes of Health, Bethesda, MD).
Cell migration was quantified as the method reported previously [16]. The angle at which each cell moved with respect to the imposed EF direction was measured. The mean direct-edness of total cell movement was calculated from the equation Σicos θi/n, where θ is the angle between the field vector and the cellular translocation direction and n is the total number of cells. The cosine of the angle would be equal to 1 if the cell moved directly along the field lines toward the cathode; 0 if the cell moved perpendicular to the field direction; -1 if the cell moved directly toward the positive pole of the field. The net displacement was the displacement of the cell migration along the field line. The cell migration velocity was calculated from the full distance of cell migration in a given time. To quantify the cell migration speed, direct-edness and displacement, 30–40 cells from four independent experiments were analysed.
Immunocytochemistry
After EFs stimulation, the EBs or the dissociated cells were fixed with 4 % paraformaldehyde and permeabilized with 0.2 % Triton X-100. Cells were exposed to blocking solution (10 % horse serum, 1 % bovine serum albumin (BSA)) for 20 min. All antibodies were diluted in PBS with 1 % BSA. The EBs were incubated with monoclonal primary antibody anti-nestin (Millipore, Billerica, MA), or polyclonal primary antibody anti-ChAT (Millipore, Billerica, MA) for 2 h at room temperature. The EBs were also labelled with Rhodamine Phalloidin (Life Technologies, Grand Island, NY). The dissociated cells were incubated with monoclonal primary antibody anti-neurofilament (NF) 160 (Sigma-Aldrich, St. Louis, MO) and polyclonal primary antibody anti-ChAT for 2 h at room temperature. The secondary antibodies were Alexa Fluor® 594 Donkey anti-goat IgG and Alexa Fluor® 488 Donkey Anti-Mouse IgG (Jackson ImmunoResearch, West Grove, PA). Nuclei were stained with DAPI.
Statistics
Statistical analysis was made using paired t-test or unpaired, two-tailed Student’s t-test. Data are expressed as mean ± SD.
Results
Cells Migrated Toward the Cathode from EBs in an Applied EF
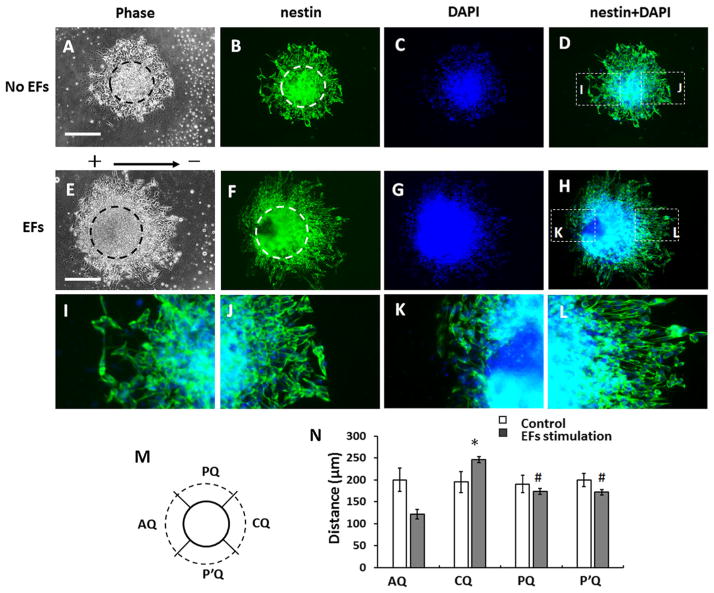
To study the migration of neural precursors from EBs, EBs were treated with motor neuron differentiation medium for 6 days, then collected and seeded in the chambers coated with poly-DL-ornithine and laminin. After 24 h, the EBs attached on the cell culture surface of the chambers and some cells grew out from the EBs (Fig. 1a, c). Then the cultured EBs in some chambers were subjected to EFs stimulation (100 mv/mm) for 20 h. For the control group, the EBs were cultured without EFs stimulation for 20 h. The cells that migrated out of the EBs were nestin-positive and we call them nestin-positive neural precursors. In the control group, the cells were symmetrically distributed around the EBs (Fig. 1b, 2a–d). For EBs that were stimulated with EFs, the area occupied by migrated cells was asymmetrical. More cells migrated out from the cathode-facing side of EBs than from the anode facing side (Fig. 1d, 2e–h).
Fig. 1.
