Abstract
The list of diseases with a known inflammatory etiology is growing. Cardiovascular disease, osteoporosis, diabetes, geriatric cachexia, and Alzheimer’s disease have all been shown to be linked to or exacerbated by aberrantly regulated inflammatory processes. Nevertheless, there is mounting evidence that those who are physically active, or who become physically active, have a reduction in biomarkers associated with chronic inflammation. There was strong early consensus that exercise-induced reductions in inflammation were explained by body mass index or body fatness, but recent studies provide support for the contention that exercise has body fat–independent anti-inflammatory effects. With few exceptions, the anti-inflammatory effects of exercise appear to occur regardless of age or the presence of chronic diseases. What remains unclear are the mechanisms by which exercise training induces these anti-inflammatory effects, but there are several intriguing possibilities, including release of endogenous products, such as heat shock proteins; selective reduction of visceral adipose tissue mass or reducing infiltration of adipocytes by macrophages; shift in immune cell phenotype; cross-tolerizing effects; or exercise-induced shifts in accessory proteins of toll-like receptor signaling. However, future research endeavors are likely to uncover additional potential mechanisms, and it could be some time before functional mechanisms are made clear. In summary, the potential anti-inflammatory influences of exercise training may provide a low-cost, readily available, and effective treatment for low-grade systemic inflammation and could contribute significantly to the positive effects of exercise training on chronic disease.
Keywords: anti-inflammatory, exercise training, chronic disease
Cardiovascular disease, diabetes, osteoporosis, and several other chronic diseases are now known to be strongly linked to inflammatory processes.1–4 Chronic diseases linked to inflammation are known to occur in greater frequency in older and sedentary individuals,5 but evidence is emerging that exercise has anti-inflammatory effects.6–9 For example, there is a substantial reduction in biomarkers of low-grade systemic inflammation in physically active adults or adults who undertake exercise training.10–14
Chronic low-grade inflammation, “persistent but more subtle than the acute phase response,”15 is frequently assessed by measurement of circulating C-reactive protein (CRP) and, somewhat less frequently, serum amyloid A. C-reactive protein concentration is higher in those with high body mass index,16,17 metabolic syndrome and/or diabetes,1,18 below-normal high-density lipoprotein cholesterol,19 chronic infection,20 and in cigarette smokers.21 Therefore, the inflammation/ damage is diffuse in low-grade inflammation and is apparently associated with several organs and tissues such as endothelial cells and adipocytes. Conversely, light to moderate alcohol intake,22 weight loss,23 medications such as glitazones24 and statins,25 and the focus of this review—increased physical activity—are associated with lower CRP concentration and reduction in other markers of inflammation.
It has been argued that CRP is a better predictor of cardiovascular disease risk than serum cholesterol,26,27 but this contention is quite controversial.28–31 Although CRP has gained acceptance as an independent marker of cardiovascular risk,4 recent research suggests that it is more than an “innocent bystander.”32 There is evidence that CRP may contribute to damage during myocardial infarct33,34 and contribute directly to atherogenesis.32
Serum or plasma pro-/anti-inflammatory cytokines are frequently used as biomarkers of inflammation. Tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) are most commonly measured and are known to stimulate CRP release from the liver.20 TNF-α and IL-6 are also frequently linked to increased incidence of disease,2,3,35–37 physical frailty or muscle wasting,38–40 and early death.41 The anti-inflammatory cytokine IL-10 and the adipocytokine adiponectin are also being measured with increased frequency.42–47 Adiponectin appears to be released in inverse proportion to fat mass23 and has anti-inflammatory effects such as negating the influence of TNF-α on endothelial cell adhesion,48 decreasing NF B activation,49 and slowing macrophage foam-cell development.50
The mechanisms for the apparent systemic and tissue-specific anti-inflammatory effects of exercise training or increased physical activity have not been elucidated. There are several intriguing possibilities, such as exercise-induced release of heat shock proteins, shifts in immune cell phenotype, training-induced reductions in visceral adipose tissue, or reduced tissue hypoxia. These are discussed in detail later in this review.
Our recent research has been focused on finding the mechanism for our observation that resistance exercise training lowered lipopolysaccharide (LPS)–stimulated inflammatory cytokine production in women aged 65 to 85.7 Our research efforts have been focused on the pattern recognition receptor toll-like receptor 4 (TLR4)—the primary signaling receptor for LPS. We found that TLR4 was substantially lower in individuals with high levels of physical activity, and TLR4 expression was reduced by exercise interventions.7,9,10,51 However, we are uncertain to what extent these changes contribute to the overall anti-inflammatory effect of exercise training.
It appears that exercise exerts anti-inflammatory effects, independent of losses in body fat, that are available to subjects of any age and to subjects with chronic diseases such as heart failure, type 2 diabetes, and osteoporosis. Exercise is known to exert positive influences on chronic diseases, but the extent to which the anti-inflammatory influence contributes to these positive effects is, as yet, unknown. Nevertheless, this is an exciting and intriguing new area of research that holds considerable promise for future researchers.
Inflammation and Chronic Disease
Recent research highlights the role that low-level systemic inflammation plays in initiating or exacerbating chronic disease.1–3 For example, cardiovascular disease was once believed to be primarily a disease of lipid deposition, but inflammation is now known to play a role in all stages of atherosclerotic plaque development.52 Further evidence of the emerging role of inflammation in chronic disease is provided by recent findings on the cholesterol-lowering (statin) drugs. Statins are now known to possess potent anti-inflammatory properties53—making it difficult to determine what proportion of cardiovascular risk reduction can be attributed to statin’s low-density lipoprotein (LDL) lowering or inflammation-lowering properties.54
There are several other interesting examples to illustrate the role of inflammation in chronic disease. Inflammatory markers effectively predicted the future development of diabetes,55–57 and elevated levels of inflammatory biomarkers were positively correlated with both insulin resistance and diabetes risk.58–61 Another example of the inflammatory influence on chronic disease is obesity, which exerts some of its comorbid influence through inflammatory processes.62 Adipocytes, or the resident macrophages in adipose tissue, are now known to produce a range of inflammatory products that contribute to insulin receptor dysfunction and related insulin insensitivity.1,8,57,63 Obese and overweight individuals have higher concentrations of inflammatory markers than normal-weight individuals;64,65 however, as will be discussed later, there is some disagreement in the literature whether it is the exercise training per se or body fat loss that elicits the decrease in inflammatory markers in studies that employ exercise, caloric restriction, or exercise and caloric restriction in combination to induce weight loss.57,64,66,67
Inflammation has been linked to several other chronic diseases, such as osteoporosis3,68–70 and muscle/lean tissue loss in geriatric cachexia,11,71 that are associated with disuse and inactivity. Furthermore, inflammation may suppress protein synthesis,12,72 and inflammatory biomarkers such as IL-6, TNF-α, and CRP are often used to predict subsequent mobility limitations or physical frailty.38–40,73 As will be discussed below, there is evidence that adaptations to exercise are preceded by an exercise training-induced blunting of inflammation.13 Therefore, exercise training has the potential to ameliorate inflammation and permit adaptations that can enhance strength and other aspects of physical function.
Collectively, as the detrimental effects of low-grade systemic inflammation become evident, our ability to control these inflammatory processes is growing in importance. A newfound emphasis on inflammation and chronic disease has led to the search for interventions that could lessen the impact of low-level systemic inflammation on health. Increased physical activity potentially provides a low-cost, readily available, positive side effect treatment for reducing inflammation.
Anti-Inflammatory Influences of Exercise
Acute Exercise
The focus of this review is to examine and discuss the potential anti-inflammatory influences of exercise training, but acute exercise can also stimulate an increase in anti-inflammatory agents.74–78 This topic was the focus of a recent review79 and will only be dealt with briefly herein.
Acute exercise stimulates an increase in plasma pro-inflammatory cyto-kines.75,77,78,80,81 For example, cycling exercise (80% VO2 max; 30 minutes) increased serum TNF-α and IL-1α,77 whereas plasma TNF-α and IL-1β increased modestly, yet significantly, following a marathon.81 Acute exercise also increases anti-inflammatory cytokines, such as IL-10, IL-1ra, and IL-4.74–78,81 Several researchers reported that acute exercise did not increase plasma concentration of TNF-α or IL-1β.75,82–85 Therefore, it appears that the pro-/anti-inflammatory balance during and immediately following exercise is dependant on several factors. These include subject health status,80,86 intensity or duration of exercise,76,77,82,87,88 glucose availability,87–90 and sampling time.74,75,91 For example, TNF-α was increased during moderate exercise in patients with chronic obstructive pulmonary disease but not in age-matched healthy controls.80 Intensity also plays a role, such that high-intensity cycling at ~80% VO2 max increased serum TNF-α, IL-1α, and IL-6, as well as intracellular (monocyte) interferon-gamma (IFN-β) and IL-4.77 Long-duration, lower intensity, 2-legged exercise (120 minutes, 55% VO2 max) did not increase plasma TNF-α, but IL-6 increased progressively throughout exercise.82 Furthermore, IL-1ra and IL-10 were inversely correlated with plasma glucose levels at the end of a competitive marathon.88 In addition, IL-1ra and IL-10 levels were higher in marathon runners who consumed an artificially sweetened placebo beverage compared to those consuming a sports drink.88 Finally, sampling time is important when interpreting the current literature because IL-10 is typically not elevated until the postexercise period.74,75
The traditional view of IL-6 is pro-inflammatory, with IL-6 linked to fever,92,93 induction of acute phase proteins such as CRP,20,93 and increased monocyte and macrophage production of inflammatory cytokines.94,95 Recent research has shown IL-6 release from skeletal muscle during exercise,82,83,96,97 with IL-6 exerting endocrine-like effects, such as the ability to alter carbohydrate (CHO) and lipid metabolism.98 IL-6 infusion increased production of IL-10 and IL-1ra and blunted the inflammatory response to subsequent endotoxin.99,100 Specifically, Starkie et al100 showed that either low-intensity exercise (180 minutes) or recombinant human IL-6 infusion equally dampened TNF-α production in response to endotoxin infusion.100 These authors suggested that IL-6 release during exercise modulated the subsequent response to endotoxin in the same fashion as the infused IL-6.100 Further evidence for the potential anti-inflammatory influence of IL-6 was shown by Steensberg et al,99 such that IL-6 infusion elicited the appearance of plasma IL-1ra and IL-10.99 Therefore, IL-6 is garnering support as an anti-inflammatory cytokine79,99,100 or as the first myokine.101 IL-6 is shown to have acute anti-inflammatory properties, and the cyclic increases in IL-6 that would occur during exercise training may help explain some of the mechanisms for the anti-inflammatory effect of exercise.
