SUMMARY
A high glucose concentration in the reproductive tract during early development may result in aberrant embryo or fetal development, with effects that could have a greater impact on one sex than the other. Here, we determine whether a high glucose concentration impacts embryo development and pregnancy outcomes in a sex-specific manner in the mouse. Zygotes were cultured in KSOM medium, which typically contains 0.2 mM D-glucose with and without additional glucose supplementation to a concentration of 28 mM. Zygote cleavage and blastocyst rate did not differ between treatments but total and trophectoderm cell counts were reduced in blastocysts cultured in a high glucose. No differences between sexes nor inner cell mass cell number were observed within each treatment. Blastocysts developed in both media were transferred to recipients. The percentage of blastocysts resulting in viable pups was significantly reduced when the blastocysts were cultured in 28 mM glucose (74±4 %, controls vs 55.8±7.1 %, 28 mM glucose), but conceptus loss affected both sexes equally, as litter sex ratio did not differ between treatments (52.7 % and 52.2 % males for controls and high glucose, respectively). Pup body weight at birth was higher for males than females, but was not affected by earlier culture in high glucose. In conclusion, in vitro culture in medium with a glucose concentration approximating that of diabetic serum reduces total and trophectoderm cell numbers at the blastocyst stage and conceptus development to term, but these detrimental effects are not sex-specific.
INTRODUCTION
Preimplantation embryo development requires optimal regulation of cellular metabolism, and an excessive availability of nutrients may perturb metabolic homeostasis (Leese et al., 2008) resulting in either embryo mortality (Dumollard et al., 2009) or epigenetic alterations leading to reduced implantation, fetal malformations and long term health consequences in the offspring (Wyman et al., 2008). Hyperglycemia is commonly associated with various metabolic disorders that are usually accompanied by a decreased fertility. Among other causes, female infertility associated with diabetes mellitus has been attributed to detrimental effects of high glucose levels on the developing embryo (Doblado and Moley, 2007; Pampfer, 2000). Consistent with this hypothesis, in vivo studies in animal models for diabetes have observed a delay in embryo development in mice (Beebe and Kaye, 1990; Diamond et al., 1989; Moley et al., 1991), rats (Vercheval et al., 1990) and rabbits (Ramin et al., 2010).
Evidence for a negative effect of an excessive concentration of glucose has also been provided by in vitro studies. Although there is some controversy about the glucose concentration that exists in the reproductive tract milieu where embryos develop and the concentration that provides optimal development when embryos are cultured in vitro (Biggers and McGinnis, 2001), glucose concentrations 3 or 4 times higher than in normoglycemic human serum (5.56 mM) have been found to impair embryo development. In the mouse model, the culture of preimplantation embryos in media containing 15 to 27 mM glucose (2 to 3 times that of serum from normal mice) impaired blastocyst expansion and hatching (Fraser et al., 2007; Pantaleon et al. 2010; Diamond et al., 1991), whereas a very high concentration (52 mM) resulted in higher rates of resorptions after embryo transfer (Wyman et al., 2008).
During preimplantation development, the differences in sex chromosome dosage between males and females and the incomplete X-chromosome inactivation in the latter (Bermejo-Alvarez et al., 2011b) can lead to transcriptional sexual dimorphism affecting large numbers of both sex-chromosome- and autosome-encoded genes (Kobayashi et al., 2006; Bermejo-Alvarez et al., 2010c) that, in turn, may affect different epigenetic, regulatory or metabolic pathways. Glucose metabolism has been proposed to differ between male and female embryos mainly because the enzyme catalyzing the first and rate limiting step of the pentose phosphate pathway (PPP), glucose-6-phosphate dehydrogenase (G6PD), is encoded by the X-chromosome and more highly expressed in female blastocysts compared with their male counterparts in mice (Kobayashi et al., 2006), bovine (Gutierrez-Adan et al., 2000; Wrenzycki et al., 2002; Jimenez et al., 2003) and human (Taylor et al., 2001). In agreement with the putative sex related differences in glucose metabolism, total glucose metabolism has been reported to be two-fold higher in males relative to females, and the activity of the pentose phosphate pathway (PPP) to be four times greater in female than in male bovine blastocysts (Tiffin et al., 1991). There is some dispute about these differences, however, as in humans a higher pyruvate and glucose uptake was initially reported for male embryos (Ray et al., 1995), whereas a recent study has observed that female embryos consume significantly more glucose than males (Gardner et al., 2011).
If a significant sexual dimorphism in glucose metabolism occurs, it may result in sex specific responses to increased glucose availability. In that case, one sex may be affected to a greater extent than the other, resulting in differential embryo mortality and altered sex ratio. Although attractive, this concept remains controversial, as the available data show conflicting results. In cattle, glucose has been proposed to accelerate the development of males and delay the development of female embryos during in vitro development (Bredbacka and Bredbacka, 1996), and, as the glucose concentration is raised, there appears to be an skew of the sex ratio towards males (Gutierrez-Adan et al., 2001; Kimura et al., 2005; Larson et al., 2001). In contrast, even higher glucose concentrations provided during in vitro culture have been reported to reduce the percentage of males in both mice and cattle (Jimenez et al., 2003). Complicating matters further, maternal diabetes mellitus has been suggested to result in a higher proportion of daughters than sons (Rjasanowski et al., 1998), although these results remain controversial (James, 2006). Finally, pregnant diabetic mice appear to give birth to more males than females (Machado et al., 2001). Clearly, there is no clear consensus on the involvement of glucose favoring the development of one sex over another, yet glucose concentrations in the reproductive tract has been proposed to be a key determinant for sex ratio adjustment in a range of species (Grant and Chamley, 2010).
