Abstract
Objective
To study molecular mechanisms involved in hematopoietic stem cell (HSC) mobilization after liver resection and determine impacts on liver regeneration.
Background
Extracellular nucleotide-mediated cell signaling has been shown to boost liver regeneration. Ectonucleotidases of the CD39 family are expressed by bone marrow–derived cells, and purinergic mechanisms might also impact mobilization and functions of HSC after liver injury.
Methods
Partial hepatectomy was performed in C57BL/6 wild-type, Cd39 ectonucleotidase-null mice and in chimeric mice after transplantation of wild-type or Cd39-null bone marrow. Bone marrow–derived HSCs were purified by fluorescence-activated cell sorting and administered after hepatectomy. Chemotactic studies were performed to examine effects of purinergic receptor agonists and antagonists in vitro. Mobilization of human HSCs and expression of CD39 were examined and linked to the extent of resection and liver tests.
Results
Subsets of HSCs expressing Cd39 are preferentially mobilized after partial hepatectomy. Chemotactic responses of HSCs are increased by CD39-dependent adenosine triphosphate hydrolysis and adenosine signaling via A2A receptors in vitro. Mobilized Cd39high HSCs boost liver regeneration, potentially limiting interleukin 1β signaling. In clinical studies, mobilized human HSCs also express CD39 at high levels. Mobilization of HSCs correlates directly with the restoration of liver volume and function after partial hepatectomy.
Conclusions
We demonstrate CD39 to be a novel HSC marker that defines a functionally distinct stem cell subset in mice and humans. HSCs are mobilized after liver resection, limit inflammation, and boost regeneration in a CD39-dependent manner. These observations have implications for monitoring and indicate future therapeutic avenues.
Keywords: CD133, CD39, liver regeneration, stem cells, vascular inflammation
Liver regeneration is a highly regulated response to injury and involves recruitment of extrahepatic, bone marrow-derived hematopoietic stem cells (HSCs) and other progenitor cells.1–10 Others and we have shown that an early subset of HSCs, defined by the antigen CD133, enhances liver regeneration in both animal models and humans.7–12 Multipotential stem cells accelerate liver repair by producing various growth factors and generating hepatocytes and liver endothelial cells.7,13,14 Furthermore, stem cells have recently been shown to exhibit immunosuppressive and anti-inflammatory properties that might further promote liver regeneration.15–18
Purinergic signaling might be important in stem cell–mediated liver repair, as it has been linked to chemotaxis of HSCs, regulation of inflammation, and organ restoration. CD39, known as ectonucleoside triphosphate diphosphohydrolase 1, hydrolyzes proinflammatory extracellular adenosine triphosphate and adenosine diphosphate to adenosine monophosphate, which is further catalyzed by CD73, 5′-nucleotidase, to form anti-inflammatory adenosine.19 CD39 is highly expressed by vascular endothelium and T regulatory cells and significantly contributes to chemotactic and anti-inflammatory properties.20–26 We have previously shown that global deletion of CD39 inhibits angiogenesis and substantially impairs liver regeneration.4,27 Purine derivatives have also been linked to stem cell expansion.28
We demonstrate here that CD39 is highly expressed by a subset of murine and human HSCs and is critical for chemotaxis and recruitment of these cells from bone marrow to the liver. CD39high HSCs decrease inflammation, at least in part, as a consequence of adenosine triphosphate scavenging, which results in decreased levels of interleukin 1β, promoting liver regeneration.
METHODS
Animal Studies
Animals
Wild-type C57Bl/6 (Taconic, Germantown, NY), Cd39-null, and DsRed transgenic male mice (Taconic), age 7 to 9 weeks, were used in accordance with the guidelines from the American Association for Laboratory Animal Care. Cd39-null mice were derived as previously described.4,29 The Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committees approved all animal research protocols.
Isolation of Bone Marrow Mononuclear Cells
Bone marrow was flushed out from the hind legs with RMPI 1640 medium, filtered through BD Falcon cell strainers (70 μM), layered over histopaque-1083 (Sigma Aldrich), and centrifuged (30 minutes, 500 rpm, 4°C). The buffy coat was washed twice (10 minutes, 500 rpm, 4°C).
Gene Expression Analysis
Total Ribonucleic acid (RNA) was isolated from fluorescence-activated cell sorting-purified CD133+/CD45+ HSCs, reverse transcribed, and quantitative polymerase chain reaction amplification performed in duplicate, as previously described.24 β-Actin was used as the internal control.
Ectonucleotidase Assays
Fluorescence-activated cell sorting -sorted CD133+/CD45+ HSCs were resuspended in Hanks balanced salt solution (30 minutes, 37°C) and incubated with adenosine triphosphate for 3 minutes. Extracellular nucleotides were analyzed by high-performance liquid chromatography, as described elsewhere.22
Liver Injury Models
Partial hepatectomy (70%) and sham surgery were performed as described.4
Chimeric Mice
Total body irradiation and subsequent bone marrow transplantation was performed as described elsewhere.24
Fluorescence-Activated Cell Sorting
Fluorescence-activated cell sorting and analysis of CD133+/CD45+ HSCs from bone marrow and peripheral blood mononuclear cells was performed as previously described30 and enrichment validated by fluorescence-activated cell sorting analysis (to 94.0 ± 3.0%). Antibodies were CD133-PE and CD133-APC (clone MB9-3G8; Miltenyi Biotec, Germany), Sca-1-PE (clone D7; Miltenyi Biotec), CD45-FITC (clone 30-F11; BD Biosciences), CD39-PE (clone 24DMS1; eBioscience), CD73-PE (clone TY/23; BD Biosciences), and VEGFR-2-APC (clone Avas12a1; eBioscience).
Administration of HSCs
Wild-type mice received intravenous fluorescence-activated cell sorting-purified CD133+/CD45+ HSCs (3 × 105 cells in 200 μL of phosphate-buffered saline) or phosphate-buffered saline (200 μL) alone as a control 24 hours after a 70% hepatectomy. Animals were killed 72 hours and 5 days after 70% hepatectomy.
Enzyme-Linked Immunosorbent Assay
Blood was processed4 and plasma levels of vascular endothelial growth factor and interleukin-1β (R&D Systems) were determined using manufacturer's specifications.
Liver Sinusoidal Endothelial Cell Studies
Wild-type liver sinusoidal endothelial cells were isolated, cultured,4 and stimulated with or without exogenous adenosine triphosphate for 20 minutes, washed, and then cultured for 5 hours.