Confusion is created by the fact that IL-6 has been linked to cardiovascular disease,35–37 morbidity and mortality,102,103 sarcopenia, and physical frailty.38–40 Ridker et al37 found the highest rate of myocardial infarction in subjects who were in the highest quartile of plasma IL-6 concentrations. IL-6 was also linked to muscle atrophy and may be a cause of muscle wasting.38–40,71 One possible explanation for this apparent paradoxical role of IL-6 is that IL-6 merely reflects or reacts to the presence of TNF-α.104–106 That is, the IL-6 response to TNF-α is an attempt to initiate an anti-inflammatory response. Plasma TNF-α is correlated with plasma IL-6104 and has been found to increase IL-6 in vitro.106,107 Because TNF-α is apparently not required for exercise-induced IL-6 release,74,78 it may be that the stimulus for IL-6 release helps to explain the apparent paradox.35–37,99,100 Simply put, contraction-induced IL-6 may exert positive, anti-inflammatory actions, whereas IL-6 release in response to TNF-α may reflect disease, injury, or chronic infection.
Increased Physical Activity or Exercise Training
A rather impressive array of research is available to show that high levels of physical activity or exercise training elicit anti-inflammatory effects. Although there are dissenters,45,47,67,107–109 several reports show the potential anti-inflammatory influence of increased physical activity or formal exercise training. Researchers employing cross-sectional designs regularly observe lower plasma IL-6,42,110–114 TNF-α,110,112,114 CRP;6,14,18,112–119 increased IL-10;42,43 and increased adiponectin levels43 in physically active/physically fit individuals compared to physically inactive/low-fit individuals. Exercise interventions have also been shown to reduce in vitro inflammatory cytokine production7,120 and other markers of systemic inflammation,43,66,114,121–126 along with documented tissue-specific effects.11,12,127,128 As an example of the latter, exercise training lowered skeletal muscle TNF-α,11,12 IL-6,11 and IL-1β11 expression in older adults. It is of interest to note that periods of intensive training or overreaching did not elevate123 and even decreased14,129 biomarkers of inflammation.
In some studies, exercise combined with dietary restriction more effectively reduced visceral fat,128 abdominal adipocyte size,127 and markers of inflammation66 than dietary restriction alone. Nevertheless, it appears that both surgical/dietary restriction-induced fat loss and exercise training, without fat losses, have positive influences on markers of inflammation.23,43,64,66,67,109,121,130 It is not known to what extent the anti-inflammatory effects of exercise training contribute to the positive influence that exercise has on chronic disease, but promising new findings lead us to speculate that the anti-inflammatory influence of exercise could explain a significant portion of the therapeutic influence of exercise on chronic disease states.13,131
An attempt to find a consistent pattern among those studies showing no anti-inflammatory effects of exercise training45–47,67,107,108,132 was unsuccessful. These researchers studied a range of age groups,45,47,108 included overweight subjects and subjects with disease,46,67,107,108 imposed long-term interventions46,67 and short-term interventions,107,108 and conducted population studies assessing the amount of leisure time physical activity.47 One consistent finding among this group of studies was the lack of an adiponectin response to exercise training.44–46,107,108 This is not particularly surprising because adiponectin is strongly linked to fat mass23 and may not change when weight loss is small.46 However, short-term exercise training (4 weeks) has been shown to increase plasma adiponectin levels.43 In addition, exercise training increased adiponectin mRNA expression in diabetic rats133 and increased adiponectin receptor expression in mice.134
Anti-Inflammatory Influence of Exercise Training: Link to Body Fat?
There is evidence to suggest that exercise training-induced lowering of inflammatory biomarkers and relationships between physical activity and inflammatory biomarkers are linked to body fat or body mass index (BMI).16,17,67,111,134 Hammett et al,134 for example, reported that baseline associations between VO2 max and inflammatory markers in female smokers were largely explained by differences in BMI. Furthermore, there were no additional reductions in inflammatory markers when these women completed a 12-week (3 days per week, 45 minutes per session) low-intensity (60%–70% estimated maximal heart rate) endurance exercise program. Additional evidence for the lack of an exercise-only anti-inflammatory effect was provided by a randomized control trial employing a large number of patients with documented osteoarthritis (n > 200).67 Nicklas et al67 found that exercise had neither an independent anti-inflammatory effect nor an additive effect when exercise was combined with dietary restriction-induced weight loss. These findings were surprising in light of the fact that these subjects completed an 18-month diet, exercise, or diet and exercise intervention period. Exercise has been shown to be effective as a combined therapy in other research, such that exercise had an additive anti-inflammatory effect when combined with statin treatment135—a cholesterol-lowering drug with potential anti-inflammatory properties.25
There is a growing body of evidence that exercise training interventions have anti-inflammatory effects independent of changes in body fat.43,51,66,121,125,129 In contrast to the Nicklas et al67 study described above, Giannopoulou et al121 found that 14 weeks of moderate endurance exercise training (walking 3–4 days per week; 65%–70% VO2 peak) led to only slight weight/fat losses but lowered CRP levels to a similar extent (~15%) as diet alone or diet and exercise combined (–4.5-kg change in body weight). However, other inflammatory markers and adipokines were not substantially altered by any of the 3 interventions. Substantial anti-inflammatory effects have also been observed when short, intensive exercise interventions were employed. Oberbach et al43 found that only 4 weeks of exercise training (60 minutes per day; 4 days per week) significantly reduced high-sensitivity CRP (hsCRP) and increased adiponectin in type 2 diabetics, subjects with impaired glucose tolerance, and subjects with normal glucose tolerance. There were slight, but significant, changes in BMI and body fat, but these authors argued that the changes in adiponectin and CRP were “disproportionally higher than expected from the improvement in percent body fat, VO2 max, and insulin sensitivity, suggesting additional beneficial effects of exercise on plasma concentrations of these parameters.”43[*PAGE NUMBER?*]
There are a number of cross-sectional studies showing the disconnect between body fat and the anti-inflammatory effects of high levels of physical activity.14,115,116 For example, Tomaszewski et al14 reported that both lean (BMI < 25 kg/m2) and non-lean (BMI > 25 kg/m2) ultra-distance runners had lower hsCRP levels than lean and nonlean untrained subjects. Aronson et al115 suggested that physical fitness has an important independent impact, such that hsCRP levels (n = 892) were decreased with increasing levels of fitness (quartiles) after adjusting for age, gender, tobacco use, BMI, diabetes and hypertension, lipid levels, and hormone replacement, anti-inflammatory drug, and statin use.
Greater weight could be given to the Nicklas et al67 study described above because of high subject number and sound research design, but there are several interesting points of comparison that may help to explain the differences between conflicting studies. First, subjects in the Nicklas et al67 study were allowed to opt for home-based exercise after the first 4 months. Roughly 36% of the subjects opted for some or all home-based exercise. Home-based exercise may not provide the same stimulus for physiological adaptation as supervised or group exercise,136–138 but home-based exercise reduced inflammatory markers in other studies.13 Second, baseline CRP levels were quite high (~6.5 mg/L) in the osteoarthritis patients in the Nicklas et al67 study, and the relative change in CRP—even in the diet-only or diet and exercise treatment groups that lost large amounts of body weight—was relatively small (~3%). It is possible that patients with osteoarthritis do not respond in the same fashion as a healthy group of older patients. For example, CRP was reduced by roughly 35% in a small group of obese (BMI 35 kg/m2) but otherwise healthy women who underwent a 2-year diet and exercise intervention.139 In another study, an aggressive 2-week low-fat/high-fiber ad libitum diet and exercise program was shown to reduce CRP levels by 45% in postmenopausal women.140 Declines in CRP concentration in short-term focused exercise training studies generally range from 15% to 50%.43,121,140 Thus, although the Nicklas et al67 study may provide an example of what can be expected when large groups of diseased patients are encouraged to exercise, focused exercise training frequently provides a significant anti-inflammatory effect.7,12,13,43,66,121,122,129
Tissue-Specific Responses
Fewer studies have been conducted to examine the tissue-specific anti-inflammatory response to exercise training. Greiwe et al12 found that 12 weeks of resistance exercise training significantly reduced skeletal muscle TNF-α mRNA expression and TNF-α protein content in frail older subjects (mean age 81 years). In fact, training reduced these variables to the same level as the healthy young controls (mean age 23 years) and increased protein synthesis in inverse proportion to the change in TNF-α protein content. Exercise training-induced reductions in skeletal muscle inflammatory protein content were also reported by Gielen et al,11 who found that 1 year of training, leading to a 30% increase in VO2 peak, significantly reduced skeletal muscle TNF-α, IL-6, and IL-1α content in men with coronary heart failure. It is of interest that these effects occurred without substantial corresponding changes in serum inflammatory markers.11
Greiwe and coworkers’ contention12 that training may blunt inflammation, enhance protein synthesis, and fight wasting disease in older subjects is supported by findings from several studies.71,72,141,142 Lang et al72 found that TNF-α blunted protein synthesis in both skeletal and heart muscle—largely by an impaired translational initiation. TNF-α is known to lower circulating insulin-like growth factor-1 (IGF-1),141 reduce appetite,143 and increase insulin resistance144—all of which could negatively affect muscle growth.
Bruun et al65 recently found that a combination of diet and exercise training reduced macrophage infiltration into adipose tissue. Morbidly obese subjects (mean BMI 45.8 kg/m2) were placed on a hypocaloric diet, designed to reduce body weight by 1% per week, and an aggressive exercise program (2–3 hours of moderate physical activity 5 days per week) for 15 weeks. Adipose tissue expression of macrophage markers CD68 and CD14 declined significantly compared to baseline measures (Figure 1), as did plasma CRP (~28%), IL-6 (~26%), and monocyte chemoattractant protein (~16%). Plasma adiponectin levels were significantly increased (~33%), but TNF-α was unchanged. Unfortunately, the study design does not allow us to distinguish between the influence of substantial fat loss and the influence of the aggressive exercise program. To our knowledge, there are no other published exercise intervention studies in which the macrophage infiltration of adipose tissue has been examined.