The aim of the present work was to determine whether a high concentration of glucose during in vitro culture impairs blastocyst development and subsequent pregnancy development in a sex specific manner. For this purpose we tested two glucose concentrations (control 0.2 mM or high 28 mM) during in vitro culture and analyzed embryo development and blastocyst cell number. Then, after embryo transfer, pregnancy rates, sex ratio and pup weight were determined.
RESULTS
Embryo development and cell number
The development of more than 400 presumptive zygotes was followed to determine the effect of a high versus low glucose concentration on embryo development. A high glucose concentration during mouse in vitro culture (IVC) did not affect cleavage rate (0.2 mM 91.6±1.9 % vs 28 mM 95.1±2.1 %) and blastocyst yield (0.2 mM 84.4±2.9 vs 28 mM 77.9±4.2; Table 1). As exposure to 28 mM glucose did not reduce the number of blastocysts that formed, a sexually-biased embryo loss could not have occurred. Therefore, the sex ratio at blastocyst (0.2 mM 53.5 % males vs 28 mM 52.4 % males) reflected that among zygotes and did not differ across treatments. However, blastocysts produced under high glucose concentration appeared less expanded than those cultured in standard KSOM. This subjective observation was confirmed by cell number analysis (Table 2). Irrespective of the sex, blastocysts cultured in 28 mM glucose displayed a significantly lower total cell number (0.2 mM glucose: male, 76.3±4.6; female 76.3±4 vs. 28 mM glucose: male 61.1±3.8; female 54.8±3.9) and less trophectoderm (TE) cells (0.2 mM glucose: male 60.8±4; female 61.9±4.8 vs 28 mM glucose: male 45.8±3.1; female 38.6±3.6) than those developed in 0.2 mM glucose (ANOVA, P<0.05). ICM cell number was not altered between the treatments groups or sexes. Two-way ANOVA confirmed the statistical relation between glucose concentration and both total or TE cell numbers (P<0.05). No sex related differences were observed either by two-way ANOVA or within each treatment by one-way ANOVA, suggesting that glucose affects embryo development equally in both sexes.
Table 1.
Effect of glucose concentration during mouse IVC on embryo development. No significant differences were found based on ANOVA (P<0.05).
| Glucose (mM) | Presumptive zygotes number | % cleaved mean ± s.e.m. (n) |
% blastocysts mean ± s.e.m. (n) |
|---|---|---|---|
| 0.2 | 185 | 91.6 ± 1.9 (169) | 84.4 ± 2.9 (153) |
| 28 | 233 | 95.1 ± 2.1 (221) | 77.9 ± 4.2 (188) |
Table 2.
Effect of glucose concentration during mouse IVC on embryo cell number according to sex. Different letters (a, b, c and d) indicate significant differences within each column based on one-way ANOVA (P<0.05). Two-way ANOVA also detected a significant correlation between glucose concentration and both total number of embryonic cells and trophectoderm (TE) cells (P<0.05) but no differences were observed between sexes or in ICM cell numbers.
| Glucose (mM) | Sex | Total cells mean ± s.e.m. |
Trophectoderm mean ± s.em. |
Inner cell mass mean ± s.e.m. |
|---|---|---|---|---|
| 0.2 | Male | 76.3 ± 4.6 a | 60.8 ± 4 c | 15.4 ± 1.2 |
| 0.2 | Female | 76.3 ± 4 a | 61.9 ± 4.8 c | 14.4 ± 1.4 |
| 28 | Male | 61.1 ± 3.8 b | 45.8 ± 3.1 d | 15.3 ± 1.5 |
| 28 | Female | 54.8 ± 3.9 b | 38.6 ± 3.6 d | 16.1 ± 1.3 |
Survival to term, litter sex ratio and pup weight
In order to determine the effect of the exposure to a high glucose concentration during preimplantation development on subsequent implantation and fetal development, 22 embryo transfers were performed (Table 3). All the transfers resulted in viable pups irrespective of the glucose concentration used in IVC, but the survival to term, i.e. the percentage of blastocysts that gave rise to viable pups, was significantly reduced for blastocysts that had developed under 28 mM glucose compared to those exposed to 0.2 mM (0.2 mM 74±4 % vs 28 mM 55.8±7.1 %). In agreement with the lack of a sex-specific effect of glucose on blastocyst cell count, embryo loss affected both sexes equally, as the sex ratio of pups was similar in both groups (0.2 mM 52.7 % males vs 28 mM 52.2 % males). Male pups weighed significantly more than females (two-way ANOVA, P<0.05), but there was no differences across treatments (Table 3). One-way ANOVA detected a significantly higher body weight in the males obtained from blastocysts cultured in 0.2 mM compared with the females cultured in either glucose concentration (0.2 mM males 2.00±0.04 g vs 0.2 mM females 1.76±0.05 g and 28 mM females 1.78±0.05 g; P<0.05), but the weight of the males resulting from blastocysts developed in 28 mM glucose (1.88±0.05 g) did not differ significantly from the other groups.
Table 3.