Homing Assays
Wild-type mice received intravenous fluorescence-activated cell sorting-purified DsRed transgenic CD133+/CD45+ HSCs (3 × 105 cells in 200 μL of phosphate-buffered saline) 24 hours after×a 70% hepatectomy and were killed 10 days later. Hepatocytes were isolated from the nonparenchymal cell fraction and purified by modified Percoll centrifugation as described elsewhere.4,31 DsRed cells were detected by fluorescence microscopy and fluorescence-activated cell sorting analysis.
Immunohistochemistry
Tissue was fixed with zinc and formalin 10%, respectively, and paraffin imbedded. Immunohistochemistry was performed as described elsewhere.4,29
Chemotaxis Assays
Transwell assays were performed with a 96-well MultiScreen MIC plate (pore size 5.0 μm; Millipore, Billerica, MA).22,32 Bone marrow mononuclear cells (3 × 105 cells/75 μL Iscove's Modified Dulbecco's Medium + Fetal Calf Serum 10%; Sigma) were added into the upper chamber. After 4 hours of incubation at 37°C, the proportion of CD133+/CD45+ HSCs that transmigrated to the lower chamber was determined.
Clinical Studies
Patients
We studied 24 patients ( female = 9/male 15; age 65 ± 25 years) undergoing hepatic resections for liver malignancies at the Department of Surgery, University Hospital of Düsseldorf, Germany, and 20 healthy volunteers. Written informed consent was obtained from all patients. The study was approved by the ethics committee of the Heinrich-Heine-University of Düsseldorf (institutional review board nos. 2852, 2853, and 2916).
Magnetic Activated Cell Sorting
CD133+ HSC purification from bone marrow mononuclear cells was performed using magnetic activated cell sorting (Miltenyi Biotec) according to manufacturer's specifications. Purity was assessed by fluorescence-activated cell sorting to be 83.6 ± 3.9%.
Gene Expression Analysis
Total RNA was isolated from magnetic activated cell sorting-purified CD133+ HSCs, reverse transcribed, and quantitative polymerase chain reaction amplification done, as described22 β-Actin was used as the internal control.22,26
Indocyanine Green LIMON
Measurement of indocyanine green-plasma disappearance rate %/minute and 15-minute retention rates (R15%) was performed using a LIMON module (PULSION Medical Systems, Germany).
Fluorescence-Activated Cell Sorting
CD133+/CD45+ HSCs were analyzed on a BD FACSCanto. Antibodies used were anti-CD133-PE (clone 293C3, Miltenyi Biotec), CD45-APC (clone 2D1; BD Bioscience), CD34-PE-Cy7 (clone 581; BD Bioscience), and CD39-FITC (clone BU61; BD Bioscience).
Enzyme-Linked Immunosorbent Assay
Plasma levels of Vascular Endothelial Growth Factor (R&D Systems) were determined according to manufacturer's specifications.
Hepatic Volumetry
Data sets for liver volumetry were obtained from helical computerized tomography of the upper abdomen before and 24 hours, 7 days, and 21 days after hepatectomy.9
Statistics
Results are reported as mean ± standard error of mean. The Student t test, the Welch test, and analysis of variance were used to test significance where appropriate. Values were considered significant when P < 0.05.
RESULTS
Transcriptional Profiles of Purinergic Enzymes and Receptors in HSCs
We first measured messenger ribonucleic acid (mRNA) levels of ectonucleotidases and purinergic receptors in wild-type mouse HSCs, as defined by a high-level expression of CD133 and CD45 and Sca-1. Highest relative mRNA expression levels were noted for Cd39, among all the other ectonucleotidases (NTPDases) and ectonucleotide pyrophosphatases/phosphodiesterases (NTPDases 2–4 and 6–8, Cd73, and ENPP 1–3). High mRNA levels were detected for adenosine P1 receptor A3 and nucleotide P2 receptors P2Y1, P2Y2. A2A, P2X1, P2Y4, P2Y12, and P2Y14 were expressed at lower levels in HSCs from mouse (data not shown).
HSCs Express Functional CD39
Fluorescence-activated cell sorting analysis further revealed 2 distinct HSC populations of approximately equal proportions being Cd39high or Cd39low. We noted that 15% to 20% of Cd39high HSCs co-expressed Cd73. Apyrase ectonucleotidase activity was significantly decreased in purified Cd39-null HSCs (40.9 nmol of Pi/min/105 cells) when compared with their wild-type counterparts (67 nmol of Pi/min/105 cells; P = 0.0009) (data not shown).
CD39-Dependent Mobilization of HSCs After Liver Injury
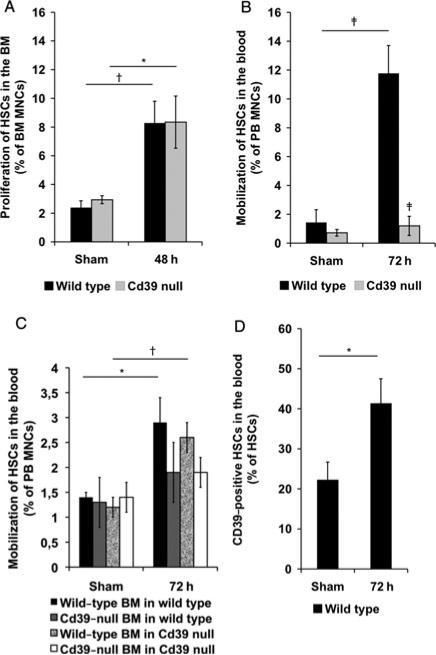
We next investigated characteristics of HSCs in the bone marrow and mobilization into the blood after partial hepatectomy in wild-type and Cd39-null mice. HSCs proliferate in the bone marrow, increasing 4-fold, in response to partial hepatectomy, comparable in both wild-type and Cd39-null mice (Fig. 1A). In contrast, dramatic differences in the mobilization potential were noted between wild-type and Cd39-null mice. When compared with wild-type mice, Cd39-null mice exhibited markedly impaired HSC mobilization into the blood (72 hours, P = 0.0004) (Fig. 1B).
Figure 1.