Figure 1.
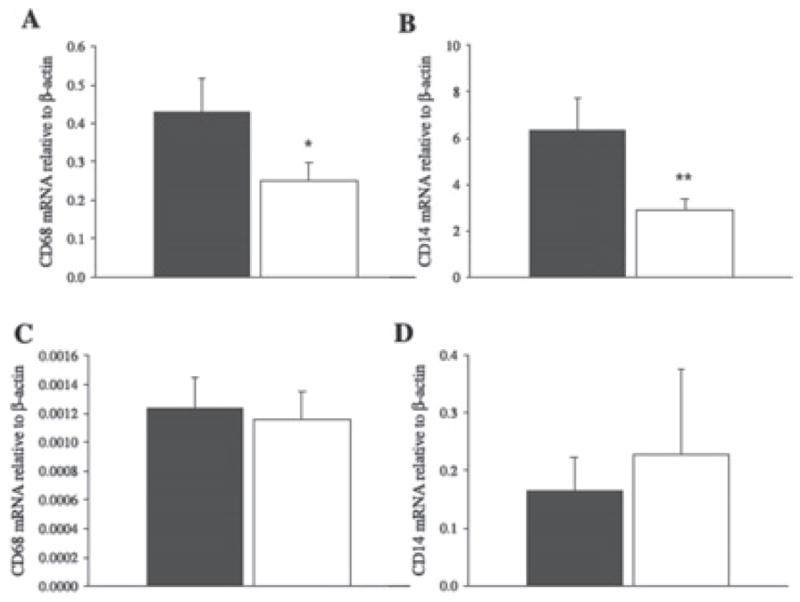
CD68 and CD14 expression changes in adipose tissue (n = 19; panels A and B, respectively) and skeletal muscle (n = 14; panels C and D, respectively) biopsies obtained from obese patients before (dark bars) and after (open bars) a 15-week diet and exercise intervention leading to significant fat losses in body fat. Mean ± SE; *P < .05 and **P < .001 compared to baseline.65
It has been suggested that macrophages residing in adipose tissue are responsible for the majority of adipokine production and that visceral adipose tissue is a more aggressive producer of inflammatory cytokines than other fat depots.63,145–148 A potential mechanism for exercise training-induced anti-inflammatory effects that occur in the absence of whole body fat loss is a selective reduction in visceral adipose tissue.128,149,150 Indeed, Giannopoulou et al128 found that exercise and diet combined and exercise alone significantly reduced visceral adipose tissue mass; however, inducing significant total body fat losses with diet alone did not change visceral adipose tissue mass. There are researchers who reported that exercise did not augment visceral fat losses when combined with diet.151,152 Nevertheless, the ability of exercise to reduce visceral fat, without influencing total body fat, could help explain exercise’s anti-inflammatory effect. Weight loss by diet alone can be problematic, such that a significant amount of skeletal muscle mass can be lost when diet is not combined with exercise.152
Exercise and diet combined was also reported to significantly reduce abdominal adipocyte size, but this did not occur with diet alone.127 It is generally accepted that reductions in abdominal fat significantly reduce disease risk.62,151,153,154
Inflammation and Training Adaptations
Inflammation appears to blunt protein synthesis,12,72 which leads to the speculation that exercise training must first blunt inflammation before skeletal muscle hypertrophy can occur. This speculation is supported by novel research from Gielen’s group,13 but with a twist—Gielen et al13 suggested that the anti-inflammatory responses were required before their subjects could make adaptations to endurance training. These authors found negative correlations between inducible nitric oxide synthase (iNOS) and cytochrome-c oxidase activity in coronary heart failure (CHF) patients who improved their VO2 peak by 29% after 6 months of training. Their interpretation was that anti-inflammation must precede metabolic adaptation. That is, because the change in cytochrome-c oxidase was strongly correlated to VO2 peak, the reductions in iNOS, putatively reducing mitochondrial suppression, allowed aerobic adaptations in their patients. Adamopoulos et al155 reported similar findings after training by CHF patients but did not observe an effect in normal controls, leaving open the possibility that these mechanisms may be active only in persons with chronic disease. Finally, Sriwijitkamol et al131 showed that 8 weeks of endurance training reversed the diabetes-induced suppression of “NF B inhibitors,” which was concomitant with a 37% increase in insulin sensitivity and a significant decline in TNF-α. These novel and intriguing “which comes first” scenarios may further our understanding of the benefits of exercise’s anti-inflammatory effects and underscore the importance of elucidating mechanisms to determine the role that these adaptations may play in chronic disease.
Inflammation and Aging: Effects of Exercise
Inflammatory markers are often increased with advancing age,12,41,156 but the anti-inflammatory effects of exercise training do not appear to have an upper age limit. Samples taken from subjects with high physical activity levels or following exercise interventions show lower serum IL-6 concentration,21,42,113 serum CRP,112,113,157 LPS-stimulated inflammatory cytokine production,7,9,10 and skeletal muscle TNF-α protein content and mRNA11,12 in persons older than age 65. In fact, exercise has been shown to have anti-inflammatory effects in healthy older subjects,7,122 subjects with coronary heart failure and coronary artery disease,11,155,158 breast cancer survivors,126 subjects taking beta-blockers,114 and those with metabolic syndrome.135 Therefore, the findings from several studies lead to the conclusion that neither age nor health status appear to limit the ability of exercise training to reduce inflammation.
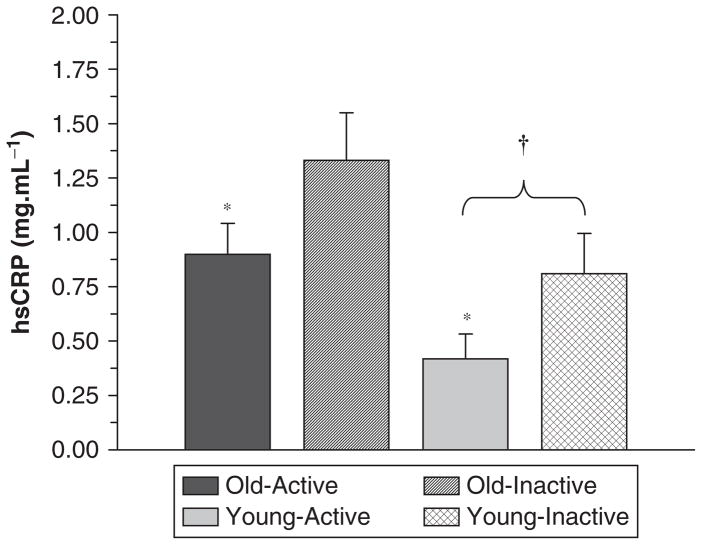
Beharka et al159 argued that age-associated differences in inflammation disappear when those with acute infections or other diseases are removed from the data set. Similarly, when we compared healthy physically active and healthy inactive younger and old subjects, we found that activity level was more important than age. Specifically, we found that physical activity level (physically active vs physically inactive) had a significant influence on CRP, LPS-stimulated cytokine production (Figure 2), and TLR4 expression, whereas age group (65–80 vs 18–35) had no effect on TLR4 or cytokine production and only a modest effect on CRP10 (Figure 3). Older subjects who were physically inactive had somewhat higher CRP levels than physically inactive younger subjects, but obligate activity could help to explain these differences. Our younger subjects were students who walked a considerable distance during the school week to attend classes. That type/amount of physical activity would have significantly increased the activity levels of our inactive older population. Thus, the apparent age-related difference in CRP could be due, in part, to higher physical activity levels in the young group. With respect to the ability of exercise training to exert body fat–independent anti-inflammatory effects, the older physically active individuals with relatively high body fat had similar CRP and TLR4 levels as young inactive subjects with substantially lower body fat10 (Figure 3). In a subsequent study, inactive younger and older subjects trained for 12 weeks. After training, CRP was reduced to the same level as groups of age-matched physically active subjects (unpublished data: Figure 4).
Figure 2.
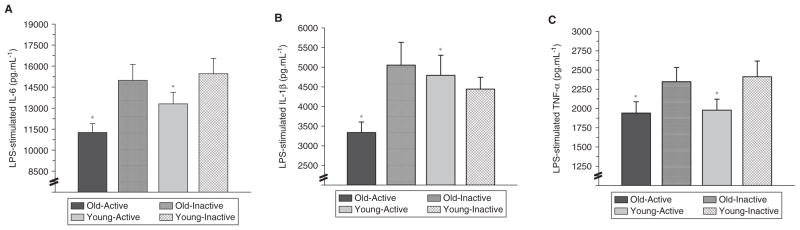
Lipopolysaccharide (LPS)–stimulated (A) interleukin-6 (IL-6), (B) IL-1β, and (C) tumor necrosis factor-alpha (TNF-α) production from whole-blood cultures that were obtained from young inactive (18–35 years; n = 19), young physically active (n = 21), old inactive (65–80 years; n = 21), and old physically active subjects (n = 23).
*P < .05 for group (physically active vs inactive).10
Figure 3.
High-sensitivity C-reactive protein (hsCRP) levels (mg/dL) from young inactive (18–35 years; n = 19), young physically active (n = 21), old inactive (65–80 years; n = 21), and old physically active subjects (n = 23). *P < .05 for group (physically active vs inactive). †P < .05 (age effect; young lower than old).10
Figure 4.
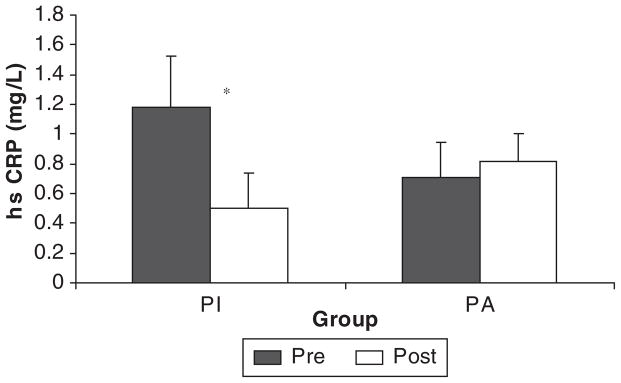
High-sensitivity C-reactive protein (hsCRP) before and after 12 weeks of endurance (20 minutes of treadmill walking, 3 days per week) and resistance exercise (2 sets of 8 exercises, 3 days per week) training in previously inactive subjects (PI) and a control group of physically active (PA) subjects who maintained normal activity. Data collapsed across age groups (PI, young physically active and old physically inactive combined; PA, young physically active and old physically active) (unpublished data). *Significant difference after training for PI (P < .05).