Effect of glucose concentration during mouse IVC on survival to term, litter sex ratio and pup body weight at the day of birth. Ten embryos were transferred to each recipient (in total 100 embryos for 0.2 mM and 120 for 28 mM). Different letters (a and b) indicate significant differences between both treatments based on one-way ANOVA (P<0.05). Superscripts α and β indicate significant differences between sexes in pup weight based on two-way ANOVA (P<0.05).
| Glucose (mM) | Embryo transfers | % of survival to term mean ± s.em. (n) |
% of males (n) | Male pup weight (g) mean ± s.e.m. α |
Female pup weight (g) mean ± s.e.m. β |
|---|---|---|---|---|---|
| 0.2 | 10 | 74.0 ± 4 (74) a | 52.7 (39) | 2.00 ± 0.04 a | 1.76 ± 0.05 b |
| 28 | 12 | 55.8 ± 7.1 (67) b | 52.2 (35) | 1.88 ± 0.05 a,b | 1.78 ± 0.05 b |
DISCUSSION
Evidence for a detrimental effect of a high glucose concentration during preimplantation embryo development has been documented in several species including mice (Fraser et al., 2007; Pantaleon et al., 2010; Diamond et al., 1991; Leunda-Casi et al., 2001), rats (Pampfer et al., 1997), rabbits (Ramin et al., 2010) and bovine (Jimenez et al., 2003; Larson et al., 2001; Cagnone et al., 2011), but here we report for the first time that glucose exposure during preimplantation development reduces the subsequent number of pups born. The earlier reports concluded that culture in high glucose medium was accompanied by either a delay in embryo development or a reduced rate of blastocyst expansion or hatching as assessed at a fixed time point, and were, therefore, in general agreement with the differences in cell count noted here. Others have reported more severe effects of such high concentrations of glucose during IVC of mouse embryos, namely reduced development to blastocyst stage (Pantaleon et al., 2010), which was not observed in our study. However, our experiments employed a later time for assessing blastocyst number and employed a higher oxygen level (atmospheric vs 5 %). Low oxygen tension enhances anaerobic glycolysis and carbon flux through the oxidative arm of the PPP (Bermejo-Alvarez et al., 2010b) and thereby may amplify the toxic effect of an excess of glucose.
Several molecular mechanisms have been linked to the toxicity of glucose in respect to embryo development, including an alteration in glucose transport (Moley et al., 1998), an increase in apoptosis mediated by Bax overexpression (Moley et al., 1998) or downregulation of the antiapoptotic gene Bcl-x (Ramin et al., 2010), activation of autophagy (Adastra et al., 2011), an increase in O-GlcNAcylation (Pantaleon et al., 2010), increased generation and oxidation of NADPH (Kimura et al., 2005), enhancement of hexosamine pathway-related genes and mitochondrial maturation (Cagnone et al., 2011), and a FGF-4 related dysregulation of trophoblast differentiation (Leunda-Casi et al., 2001). However, there is scant information about the consequences of these abnormalities originating at the blastocyst stage on later development. The metabolic alterations listed above may result in an increase of reactive oxygen species (ROS) production and apoptosis, either of which could be responsible for the reduced TE numbers observed in our study, thereby impairing embryo development after implantation. One study observed that culture of mouse embryos in 52 mM glucose provided lower implantation rates, higher numbers of resorptions and smaller fetuses at day 14.5, with no increase in malformations relative to control embryos (Wyman et al., 2008). As the incidence of some features of glucose-induced embryophathy, e.g. apoptosis, seems to plateau above 20 mM glucose concentration (Adastra et al., 2011), we decided to use a glucose concentration closer to that in rodent diabetic serum (estimated in 23.3±0.05 by (Pampfer et al., 1997)), although it there is some controversy about whether glucose concentration in the reproductive tract mimics that of serum (Harris et al., 2005; Igosheva et al., 2010).
In the experiments reported in the present paper, in which embryos were cultured in 28 mM glucose rather than the normal 0.2 mM of standard KSOM, there was a significant reduction in subsequent blastocyst development to term after transfer to surrogate mothers, thereby providing a link between the diabetes induced embryopathy and pregnancy outcomes (Pampfer, 2000). Nevertheless, despite the reduction in litter size, surviving pups seemed normal and pup weight did not differ among treatments. However, we cannot exclude the possibility that long term alterations might have arisen later, as has been reported for gestational diabetes (Martin-Gronert et al., 2007).
Male embryos have been reported to develop faster than females in several species (Xu et al., 1992; Avery et al., 1991), but when more reliable sexing techniques and optimized culture conditions were used, sex-related differences were not observed (Rizos et al., 2008; Bermejo-Alvarez et al., 2010a; Weston et al., 2009). The lack of differences among sexes in cell counts observed here in the control group (0.2 mM glucose) is consistent with this notion, but it does not rule out that under suboptimal conditions, where one sex may be more affected that the other, the less affected sex may develop faster. Both female and male mouse embryos were equally affected by culture in 28 mM glucose, each experiening a reduction in total and TE cell numbers. Moreover, when such blastocysts were transferred to surrogate dams, no difference in sex ratio of pups born was observed compared to those litters that had been derived from blastocyst cultured under standard conditions, despite the fact that litter size was reduced. Therefore, there was no selective loss of embryos of one sex relative to the other in relation to earlier high glucose exposure, arguing against a sex-specific effect of glucose during preimplantation development in mice.