HSC proliferation and mobilization after partial hepatectomy in mice. A, HSC levels in the bone marrow of sham-operated wild-type and Cd39-null mice and 48 hours after 70% hepatectomy (% HSCs of bone marrow mononuclear cells; sham operation, n = 5; 70% hepatectomy, n = 8). B, HSC levels in the blood of sham-operated wild-type and Cd39-null mice and 72 hours after 70% hepatectomy (% HSCs of peripheral blood mononuclear cells; sham operation, n = 5; hepatectomy, n = 5–9). C, HSC levels in the blood of sham-operated mice chimeric with wild-type or Cd39-null bone marrow, as described earlier (% HSCs of peripheral blood mononuclear cells; sham operation, n = 2–3; 70% hepatectomy, n = 6–10). D, Cd39 expression by=HSCs in the blood of sham-perated wild-type mice and 72 hours after 70% hepatectomy (% Cd39-positive HSCs of total wild-type HSCs; n = 3). Error bars represent standard error of mean. *P < 0.05, †P < 0.01, ‡P < 0.001.
To determine whether this effect was intrinsic to HSCs or due to Cd39 deletion in the liver vasculature, we generated chimeric mice, in which wild-type mice expressed Cd39-null bone marrow, or vice versa. HSC levels in the blood were significantly increased 72 hours after a 70% hepatectomy in wild-type (P = 0.016) and Cd39-null mice (P = 0.002) chimeric with wild-type bone marrow when compared with sham-operated chimera (Fig. 1C). HSCs were not significantly mobilized in the blood of mice grafted with Cd39-null bone marrow when compared with baseline values (Fig. 1C). HSC mobilization rates after hepatectomy were significantly higher in Cd39-null mice when reconstituted with wild-type bone marrow (120% 31 HSC increase vs sham) than in Cd39-null mice grafted with±Cd39-null bone marrow (36% ± 24 HSC increase vs sham; P = 0.007; data not shown).
CD39-Positive HSCs Show Increased Rates of Proliferation and Mobilization
We next examined the proportion of Cd39high wild-type HSC subsets before and after partial hepatectomy. The percentage of Cd39high HSCs significantly increased in the bone marrow from 56.9 ± 4.5% in sham-operated wild-type mice to 80.3 ± 2.8% (72 hours, P = = 0.016) after a 70% hepatectomy (data not shown). The percentage of Cd39high wild-type HSCs increased significantly in the blood (P = 0.025), 72 hours after 70% hepatectomy, when compared with shams (Fig. 1D).
HSCs Boost Liver Regeneration in a CD39-Dependent Manner
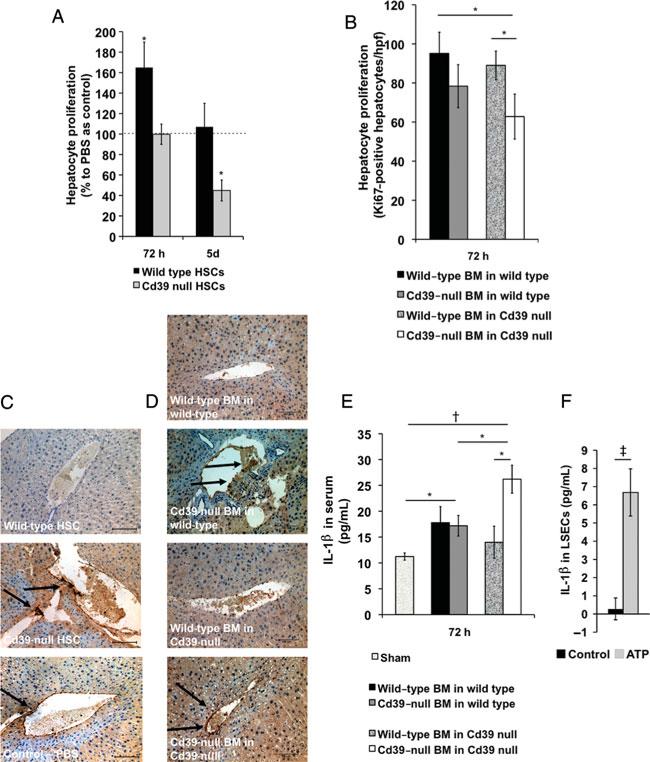
We next administered fluorescence-activated cell sorting-purified wild-type or Cd39-null HSCs into wild-type mice 24 hours after partial hepatectomy. Wild-type mice receiving wild-type HSCs had significantly increased hepatocyte proliferation rates when compared with control mice (P = 0.035) (Fig. 2A). In contrast, administration of HSCs from Cd39-null mice into wild-type mice inhibited hepatocyte proliferation rate (P = 0.0048) (Fig. 2A). In agreement, chimeric mice bearing wild-type=bone marrow had higher hepatocyte proliferation than those with Cd39-null bone marrow after partial hepatectomy (Fig. 2B).
FIGURE 2.
Bone marrow- and HSC-dependent liver regeneration. A, Hepatocyte proliferation in regular wild-type mice receiving 3 105 wild-type or Cd39-null HSCs 24 hours×after 70% hepatectomy (% Ki67-positive hepatocyte nuclei/high-power field relative to wild-type mice receiving phosphate-buffered saline as control; n = 6–8). B, Hepatocyte proliferation in bone marrow-irradiated mice chimeric with wild-type or Cd39-null bone marrow 72 hours after 70% hepatectomy (Ki67-positive hepatocyte nuclei/hpf; 70% hepatectomy, n = 5–10). C, Representative immunostaining for vascular P-selectin in livers of regular wild-type mice receiving 3 × 105 wild-type, Cd39-null HSCs or phosphate-buffered saline as control 24 hours after 70% hepatectomy (sham operation, n = 3; 70% hepatectomy, n = 6–8). Scale bars: 100 μm. D, Representative immunostaining for vascular P-selectin in livers of bone marrow-irradiated mice chimeric with wild-type or Cd39-null bone marrow 72 hours after 70% hepatectomy (n 6–10). Scale bars: 100 μm. E, interleukin-1β=plasma concentration (pg/mL) in bone marrow-irradiated mice chimeric with wild-type or Cd39-null bone marrow at 72 hours (sham operation, n = 3; 70% hepatectomy, n = 6–8). F, interleukin-1β concentrations (pg/mL) in liver sinusoidal endothelial cells, cultured for 5 hours with/without preincubation with adenosine triphosphate (5 mM) for 20 minutes. Error bars represent standard error of mean. *P < 0.05, †P < 0.01, ‡P < 0.001.