Potential Mechanisms for the Anti-Inflammatory Effect of Exercise
Physically active individuals have lower indexes of chronic inflammation, which reflects a reduced risk of developing metabolic syndrome, cardiovascular disease, and other related disorders.7,10,120,122,160 The anti-inflammatory actions of regular exercise have been well documented; however, the mechanism underlying these effects has not been elucidated.7,161 Nevertheless, based on the current literature, several viable mechanisms exist. These potential mechanisms could directly or indirectly alter whole-body inflammation. The remainder of this section is devoted to further exploring the specifics of mechanisms for the anti-inflammatory influence of exercise training.
Anti-Inflammatory Effects of Exercise: Does It Take a Toll?
In work from our laboratory,7,9,10,121,162 higher levels of physical activity or exercise intervention resulted in blunted inflammation and lower monocyte toll-like receptor 4 expression. Toll-like receptors (TLRs) are pattern recognition receptors, with TLR4 recognized as the primary signaling molecule for lipo-polysaccharide.163,164 The TLR4 signaling activates innate immunity and other inflammatory processes.164
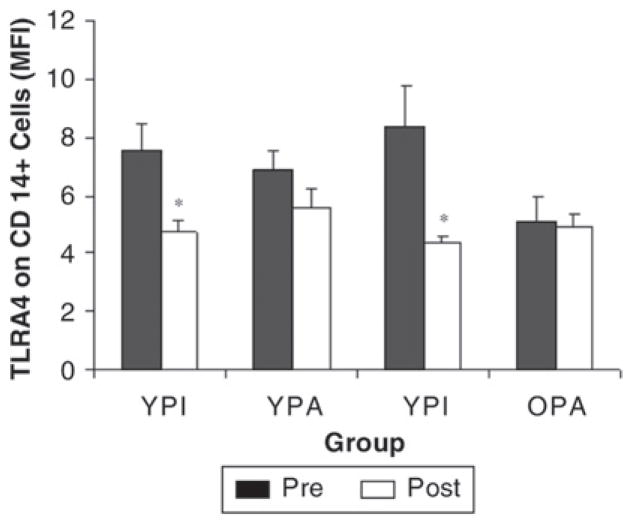
Our interest in TLR4 stemmed from the finding that 10 weeks of resistance exercise training blunted LPS-stimulated inflammatory cytokine production in whole-blood cultures from older women.7 The details of our TLR work can be found in a recent review.162 We found that TLR4 was lower in physically active young and old subjects compared to age-matched inactive subjects,10 and TLR4 was lower after 12 weeks of exercise training in previously inactive younger and older subjects.122 In fact, the training reduced TLR4 to levels similar to subjects who had been physically active for several months to years (Figure 5). We also found that TLR4 expression was related to LPS-stimulated production of inflammatory cytokines.7,10 Although it appears that training-induced declines in TLR4 may help to explain the training-associated blunting of mitogen-stimulated cytokine production, it is not, at present, known to what extent TLR4 downregulation contributes to the anti-inflammatory actions of exercise training.
Figure 5.
CD14+ cell surface expression of TLR4 from young (18–35 years) and old (65–80 years) physically inactive (YPI and OPI) and young and old active (YPA and OPA) subjects. Measurements were made before (PRE) and after (POST) 12 weeks of endurance and resistance training for physically inactive (YPI and OPI) or before and after 12 weeks of maintaining a physically active lifestyle for physically active subjects (YPA and OPA). That is, the physically inactive subjects trained for 12 weeks, and the physically active subjects maintained their activity levels as an active control group.122 *P < .05 for training effect.122 MFI, mean fluorescence intensity.
Inflammatory Monocytes
Blood monocytes have the capacity to influence whole-body inflammation by producing and releasing pro- and anti-inflammatory cytokines in response to a variety of endogenous and exogenous ligands.91,120,164,165 Recent evidence suggests that a subset of monocytes, referred to as inflammatory monocytes (CD14+CD16+), is responsible for the majority of monocyte-derived pro-inflammatory cytokines.91,166 Inflammatory monocytes also express higher levels of TLR2 than classical (CD14+CD16–) monocytes,167 are higher in patients with rheumatoid arthritis,167 and are expanded in sepsis.168
Acute exercise (cycling at 400 W for 60 seconds) was reported by Steppich et al166 to induce a several-fold increase in the circulating inflammatory monocyte number. Because some of the increase can be blocked by propanolol, the authors attributed the changes to an epinephrine-induced release of inflammatory monocytes from the marginal pool.166 To our knowledge, no published studies have examined the effect of exercise training on inflammatory monocytes, nor are we aware of reduced inflammatory monocyte concentration or activity being used as a means of explaining the anti-inflammatory effects of exercise. However, in a recent study from our lab, we found that older physically active individuals had a lower number of circulating inflammatory monocytes than physically inactive subjects of the same age (unpublished data). We are in the process of determining whether exercise training can lower inflammatory monocyte concentration in previously sedentary older subjects. The underlying stimulus by which chronic exercise training could alter monocyte phenotype is not known, but it has been reported that the inflammatory monocyte population is nearly depleted by high-dose glucocorticoid treatment.169 Therefore, it is possible that pulsatile changes in glucocorticoids associated with daily exercise sessions have a similar effect on inflammatory monocytes.
Th1/Th2 Balance
Type 1 helper T cells (Th1) play an important role in mediating the actions of the innate immune system. The local and systemic actions of blood monocytes and adipose tissue macrophages are controlled by cytokines, released from helper T cells (Th). Type 1 helper T cells activate components of the innate immune system by releasing type 1 cytokines: IFN-γ and IL-2.93,161,170 Type 2 helper T cells (Th2) activate selective components of the adaptive immune system by releasing type 2 cytokines: IL-4 and IL-10.9,93,170,171 Activation of Th1-mediated immunity (blood monocytes, peripheral tissue macrophages, and other phagocytes) enhances the leukocyte inflammatory capacity; however, activation of Th2 suppresses inflammation by activating the adaptive immune system.
Both acute exercise and a short period of intensified training have been reported to induce Th2 dominance and decrease the circulating concentration of Th1 cells, thus decreasing inflammatory capacity.161,170,171 Steensberg et al,171 for example, reported a significant decline in Th1 cells after 2.5 hours of treadmill running (75% VO2 max). The shift in Th1:Th2 was largely due to changes in Th1 because the Th2 cells were unchanged with exercise.171 We believe it is possible that the inflammatory capacity of blood monocytes and peripheral tissue macrophages is decreased following a period of exercise training due to alterations in the relative balance of Th1 and Th2 cells, such that anti-inflammatory processes are favored.
Adipocytes
Exercising individuals tend to experience a redistribution of body composition, such that their adipose tissue atrophies.61,95,172 Adipocyte atrophy has been linked to a reduction in the inflammatory capacity of adipose tissue.172 Adipose tissue is a rich, metabolically active organ that has far-reaching physiological influences.94 Obesity and physical inactivity are believed to negatively alter the coordinated regulation of energy balance and other processes.67,93,94 For instance, IL-6 release from the adipose tissue of obese patients increased monocyte and macrophage production of inflammatory cytokines (IL-6, TNF-α, IL-1β, and IL-8).94,95
It was originally believed that adipocytes were the primary source of inflammatory cytokines released from adipose tissue; however, recent evidence suggested that adipose tissue macrophages produce all of the TNF-α and roughly one third of the IL-6 from adipose tissue.95,173,174 Accumulation of adiposity is associated with an increased migration of monocytes from the blood to adipose tissue, which further exacerbates inflammatory cytokine release.95,173,174 Monocyte infiltration of adipose tissue is minimized in nonobese individuals by the hormone ghrelin, suggesting that ghrelin could have anti-inflammatory effects.95,174,175 It is not known whether ghrelin interacts with the TLR4 pathway or an alternate pathway to induce its anti-inflammatory action. Obese individuals have low plasma ghrelin and elevated plasma leptin, a combination that stimulates a rapid transmigration of monocytes from the blood to adipose tissue.95,175,176 Given the regulatory influence of leptin and ghrelin on monocytes, it is possible that these proteins are responsible for the anti-inflammatory effects of exercise-induced weight loss. Adipocyte production and release of leptin can alter inflammation at both the local (ie, adipose tissue and recruitment of monocytes) and systemic levels (alteration of blood monocytes).177 Excessive release of TNF-α from adipose tissue macrophages during weight gain is believed to be partially responsible for the development of insulin resistance, which, incidentally, may be a response to prevent additional weight gain.95,177,178 Exercise training is associated with a redistribution of body composition, such that adipocyte atrophy occurs. Adipocyte atrophy has been reported to decrease monocyte accumulation in adipose tissue and subsequent release of TNF-α.95,177,178 Reduction in TNF-α release would be consistent with an anti-inflammatory effect localized to adipose tissue.
Heat Shock Proteins
The anti-inflammatory effects of exercise training may be linked to the production and release of heat shock proteins (HSP) during a period of exercise training.165,179 Repeated bouts of eccentric exercise have been shown to increase intramuscular stores of heat shock proteins, which may be responsible for skeletal muscle-specific anti-inflammatory effects following a period of chronic resistance exercise training.180,181 Acute bouts of eccentric exercise damage skeletal muscle, which results in a release of HSP60 and HSP70 into the circulation.182,183 During repeated bouts of eccentric exercise, separated by 2 weeks, skeletal muscle inflammatory cytokine release decreases,180,181 which is consistent with an anti-inflammatory adaptation. Heat shock proteins have been implicated as a possible cross-tolerance signal, which influence blood monocyte/peripheral macrophage LPS-stimulated inflammatory cytokine production and hepatic acute phase protein production.165 Pretreatment of monocytic cell lines in vitro with HSP60 significantly decreased cellular responses to LPS and cell surface expression of TLR4, HLA-DR, and CD86184—suggesting that HSP can induce cross-tolerance against LPS at the systemic level. Therefore, an increase in intramuscular HSP may provide protection against future eccentric insults and tolerize monocytes, which may explain local and systemic anti-inflammatory effects. Heat shock protein may exert its effects through TLR or another yet to be identified pathway. Evidence of exercise-induced tolerance to endotoxin (LPS) exists, such that Starkie et al100 found that both 3 hours of cycling exercise (75% VO2 max) and IL-6 infusion blunted subsequent TNF-α responses to endotoxin infusion.