Glucose concentration in the culture media has been suggested to influence the sex ratio, but the available published data is far from consensus. High glucose concentrations during in vitro culture have been reported to result in a higher proportion of males at the blastocyst stage (Gutierrez-Adan et al., 2001; Kimura et al., 2005; Larson et al., 2001), due to females being unable to pass efficiently through the morula to blastocyst transition, but there are some reports that conflict with this outcome (Jimenez et al., 2003; Rjasanowski et al., 1998; Bredbacka et al., 1996). However, the reported distortion of sex ratio has been modest, with the favoured sex rarely exceeding 60 % of the total (Jimenez et al., 2003; Kimura et al., 2005). Estimates of sex differences in glucose metabolism across the sexes have also yielded conflicting results (Ray et al., 1995; Gardner et al., 2011), and while an upregulation of G6PD in female blastocysts is consistently observed for several species (Kobayashi et al., 2006; Gutierrez-Adan et al., 2000; Wrenzycki et al., 2002; Jimenez et al., 2003; Taylor et al., 2001), other genes implicated either directly or indirectly in glucose metabolism do not appear to be overrepresented on the X-chromosome (Berletch et al., 2011). Moreover, gene ontology analyses of global transcriptional differences between sexes have failed to detect glucose metabolism as being sex-specific pathway (Bermejo-Alvarez et al., 2010c; Kobayashi et al., 2006). A more focused analysis of the transcriptional dimorphism of the genes encoding enzymes of the PPP and anaerobic glycolysis pathways have also shown no evidence for sex bias (Bermejo-Alvarez et al., 2011a), and hyperglycemia-related genes do not significantly overlap with sex-related genes (Cagnone et al., 2011).
In conclusion, preimplantation embryos subjected to a glucose concentration approximating that of serum from diabetic mice (28 mM) exhibited lower total and trophectoderm cell counts at the blastocyst stage compared with the control (0.2 mM), and showed poorer development to term. The detrimental effects of high glucose availability on embryo development and pregnancy outcomes affected embryos of both sexes equally.
MATERIALS AND METHODS
Embryo collection and culture
All animal experiments were approved by the University of Missouri ACUC committee and performed in accordance with NIH Animal Care and Use Guidelines (ACUC Protocol Number 6154). Outbred mice (CD1 strain, Harlan) were maintained in a 12:12 light cycle with ad libitum access to water and food (5001 -maintenance diet- or 5015 -breeder diet after embryo transfer-, Harlan). Donor females 6 to 9 weeks old were superovulated by an i.p. injection of 5 IU PMSG (Sigma G4877) per mouse followed by a second i.p. injection 48 h later of 5 IU hCG (Sigma C1063). After injection with hCG females were mated with CD1 males of proven fertility. Mate was assessed by plug detection on the following morning of hCG injection (0.5 day post-coitum –dpc-) and presumptive zygotes were collected 22 h after hCG injection. Mated females were humanely euthanized in accordance with the recommendations of the Panel of Euthanasia of the American Veterinary Medical Association (http://www.avma.org/issues/animal_welfate/euthanasia.pdf), and fertilized cumulus oocyte-complexes were extracted from the oviduct in pre-warmed (37 °C) CZBH medium. Cumulus cells were disaggregated with a 0.1 mg/ml hyaluronidase solution (Sigma H4272) in CZBH and the presumptive zygotes from each female were divided in two groups and cultured in either regular KSOM (Millipore MR-121-D, 0.2 mM glucose) or KSOM supplemented to a glucose concentration of 28 mM. Culture was performed in 50 μl microdrops under mineral oil at 37 °C in a humidified atmosphere of 5 % CO2 in air. Special care was taken to assure periodically a correct CO2 concentration, since this parameter has a major impact on both media pH and cell glucose metabolism (Longmore et al., 1968). Embryo development was assessed by cleavage rate at 1.5 dpc and blastocyst rate at 4.5 dpc.
Differential TE/ICM staining, cell count and embryo sexing
The resulting Day 4.5 blastocysts produced as described above had their zona removed with Tyrode’s acidic solution (Sigma T1788) and then fixed in 4 % paraformaldehyde (Electron Microscopy Sciences 15710) in PBS supplemented with 1 % BSA (PBS+1%BSA) for 10 minutes at room temperature (RT). After fixation, embryos were washed 3 times in PBS+1%BSA and kept in that medium at 4 °C until analysis. For immunostaining, cells were permeabilized in PBS plus 5% goat serum (Sigma G9023) (IF buffer) with 1 % Triton X-100 (Sigma X100) for 45 minutes at RT. Blastocysts were then incubated overnight at 4 °C in primary antibody solution consisting of PBS+1% BSA, 20 % IF buffer and 1:1000 mouse monoclonal anti-CDX2 antibody (Biogenix, CDX2-88). Following incubation, they were washed twice in PBS+1%BSA and incubated in the secondary antibody solution consisting in PBS+1%BSA, 20 % IF buffer, 1:3000 alexaFluor goat antimouse 488 (Invitrogen A11029) and 0.01 mg/ml DAPI (Sigma D8417) for 2 h at RT. Finally, embryos were washed three times in PBS+1%BSA and placed individually in numbered microdrops on a cover glass overlayed with an incubation chamber (Sigma Z37,9467). Microdrops were made by drawing 3 to 4 mm circles a with PAP pen (Zymed laboratories) and by adding 6 μl of PBS+1%BSA to each circle. This approach prevented the mounting medium from spreading away from the circles and causing either mixing or loss of blastocysts when the incubation chamber was placed over the cover glass containing the specimens. It also allowed subsequent recovery of the embryos for PCR sexing, following microscopic observation. Stained blastocysts were analyzed by a 5LIVE Zeiss confocal microscope. Z-stack sections of 10 μm were taken in the 405 nm (DAPI positive cells, total cell number) and 488 nm (CDX2-positive cells, trophectoderm cells –TE-) channels (Figure 1A). Twenty blastocysts were examined for each glucose concentration and sex.
Figure 1.