Migration of neural progenitor cells from embryoid bodies (EBs) in direct current electric fields (EFs). a, c EBs after first 24 h of culture; (b, d) EBs after 44 h. Note that the EBs adhered to the tissue culture plate and that several cells migrated away from the outside of the EB, with more cells migrating away over time in culture. When cultured without the electric field (EF), the neural precursors extensively migrated out of the EB and were symmetrically distributed around it (a–b). c–d When EBs were stimulated with EFs (100 mV/mm) for 20 h after the initial 24-hour culture, more cells migrated out from the cathode facing side (right side of panel) of EB than the anode facing side (left). Scale bar: 200 μm
Fig. 2.
Analysis of cell migration from embryoid bodies (EBs). a–d Symmetrical distribution of neural precursors around an EB without EFs stimulation. e–h Asymmetrical distribution of neural precursors around an EB exposed to EF for 20 h. The dash cycle lines in (a) and (e) show the core area of the EBs. b, f The cells were labelled with anti-nestin antibody (green). c, g DNA stained with DAPI (blue). d Overlaid image of (b) and (c). h Overlaid image of (f) and (g). i, j Magnified images of inset indicated in (d). k, l Magnified images of insets indicated in in h. m A diagram shows the division of quadrants around an EB. CQ, the cathode facing quadrant. AQ, the anode facing quadrant. PQ and P’Q, the quadrants that are perpendicular to the field line of EFs. Solid circle line represents the edge of the EB core. The dash circle line represents the frontier of migrated cells. n Analysis of the cell migration distance from the EBs. The *, p<0.01, compared with the anode facing quadrant and the quadrants that are perpendicular to the field line of EFs. #, p<0.01, compared with the anode facing quadrant
Brightfield images of the EBs were taken after EBs were cultured for 24 h (t=0) and after culturing for additional 20 h (t=20 h) with or without EFs stimulation. The EB core was determined from the brightfield image and immunostaining with anti-Nestin antibody and DAPI. The core of an EB was the part of the highest density in the brightfield image. The overlapped images of the Nestin and DAPI confirmed the observation in brightfield image. To quantify the distribution of migrated cells, the area surrounding each EB was divided into four quadrants (Fig. 2m). Quadrant C (CQ) was the cathodal facing quadrant, while quadrant A (AQ) was the anodal facing quadrant. The distance between the EB core and the frontier of migrated cells in each quadrant was measured. In either control group or EFs stimulation group, at least 50 EBs from 3 independent experiments were quantified. In the EF stimulation group, the average migration distance of the cells in the cathode facing quadrant was 245.8±6.5 μm, which is significantly higher than that of anode facing side (121.5±11.3 μm, p<0.01) and the that of corresponding side of the control group (195.2±23.5, p<0.05). However, the average migration distance of the cells in the anode facing quadrant is significantly lower than that of corresponding side of the control group (200.2±26.5, p<0.05) (Fig. 2n). In the control group, the average cell migration distance in the right (cathode facing side in EFs study) quadrant was not different from the left (anode facing side in EFs study) quadrant. This suggested that the EFs stimulation promoted the polarized cell migration from EBs towards the cathode.
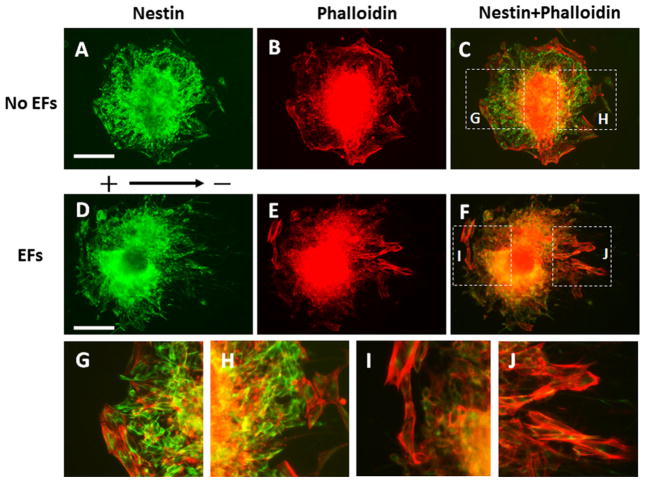
The cells outside the EBs were labelled with anti-nestin antibody and Rhodamine Phalloidin to study their morphology and orientation (Fig. 3). The cells showed random orientation outside the EBs without EFs stimulation (Fig. 3a–c,g,h). We observed that the cells re-oriented on the anode-facing side and most cells were perpendicular to the EFs on the anode-facing side of the EBs that were subjected applied EFs. In contrast, most cells were moving parallel to the field line on the side facing the cathode pole (Fig. 3d–f, i, j; Supplemental Fig. 1).