Reduction of Local Hypoxia/ Cross-Tolerance
Exercise training has been reported to decrease local hypoxia and ischemia, which may reduce inflammation.185,186 Hypoxia and ischemia cause the release of intracellular substances (ie, organelles, heat shock proteins, ATPγ [*PLS. SPELL OUT*], etc) that may play a role in promoting inflammation.185,186 Ischemia and hypoxia are physiological responses associated with both exercise-induced damage to skeletal muscle and adipocytes. Kariko et al186 speculated that ischemic products have the capacity to induce a cross-tolerance with TLR that is similar to endotoxin tolerance (induced by LPS or another ligand). Ischemia is associated with tissue damage, release of intracellular proteins, and exposure of circulating blood monocytes and tissue macrophages to endogenous ligands.185 Ischemia also occurs following damage to skeletal muscle as blood supply is disrupted, and thus the effect may be similar to ischemic stroke.
Kaufmann et al185 reported that tissue damage resulted in the release of ATPγs from damaged cells, which stimulated monocyte transmigration from the blood and decreased the ability of TLR2 and TLR4 to respond to lipoteichoic acid and LPS, respectively. Thus, ATPγ has been shown to induce cross-tolerance of TLR2 and TLR4 against other endogenous and exogenous ligands.187
Accessory Proteins
In our previous work, we found that the TLR4 signaling pathway may play an important role in mediating exercise training-induced changes in LPS-stimulated inflammatory cytokine production.7,10 The TLR accessory proteins play a critical role in mediating TLR responsiveness to LPS and other ligands, both endogenous and exogenous. Soluble CD14 (sCD14) has a similar molecular structure as membrane-bound CD14; however, it allows cells that lack CD14 (ie, vascular endothelial, smooth muscle cells, adipocytes, skeletal muscle cells, etc) to use TLR4 pathways to respond to the challenge from various ligands.187 Soluble CD14 has been shown to increase cellular responses to LPS both in vivo and in vitro.188 Inflammatory atherosclerotic disease has been reported to significantly elevate plasma sCD14 concentration and markers of chronic inflammation compared to normal individuals.189–191 Plasma sCD14 concentration has also been linked to the pathophysiology of other inflammatory disease states.192–195 To our knowledge, only 1 group has examined the effect of exercise training on sCD14.192 Exercise training did not alter sCD14; however, these researchers employed a modest subject number (n = 18) and only 8 weeks of exercise.192
Lipopolysaccharide binding protein (LBP), an accessory protein required for LPS binding to CD14 or sCD14,196 has a similar link to inflammatory disease as sCD14.187 However, there is no research to document the LBP response to exercise or exercise training. The lipopolysaccharide binding protein is also an acute phase protein,187 and circulating levels could decrease with training, in a similar fashion to CRP. LBP:sCD14 may also play a role, such that Stoll et al197 found that lower LBP:sCD14 increased LPS-stimulated cellular activation.
Summary and Future Directions
Previous research provides evidence that a physically active lifestyle is associated with lower whole-body inflammatory biomarkers.42,110–114 The anti-inflammatory actions of chronic exercise training are evident after as little as 2 to 12 weeks of supervised exercise training.7,43,122,140 Anti-inflammatory effects of exercise training have been reported by our group and several others,7,43,66,114,120–122,124–126 but a number of researchers, conducting exercise intervention studies, report minimal or no anti-inflammatory effect of exercise.45,67,107,108,132 The interpretations are complicated by results from cross-sectional studies showing that when covariates (eg, BMI, body fat, etc) are included in the analysis, the inverse relationship between physical activity and inflammatory biomarkers disappears.16,17,111,132 Although the weight of evidence appears to be in favor of a body fat–independent influence of increased physical activity, more research is needed to settle this debate. Unique designs that can separate the influence of diet and exercise—such as those that institute dietary restriction-induced weight loss after an extended period of exercise training without weight loss—are required to solve this problem. Also, use of animal models may be an effective way to control key independent variables that may influence the degree of reduction in whole-body inflammation following a period of exercise training. These study designs may also be useful in identifying the mechanism(s) by which chronic exercise training exerts its effects.
In this review, we presented a number of mechanisms that may underlie the anti-inflammatory effects of chronic exercise training. Among these, our group has published descriptive findings showing that subjects with high physical activity level had significantly lower TLR4 expression.9,10 We also found that exercise training reduced TLR4 expression in previously sedentary subjects.7,122 The next logical step is to complete a detailed mechanistic study of the toll-like receptor pathway and attempt to identify how its intermediates are changed following exercise training. It is possible that release of endogenous ligands (ie, heat shock protein, ATPγ, etc) from contracting skeletal muscle or atrophied adipose tissue may mediate changes in TLR pathway intermediates. Other possible mechanisms of the anti-inflammatory influence of exercise that we are presently exploring include the influence of exercise training on Th1:Th2 and inflammatory monocytes. To our knowledge, none of the above mechanisms has been scrutinized as possible explanations for the anti-inflammatory actions of exercise training. We proposed several potential mechanisms in this review but acknowledge that the anti-inflammatory influences of exercise training are likely mediated by a complex interaction among multiple pathways.
Identification of the mechanism(s) underlying the anti-inflammatory effects of exercise training is important because these pathways may serve as targets for future pharmaceutical treatments. It is also possible that a better understanding of the mechanisms that are responsible for exercise-induced lowering of whole-body inflammation may allow physicians and scientists to develop more effective exercise countermeasures. Nevertheless, a number of important research questions need to be addressed to develop a comprehensive understanding of the link between low-grade chronic inflammation and physical activity.
References
- 1.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher A, Seljeflot I, Sommervoll L, Christensen B, Otterstad JE, Arnesen H. Increased levels of markers of vascular inflammation in patients with coronary heart disease. Scand J Clin Lab Invest. 2002;62:59–68. doi: 10.1080/003655102753517217. [DOI] [PubMed] [Google Scholar]
- 3.Saidenberg-Kermanac’h N, Corrado A, Lemeiter D, deVernejoul MC, Boissier MC, Cohen-Solal ME. TNF-alpha antibodies and osteoprotegerin decrease systemic bone loss associated with inflammation through distinct mechanisms in collagen-induced arthritis. Bone. 2004;35:1200–1207. doi: 10.1016/j.bone.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Koenig W, Lowel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based on the Framingham score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004;109:1349–1353. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- 5.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000 [see comment] JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 6.Albert MA, Glynn RJ, Ridker PM. Effect of physical activity on serum C-reactive protein. Am J Cardiol. 2004;93:221–225. doi: 10.1016/j.amjcard.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Flynn MG, McFarlin BK, Phillips MD, Stewart LK, Timmerman KL. Toll-like receptor 4 and CD14 mRNA expression are lower in resistive exercise-trained elderly women. J Appl Physiol. 2003;95:1833–1842. doi: 10.1152/japplphysiol.00359.2003. [DOI] [PubMed] [Google Scholar]
- 8.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2005 doi: 10.1152/ajpendo.00506.2005. ePub ahead of print. Available at: http://ajpendo.physiology.org/cgi/reprint/00506.2005v1. [DOI] [PubMed]
- 9.McFarlin BK, Flynn MG, Campbell WW, Stewart LK, Timmerman KL. TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med Sci Sports Exerc. 2004;36:1876–1883. doi: 10.1249/01.mss.0000145465.71269.10. [DOI] [PubMed] [Google Scholar]
- 10.McFarlin BK, Flynn MG, Campbell WW, et al. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol. 2006;61:388–393. doi: 10.1093/gerona/61.4.388. [DOI] [PubMed] [Google Scholar]
- 11.Gielen S, Adams V, Mobius-Winkler S, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42:861–868. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 12.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. Faseb J. 2001;15:475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- 13.Gielen S, Adams V, Linke A, et al. Exercise training in chronic heart failure: correlation between reduced local inflammation and improved oxidative capacity in the skeletal muscle. Eur J Cardiovasc Prev Rehabil. 2005;12:393–400. doi: 10.1097/01.hjr.0000174824.94892.43. [DOI] [PubMed] [Google Scholar]
- 14.Tomaszewski M, Charchar FJ, Przybycin M, et al. Strikingly low circulating CRP concentrations in ultramarathon runners independent of markers of adiposity: how low can you go? Arterioscl Thromb Vasc Biol. 2003;23:1640–1644. doi: 10.1161/01.ATV.0000087036.75849.0B. [DOI] [PubMed] [Google Scholar]
- 15.Economou EV, Malamitsi-Puchner AV, Pitsavos CP, Kouskouni EE, Magaziotou-Elefsinioti I, Creatsas G. Low-grade systemic inflammation profile, unrelated to homocysteinemia, in obese children. Mediators Inflamm. 2005;2005:337–342. doi: 10.1155/MI.2005.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aronson D, Bartha P, Zinder O, et al. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int J Obesity Related Metab Disord. 2004;28:674–679. doi: 10.1038/sj.ijo.0802609. [DOI] [PubMed] [Google Scholar]
- 17.Rawson ES, Freedson PS, Osganian SK, Matthews CE, Reed G, Ockene IS. Body mass index, but not physical activity, is associated with C-reactive protein. Med Sci Sports Exerc. 2003;35:1160–1166. doi: 10.1249/01.MSS.0000074565.79230.AB. [DOI] [PubMed] [Google Scholar]