Anti-Inflammatory Properties of CD39-Positive Bone Marrow-Derived Cells
Vascular inflammation, as detected by vascular expression of P-selectin,33,34 appeared lower in wild-type mice receiving wild-type HSCs (Fig. 2C) 3 days after partial hepatectomy, than in control mice. In contrast, features of high vascular activation were detected in wild-type mice receiving Cd39-null HSCs. In agreement, regenerating livers of mice chimeric with Cd39-null bone marrow 3 days after partial hepatectomy were characterized by higher vascular expression of P-selectin than mice bearing wild-type bone marrow 72 hours after partial hepatectomy (Fig. 2C). Together, these data suggest that Cd39 expressed by bone marrow-derived cells, including HSCs, suppresses vascular inflammation at injury sites.
Wild-type and Cd39-null mice transplanted with Cd39-null bone marrow had increased plasma levels of interleukin-1β, an important proinflammatory cytokine in liver regeneration,35 when compared with sham-operated mice (P < 0.05) (Fig. 2E). Furthermore, plasma levels of interleukin-1β in Cd39-null mice chimeric with wild-type bone marrow were significantly decreased when compared with Cd39-null mice chimeric with Cd39-null bone marrow (P = 0.014). These data suggest that bone marrow-associated Cd39 regulates systemic inflammatory responses after partial hepatectomy.
Cd39high HSCs are functionally active and actively hydrolyze proinflammatory extracellular adenosine triphosphate. To test modulatory effects on liver sinusoidal endothelial cells during liver regener ation, we examined the effect of exogenous adenosine triphosphate on interleukin-1β production in liver sinusoidal endothelial cells in vitro. Liver sinusoidal endothelial cells treated with adenosine triphosphate (5 mM) for 20 minutes in vitro significantly increased interleukin-1β production (P = 0.0009) (Fig. 2F).
Progenitor Properties of HSCs
To further investigate possible mechanisms by which HSCs modulate liver proliferation, we administered fluorescence-activated cell sorting-purified HSCs from DsRed transgenic wild-type mice into wild-type mice 24 hours after partial hepatectomy. We detected DsRed transgenic hepatocytes and liver endothelial cells by fluorescence microscopy and fluorescence-activated cell sorting analysis, indicating that HSCs may differentiate into or fuse with liver cells (n = 5).However, the percentage of DsRed cells 10 days after partial hepatectomy was less than 0.01% of total liver endothelial cells and hepatocytes, respectively, suggesting a minor role in postsurgical liver regeneration (see Supplementary Digital Content 1, available at: http://links.lww.com/SLA/A293).
Vascular Endothelial Growth Factor Triggers Mobilization of HSCs
HSCs express vascular endothelial growth factor receptor 2, also known as Flk-1, as determined by fluorescence-activated cell sorting analysis (see Supplementary Digital Content 2a, available at: http://links.lww.com/SLA/A294). Vascular endothelial growth factor is a chemoattractant36 induced and upregulated in vascular cells in response to extracellular adenosine.37,38 Vascular endothelial growth factor resistance in Cd39-null mice post–liver re-section was noted with increased plasma levels when compared with wild-type mice (see Supplementary Digital Content 2b, available at: http://links.lww.com/SLA/A294).4
Vascular Endothelial Growth Factor-Induced Chemotaxis of HSCs
Using a Boyden chamber chemotaxis Transwell assay, we examined the impact of Cd39 deletion on HSC migration toward vascular endothelial growth factor (50 ng/mL). Wild-type and Cd39-null HSCs (upper chamber) both migrated toward vascular endothelial growth factor (lower chamber); however, migration of Cd39-null cells was significantly lower than wild-type HSCs (P = 0.026) (see Supplementary Digital Content 2c, available at: http://links.lww.com/SLA/A294).
Phosphohydrolysis of Extracellular Adenosine Triphosphate by CD39 Modulates Vascular Endothelial Growth Factor-Triggered Migration of HSCs
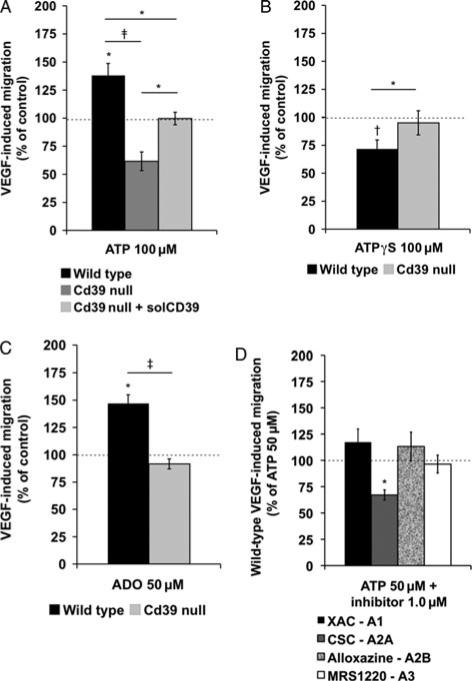
Migration rates of wild-type HSCs toward vascular endothelial growth factor (50 ng/mL, lower chamber) were significantly improved after preincubation of these cells with adenosine triphosphate (100 μM, upper chamber, P < 0.024), whereas preincubation with adenosine triphosphate decreased migration rates of Cd39-null HSCs (Fig. 3A). Preincubation with apyrase (5 U/mL, upper chamber), a soluble form of CD39, abolished adenosine triphosphate-induced inhibitory effects on Cd39-null HSCs (100 μM, upper chamber, P < 0.011) (Fig. 3A). Nonphosphohydrolyzable ATPγ S (100 μM, upper chamber) did not increase migration rates of Cd39-null HSCs toward vascular endothelial growth factor (50 ng/mL, lower chamber) and significantly impaired migration rates of wild-type HSCs (P < 0.005) (Fig. 3B). In parallel, adenosine triphosphate in the lower chamber, instead of vascular endothelial growth factor, had no primary chemoattractant capacity for HSCs (data not shown).
FIGURE 3.