Fig. 3.
Orientation of the cells that migrated out of the embryoid bodies (EBs). The EBs were labelled with anti-nestin antibody (green) and Rhodamine Phalloidin (red). a–c The symmetrical distribution and random orientation of the cells that migrated out the EB without direct current electric fields (EFs) stimulation. g, h The magnified images show the dash line labelled areas in (c). d–f The asymmetrical distribution of cells that migrated out of the EB. On the cathode facing side (right), the cells aligned parallel to the field line of EFs. On the anode facing side (left) the cells oriented perpendicular to the field line of EFs. i, j Magnified images show the dash line labelled areas (f). Scale bar: 200 μm
ESC-derived Presumptive Motor Neurons Migrated Cathodally In An Applied EF
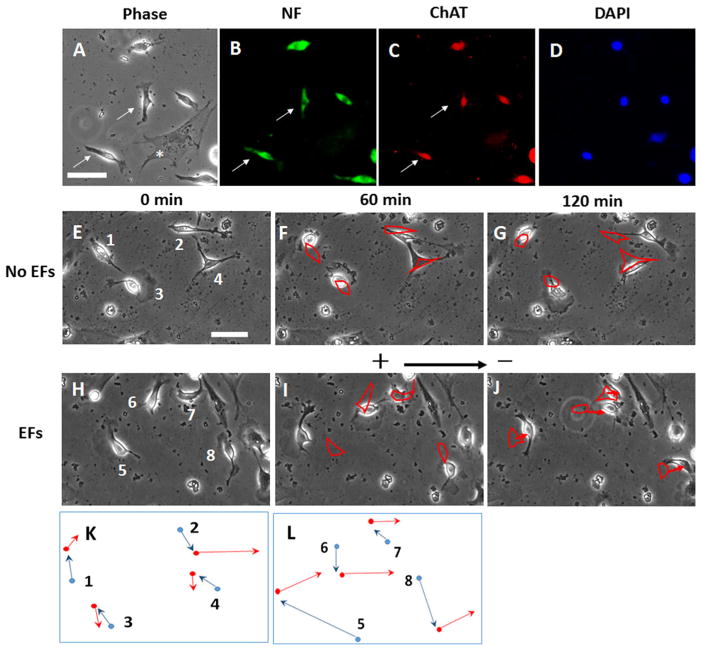
Anti-neurofilament and anti-ChAT antibodies were used to label the dissociated presumptive motor neurons. Most presumptive motor neurons extended short processes and showed bipolar morphology (Fig. 4a–d). The time-lapse images showed that the presumptive motor neurons in the control group migrated randomly during the 2-hour recording period (Fig. 4e–g). In the EF-stimulated group, the migration of presumptive motor neurons was recorded for 2 h (Fig. 4e–g). The first hour was the control period (before EF was applied) and then EFs were applied to the cells and migration was recorded for the second hour. The cells showed random migration in the first hour of recording (Fig. 4h), while the cells showed a clear trend of migration toward the cathode in an applied EF of 100 mv/mm in the second hour (Fig. 4i,j). Here, we observed that cell debris were generated during the differentiation process. We attribute this to the large number of cells that died during the cell differentiation process (Fig. 4e–j).
Fig. 4.