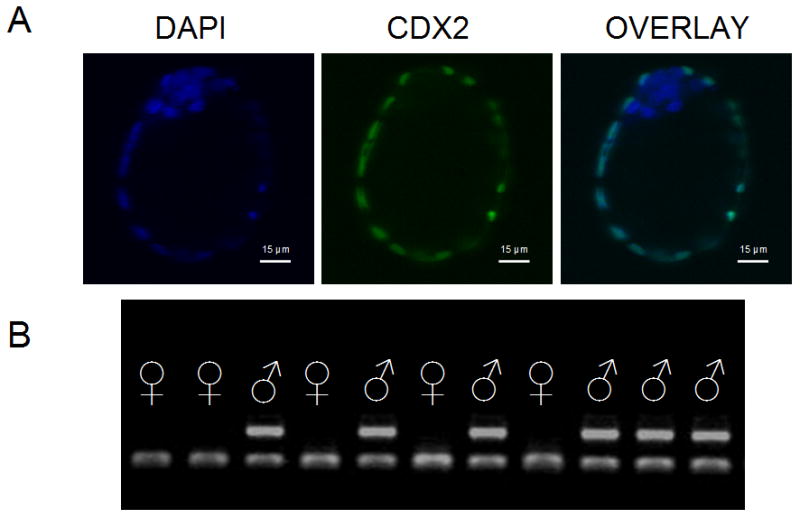
Representative images of blastocyst cell count and sexing. A: 10 μm Z-stack section of a blastocyst. Total cell number were determined based on DAPI nuclei staining (right image), TE cells were detected by antiCDX2 (middle image) and DAPI positive CDX2 negative cells correspond to ICM (left image). B: Result of the PCR sexing of analyzed blastocysts after agarose gel electrophoretic separation. Female embryos exhibited only one band corresponding to Rn18S sequence, whereas males also amplify a Y chromosome-specific product (DYzEms3).
Embryo sexing
After confocal analysis, the sealed incubation chamber, which was employed to minimize evaporation, was opened and each blastocyst placed individually in the bottom of a 0.2 ml PCR tube with a minimal amount of medium. To increase PCR efficiency, embryos were digested with 8 μl of a 100 μg/ml proteinase K (Sigma P8044) solution in water at 55 °C overnight as described in (Bermejo-Alvarez et al., 2008). After digestion, proteinase K was inactivated at 95 °C for 10 min. Due to the small amount of DNA present in the mouse blastocyst, a method based on repeated sequences, i.e. with more than 2 copies per cell, was developed. Embryos were sexed by a duplex PCR amplification of a Y chromosome specific repeated sequence (DYzEms3, primers 211–212 (Navin et al., 1996)), and the autosomal gene Rn18s as control. After optimization trials, the PCR reactions were conduced in a total volume of 25 μl containing 8 μl of the proteinase K-digested sample, 1X Gotaq Flexi buffer (Promega), 1 IU of Gotaq (Promega), 2.5 mM MgCl2, 0.1 mM dNTP and 0.2 μM of each of the four primers (Table 4). The PCR was performed with an initial denaturalization step at 95 °C for 3 min followed by 40 cycles at 95 °C 30 sec, 52 °C 45 secs and 72 °C 45 sec. Products were visualized on an ethidium bromide stained 2 % agarose gel in TBE buffer under ultraviolet illumination. A 91 bp band for Rn18s genomic sequence was present in both males and females, whereas the Y-specific 254 bp band for DYzEms3 was only present in male samples (Figure 1B). The sensitivity of the method was tested by sexing each of the cells of 20 2-cell embryos (40 reactions): all samples were sexed and in all cases the sex of both cells from the same embryo was identical. Every PCR was carried out with three controls: male genomic DNA, female genomic DNA and a negative control.
Table 4.
Details of primers used for embryo sexing.
| Primer | Sequence (5′-3′) |
|---|---|
| 211 | TAGGATGGTAAGCCCAATGC |
| 212 | TTGGTTGGTTAATTGTTTGGG |
| 18S rRNA F | AGAAACGGCTACCACATCCAA |
| 18S rRNA R | CCTGTATTGTTATTTTTCGTCACTACCT |
Embryo transfer and litter analysis
CD1 females 10–15 weeks old were used as embryo recipients. Females were mated with vasectomized CD1 males that had been previously tested for infertility by pairing with other females, to induce pseudopregnancy, and successful mating confirmed by plug detection on the following morning. The blastocyst obtained under the in vitro culture conditions previously described were randomly selected, transferred to pre-warmed CZBH medium and then to the uterus of a pseudopregnant female on day 2.5 pc following the bilateral utero-tubal method described in (Chin and Wang, 2001) with minor modifications. In our experiments, The recipients were anesthesized with an i.p. combination of ketamine (0.1 mg/g) and xylazine (0.01 mg/g), supplemented with a s.c. injection of buprenorphine (0.1 μg/g) as premedication and postoperative analgesic. A small incision was made in the lateral abdominal wall to expose the ovary, oviduct and the cranial part of the uterus. The oviduct was punctured with a 30-gauge needle near the utero-tubal junction and a fire-polished glass pipette was inserted through the punctured hole and allowed to penetrate through the utero-tubal junction and extend into the uterine cavity, where 5 blastocysts were released with a minimal amount of medium. Ten blastocysts were transfer bilaterally to each recipient (5 in each uterine horn). On the day they were born, pups were weighed and sexed. In order to avoid missed or erroneous data due to pup mortality or mistakes in sex determination by ano-genital distance, pups were sacrificed the day of birth, the abdomen opened and the gonads and reproductive tract verified as being either male or female.