Chemotaxis of murine HSC. A, Migration rates of wild-type and Cd39-null HSCs (3 × 105 bone marrow mononu-clear cells) to vascular endothelial×growth factor (50 ng/mL) 4 hours after preincubation with adenosine triphosphate (100 μM) or adenosine triphosphate (100 μM) + soluble CD39 (solCD39) (5 U/mL; n = 5–6). B, Migration rates of wild-type and Cd39-null HSC toward vascular endothelial growth factor (50 ng/mL) after preincubation with ATPγS (100 μM; n = 6). C, Migration rates of wild-type and Cd39-null HSCs to vascular endothelial growth factor (50 ng/mL) after preincubation with adenosine (50 μM; n = 6). D, Migration rates of wild-type HSCs to vascular endothelial growth factor (50 ng/mL) after preincubation with adenosine triphosphate (50μM) and selective adenosine receptor inhibitors [XAC-A1; CSC-A2A; alloxazine-A2B; or MRS1220-A3: (each 1.0 μM)] (n = 5–6). Error bars represent standard error of mean. †P < 0.01, ‡P < 0.001. 100% equals a mean cell number of n = ~ 1700.
Adenosine Modulates Vascular Endothelial Growth Factor-Triggered Migration of HSCs
As expected, migration rates of wild-type HSCs toward vascular endothelial growth factor (50 ng/mL, lower chamber) were significantly improved after preincubation with adenosine (50 μM, upper chamber, P = 0.023) (Fig. 3C). Adenosine (100 μM, upper chamber) significantly improved migration rates of Cd39-null HSCs (P = 0.022, data not shown). Preincubation with adenosine (50 μM, upper chamber) together with adenosine triphosphate (25 μM, upper chamber) significantly improved migration rates of wild-type HSCs (P = 0.0038) when compared with adenosine alone. How-ever, this combination failed to increase the migration of Cd39-null HSCs (see Supplementary Digital Content 2d, available at: http://links.lww.com/SLA/A294). As with adenosine triphosphate, adenosine in the lower chamber alone did not show chemoattractive effects on HSC migration (data not shown).
Adenosine Triphosphate Effects on Vascular Endothelial Growth Factor Triggered Migration of HSCs Are Mediated by A2A Receptors
To test whether the migration effects noted earlier are dependent on nucleoside binding to adenosine P1 receptors, we next preincubated wild-type HSCs with adenosine triphosphate in combination with selective P1 receptor inhibitors. Migration rates of wild-type HSCs toward vascular endothelial growth factor (50 ng/mL, lower chamber) were significantly impaired after preincubation with adeno-sine triphosphate (50 μM, upper chamber) and CSC, a selective A2A receptor inhibitor (1.0 μM, upper chamber, P = 0.022), when compared with incubation with adenosine triphosphate (50 μM, upper chamber) alone (Fig. 3D). In parallel, coincubation with adenosine triphosphate (50 μM, upper chamber) and selective A1, A2B, or A3 receptor inhibitors (1.0 μM, upper chamber) did not impact adeno-sine triphosphate-stimulated HSC migration toward vascular endothelial growth factor (Fig. 3D). These studies indicate that chemotaxis of HSCs toward vascular endothelial growth factor is mediated via stimulation of A2A receptor responses by adenosine triphosphate-derived adenosine and that is tightly controlled by intrinsic CD39 expression on HSCs.
Clinical Studies
Human Bone Marrow-Derived HSCs Express Purinergic Enzymes and Receptors
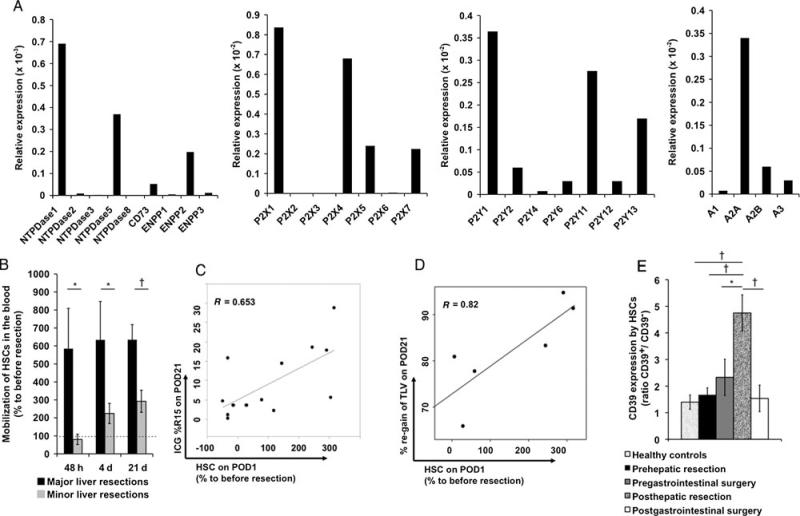
Human HSCs, as defined by CD133 with coexpression of CD34 and CD45, express both purinergic ectoenzymes and purinergic receptors at the transcriptional level (Fig. 4A). CD39 appeared to be dominant, whereas ENTPDase5, ectonucleotide pyrophosphatase/ phosphodiesterase 2, and CD73 mRNA levels were present as well but at lower levels (Fig. 4A).
Figure 4.
Purinergic profiles of human CD133+ HSCs. A, mRNA expression of ectonucleotidases and ecto-nucleotide pyrophosphatases/ phosphodiesterases, purinergic P2X, P2Y, and P1 receptors by human HSCs relative to β-actin (real-time reverse transciptase-polymerase chain reaction). B, Proportion of human blood HSCs (defined as % HSCs of peripheral blood mononuclear cells) 48 hours, 4 and 21 days after partial hepatectomy relative to levels before resection. Extent of liver resection was measured by computerized tomography-volumetry and defined as major liver resection (>30% of total liver volume; n=6) and minor liver resection (<20% of total liver volume; n = 18). C, Correlation of HSC blood levels 24 hours after partial hepatectomy with the indocyanine green retention rate (15%) on postoperative day 21 (n = 13). D, Correlation of HSCs in the blood 24 hours after major hepatectomy (>30% of total liver volume) with reconstituted liver volume on postoperative day 21 (n = 6). E, CD39 expression by HSCs in the blood of patients after major liver resections (n = 5, >30% of total liver volume), gastrointestinal, that is, extrahepatic surgery (n = 5), or of healthy controls (n = 14). Data presented as CD39-positive to CD39-null ratio. Error bars represent standard error of mean. *P < 0.05, †P < 0.01, ‡P < 0.001.
High mRNA levels were detected for P2 receptors P2X1, P2X4, P2Y1, and P2Y11. Among human P1 receptors, A2A was expressed at the highest level (Fig. 4A). Interestingly, A2A was noted before to be involved in migration of murine HSCs.