Dissociated presumptive motor neurons migrated toward the cathode in a direct current electric field (EF). a–d The same field of choline acetyl transferase staining (ChAT, marker of cholinergic neural cells) are shown. In A, phase contrast image. In B, cells were immuno-cytochemically labelled with neural filament (NF) antibody (green), In C, cells labelled with ChATantibody (red) and, in D, the nuclei were labelled using DAPI. The arrows indicate the ChAT- and NF-positive cells and they show the typical bipolar morphology. Phase dark (flattened) cells indicated by asterisk (*) denotes a mitotically inactivated mouse embryonic fibroblast. Panels E through J show time-lapse images of single neural cells migrating. e–g The random migration of cells during 2-hour period without EFs stimulation. See also Supplementary Material Video 1. h–j ChAT neural cells that migrate in response to an applied EF (100 mV/mm). The cell migrated randomly without EFs stimulation in the first hour. The cells migrated to the cathode (right) with EFs stimulation in the second hour. See also Supplementary Material Video 2. Outlines of the labelled cells in (f) and (g) highlight cell positions in (e) and (f), respectively. Outlines of the labelled cells in (i) and (j) highlight cell positions in (h) and (i), respectively. The arrows showed the directional migration of the cells in EFs. Scale bar: 50 μm. Figure 4k and l are schematics showing the relative cell translocation and migration direction demonstrated in figure (e–g) and (h–j), respectively. The blue lines indicate the cell migration in the first hour and the red lines indicate the cell migration in the second hour. Dot: cell center; arrow: cell migration direction
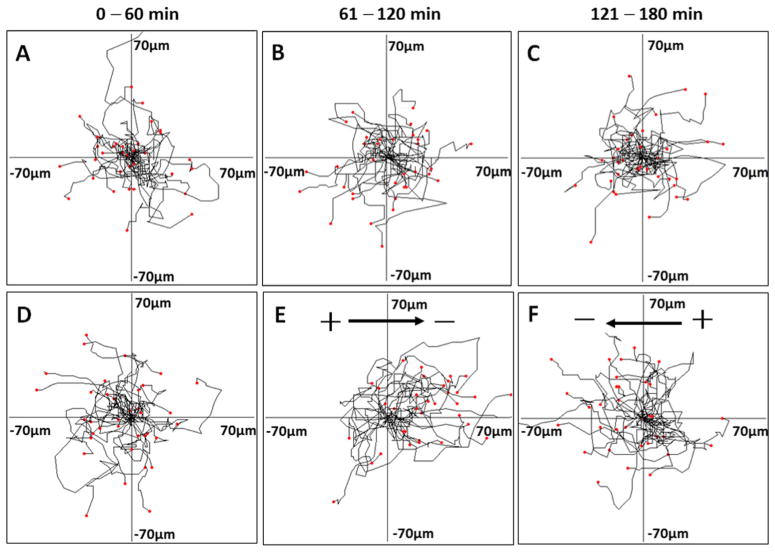
In Fig. 5, each frame shows the superimposed migration tracks of 30–40 presumptive motor neurons from four different experiments. The position of all the cells at t=0 min is represented by the origin (0, 0). Each line represents the migration track of one single cell over 1-hour period. Cells that were not subjected to EFs stimulation migrated randomly in the control studies (Fig. 5a–c). In the EFs stimulation study, after 1 h of apparently random migration, the cells subjected to an applied EF (100 mV/mm) showed clear cathodal migration. To confirm the response of the cells to EF application, the EF polarity was reversed after the cells had been exposed to EFs for 1 h. After reversal of field polarity, cells migrated toward the new cathode (Fig. 5d–f). This reversal of migration direction again indicates that their migration was directed by the EF. Histograms show the frequency distribution of the angles of the cells after the first hour and the second hour migration. Histograms showing the frequency distribution of the angles of the cells after the first hour and the second hour migration are found in Supplemental Fig. 2. After the cells migrated for 1 h in an applied EF of 100 mV/mm, the histograms indicate an asymmetrical distribution with more cells falling in the sectors near 0° than elsewhere (see Supplemental Fig. 2).
Fig. 5.