- 18.Aronson D, Sella R, Sheikh-Ahmad M, et al. The association between cardiorespiratory fitness and C-reactive protein in subjects with the metabolic syndrome. J Am Coll Cardiol. 2004;44:2003–2007. doi: 10.1016/j.jacc.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Hansel B, Kontush A, Giral P, Bonnefont-Rousselot D, Chapman MJ, Bruckert E. One third of the variability in HDL-cholesterol level in a large dyslipidaemic population is predicted by age, sex and triglyceridaemia: the Paris La Pitie Study. Curr Med Res Opin. 2006;22:1149–1160. doi: 10.1185/030079906X104821. [DOI] [PubMed] [Google Scholar]
- 20.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 22.Volpato S, Pahor M, Ferrucci L, et al. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitor-1 in well-functioning older adults: the Health, Aging, and Body Composition study. Circulation. 2004;109:607–612. doi: 10.1161/01.CIR.0000109503.13955.00. [DOI] [PubMed] [Google Scholar]
- 23.Kopp HP, Krzyzanowska K, Mohlig M, Spranger J, Pfeiffer AF, Schernthaner G. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int J Obes (Lond) 2005;29:766–771. doi: 10.1038/sj.ijo.0802983. [DOI] [PubMed] [Google Scholar]
- 24.Esposito K, Ciotola M, Carleo D, et al. Effect of rosiglitazone on endothelial function and inflammatory markers in patients with the metabolic syndrome. Diabetes Care. 2006;29:1071–1076. doi: 10.2337/diacare.2951071. [DOI] [PubMed] [Google Scholar]
- 25.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 26.Verma S, Szmitko PE, Ridker PM. C-reactive protein comes of age. Nat Clin Pract Cardiovasc Med. 2005;2:29–36. doi: 10.1038/ncpcardio0074. quiz 58. [DOI] [PubMed] [Google Scholar]
- 27.Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality: a population-based, prospective study. Thromb Haemost. 2006;95:511–518. doi: 10.1160/TH05-08-0571. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd-Jones DM, Liu K, Tian L, Greenland P. Narrative review: assessment of C-reactive protein in risk prediction for cardiovascular disease. Ann Intern Med. 2006;145:35–42. doi: 10.7326/0003-4819-145-1-200607040-00129. [DOI] [PubMed] [Google Scholar]
- 29.Olsen MH, Christensen MK, Hansen TW, et al. High-sensitivity C-reactive protein is only weakly related to cardiovascular damage after adjustment for traditional cardiovascular risk factors. J Hypertens. 2006;24:655–661. doi: 10.1097/01.hjh.0000217847.03208.ba. [DOI] [PubMed] [Google Scholar]
- 30.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 31.Wilson PW, Nam BH, Pencina M, D’Agostino RB, Benjamin EJ, O’Donnell CJ. C-reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med. 2005;165:2473–2478. doi: 10.1001/archinte.165.21.2473. [DOI] [PubMed] [Google Scholar]
- 32.Labarrere CA, Zaloga GP. C-reactive protein: from innocent bystander to pivotal mediator of atherosclerosis. Am J Med. 2004;117:499–507. doi: 10.1016/j.amjmed.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 33.Lagrand WK, Visser CA, Hermens WT, et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100:96–102. doi: 10.1161/01.cir.100.1.96. [DOI] [PubMed] [Google Scholar]
- 34.Lagrand WK, Niessen HW, Wolbink GJ, et al. C-reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation. 1997;95:97–103. doi: 10.1161/01.cir.95.1.97. [DOI] [PubMed] [Google Scholar]
- 35.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 38.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526, e9–17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 39.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen M, Bruunsgaard H, Weis N, et al. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev. 2003;124:495–502. doi: 10.1016/s0047-6374(03)00027-7. [DOI] [PubMed] [Google Scholar]
- 41.Bruunsgaard H. Effects of tumor necrosis factor-alpha and interleukin-6 in elderly populations. Eur Cytokine Network. 2002;13:389–391. [PubMed] [Google Scholar]
- 42.Jankord R, Jemiolo B. Influence of physical activity on serum IL-6 and IL-10 levels in healthy older men. Med Sci Sports Exerc. 2004;36:960–964. doi: 10.1249/01.mss.0000128186.09416.18. [DOI] [PubMed] [Google Scholar]
- 43.Oberbach A, Tonjes A, Kloting N, et al. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Eur J Endocrinol. 2006;154:577–585. doi: 10.1530/eje.1.02127. [DOI] [PubMed] [Google Scholar]
- 44.Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P, Gautier JF. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur J Endocrinol. 2003;149:421–424. doi: 10.1530/eje.0.1490421. [DOI] [PubMed] [Google Scholar]
- 45.Hulver MW, Zheng D, Tanner CJ, et al. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab. 2002;283:E861–E865. doi: 10.1152/ajpendo.00150.2002. [DOI] [PubMed] [Google Scholar]
- 46.Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord. 2003;27:1066–1071. doi: 10.1038/sj.ijo.0802387. [DOI] [PubMed] [Google Scholar]
- 47.Verdaet D, Dendale P, De Bacquer D, Delanghe J, Block P, De Backer G. Association between leisure time physical activity and markers of chronic inflammation related to coronary heart disease. Atherosclerosis. 2004;176:303–310. doi: 10.1016/j.atherosclerosis.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 49.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 50.Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 51.Stewart LK, Flynn MG, Campbell WW, et al. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun. 2005;19:389–397. doi: 10.1016/j.bbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 53.Mora S, Ridker PM. Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)—can C-reactive protein be used to target statin therapy in primary prevention? Am J Cardiol. 2006;97:33A–41A. doi: 10.1016/j.amjcard.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 55.Festa A, D’Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 56.Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care. 1999;22:1971–1977. doi: 10.2337/diacare.22.12.1971. [DOI] [PubMed] [Google Scholar]
- 57.Ryan AS, Nicklas BJ. Reductions in plasma cytokine levels with weight loss improve insulin sensitivity in overweight and obese postmenopausal women. Diabetes Care. 2004;27:1699–1705. doi: 10.2337/diacare.27.7.1699. [DOI] [PubMed] [Google Scholar]
- 58.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 59.Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 60.Spranger J, Kroke A, Mohlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 61.Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32(suppl 3):24–34. doi: 10.1046/j.1365-2362.32.s3.4.x. [DOI] [PubMed] [Google Scholar]
- 62.You T, Yang R, Lyles MF, Gong D, Nicklas BJ. Abdominal adipose tissue cytokine gene expression: relationship to obesity and metabolic risk factors. Am J Physiol Endocrinol Metab. 2005;288:E741–E747. doi: 10.1152/ajpendo.00419.2004. [DOI] [PubMed] [Google Scholar]
- 63.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance [see comment] J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 65.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961–E967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 66.You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89:1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 67.Nicklas BJ, Ambrosius W, Messier SP, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79:544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 68.Gur A, Denli A, Nas K, et al. Possible pathogenetic role of new cytokines in postmenopausal osteoporosis and changes during calcitonin plus calcium therapy. Rheumatol Int. 2002;22:194–198. doi: 10.1007/s00296-002-0223-x. [DOI] [PubMed] [Google Scholar]
- 69.Gur A, Denli A, Cevik R, Nas K, Karakoc M, Sarac AJ. The effects of alendronate and calcitonin on cytokines in postmenopausal osteoporosis: a 6-month randomized and controlled study. Yonsei Med J. 2003;44:99–109. doi: 10.3349/ymj.2003.44.1.99. [DOI] [PubMed] [Google Scholar]
- 70.Abrahamsen B, Bonnevie-Nielsen V, Ebbesen EN, Gram J, Beck-Nielsen H. Cytokines and bone loss in a 5-year longitudinal study—hormone replacement therapy suppresses serum soluble interleukin-6 receptor and increases interleukin-1-receptor antagonist: the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2000;15:1545–1554. doi: 10.1359/jbmr.2000.15.8.1545. [DOI] [PubMed] [Google Scholar]
- 71.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 72.Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC. TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol. 2002;282:E336–E347. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- 73.Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 74.Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol. 1998;513(pt 3):889–894. doi: 10.1111/j.1469-7793.1998.889ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999;515(pt 1):287–291. doi: 10.1111/j.1469-7793.1999.287ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peake JM, Suzuki K, Hordern M, Wilson G, Nosaka K, Coombes JS. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur J Appl Physiol. 2005;95(5–6):514–521. doi: 10.1007/s00421-005-0035-2. [DOI] [PubMed] [Google Scholar]
- 77.Zaldivar F, Wang-Rodriguez J, Nemet D, et al. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J Appl Physiol. 2006;100:1124–1133. doi: 10.1152/japplphysiol.00562.2005. [DOI] [PubMed] [Google Scholar]
- 78.Suzuki K, Yamada M, Kurakake S, et al. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur J Appl Physiol. 2000;81:281–287. doi: 10.1007/s004210050044. [DOI] [PubMed] [Google Scholar]
- 79.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 80.Rabinovich RA, Figueras M, Ardite E, et al. Increased tumour necrosis factor-{alpha} plasma levels during moderate-intensity exercise in COPD patients. Eur Respir J. 2003;21:789–794. doi: 10.1183/09031936.03.00042702. [DOI] [PubMed] [Google Scholar]
- 81.Nieman DC, Henson DA, Smith LL, et al. Cytokine changes after a marathon race. J Appl Physiol. 2001;91:109–114. doi: 10.1152/jappl.2001.91.1.109. [DOI] [PubMed] [Google Scholar]
- 82.Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E1272–E1278. doi: 10.1152/ajpendo.00255.2002. [DOI] [PubMed] [Google Scholar]
- 83.Febbraio MA, Steensberg A, Starkie RL, McConell GK, Kingwell BA. Skeletal muscle interleukin-6 and tumor necrosis factor-alpha release in healthy subjects and patients with type 2 diabetes at rest and during exercise. Metabolism. 2003;52:939–944. doi: 10.1016/s0026-0495(03)00105-7. [DOI] [PubMed] [Google Scholar]
- 84.Drenth JP, Van Uum SH, Van Deuren M, Pesman GJ, Van der Ven-Jongekrijg J, Van der Meer JW. Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-alpha and IL-1 beta production. J Appl Physiol. 1995;79:1497–1503. doi: 10.1152/jappl.1995.79.5.1497. [DOI] [PubMed] [Google Scholar]
- 85.Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol (Lond) 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abad LW, Schmitz HR, Parker R, Roubenoff R. Cytokine responses differ by compartment and wasting status in patients with HIV infection and healthy controls. Cytokine. 2002;18:286–293. doi: 10.1006/cyto.2002.0893. [DOI] [PubMed] [Google Scholar]