Human HSCs Are Mobilized in the Blood After Liver Injury
Mobilization of human HSCs was noted 2 days after hepatectomy and reached highest levels at postoperative day 4, comparable with the time of recruitment in wild-type mice (day 3) (Fig. 4B). Human HSC levels in the blood were persistently elevated up to 21 days after liver resection. The extent of mobilization of human HSCs correlated directly with plasma vascular endothelial growth factor levels 2 days after hepatectomy (R = 0.72, P < 0.001, data not shown).
Mobilization of Human HSCs Is Associated With the Extent of Resection and Liver Growth After Hepatic Resection
Mobilization of human HSCs was directly associated with the extent of liver resection. Significantly higher levels were evident after major liver resections (Fig. 4B). These results were in keeping with functional liver studies, as determined by the plasma disappearance of indocyanine green (%/min) and 15-minute retention rates. The plasma disappearance rate of indocyanine green -%/min was significantly decreased and the indocyanine green 15-minute retention rate% was significantly elevated in patients with a liver re-section volume of 30% to 65% when compared with patients with a liver resection volume of 10% to 20% and less than 10% (data not shown). Indocyanine green 15-minute retention rate% 21 days after hepatectomy was significantly correlated with levels of HSCs on postoperative day 1 (% of day 0; R = 0.653, P = 0.016), which indicates direct correlations between “liver functions” and the mobilization of HSCs from the bone marrow after liver resection (Fig. 4C).
Blood levels of human HSCs on postoperative day 1 were significantly correlated with the individual restoration of liver volume in patients undergoing major liver resections, as further determined by computerized tomography–based volumetry 21 days after hepatectomy (Fig. 4D).
CD39-Expressing HSCs Are Preferentially Mobilized After Surgical Liver Injury
Ratios of CD39high- to CD39low-expressing HSCs in the blood were significantly higher in patients after extended partial hepatectomy (≥30% of total liver volume) than preoperative ratios (P = 0.008) or ratios noted in healthy controls (P < 0.0054) (Fig. 4E). Ratios seen in patients after major abdominal, extrahepatic surgery did not significantly differ from preoperative ratios or ratios noted in healthy controls (Fig. 4E).
Interestingly, granulocyte colony-stimulating factor–mobilized peripheral blood stem cells in apheresis from healthy stem cell donors (n = 6) were characterized by lower CD39 numbers (ratio = 0.085 ± 0.03) than from healthy controls (n = 14; ratio 1.4 ± 0.27; P = 0.0003).
DISCUSSION
We have identified subsets of HSCs that highly express functional active CD39 and noted that these cells are preferentially mobilized after liver resection. This population of CD39high HSCs scavenges extracellular adenosine triphosphate, which, in turn, promotes liver regeneration by inhibiting interleukin-1β–driven vascular inflammation. We note that extracellular adenosine signaling is critically involved in vascular endothelial growth factor-induced chemo-taxis of HSCs. Clinical studies indicate that preferential mobilization of CD39high HSCs occurs after liver resection in humans and suggest positive associations with the restoration of liver volume.
CD39 is expressed at high levels in murine HSCs. Coexpression of CD73 and CD133 is considered indicative of early and undifferentiated stem cell subsets.12 CD39 is the rate-limiting ectonucleotidase of HSCs, which dictates nucleotide hydrolysis. Higher levels of adenosine productions are expected for HSC subsets that coexpress CD39 and CD73. Feed-forward inhibition of Cd73-mediated adeno-sine monophosphatase activity can occur with high levels of extra-cellular adenosine triphosphate.29,39 The production of extracellular adenosine by HSCs in combination with CD39+CD73+ regulatory T cells that provide immune privileges in bone marrow stem cell niches40 is also possible.
HSCs proliferate in the bone marrow and are mobilized into the blood after partial hepatectomy in mice. Mobilization of HSCs is significantly decreased in Cd39-null mice when compared with in wild-type mice (Fig. 1B). Experiments in mice chimeric with either wild-type or Cd39-null bone marrow show that impaired mobilization of HSCs in Cd39-null mice is due to the deficiency of Cd39 bioactivity in bone marrow-derived cells (Fig. 1C). Further studies in wild-type mice revealed that HSCs are mobilized after partial hepatectomy in proportion to the levels of Cd39 expression (Fig. 1D).
We further show that murine HSCs express vascular endothelial growth factor receptor 2 and migrate toward vascular endothelial growth factor in vitro. Chemotaxis to vascular endothelial growth factor is modulated by regulated phosphohydrolysis of extracellular nucleotides to adenosine, which is clearly defective in Cd39-null cells. This might explain, at least in part, vascular endothelial growth factor resistance in Cd39-null mice.4 Studies with selective adenosine type P1 receptor inhibitors indicate that boosted vascular endothelial growth factor chemotaxis is dependent on HSC A2A receptor responses (Figs. 3D, 5). Adenosinergic effects have already been shown to have beneficial effects in liver injury models and are being studied in this and other settings.41–43
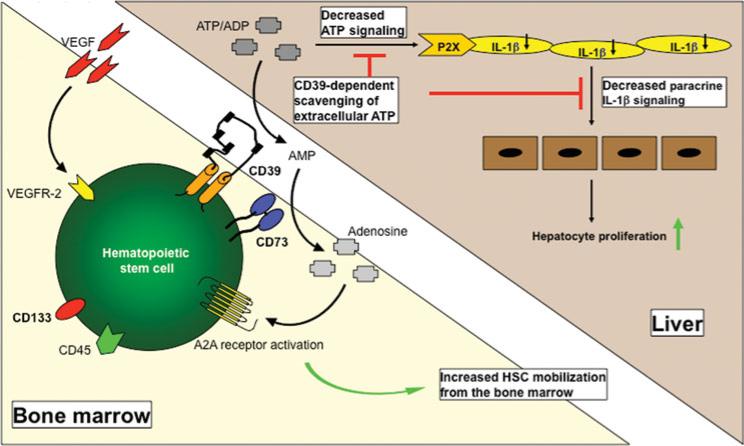
FIGURE 5.
Proposed mechanism of action. Chemotaxis of HSCs toward vascular endothelial growth factor is modulated by phosphohydrolysis of extracellular adeno-sine triphosphate by CD39 and stimulation of A2A receptor responses. CD39-positive HSCs are preferentially mobilized from the bone marrow to the liver. Decreases in adenosine triphosphate-driven paracrine interleukin-1β signaling between liver sinusoidal endothelial cells and regenerating hepatocytes promote, at least in part, the observed increased hepatocyte turnover.