The net translocation of embryonic stem cells (ESC)-derived neural cells during 3-hour migration period. Migration paths were determined by video monitor tracings. The position of all cells at t=0 min is represented by the origin position (the center of the frame) with the migratory track of each cell at 60 min plotted as a single line on the graph. Each arm of the axes represents 70 μm of translocation distance. a–c Random cell migration without EFs stimulation during the 3-hour recording period. d–f Presumptive motor neurons migration in an applied EF (100 mV/mm). d The cells migrated apparently randomly without EFs stimulation in the first hour. e Most cells migrated to the cathode with EFs stimulation in the second hour. See also Supplementary Material Video 3. f The cells migrated to the opposite direction after the EFs polarity was reversed in the third hour. See also Supplementary Material Video 4
EFs Directed Cells Migration Showed a Voltage Dependent Manner
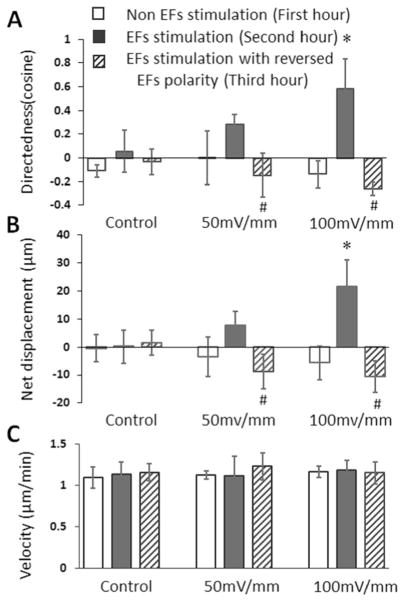
In the control study, the migration of the cells was apparently random during the 3-hour recording period. In the EFs group, the migration of the cells was random in the first hour when no EF was present as indicated by the cosine which indicates directedness of 0.00±0.22 (total cell number=32) at the end of the migration period. After 1 h exposure to EF stimulation of 50 mV/mm, the cells showed a trend of cathodal migration (directedness=0.29±0.08), however, the directional migration was not significantly different with the random migration in the first hour (Fig. 6a). After the EFs polarity was reversed, the cells migrated to the new cathode pole (directedness, −0.15± 0.18), which was significantly different with the directedness of the previous hour (Fig. 6a). The directedness of the cells in the EFs of 100 mV/mm was 0.58±0.25 (total cell number= 36), which was significantly higher than that of the first hour random migration (directedness= −0.14±0.11) and the directedness in the EFs of 50 mV/mm. The reversal of the 100 mV/mm EF polarity reversed the cell migration direction (directedness= −0.26±0.06) (Fig. 6a).
Fig. 6.
The directedness, net displacement and migration speed of embryonic stem cell (ESC)-derived neural cells migration in EFs. a The average cosine (directedness) increased when the field strength was increased from 50 mV/mm to 100 mV/mm. Reversal of the EFs polarity reversed cell migration direction. b The net displacement of the cells along the field line of EFs. The net cathodal displacement of the cells became more significant when the EFs were increased from 50 mV/mm to 100 mV/mm. Reversal of the EFs polarity reversed cell displacement along the field line of EFs. c Analysis of cell migration speed in EFs. The EFs did not change the cell migration velocity
The quantification of net displacement of the cells along the field line also showed cathodal migration pattern of the cells. The net displacement of cells in the EFs of 50 mV/mm and 100 mV/mm was 7.64±5.11 and 21.55±9.70 μm per hour, respectively (Fig. 6b). After the EFs polarity was reversed, the net displacement of the cells in EFs of 50 mV/mm and 100 mV/mm was −8.73±6.11 and −10.59±5.54 μm per hour, respectively (Fig. 6b), and showed that the reversal of the EFs polarity guided the cell migration in the opposite direction and towards the cathode. These results demonstrate that the directedness and displacement of cathodal migration increased when the EFs strength increased.
EFs Did Not Influence the Cell Migration Speed
The cell migration velocity during the control period, exposure to EFs and exposure to reversed EFs were quantified. In the control study, the cell migration velocity was not significantly different over the three one hour periods (when no EF was applied). In the EFs stimulation study, the migration speeds of the cells in EFs of 50 mV/mm and in reversed EFs were 1.11±0.24 μm/min and 1.23±0.16 μm/min respectively, which were not significantly different with that of the first hour random migration (1.12±0.04 μm/min). In the EFs of 100 mV/mm and the reversed EFs, the migration speeds of the cells were 1.18±0.12 μm/min and 1.14±0.13 μm/min, respectively, which were not significantly different with that of the first hour random migration (1.16±0.06 μm/min) (Fig. 6c). These results demonstrate that cell migration speed did not change when the cells migrated in response to an EF.
Discussion
In this study, the effect of an applied EF on guiding migration of ESC-derived nestin-positive neural progenitors and presumptive motor neurons was investigated and we made four new observations. First, we found that the cell migration was toward the cathode pole of the applied EFs. Second, we found that this finding was specific since the reversal of EFs polarity reversed the migration direction of the cells. Third, we found that the directedness and displacement of cathodal migration increased when the strength of EFs was increased from 50 mV/mm to 100 mV/mm. Fourth, we found that exposure to EFs did not influence cell migration speed over the three hour observation period.