- 87.Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in humans—effect of intensity of exercise. Eur J Appl Physiol Dec. 2000;83(6):512–515. doi: 10.1007/s004210000312. [DOI] [PubMed] [Google Scholar]
- 88.Nieman DC, Henson DA, Smith LL, et al. Cytokine changes after a marathon race. J Appl Physiol. 2001;91:109–114. doi: 10.1152/jappl.2001.91.1.109. [DOI] [PubMed] [Google Scholar]
- 89.Nieman DC, Davis JM, Henson DA, et al. Muscle cytokine mRNA changes after 2.5 h of cycling: influence of carbohydrate. Med Sci Sports Exerc. 2005;37:1283–1290. doi: 10.1249/01.mss.0000175054.99588.b1. [DOI] [PubMed] [Google Scholar]
- 90.Keller C, Keller P, Marshal S, Pedersen BK. IL-6 gene expression in human adipose tissue in response to exercise: effect of carbohydrate ingestion. J Physiol (Lond) 2003;550:927–931. doi: 10.1113/jphysiol.2003.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Belge KU, Dayyani F, Horelt A, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 92.Opp M, Obal F, Jr, Cady AB, Johannsen L, Krueger JM. Interleukin-6 is pyrogenic but not somnogenic. Physiol Behav. 1989;45:1069–1072. doi: 10.1016/0031-9384(89)90239-4. [DOI] [PubMed] [Google Scholar]
- 93.Janeway C. Immunobiology: The Immune System in Health and Disease. 5. New York: Garland; 2001. [Google Scholar]
- 94.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277 (6 pt 1):E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 95.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 96.Febbraio MA, Steensberg A, Keller C, et al. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J Physiol. 2003;549(pt 2):607–612. doi: 10.1113/jphysiol.2003.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steensberg A, Febbraio MA, Osada T, et al. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol. 2001;537(pt 2):633–639. doi: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Hall G, Steensberg A, Sacchetti M, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88:3005–3010. doi: 10.1210/jc.2002-021687. [DOI] [PubMed] [Google Scholar]
- 99.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 100.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. Faseb J. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 101.Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 102.Soubrane C, Rixe O, Meric JB, Khayat D, Mouawad R. Pretreatment serum interleukin-6 concentration as a prognostic factor of overall survival in metastatic malignant melanoma patients treated with biochemotherapy: a retrospective study. Melanoma Res. 2005;15:199–204. doi: 10.1097/00008390-200506000-00009. [DOI] [PubMed] [Google Scholar]
- 103.Nakashima J, Tachibana M, Horiguchi Y, et al. Serum Interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–2706. [PubMed] [Google Scholar]
- 104.Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 105.Shimada M, Andoh A, Hata K, et al. IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J Immunol. 2002;68:861–868. doi: 10.4049/jimmunol.168.2.861. [DOI] [PubMed] [Google Scholar]
- 106.Aloisi F, Care A, Borsellino G, et al. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–2366. [PubMed] [Google Scholar]
- 107.Marcell TJ, McAuley KA, Traustadottir T, Reaven PD. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism. 2005;54:533–541. doi: 10.1016/j.metabol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 108.Nassis GP, Papantakou K, Skenderi K, et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54:1472–1479. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 109.Hammett CJ, Oxenham HC, Baldi JC, et al. Effect of six months’ exercise training on C-reactive protein levels in healthy elderly subjects. J Am Coll Cardiol. 2004;44:2411–2413. doi: 10.1016/j.jacc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 110.Panagiotakos DB, Kokkinos P, Manios Y, Pitsavos C. Physical activity and markers of inflammation and thrombosis related to coronary heart disease. Prev Cardiol. 2004;7:190–194. doi: 10.1111/j.1520-037x.2004.03539.x. [DOI] [PubMed] [Google Scholar]
- 111.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obes Res. 2003;11:1055–1064. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
- 112.Colbert LH, Visser M, Simonsick EM, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 113.Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE. The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2003;51:1125–1130. doi: 10.1046/j.1532-5415.2003.51380.x. [DOI] [PubMed] [Google Scholar]
- 114.Kohut ML, McCann DA, Russell DW, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20:201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 115.Aronson D, Sheikh-Ahmad M, Avizohar O, et al. C-reactive protein is inversely related to physical fitness in middle-aged subjects. Atherosclerosis. 2004;176:173–179. doi: 10.1016/j.atherosclerosis.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 116.Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22:1869–1876. doi: 10.1161/01.atv.0000036611.77940.f8. [DOI] [PubMed] [Google Scholar]
- 117.Ichihara Y, Ohno J, Suzuki M, Anno T, Sugino M, Nagata K. Higher C-reactive protein concentration and white blood cell count in subjects with more coronary risk factors and/or lower physical fitness among apparently healthy Japanese. Circ J. 2002;66:677–684. doi: 10.1253/circj.66.677. [DOI] [PubMed] [Google Scholar]
- 118.LaMonte MJ, Durstine JL, Yanowitz FG, et al. Cardiorespiratory fitness and C-reactive protein among a tri-ethnic sample of women. Circulation. 2002;106:403–406. doi: 10.1161/01.cir.0000025425.20606.69. [DOI] [PubMed] [Google Scholar]
- 119.Stauffer BL, Hoetzer GL, Smith DT, DeSouza CA. Plasma C-reactive protein is not elevated in physically active postmenopausal women taking hormone replacement therapy. J Appl Physiol. 2004;96:143–148. doi: 10.1152/japplphysiol.00360.2003. [DOI] [PubMed] [Google Scholar]
- 120.Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999;281:1722–1727. doi: 10.1001/jama.281.18.1722. [DOI] [PubMed] [Google Scholar]
- 121.Giannopoulou I, Fernhall B, Carhart R, et al. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–875. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 122.Stewart LK, Flynn MG, Campbell WW, et al. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun. 2005;19:389–397. doi: 10.1016/j.bbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 123.Fallon KE, Fallon SK, Boston T. The acute phase response and exercise: court and field sports. Br J Sports Med. 2001;35:170–173. doi: 10.1136/bjsm.35.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caulin-Glaser T, Falko J, Hindman L, La Londe M, Snow R. Cardiac rehabilitation is associated with an improvement in C-reactive protein levels in both men and women with cardiovascular disease. J Cardiopulm Rehabil. 2005;25:332–336. doi: 10.1097/00008483-200511000-00003. quiz 337–338. [DOI] [PubMed] [Google Scholar]
- 125.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056–1061. doi: 10.1016/j.jacc.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 126.Fairey AS, Courneya KS, Field CJ, et al. Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain Behav Immun. 2005;19:381–388. doi: 10.1016/j.bbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 127.You T, Murphy KM, Lyles MF, Demons JL, Lenchik L, Nicklas BJ. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. Int J Obes (Lond) 2006;30:1211–1216. doi: 10.1038/sj.ijo.0803245. [DOI] [PubMed] [Google Scholar]
- 128.Giannopoulou I, Ploutz-Snyder LL, Carhart R, et al. Exercise is required for visceral fat loss in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1511–1518. doi: 10.1210/jc.2004-1782. [DOI] [PubMed] [Google Scholar]
- 129.Mattusch F, Dufaux B, Heine O, Mertens I, Rost R. Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int J Sports Med. 2000;21:21–24. doi: 10.1055/s-2000-8852. [DOI] [PubMed] [Google Scholar]
- 130.Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 131.Sriwijitkamol A, Christ-Roberts C, Berria R, et al. Reduced skeletal muscle inhibitor of kappaB beta content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes. 2006;55:760–767. doi: 10.2337/diabetes.55.03.06.db05-0677. [DOI] [PubMed] [Google Scholar]
- 132.Hammett CJ, Prapavessis H, Baldi JC, et al. Effects of exercise training on 5 inflammatory markers associated with cardiovascular risk. Am Heart J. 2006;151:367.e7–367.e16. doi: 10.1016/j.ahj.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 133.Tang Z, Yuan L, Gu C, Liu Y, Zhu L. Effect of exercise on the expression of adiponectin mRNA and GLUT4 mRNA in type 2 diabetic rats. J Huazhong Univ Sci Technolog Med Sci. 2005;25:191–193. 201. doi: 10.1007/BF02873574. [DOI] [PubMed] [Google Scholar]
- 134.Huang H, Iida KT, Sone H, Yokoo T, Yamada N, Ajisaka R. The effect of exercise training on adiponectin receptor expression in KKAy obese/diabetic mice. J Endocrinol. 2006;189:643–653. doi: 10.1677/joe.1.06630. [DOI] [PubMed] [Google Scholar]
- 135.Troseid M, Lappegard KT, Claudi T, et al. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur Heart J. 2004;25:349–355. doi: 10.1016/j.ehj.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 136.Timonen L, Rantanen T, Ryynanen OP, Taimela S, Timonen TE, Sulkava R. A randomized controlled trial of rehabilitation after hospitalization in frail older women: effects on strength, balance and mobility. Scand J Med Sci Sports. 2002;12:186–192. doi: 10.1034/j.1600-0838.2002.120310.x. [DOI] [PubMed] [Google Scholar]
- 137.Capodaglio P, Facioli M, Burroni E, Giordano A, Ferri A, Scaglioni G. Effectiveness of a home-based strengthening program for elderly males in Italy: a preliminary study. Aging-Clin Exp Res. 2002;14:28–34. doi: 10.1007/BF03324414. [DOI] [PubMed] [Google Scholar]
- 138.Van der Bij AK, Laurant MG, Wensing M. Effectiveness of physical activity interventions for older adults: a review. Am J Prev Med. 2002;22:120–133. doi: 10.1016/s0749-3797(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 139.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 140.Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism. 2004;53:377–381. doi: 10.1016/j.metabol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 141.Sarzi-Puttini P, Atzeni F, Scholmerich J, Cutolo M, Straub RH. Anti-TNF antibody treatment improves glucocorticoid induced insulin-like growth factor 1 (IGF1) resistance without influencing myoglobin and IGF1 binding proteins 1 and 3. Ann Rheum Dis. 2006;65:301–305. doi: 10.1136/ard.2005.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Williamson DL, Kimball SR, Jefferson LS. Acute treatment with TNF-alpha attenuates insulin-stimulated protein synthesis in cultures of C2C12 myotubes through a MEK1-sensitive mechanism. Am J Physiol Endocrinol Metab. 2005;289:E95–E104. doi: 10.1152/ajpendo.00397.2004. [DOI] [PubMed] [Google Scholar]