Statistical analysis
Data were analyzed by using the SigmaStat (Jandel Scientific, San Rafael, CA) software package. Differences between treatments or sexes in cleavage rates at 1.5 dpc, day 4.5 blastocyst rates and cell number, survival to term and pups weights were analyzed by one-way analysis of variance (ANOVA, P<0.05). The effect of two independent factors (sex and glucose concentration) on cell numbers and pups weight was also analyzed by two-way ANOVA (P<0.05). Sex ratio of the offspring was analyzed by a chi-square test.
Acknowledgments
Grant sponsor: Lalor Foundation Postdoctoral Fellowship to PBA, and NIH grants HD21896 (RMR), HD 44042 (RMR&CSR) and RC1 ES018195 to CSR.
We acknowledge Alfonso Gutierrez-Adan (INIA, Spain) for his help in the search of Y-chromosome sequences for PCR sexing, Dr Jiude Mao for initiating the study on glucose effects, and Laura Schultz, Alexandr Jurkevic and Joe Mercurio (University of Missouri, USA) for their advice in embryo immunohistochemestry and confocal microscopy.
ABREVIATIONS
Abbreviate measurements according to Style Manual for Biological journals, American Institute for Biological Sciences, 3900 Wisconsin Avenue, N.W., Washington DC
- NIH
National institutes of Health
- KSOM
Potasium simple optimized medium
- PPP
Pentose Phosphate Pathway
- IVC
In vitro culture
- TE
Trophectoderm
- ICM
Inner cell mass
- ANOVA
Analysis of variance
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- ROS
Radical Oxygen Species
- ACUC
Animal Care and Use Guidelines
- IU
International Units
- PMSG
Pregnant Mare Serum Gonadotropin
- i.p
Intraperitoneal
- hCG
Human Chorionic Gonadotropin
- CZBH
Chatot-Ziomek-Bavister Hepes buffered medium
- Dpc
Days post coitum
- PBS
Phosphate Buffered Saline
- BSA
Bovine Serum Albumin
- IF
Immunofluorescence buffer
- DAPI
4′,6-diamidino-2-phenylindone
- s.c
Subcutaneous
- PCR
Polymerase Chain Reaction
- DNA
Deoxyribonucleic acid
- bp
Base pairs
- dNTP
Deoxyribonucleotide triphosphate
- TBE
Tris-Boric-EDTA buffer
References
- Adastra KL, Chi MM, Riley JK, Moley KH. A differential autophagic response to hyperglycemia in the developing murine embryo. Reproduction. 2011;141(5):607–615. doi: 10.1530/REP-10-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery B, Madison V, Greve T. Sex and development in bovine in-vitro fertilized embryos. Theriogenology. 1991;35(5):953–963. doi: 10.1016/0093-691x(91)90306-x. [DOI] [PubMed] [Google Scholar]
- Beebe LF, Kaye PL. Preimplantation development in the streptozotocin-induced diabetic mouse. Reproduction, fertility, and development. 1990;2(4):407–412. doi: 10.1071/rd9900407. [DOI] [PubMed] [Google Scholar]
- Berletch JB, Yang F, Xu J, Carrel L, Disteche CM. Genes that escape from X inactivation. Human Genetics. 2011;130(2):237–245. doi: 10.1007/s00439-011-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Lonergan P, Rath D, Gutierrez-Adan A, Rizos D. Developmental kinetics and gene expression in male and female bovine embryos produced in vitro with sex-sorted spermatozoa. Reproduction, fertility, and development. 2010a;22(2):426–436. doi: 10.1071/RD09142. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Lonergan P, Rizos D, Gutierrez-Adan A. Low oxygen tension during IVM improves bovine oocyte competence and enhances anaerobic glycolysis. Reproductive biomedicine online. 2010b;20(3):341–349. doi: 10.1016/j.rbmo.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Lonergan P, Gutierrez-Adan A. Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease. Reproduction. 2011a;141(5):563–570. doi: 10.1530/REP-10-0482. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Lonergan P, Gutierrez-Adan A. Transcriptional sexual dimorphism in elongating bovine embryos: implications for XCI and sex determination genes. Reproduction. 2011b;141(6):801–808. doi: 10.1530/REP-11-0006. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. Can bovine in vitro-matured oocytes selectively process X- or Y-sorted sperm differentially? Biol Reprod. 2008;79(4):594–597. doi: 10.1095/biolreprod.108.070169. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proceedings of the National Academy of Sciences of the United States of America. 2010c;107(8):3394–3399. doi: 10.1073/pnas.0913843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggers JD, McGinnis LK. Evidence that glucose is not always an inhibitor of mouse preimplantation development in vitro. Human reproduction (Oxford, England) 2001;16(1):153–163. doi: 10.1093/humrep/16.1.153. [DOI] [PubMed] [Google Scholar]
- Bredbacka K, Bredbacka P. Glucose controls sex-related growth rate differences of bovine embryos produced in vitro. J Reprod Fertil. 1996;106(2):169–172. doi: 10.1530/jrf.0.1060169. [DOI] [PubMed] [Google Scholar]
- Cagnone GL, Dufort I, Vigneault C, Sirard MA. Differential Gene Expression Profile in Bovine Blastocysts Resulting from Hyperglycemia Exposure During Early Cleavage Stages. Biology of Reproduction. 2011 doi: 10.1095/biolreprod.111.094391. [DOI] [PubMed] [Google Scholar]
