Abstract
Visceral leishmaniasis (VL), the second-most dreaded parasitic disease after malaria, is currently endemic in 88 countries. Dramatic increases in the rates of infection, drug resistance, and non-availability of safe vaccines have highlighted the need for identification of novel and inexpensive anti-leishmanial agents from natural sources. In this study, we showed the leishmanicidal effect of essential oil from Artemisia annua leaves (AALEO) against Leishmania donovani in vitro and in vivo. AALEO was extracted by hydrodistillation and characterized by GC-MS, the most abundant compounds were found to be camphor (52.06 %) followed by β-caryophyllene (10.95 %). AALEO exhibited significant leishmanicidal activity against L. donovani, with 50 % inhibitory concentration of 14.63 ± 1.49 μg ml-1 and 7.3 ± 1.85 μg ml-1, respectively, against the promastigotes and intracellular amastigotes. The effect was mediated through programmed cell death as confirmed by externalization of phosphatidylserine, DNA nicking by TdT-mediated dUTP nick-end labeling assay, dyskinetoplastidy, cell cycle arrest at sub-G0–G1 phase, loss of mitochondrial membrane potential and reactive oxygen species generation in promastigotes and nitric oxide generation in ex vivo model. AALEO presented no cytotoxic effects against mammalian macrophages even at 200 μg ml-1. Intra-peritoneal administration of AALEO (200 mg/ kg.b.w.) to infected BALB/c mice reduced the parasite burden by almost 90% in the liver and spleen with significant reduction in weight. There was no hepato- or nephro-toxicity as demonstrated by normal levels of serum enzymes. The promising antileishmanial activity shown by camphor-rich AALEO may provide a new lead in the treatment of VL.
Keywords: leishmaniasis, visceral, essential oil, Artemisia annua, leishmanicidal, apoptosis, therapeutic efficacy
INTRODUCTION
Visceral leishmaniasis (VL), also known as kala azar is a fatal vector-borne illness caused by Leishmania donovani. The disease remains a public health problem worldwide causing considerable morbidity and mortality. The chemotherapy of VL has been undermined by drug resistance, variable efficacy, toxicity, parenteral administration, and requirement for long courses of treatment. In India, sodium antimony gluconate (SAG) is no longer useful as a drug because more than 64% of VL patients fail to respond or promptly relapse due to development of resistance in the parasites (Sundar, 2003). Alternative chemotherapeutic measures with amphotericin B (AMB) and its lipid formulations have severe limitations due to their toxic effects and prohibitive high cost. VL has also emerged as an opportunistic infection in individuals carrying HIV infection (Murray, 2004). Thus, there is an urgent need for new and promising compounds for the treatment of this debilitating disease, which mainly affects the rural population.
Essential oils (EO) are volatile mixtures of compounds obtained from spices, aromatic herbs, fruits, and flowers and have been traditionally used in folk medicine to treat several diseases. Various research groups have demonstrated that EO and their main components possess a wide spectrum of biological activities, which may be of great importance in several fields ranging from food chemistry to pharmacology and pharmaceutics. These properties have been attributed to the presence of characteristic constituents, the most abundant being the terpenoids, specially the monoterpenes and sesquiterpenes (Schelz et al., 2010). EO are known to have antibacterial and antifungal properties against human pathogenic microorganisms (Fyfe et al., 1997). EO obtained from Achillea millefolium and Ocimum basilicum (Santoro et al., 2007) exhibited toxic effects against trypanosomes, the protozoal species closely related to Leishmania. A plethora of studies indicate promising leishmanicidal effect of EO from various plants including Croton cajucara (Rosa et al., 2003), Ocimum gratissimum (Ueda-Nakamura et al., 2006), Artemisia abyssinica (Tariko et al., 2010), A. absinthium L. (Tariko et al., 2011), and A. herba-alba (Mohamed et al., 2010). A. annua (Asteraceae), a well-known traditional medicinal plant, has been extensively used as antimalarial and anticancer agent. EO from A. annua has been reported to exhibit antifungal, antibacterial (Juteaua et al., 2002) and acaricidal activities (Pirali-Kheirabadi and Teixeira da Silva, 2011).
In this study, we demonstrated the leishmanicidal effect of EO from the leaves of A. annua (AALEO) on the promastigotes and amastigotes of L. donovani with negligible toxic effect on the host macrophages. We further established the in vivo efficacy of AALEO upon intraperitoneal administration to infected BALB/c mice with no impairment of hepatic and renal functions. Our results suggest that AALEO may be a source of novel agents for the treatment of VL.
MATERIALS AND METHODS
MATERIALS
Fetal bovine serum (FBS) was procured from Gibco-BRL, DMSO from SRL, methanol from Merck, limulus amebocyte lysate (LAL) kit from Pierce, Thermo Scientific and annexin V-FITC and the Apo- Direct kits from Roche Inc., Basel, Switzerland. All the other chemicals were from Sigma–Aldrich unless otherwise stated.
PARASITE CULTURE AND MAINTENANCE
Leishmania donovani parasites (MHOM/IN/83/AG83) were maintained in vivo in BALB/c mice. Promastigotes were routinely cultured at 22°C in M199 medium supplemented with 10% heat inactivated FBS, penicillin (100 IU ml-1), streptomycin (100 μg ml-1). Log phase promastigotes were sub-cultured every 72–96 h, the inoculum being 2 × 106 cells ml-1.
CELL CULTURE
Cell line, RAW 264.7 were grown at 37°C in RPMI-1640 medium (pH 7.4) supplemented with 10% heat-inactivated FBS for 48–72 h in a humidified atmosphere of 5% CO2 and sub-cultured in fresh RPMI-1640 medium at an average density 2 × 105 cells ml-1.