- 143.Yeh SS, Schuster MW. Geriatric cachexia: the role of cytokines. Am J Clin Nutr. 1999;70:183–197. doi: 10.1093/ajcn.70.2.183. [DOI] [PubMed] [Google Scholar]
- 144.Natali A, Toschi E, Baldeweg S, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133–1140. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 145.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 146.Lihn AS, Bruun JM, He G, Pedersen SB, Jensen PF, Richelsen B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol Cell Endocrinol. 2004;219(1–2):9–15. doi: 10.1016/j.mce.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 147.Bruun JM, Lihn AS, Madan AK, et al. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue: implication of nonadipose cells in adipose tissue. Am J Physiol Endocrinol Metab. 2004;286:E8–E13. doi: 10.1152/ajpendo.00269.2003. [DOI] [PubMed] [Google Scholar]
- 148.Fain JN, Tichansky DS, Madan AK. Most of the interleukin 1 receptor antagonist, cathepsin S, macrophage migration inhibitory factor, nerve growth factor, and interleukin 18 release by explants of human adipose tissue is by the non-fat cells, not by the adipocytes. Metabolism. 2006;55:1113–1121. doi: 10.1016/j.metabol.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 149.Lee S, Kuk JL, Davidson LE, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without type 2 diabetes. J Appl Physiol. 2005;99:1220–1225. doi: 10.1152/japplphysiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 150.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 151.Rice B, Janssen I, Hudson R, Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–691. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- 152.Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25:431–438. doi: 10.2337/diacare.25.3.431. [DOI] [PubMed] [Google Scholar]
- 153.Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord. 2001;25:1047–1056. doi: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
- 154.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 155.Adamopoulos S, Parissis J, Kroupis C, et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001;22:791–797. doi: 10.1053/euhj.2000.2285. [DOI] [PubMed] [Google Scholar]
- 156.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 157.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 158.Adamopoulos S, Parissis J, Karatzas D, et al. Physical training modulates proinflammatory cytokines and the soluble Fas/ soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:653–663. doi: 10.1016/s0735-1097(01)01795-8. [DOI] [PubMed] [Google Scholar]
- 159.Beharka AA, Meydani M, Wu D, Leka LS, Meydani A, Meydani SN. Interleukin-6 production does not increase with age. J Gerontol A Biol Sci Med Sci. 2001;56:B81–B88. doi: 10.1093/gerona/56.2.b81. [DOI] [PubMed] [Google Scholar]
- 160.Zebrack JS, Anderson JL. The role of inflammation and infection in the pathogenesis and evolution of coronary artery disease. Curr Cardiol Rep. 2002;4:278–288. doi: 10.1007/s11886-002-0063-z. [DOI] [PubMed] [Google Scholar]
- 161.McFarlin BK, Flynn MG, Stewart LK, Timmerman KL. Carbohydrate intake during endurance exercise increases natural killer cell responsiveness to IL-2. J Appl Physiol. 2004;96:271–275. doi: 10.1152/japplphysiol.00585.2003. [DOI] [PubMed] [Google Scholar]
- 162.Flynn MG, McFarlin BK. Toll-like receptor 4: link to the anti-inflammatory effects of exercise? Exerc Sport Sci Rev. 2006;34:176–181. doi: 10.1249/01.jes.0000240027.22749.14. [DOI] [PubMed] [Google Scholar]
- 163.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 164.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 165.Beg AA. Endogenous ligands of toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- 166.Steppich B, Dayyani F, Gruber R, Lorenz R, Mack M, Ziegler-Heitbrock HW. Selective mobilization of CD14(+)CD16(+) monocytes by exercise. Am J Physiol Cell Physiol. 2000;279:C578–C586. doi: 10.1152/ajpcell.2000.279.3.C578. [DOI] [PubMed] [Google Scholar]
- 167.Iwahashi M, Yamamura M, Aita T, et al. Expression of toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50:1457–1467. doi: 10.1002/art.20219. [DOI] [PubMed] [Google Scholar]
- 168.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–3176. [PubMed] [Google Scholar]
- 169.Fingerle-Rowson G, Angstwurm M, Andreesen R, Ziegler-Heitbrock HW. Selective depletion of CD14+ CD16+ monocytes by glucocorticoid therapy. Clin Exp Immunol. 1998;112:501–506. doi: 10.1046/j.1365-2249.1998.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lancaster GI, Halson SL, Khan Q, et al. Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc Immunol Rev. 2004;10:91–106. [PubMed] [Google Scholar]
- 171.Steensberg A, Toft AD, Bruunsgaard H, Sandmand M, Halkjaer-Kristensen J, Pedersen BK. Strenuous exercise decreases the percentage of type 1 T cells in the circulation. J Appl Physiol. 2001;91:1708–1712. doi: 10.1152/jappl.2001.91.4.1708. [DOI] [PubMed] [Google Scholar]
- 172.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue: regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 174.Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li WG, Gavrila D, Liu X, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–2226. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 176.Daniel JA, Elsasser TH, Morrison CD, et al. Leptin, tumor necrosis factor-alpha (TNF), and CD14 in ovine adipose tissue and changes in circulating TNF in lean and fat sheep. J Anim Sci. 2003;81:2590–2599. doi: 10.2527/2003.81102590x. [DOI] [PubMed] [Google Scholar]
- 177.Hube F, Hauner H. The role of TNF-alpha in human adipose tissue: prevention of weight gain at the expense of insulin resistance? Horm Metab Res. 1999;31:626–631. doi: 10.1055/s-2007-978810. [DOI] [PubMed] [Google Scholar]
- 178.Hauner H, Bender M, Haastert B, Hube F. Plasma concentrations of soluble TNF-alpha receptors in obese subjects. Int J Obes Relat Metab Disord. 1998;22:1239–1243. doi: 10.1038/sj.ijo.0800773. [DOI] [PubMed] [Google Scholar]
- 179.Tsan MF, Gao B. Endogenous ligands of toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 180.Thompson HS, Clarkson PM, Scordilis SP. The repeated bout effect and heat shock proteins: intramuscular HSP27 and HSP70 expression following two bouts of eccentric exercise in humans. Acta Physiol Scand. 2002;174:47–56. doi: 10.1046/j.1365-201x.2002.00922.x. [DOI] [PubMed] [Google Scholar]
- 181.Willoughby DS, McFarlin B, Bois C. Interleukin-6 expression after repeated bouts of eccentric exercise. Int J Sports Med. 2003;24:15–21. doi: 10.1055/s-2003-37197. [DOI] [PubMed] [Google Scholar]
- 182.Febbraio MA, Ott P, Nielsen HB, et al. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544(pt 3):957–962. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:eishih>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kilmartin B, Reen DJ. HSP60 induces self-tolerance to repeated HSP60 stimulation and cross-tolerance to other pro-inflammatory stimuli. Eur J Immunol. 2004;34:2041–2051. doi: 10.1002/eji.200425108. [DOI] [PubMed] [Google Scholar]
- 185.Kaufmann A, Musset B, Limberg SH, et al. “Host tissue damage” signal ATP promotes non-directional migration and negatively regulates toll-like receptor signaling in human monocytes. J Biol Chem. 2005;280:32459–32467. doi: 10.1074/jbc.M505301200. [DOI] [PubMed] [Google Scholar]
- 186.Kariko K, Weissman D, Welsh FA. Inhibition of toll-like receptor and cytokine signaling—a unifying theme in ischemic tolerance. J Cereb Blood Flow Metab. 2004;24:1288–1304. doi: 10.1097/01.WCB.0000145666.68576.71. [DOI] [PubMed] [Google Scholar]
- 187.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11:225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 188.Amar J, Ruidavets JB, Bal dit Sollier C, et al. CD14 C(−260)T gene polymorphism, circulating soluble CD14 levels and arteriosclerosis. J Hypertens. 2004;22:1523–1528. doi: 10.1097/01.hjh.0000133724.16947.a3. [DOI] [PubMed] [Google Scholar]
- 189.Albert MA, Glynn RJ, Ridker PM. Plasma concentration of C-reactive protein and the calculated Framingham Coronary Heart Disease Risk Score. Circulation. 2003;108:161–165. doi: 10.1161/01.CIR.0000080289.72166.CF. [DOI] [PubMed] [Google Scholar]
- 190.Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 is an acute-phase protein. J Immunol. 2004;172:4470–4479. doi: 10.4049/jimmunol.172.7.4470. [DOI] [PubMed] [Google Scholar]
- 191.Ford ES, Giles WH, Myers GL, Rifai N, Ridker PM, Mannino DM. C-reactive protein concentration distribution among US children and young adults: findings from the National Health and Nutrition Examination Survey, 1999–2000. Clin Chem. 2003;49:1353–1357. doi: 10.1373/49.8.1353. [DOI] [PubMed] [Google Scholar]
- 192.Niebauer J, Clark AL, Webb-Peploe KM, Coats AJ. Exercise training in chronic heart failure: effects on pro-inflammatory markers. Eur J Heart Fail. 2005;7:189–193. doi: 10.1016/j.ejheart.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 193.Fernandez-Real JM, Vendrell J, Ricart W, et al. Polymorphism of the tumor necrosis factor-alpha receptor 2 gene is associated with obesity, leptin levels, and insulin resistance in young subjects and diet-treated type 2 diabetic patients. Diabetes Care. 2000;23:831–837. doi: 10.2337/diacare.23.6.831. [DOI] [PubMed] [Google Scholar]
- 194.Fernandez-Real JM, Broch M, Richart C, Vendrell J, Lopez-Bermejo A, Ricart W. CD14 monocyte receptor, involved in the inflammatory cascade, and insulin sensitivity. J Clin Endocrinol Metab. 2003;88:1780–1784. doi: 10.1210/jc.2002-020173. [DOI] [PubMed] [Google Scholar]
- 195.Fernandez-Real JM, Lopez-Bermejo A, Castro A, et al. Opposite relationship between circulating soluble CD14 concentration and endothelial function in diabetic and nondiabetic subjects. Thromb Haemost. 2005;94:615–619. doi: 10.1160/TH04-12-0831. [DOI] [PubMed] [Google Scholar]
- 196.Dunzendorfer S, Lee HK, Soldau K, Tobias PS. Toll-like receptor 4 functions intracellularly in human coronary artery endothelial cells: roles of LBP and sCD14 in mediating LPS responses. Faseb J. 2004;18:1117–1119. doi: 10.1096/fj.03-1263fje. [DOI] [PubMed] [Google Scholar]
- 197.Stoll LL, Denning GM, Li WG, et al. Regulation of endotoxin-induced proinflammatory activation in human coronary artery cells: expression of functional membrane-bound CD14 by human coronary artery smooth muscle cells. J Immunol. 2004;173:1336–1343. doi: 10.4049/jimmunol.173.2.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]