- Chin HJ, Wang CK. Utero-tubal transfer of mouse embryos. Genesis. 2001;30(2):77–81. doi: 10.1002/gene.1036. [DOI] [PubMed] [Google Scholar]
- Diamond MP, Moley KH, Pellicer A, Vaughn WK, DeCherney AH. Effects of streptozotocin- and alloxan-induced diabetes mellitus on mouse follicular and early embryo development. J Reprod Fertil. 1989;86(1):1–10. doi: 10.1530/jrf.0.0860001. [DOI] [PubMed] [Google Scholar]
- Diamond MP, Pettway ZY, Logan J, Moley K, Vaughn W, DeCherney AH. Dose-response effects of glucose, insulin, and glucagon on mouse pre-embryo development. Metabolism: clinical and experimental. 1991;40(6):566–570. doi: 10.1016/0026-0495(91)90045-x. [DOI] [PubMed] [Google Scholar]
- Doblado M, Moley KH. Glucose metabolism in pregnancy and embryogenesis. Curr Opin Endocrinol Diabetes Obes. 2007;14(6):488–493. doi: 10.1097/MED.0b013e3282f1cb92. [DOI] [PubMed] [Google Scholar]
- Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Seminars in cell & developmental biology. 2009;20(3):346–353. doi: 10.1016/j.semcdb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Fraser RB, Waite SL, Wood KA, Martin KL. Impact of hyperglycemia on early embryo development and embryopathy: in vitro experiments using a mouse model. Human reproduction (Oxford, England) 2007;22(12):3059–3068. doi: 10.1093/humrep/dem318. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Wale PL, Collins R, Lane M. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Human reproduction (Oxford, England) 2011;26(8):1981–1986. doi: 10.1093/humrep/der143. [DOI] [PubMed] [Google Scholar]
- Grant VJ, Chamley LW. Can mammalian mothers influence the sex of their offspring peri-conceptually? Reproduction. 2010;140(3):425–433. doi: 10.1530/REP-10-0137. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Adan A, Granados J, Pintado B, De La Fuente J. Influence of glucose on the sex ratio of bovine IVM/IVF embryos cultured in vitro. Reproduction, fertility, and development. 2001;13(5–6):361–365. doi: 10.1071/rd00039. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Adan A, Oter M, Martinez-Madrid B, Pintado B, De La Fuente J. Differential expression of two genes located on the X chromosome between male and female in vitro-produced bovine embryos at the blastocyst stage. Mol Reprod Dev. 2000;55(2):146–151. doi: 10.1002/(SICI)1098-2795(200002)55:2<146::AID-MRD3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Harris SE, Gopichandran N, Picton HM, Leese HJ, Orsi NM. Nutrient concentrations in murine follicular fluid and the female reproductive tract. Theriogenology. 2005;64(4):992–1006. doi: 10.1016/j.theriogenology.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE. 2010;(4):e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James WH. Sex ratios of offspring of patients with breast cancer and other endocrine related cancers. Int J Cancer. 2006;119(11):2710–2711. doi: 10.1002/ijc.22193. author reply 2712. [DOI] [PubMed] [Google Scholar]
- Jimenez A, Madrid-Bury N, Fernandez R, Perez-Garnelo S, Moreira P, Pintado B, De la Fuente J, Gutierrez-Adan A. Hyperglycemia-induced apoptosis affects sex ratio of bovine and murine preimplantation embryos. Molecular Reproduction and Development. 2003;65:180–187. doi: 10.1002/mrd.10286. [DOI] [PubMed] [Google Scholar]
- Kimura K, Spate LD, Green MP, Roberts RM. Effects of D-glucose concentration, D-fructose, and inhibitors of enzymes of the pentose phosphate pathway on the development and sex ratio of bovine blastocysts. Mol Reprod Dev. 2005;72(2):201–207. doi: 10.1002/mrd.20342. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Isotani A, Mise N, Yamamoto M, Fujihara Y, Kaseda K, Nakanishi T, Ikawa M, Hamada H, Abe K, Okabe M. Comparison of gene expression in male and female mouse blastocysts revealed imprinting of the X-linked gene, Rhox5/Pem, at preimplantation stages. Curr Biol. 2006;16(2):166–172. doi: 10.1016/j.cub.2005.11.071. [DOI] [PubMed] [Google Scholar]
- Larson MA, Kimura K, Kubisch HM, Roberts RM. Sexual dimorphism among bovine embryos in their ability to make the transition to expanded blastocyst and in the expression of the signaling molecule IFN-tau. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(17):9677–9682. doi: 10.1073/pnas.171305398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese HJ, Baumann CG, Brison DR, McEvoy TG, Sturmey RG. Metabolism of the viable mammalian embryo: quietness revisited. Mol Hum Reprod. 2008;14(12):667–672. doi: 10.1093/molehr/gan065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunda-Casi A, De Hertogh R, Pampfer S. Decreased expression of fibroblast growth factor-4 and associated dysregulation of trophoblast differentiation in mouse blastocysts exposed to high D-glucose in vitro. Diabetologia. 2001;44(10):1318–1325. doi: 10.1007/s001250100633. [DOI] [PubMed] [Google Scholar]