In support of our observations, a previous study showed that hippocampal neurons with short processes were highly motile at the early stages of cell culture, and that the cells dynamically responded to the applied EFs and migrated under the guidance of EFs [17]. In this study, we found that most NF- and ChAT-positive cells showed short processes and the bipolar morphology after dissociation and were highly motile when they placed onto poly-DL-ornithine- and laminin-coated cell culture plates. Importantly, the dissociate hippocampal neurons migrated to the cathode pole in an applied EF, just like the ESC-derived nestin-positive neural progenitors did here. Again, similar to our observations, the cathodal migration of hippocampal neurons was confirmed by reversing the electrical polarity. In contrast to our previous observations with primary hippocampal neurons, ESC-derived nestin-positive neural progenitors were more sensitive to EF since we observed that only 50 mV/mm was sufficient to generate a directedness of 0.29 in ESC-derived cells, while a EFs strength of 120 mV/mm was needed for the hippocampal neurons to achieve the similar migration directedness [17]. Similarly, neuronal migration from micro-explants is influenced by external cues [19, 21]. We showed previously using cultured hippocampal micro-explants that neuronal migration was influenced by EFs [17]. In these experiments, too, after the explants were stimulated with EFs, the neurons migrated toward the cathode and from the cathode facing side of the explant, and neurons turned their migration direction and migrated cathodally after they moved out from the anode facing side of the explant. The migration of neural precursors from EBs in EFs showed a similar pattern as the neuron migration from hippocampal micro-explants. We observed that more cells migrated out from the cathode facing side of the EBs than the anode facing side of the EBs. The cells moved in parallel to the field line on the cathode facing side. On the anode-facing side, the cells re-oriented their migration direction to move around the EBs and then migrated toward the cathode pole. Therefore on the anode-facing side, many cells appeared perpendicular to the field line because of the reorientation of the cells in the applied EF. However, on the cathode-facing side, the cells migrate toward the cathode pole when they migrated out of the EBs. In summary, similar to what has been reported for primary neural culture, the present results indicate that EFs caused oriented cell migration in the direction of the cathode and affected the migration of cells on both cathode facing side or anode facing side of differentiating EBs.
We previously reported that hippocampal neurons migrated toward the cathode in EFs [17]. The threshold of EFs that directed primary hippocampal neuron migration was between 50 to 120 mV/mm. Other studies support this finding and showed that stem cells, and stem cell-derived cells respond to EFs and the migration of the cells can be guided in EFs. For example, bone marrow mesenchymal stromal cells (BM-MSC) migrated to the cathode in EFs. The EF threshold that induced directional migration of BM-MSCs was about 25 mV/mm [13]. The migration of human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells (hESCs) can be guided by EFs [14]. However, the migration direction of hESCs and hiPSCs was different in EFs. HiPSC migrated to the anode pole in EFs, while hESCs migrated toward cathode [14]. The EF threshold for guided hiPSC migration was lower than 30 mV/mm. Similarly, neural stem cells (NSCs) derived from hESCs migrated to the cathode in an applied EF [15]. The cathodal migration of NSCs in EFs can be induced by the field strength of 16 mV/mm or higher [15]. Additionally, the embryonic and adult neural progenitor cells migrated to the cathode pole in an applied EF with the field strength above 250 mV/mm [12]. Here, we observed that both the cells from EBs and mESC-derived presumptive motor neurons can be guided to migrate toward the cathode in EFs. We found that the presumptive motor neurons migrated toward cathode in EFs. Though the value of migration directedness (cosine) of the presumptive motor neurons in 50 mV/mm EF appeared to be higher than that of random migration, the difference was not statistically significant. However, when the EFs reversed the cell migration direction reversed and the change of the value was significantly different than that before the EFs reversal. The result suggests that the threshold of EFs for mESC-derived presumptive motor neurons should be about 50 mV/mm. This value is similar to previous findings obtained using primary hippocampal neurons, iPSC and ESC derived neural progenitor/stem cells as discussed above.