We have previously noted that Cd39-null mice have impaired liver regeneration responses.4 We show here that HSCs enhance liver regeneration in a CD39-dependent manner (Fig. 2B). Further studies are needed to investigate the importance of CD39 expression by other bone marrow-derived cells in this setting.
Our data suggest HSCs to promote liver regeneration by impairing vascular inflammation in a CD39-dependent manner. HSCs scavenge extracellular adenosine triphosphate and might thereby decrease associated interleukin-1β production by liver sinusoidal endothelial cells.44 This is of particular importance as interleukin-1β directly inhibits hepatocyte proliferation after partial hepatectomy.31,32 Such interleukin-1 receptor antagonists have already been shown to exhibit salutary effects in the setting of liver regeneration.45,46
Low numbers of transdifferentiated or fused HSCs noted in the liver after the administration of DsRed transgenic HSCs further indicate that the immunosuppressive and anti-inflammatory properties of HSCs15–18 might be more important in boosting repair processes after partial hepatectomy rather than the facile replacement of liver cells alone.
HSCs are also mobilized after partial hepatectomy in humans and remain elevated for at least 21 days after surgery. They are mobilized to a greater extent in patients after major hepatectomy, with a significantly reduced global liver function. In both mice and humans, we demonstrate that a CD39high HSC subset is preferentially mobilized from the bone marrow after major hepatic resection (Figs. 1D, 4E). These observations are in agreement with previous studies, showing that CD39 facilitates chemotactic properties of leukocytes.26,47 However, our results in granulocyte colony-stimulating factor–treated stem cell donors and patients undergoing extrahepatic surgery indicate that mobilization of CD39high HSCs may be strongly linked to liver regeneration.
We have previously shown beneficial effects to the intraportal administration of human autologous CD133+ HSCs in patients after portal venous embolization of right liver segments.8–10 These approaches have been established to expand left lateral hepatic segments into an enlarged future liver remnant volume before extended resections.8–10
Mechanisms by which extrahepatic stem cells and progenitors contribute to liver regeneration remain incompletely understood.48–50 Our observations suggest that CD39 expression on HSCs is important in promoting liver regeneration. Moreover, these findings might represent an important step toward a pharmaceutical manipulation to enhance liver regeneration in different settings of liver failure by targeting purinergic signaling pathways in HSCs in the bone marrow or at sites of injury in the liver.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Keiichi Enjyoji, for providing Cd39-null mice, and Marina Ralemska and Gulcin Demirci, for technical support. They also thank Terry Strom for his helpful comments and his always-valued advice.
We appreciate the fact that the number of authors should be limited to 8 and consequently that our manuscript exceeds the average number. However, this study combines data from experimental approaches in vivo and in vitro with data obtained in patients undergoing partial hepatectomy to strengthen the clinical importance. We believe all individuals listed are justifiably credited with authorship. May we request the editorial board of the Annals of Surgery evaluate the following list that addresses the authors’ contributions for our proposed manuscript?:
M.S.: Conception and design of the study. Generation, collection, assembly, analysis, and/or interpretation of data. Drafting and revision of the manuscript, approval of the final version of the manuscript.
C.D.: Conception and design of the study. Generation, collection, assembly, analysis, and/or interpretation of data. Drafting and revision of the manuscript, approval of the final version of the manuscript.
W.J.: Conception and design of the study. Generation, collection, assembly, analysis, and/or interpretation of data. Revision of the manuscript, approval of the final version of the manuscript.
S.S.S.: Conception and design of the study. Generation, collection, assembly, analysis, and/or interpretation of data. Revision of the manuscript, approval of the final version of the manuscript.
Y.C.: Generation, collection, assembly, analysis, and interpretation of data. Revision of the manuscript, approval of the final version of the manuscript.
Y.W.: Generation, collection, assembly, analysis, and interpretation of data. Drafting or revision of the manuscript. Revision of the manuscript, approval of the final version of the manuscript. V.T.: Generation, collection, assembly, analysis, and interpretation of data. Revision of the manuscript, approval of the final version of the manuscript.
E.C.: Generation, collection, assembly, analysis, and interpretation of data. Revision of the manuscript, approval of the final version of the manuscript. L.H.: Generation, collection, assembly, analysis, and interpretation of data. Revision of the manuscript, approval of the final version of the manuscript.
S.B.: Generation, collection, analysis, and interpretation of data. Revision of the manuscript, approval of the final version of the manuscript.
G.F.: Generation, collection, assembly, analysis, and interpretation of data. Revision of the manuscript, approval of the final version of the manuscript.
M.N.: Generation, collection, assembly, analysis, and interpretation of data. Revision of the manuscript, approval of the final version of the manuscript.
S.J.K.: Generation, collection, assembly, analysis, and interpretation of data. Revision of the manuscript, approval of the final version of the manuscript.
W.T.K.: Conception and design of the study. Generation, collection, assembly, analysis, and interpretation of data. Drafting and revision of the manuscript, approval of the final version of the manuscript.
J.S.aE.: Conception and design of the study. Generation, collection, assembly, analysis, and interpretation of data. Drafting and revision of the manuscript, approval of the final version of the manuscript.
S.C.R.: Conception and design of the study. Generation, collection, assembly, analysis, and interpretation of data. Drafting and revision of the manuscript, approval of the final version of the manuscript.
M.S. was supported by grants DFG SCHM 2661/1-1 and 2661/1-2 and from the 2011 Thomas E Starzl, MD, Postdoctoral Fellowship Award by the American Liver Foundation (ALF); M.N. by grant NIH T32 GM007592-32; J.S.aE. by grant DFG SCHU1126/4-1; S.C.R. by grants R01 HL094400, U01 AI066331, and P01 AI045897.