- Longmore WJ, Landau BR, Baker ES, Hastings AB, Lum DM, Williams HR. Effect of pH and CO2 concentration on glucose metabolism by rat adipose tissue in vitro. The American journal of physiology. 1968;215(3):582–586. doi: 10.1152/ajplegacy.1968.215.3.582. [DOI] [PubMed] [Google Scholar]
- Machado AF, Zimmerman EF, Hovland DN, Jr, Weiss R, Collins MD. Diabetic embryopathy in C57BL/6J mice. Altered fetal sex ratio and impact of the splotch allele. Diabetes. 2001;50(5):1193–1199. doi: 10.2337/diabetes.50.5.1193. [DOI] [PubMed] [Google Scholar]
- Martin-Gronert MS, Ozanne SE. Experimental IUGR and later diabetes. Journal of internal medicine. 2007;261(5):437–452. doi: 10.1111/j.1365-2796.2007.01800.x. [DOI] [PubMed] [Google Scholar]
- Moley KH, Chi MM, Knudson CM, Korsmeyer SJ, Mueckler MM. Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nature medicine. 1998;4(12):1421–1424. doi: 10.1038/4013. [DOI] [PubMed] [Google Scholar]
- Moley KH, Vaughn WK, DeCherney AH, Diamond MP. Effect of diabetes mellitus on mouse pre-implantation embryo development. J Reprod Fertil. 1991;93(2):325–332. doi: 10.1530/jrf.0.0930325. [DOI] [PubMed] [Google Scholar]
- Navin A, Prekeris R, Lisitsyn NA, Sonti MM, Grieco DA, Narayanswami S, Lander ES, Simpson EM. Mouse Y-specific repeats isolated by whole chromosome representational difference analysis. Genomics. 1996;36(2):349–353. doi: 10.1006/geno.1996.0473. [DOI] [PubMed] [Google Scholar]
- Pampfer S. Peri-implantation embryopathy induced by maternal diabetes. J Reprod Fertil Suppl. 2000;55:129–139. [PubMed] [Google Scholar]
- Pampfer S, Vanderheyden I, McCracken JE, Vesela J, De Hertogh R. Increased cell death in rat blastocysts exposed to maternal diabetes in utero and to high glucose or tumor necrosis factor-alpha in vitro. Development (Cambridge, England) 1997;124(23):4827–4836. doi: 10.1242/dev.124.23.4827. [DOI] [PubMed] [Google Scholar]
- Pantaleon M, Tan HY, Kafer GR, Kaye PL. Toxic effects of hyperglycemia are mediated by the hexosamine signaling pathway and o-linked glycosylation in early mouse embryos. Biol Reprod. 2010;82(4):751–758. doi: 10.1095/biolreprod.109.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramin N, Thieme R, Fischer S, Schindler M, Schmidt T, Fischer B, Navarrete Santos A. Maternal diabetes impairs gastrulation and insulin and IGF-I receptor expression in rabbit blastocysts. Endocrinology. 2010;151(9):4158–4167. doi: 10.1210/en.2010-0187. [DOI] [PubMed] [Google Scholar]
- Ray PF, Conaghan J, Winston RM, Handyside AH. Increased number of cells and metabolic activity in male human preimplantation embryos following in vitro fertilization. J Reprod Fertil. 1995;104(1):165–171. doi: 10.1530/jrf.0.1040165. [DOI] [PubMed] [Google Scholar]
- Rizos D, Bermejo-Alvarez P, Gutierrez-Adan A, Lonergan P. Effect of duration of oocyte maturation on the kinetics of cleavage, embryo yield and sex ratio in cattle. Reproduction, fertility, and development. 2008;20(6):734–740. doi: 10.1071/rd08083. [DOI] [PubMed] [Google Scholar]
- Rjasanowski I, Kloting I, Kovacs P. Altered sex ratio in offspring of mothers with insulin-dependent diabetes mellitus. Lancet. 1998;351(9101):497–498. doi: 10.1016/S0140-6736(05)78685-2. [DOI] [PubMed] [Google Scholar]
- Taylor DM, Handyside AH, Ray PF, Dibb NJ, Winston RM, Ao A. Quantitative measurement of transcript levels throughout human preimplantation development: analysis of hypoxanthine phosphoribosyl transferase. Mol Hum Reprod. 2001;7(2):147–154. doi: 10.1093/molehr/7.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffin GJ, Rieger D, Betteridge KJ, Yadav BR, King WA. Glucose and glutamine metabolism in pre-attachment cattle embryos in relation to sex and stage of development. J Reprod Fertil. 1991;93(1):125–132. doi: 10.1530/jrf.0.0930125. [DOI] [PubMed] [Google Scholar]
- Vercheval M, De Hertogh R, Pampfer S, Vanderheyden I, Michiels B, De Bernardi P, De Meyer R. Experimental diabetes impairs rat embryo development during the preimplantation period. Diabetologia. 1990;33(4):187–191. doi: 10.1007/BF00404794. [DOI] [PubMed] [Google Scholar]
- Weston G, Osianlis T, Catt J, Vollenhoven B. Blastocyst transfer does not cause a sex-ratio imbalance. Fertility and Sterility. 2009;92(4):1302–1305. doi: 10.1016/j.fertnstert.2008.07.1784. [DOI] [PubMed] [Google Scholar]
- Wrenzycki C, Lucas-Hahn A, Herrmann D, Lemme E, Korsawe K, Niemann H. In vitro production and nuclear transfer affect dosage compensation of the X-linked gene transcripts G6PD, PGK, and Xist in preimplantation bovine embryos. Biol Reprod. 2002;66(1):127–134. doi: 10.1095/biolreprod66.1.127. [DOI] [PubMed] [Google Scholar]
- Wyman A, Pinto AB, Sheridan R, Moley KH. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology. 2008;149(2):466–469. doi: 10.1210/en.2007-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KP, Yadav BR, King WA, Betteridge KJ. Sex-related differences in developmental rates of bovine embryos produced and cultured in vitro. Mol Reprod Dev. 1992;31(4):249–252. doi: 10.1002/mrd.1080310404. [DOI] [PubMed] [Google Scholar]