Our findings indicate that cell motility and migratory direction are two different and separately regulated processes in cell migration. Previous work has shown that the neuronal migratory directedness and motility can be controlled separately by external cues [22]. For example, the guidance molecule slit can function as a repellent without concurrent inhibition of neuronal migration [22]. EFs have a strong influence on directed migration of many cell types and the influence on the cell migration speed is variable [23–27]. EFs stimulation can increase the migration speed of some cell types [26, 27]. It was shown that EFs significantly increased the speed of hiPSCs [14]. The mechanism underlying migration and electrotaxis might be related to asymmetric redistribution of PI3K/AKT, cell surface receptors and actin [12]. Migration of adult and embryonic neural progenitor cells also depends upon growth factors EGF and FGF [12, 28]. Our findings directly compared the cell migration speeds before and after EFs stimulation, after EF stimulation of two different intensities, and the speed after EFs polarity was reversed. We observed that, similar to hippocampal neurons [17], the migration velocity for ESC-derived neural cells was about 60 μm/hour, and that EF stimulation did not change the cell migration velocity for either hippocampal neurons or ESC- derived cells. In this study, we also found the migration speed was consistent after the polarity of EFs were reversed. Further work is need to determine whether ESC-derived NSCs and EBs depend upon growth factors such as epidermal growth factor (EGF) or whether FGF and PI3K/AKT are involved in electrotaxis.
The ability to use EF to direct migration of neural stem/progenitor cells might be leveraged in the future to guide these populations to the sites of CNS injury. Thus, our work has implications for CNS repair in such conditions as spinal cord injury or similar focal neural injuries/damage, and application of EF may assist with directing endogenous neural repairs in combination with exogenous cells. Further work is needed to evaluate the role of EF following injury and disease and to determine whether EF can be useful in regenerative medicine.
Supplementary Material
Acknowledgments
This work was supported by Li Yao’s start-up funding, Wichita State University and National Center for Research Resources (P20 RR016475) and the National Institute of General Medical Sciences (P20 GM103418) from the National Institutes of Health. MLW’s laboratory is supported by the State of Kansas to the Midwest Institute of Comparative Stem Cell Biology, NSF Award: 1321261, NIH R01AR056347, the Dean’s office of the Kansas State University’s College of Veterinary Medicine, and gifts from Ronald Deffenbaugh Foundation and the Northeast Kansas Parkinson’s Association Research Fund. We acknowledge James Hong, Dr. Pavan Rajanahalli, and Dr. Hong He for advice and technical support.
Footnotes
Conflict of interest The authors indicate no potential conflicts of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s12015-014-9518-z) contains supplementary material, which is available to authorized users.
Contributor Information
Yongchao Li, Email: li.yao@wichita.edu, Department of Biological Sciences, Wichita State University, Wichita, KS 67260, USA.
Mark Weiss, Department of Anatomy and Physiology, Kansas State University, Manhattan, KS 66506, USA.
Li Yao, Department of Biological Sciences, Wichita State University, Wichita, KS 67260, USA.
References
- 1.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 2.Miles GB, Yohn DC, Wichterle H, Jessell TM, Rafuse VF, Brownstone RM. Functional properties of motoneurons derived from mouse embryonic stem cells. Journal of Neuroscience. 2004;24:7848–7858. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu CY, Whye D, Mason RW, Wang W. Efficient differentiation of mouse embryonic stem cells into motor neurons. Journal of Visualized Experiments. 2012;64:e3813. doi: 10.3791/3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotary KB, Robinson KB. Endogenous electrical currents and the resultant voltage gradients in the chick embryo. Developmental Biology. 1990;140:149–160. doi: 10.1016/0012-1606(90)90062-n. [DOI] [PubMed] [Google Scholar]
- 5.Hotary KB, Robinson KB. The neural tube of the Xenopus embryo maintains a potential difference across itself. Brain Research Developmental Brain Research. 1991;59:65–73. doi: 10.1016/0165-3806(91)90030-m. [DOI] [PubMed] [Google Scholar]
- 6.McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiological Reviews. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- 7.Rajnicek AM, Foubister LE, McCaig CD. Temporally and spatially coordinated roles for Rho, Rac, Cdc42 and their effectors in growth cone guidance by a physiological electric field. Journal of Cell Science. 2006;119:1723–1735. doi: 10.1242/jcs.02896. [DOI] [PubMed] [Google Scholar]