Footnotes
Disclosure: The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Ho KJ, Do NL, Out HH, et al. Tob1 is a constitutively expressed repressor of liver regeneration. J Exp Med. 2010;207:1197–1208. doi: 10.1084/jem.20092434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 3.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 4.Beldi G, Wu Y, Sun X, et al. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology. 2008;135:1751–1760. doi: 10.1053/j.gastro.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaudry P, Hida Y, Udagawa T, et al. Endothelial progenitor cells contribute to accelerated liver regeneration. J Pediatr Surg. 2007;42:1190–1198. doi: 10.1016/j.jpedsurg.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 6.Gehling UM, Willems M, Dandri M, et al. Partial hepatectomy induces mobilization of a unique population of haematopoietic progenitor cells in human healthy liver donors. J Hepatol. 2005;43:845–853. doi: 10.1016/j.jhep.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Harb R, Xie G, Lutzko C, et al. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology. 2009;137:704–712. doi: 10.1053/j.gastro.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulte am Esch JS, II, Knoefel WT, Klein M, et al. Portal application of autologous CD133+ bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem Cells. 2005;23:463–470. doi: 10.1634/stemcells.2004-0283. [DOI] [PubMed] [Google Scholar]
- 9.Fürst G, Schulte am Esch J, Poll LW, et al. Portal vein embolization and autologous CD133+ bone marrow stem cells for liver regeneration: initial experience. Radiology. 2007;243:171–179. doi: 10.1148/radiol.2431060625. [DOI] [PubMed] [Google Scholar]
- 10.Schulte am Esch J, Schmelzle M, Fürst G, et al. Infusion of CD133+ bone marrow-derived stem cells after selective portal vein embolization enhances functional hepatic reserves after extended right hepatectomy. Ann Surg. 2012;255:79–85. doi: 10.1097/SLA.0b013e31823d7d08. [DOI] [PubMed] [Google Scholar]
- 11.Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 12.Tondreau T, Meuleman N, Delforge A, et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 13.Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi E, Kin M, Torimura T, et al. Endothelial progenitor cell transplantation improves the survival following liver injury in mice. Gastroenterology. 2006;130:521–531. doi: 10.1053/j.gastro.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 15.Kania G, Blyszczuk P, Valaperti A, et al. Prominin-1+/CD133+ bone marrow-derived heart-resident cells suppress experimental autoimmune myocarditis. Cardiovasc Res. 2008;80:236–245. doi: 10.1093/cvr/cvn190. [DOI] [PubMed] [Google Scholar]
- 16.Ghannam S, Pène J, Torcy-Moquet G, et al. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez L, Gutierrez-Aranda I, Ligero G, et al. Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells. 2011;29:251–262. doi: 10.1002/stem.569. [DOI] [PubMed] [Google Scholar]
- 18.Jung KH, Song SU, Yi T, et al. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats [published online ahead of print December 1, 2010]. Gastroenterology. doi: 10.1053/j.gastro.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 19.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q, Yan J, Putheti P, et al. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant. 2009;9:2303–2311. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 23.Mizumoto N, Kumamoto T, Robson SC, et al. CD39 is the dominant Langer-hans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 24.Beldi G, Banz Y, Kroemer A, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beldi G, Banz Y, Kroemer A, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corriden R, Chen Y, Inoue Y, et al. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem. 2008;283:28480–28486. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart ML, Gorzolla IC, Schittenhelm J, et al. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enjyoji K, Sevigny J, Lin Y, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 30.Dwyer KM, Hanidziar D, Putheti P, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreamer BL, Staecker JL, Sawada N, et al. Use of a low-speed, iso-density Percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol. 1986;22:201–211. doi: 10.1007/BF02623304. [DOI] [PubMed] [Google Scholar]
- 32.Lévesque JP, Hendy J, Takamatsu Y, et al. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Meurs M, Wulfert FM, Knol AJ, et al. Early organ-specific endothelial activation during hemorrhagic shock and resuscitation. Shock. 2008;29:291–299. doi: 10.1097/SHK.0b013e318145a7c1. [DOI] [PubMed] [Google Scholar]
- 34.Shen XD, Ke B, Zhai Y, et al. Diannexin, a novel Annexin V homodimer, protects rat liver transplants against cold ischemia-reperfusion injury. Am J Transplant. 2007;7:2463–2471. doi: 10.1111/j.1600-6143.2007.01967.x. [DOI] [PubMed] [Google Scholar]
- 35.Boulton R, Woodman A, Calnan D, et al. Nonparenchymal cells from regenerating rat liver generate interleukin-1alpha and -1beta: a mechanism of negative regulation of hepatocyte proliferation. Hepatology. 1997;26:49–58. doi: 10.1053/jhep.1997.v26.pm0009214451. [DOI] [PubMed] [Google Scholar]
- 36.Heeschen C, Lehmann R, Honold J, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 37.Gu JW, Brady AL, Anand V, et al. Adenosine upregulates VEGF expression in cultured myocardial vascular smooth muscle cells. Am J Physiol. 1999;277:H595–H602. doi: 10.1152/ajpheart.1999.277.2.H595. [DOI] [PubMed] [Google Scholar]
- 38.Takagi H, King GL, Robinson GS, et al. Adenosine mediates hypoxic induction of vascular endothelial growth factor in retinal pericytes and endothelial cells. Invest Ophthalmol Vis Sci. 1996;37:2165–2176. [PubMed] [Google Scholar]
- 39.Gordon EL, Pearson JD, Slakey LL. The hydrolysis of extracellular adenine nucleotides by cultured endothelial cells from pig aorta. Feed-forward inhibition of adenosine production at the cell surface. J Biol Chem. 1986;261:15496–15507. [PubMed] [Google Scholar]
- 40.Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awad AS, Huang L, Ye H, et al. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–F837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 42.Lappas CM, Day YJ, Marshall MA, et al. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson SG, Peters S. Advances in pharmacologic stress agents: focus on regadenoson. J Nucl Med Technol. 2010;38:163–171. doi: 10.2967/jnmt.109.065581. [DOI] [PubMed] [Google Scholar]
- 44.Lévesque SA, Kukulski F, Enjyoji K, et al. NTPDase1 governs P2×7-dependent functions in murine macrophages. Eur J Immunol. 2010;40:1473–1485. doi: 10.1002/eji.200939741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serafín A, Roselló-Catafau J, Prats N, et al. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology. 2004;39:688–698. doi: 10.1002/hep.20089. [DOI] [PubMed] [Google Scholar]
- 46.Petrasek J, Dolganiuc A, Csak T, et al. Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice [published online ahead of print August 19, 2010]. Gastroenterology. 2011;140:697–708. doi: 10.1053/j.gastro.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goepfert C, Sundberg C, Sevigny J, et al. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 49.Willenbring H, Bailey AS, Foster M, et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med. 2004;10:744–748. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- 50.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.