- 8.Rajnicek AM, Foubister LE, McCaig CD. Growth cone steering by a physiological electric field requires dynamic microtubules, microfilaments and Rac-mediated filopodial asymmetry. Journal of Cell Science. 2006;119:1736–1745. doi: 10.1242/jcs.02897. [DOI] [PubMed] [Google Scholar]
- 9.Nuccitelli R. A role for endogenous electric fields in wound healing. Current Topics in Developmental Biology. 2003;58:1–26. doi: 10.1016/s0070-2153(03)58001-2. [DOI] [PubMed] [Google Scholar]
- 10.McKasson MJ, Huang L, Robinson KR. Chick embryonic Schwann cells migrate anodally in small electrical fields. Experimental Neurology. 2008;211:585–587. doi: 10.1016/j.expneurol.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgens RB, Blight AR, McGinnis ME. Behavioral recovery induced by applied electric fields after spinal cord hemisection in guinea pig. Science. 1987;238:366–369. doi: 10.1126/science.3659920. [DOI] [PubMed] [Google Scholar]
- 12.Meng X, Arocena M, Penninger J, Gage FH, Zhao M, Song B. PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Experimental Neurology. 2011;227:210–217. doi: 10.1016/j.expneurol.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Z, Watt C, Karystinou A, Roelofs AJ, McCaig CD, Gibson IR, et al. Directed migration of human bone marrow mesenchymal stem cells in a physiological direct current electric field. European Cells & Materials. 2011;22:344–358. doi: 10.22203/ecm.v022a26. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Calafiore M, Zeng Q, Zhang X, Huang Y, Li RA, et al. Electrically guiding migration of human induced pluripotent stem cells. Stem Cell Reviews. 2011;7:987–996. doi: 10.1007/s12015-011-9247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng JF, Liu J, Zhang XZ, Zhang L, Jiang JY, Nolta J, et al. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells. 2012;30:349–355. doi: 10.1002/stem.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, El-Hayek YH, Liu B, Chen Y, Gomez E, Wu X, Ning K, Li L, Chang N, Zhang L, Wang Z, Hu X, Wan Q. Direct-current electrical field guides neuronal stem/progenitor cell migration. Stem Cells. 2008;26:2193–2200. doi: 10.1634/stemcells.2007-1022. [DOI] [PubMed] [Google Scholar]
- 17.Yao L, Shanley L, McCaig C, Zhao M. Small applied electric fields guide migration of hippocampal neurons. Journal of Cellular Physiology. 2008;216:527–535. doi: 10.1002/jcp.21431. [DOI] [PubMed] [Google Scholar]
- 18.Yao L, McCaig CD, Zhao M. Electrical signals polarize neuronal organelles, direct neuron migration, and orient cell division. Hippocampus. 2009;19:855–868. doi: 10.1002/hipo.20569. [DOI] [PubMed] [Google Scholar]
- 19.Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao M, Agius-Fernandez A, Forrester JV, McCaig CD. Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent. Journal of Cell Science. 1996;109:1405–1414. doi: 10.1242/jcs.109.6.1405. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nature Neuroscience. 2002;5:719–720. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward M, McCann C, DeWulf M, Wu JY, Rao Y. Distinguishing between directional guidance and motility regulation in neuronal migration. Journal of Neuroscience. 2003;23:5170–5177. doi: 10.1523/JNEUROSCI.23-12-05170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naegele RJ, Lipari J, Chakkalakal D, Strates B, McGuire M. Electric field stimulation of human osteosarcoma-derived cells: a dose-response study. Cancer Biochemistry Biophysics. 1991;12:95–101. [PubMed] [Google Scholar]
- 24.Nishimura KY, Isseroff RR, Nuccitelli R. Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. Journal of Cell Science. 1996;109:199–207. doi: 10.1242/jcs.109.1.199. [DOI] [PubMed] [Google Scholar]
- 25.Chao PH, Roy R, Mauck RL, Liu W, Valhmu WB, Hung CT. Chondrocyte translocation response to direct current electric fields. Journal of Biomechanical Engineering. 2000;122:261–267. doi: 10.1115/1.429661. [DOI] [PubMed] [Google Scholar]
- 26.Farboud B, Nuccitelli R, Schwab IR, Isseroff RR. DC electric fields induce rapid directional migration in cultured human corneal epithelial cells. Experimental Eye Research. 2000;70:667–673. doi: 10.1006/exer.2000.0830. [DOI] [PubMed] [Google Scholar]
- 27.Wang E, Zhao M, Forrester JV, MCCaig CD. Reorientation and faster, directed migration of lens epithelial cells in a physiological electric field. Experimental Eye Research. 2000;71:91–98. doi: 10.1006/exer.2000.0858. [DOI] [PubMed] [Google Scholar]
- 28.Dayer AG, Jenny B, Sauvain MO, Potter G, Salmon P, Zgraggen, et al. Expression of FGF-2 in neural progenitor cells enhances their potential for cellular brain repair in the rodent cortex. Brain. 2007;130:2962–2976. doi: 10.1093/brain/awm200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.