ANIMALS
Female BALB/c mice, aged 6–8 weeks and weighing about 20–25 g were used in the present study. All the animals were individually housed in standard size polycarbonate cages with controlled conditions of temperature (23 ± 1°C), humidity (55 ± 10%), 12:12 h of light and dark cycles and fed with a standard pellet diet (Ashirwad Industries, Chandigarh, India) and filtered water ad libitum in the Central Animal House of Jamia Hamdard according to the internationally accepted principles. A prior approval (Ethical approval judgment number 459) was obtained from the Jamia Hamdard Animal Ethics Committee (JHAEC) for the study protocol. JHAEC is registered under the Committee for the purpose of control and supervision of experiments on animals (CPCSEA).
PLANT MATERIAL AND THE ESSENTIAL OIL
Fresh A. annua leaves were collected from the Herbal Garden of Jamia Hamdard. The essential oil was extracted with a modified Clevenger-type apparatus (Borosilicate) by steam distillation (Tzenkova et al., 2010). Briefly, 1 kg of the A. annua fresh leaves were boiled in 3 L of distilled water for 4 h. At the end of this procedure, the yield obtained was 0.6% of oil on a dry weight basis (4.2 g of A. annua leaves). After extraction, the essential oil obtained was carefully separated, transferred to an opaque glass vial with rubber lid and covered with aluminum foil to protect from light. The EO was stored in a refrigerator (–20°C) prior to analysis. For biological assays, EO was reconstituted aseptically to 1 mg ml-1 in DMSO (cell culture grade) and diluted further in culture medium to achieve a final DMSO concentration of not more than 0.2%.
GC AND GC-MS ANALYSIS OF THE ESSENTIAL OIL FROM A. annua LEAVES
The chemical composition and characterization of the bio-active compounds from the essential oil of AALEO was analyzed by gas chromatography (GC) and GC-mass spectrometry (GC-MS) using a SHIMADZU QP2010 with a RTX5 MS column (30 m, film 0.25 μm, ID 0.25 mm). The temperature of the column was programmed from 45 to 270°C at 5°C min-1, the injector and detector temperatures for the analysis were 250°C. Helium was used as the carrier gas at a flow rate of 1.5 ml min-1. The identification of chemical constituents was performed on the basis of retention indices (RI) determined with reference to a homologous series of n-alkanes, under identical experimental conditions, co-injected with standards (RESTEK, 110 Benner Circle, Bellefonte, PA, USA), followed by MS library search (WILEY8.LIB and NIST08.LIB Version; Mdoe et al., 2014).
PROMASTIGOTE PROLIFERATION KINETICS AND GROWTH REVERSIBILITY ASSAY
Promastigotes (2 × 106 cells ml-1) in M199 medium were incubated at 22°C with AALEO (100 μg ml-1) in M199 containing 10% FBS. Pentamidine at the same concentration served as the reference antileishmanial drug while 0.2% DMSO, which represented the highest concentration in the test oil was used as solvent control. Parasites in media alone were taken as control. Promastigotes were enumerated daily for 7 days in a hemocytometer under phase contrast microscope using 40X objective (Islamuddin et al., 2014). Treated and untreated parasites after 7 days in culture, were washed and re-suspended in fresh medium, and allowed to grow for a further 4 days at 22°C. The viability of the parasites was determined microscopically to affirm the leishmanicidal effect of AALEO (Afrin et al., 2001).
DETERMINATION OF PROMASTIGOTE CELLULAR MORPHOLOGY
Changes in the cellular morphology of parasites as a result of AALEO treatment was observed microscopically. Briefly, the promastigotes (2 × 106 cells ml-1) were incubated at 22°C in the absence or presence of AALEO and pentamidine for 72 h at a concentration of 100 μg ml-1 and observed under 40X objective using a phase-contrast microscope. At least 20 microscopic fields were examined for each sample. Data were recorded using NIS-Elements imaging software (Paris et al., 2004).
DOSE-DEPENDENT EVALUATION OF ANTI-PROMASTIGOTE ACTIVITY AND DETERMINATION OF IC50
Promastigotes at a density of 2 × 106 cells ml-1 were incubated in the absence and or presence of AALEO at serial three fold dilutions starting at 100 μg ml-1 (100, 33.33, 11.11, 3.70, 1.23, and 0.41 μg ml-1) for 72 h at 22°C. Pentamidine was used as a standard antileishmanial drug. The mean percentage viability was calculated as follows: (Mean cell number of treated parasites/Mean cell number of untreated parasites) X 100. The 50 % inhibitory concentration (IC50) i.e., the concentration that decreased the cell growth by 50 % was determined by graphical extrapolation after plotting the graph of percent (%) viability versus concentration of the drug (Islamuddin et al., 2014).
ANTI-AMASTIGOTE ACTIVITY AND NITRITE PRODUCTION IN AN EX-VIVO MACROPHAGE–MODEL
Macrophages from the RAW 264.7 cell line (1 × 106 cells ml-1) were allowed to adhere to round glass cover slips in a 24-well tissue culture plate and incubated for 12 h at 37°C in a carbon dioxide incubator (5% CO2). After removal of the non-adherent macrophages, the cells were infected with stationary phase promastigotes using a cell to parasite ratio of 1:10 and incubated at 37°C for 24 h. The non-phagocytosed parasites were removed by washing, and the infected macrophages incubated with AALEO (0–100 μg ml-1) for an additional 48 h. The cover slips were fixed in methanol and giemsa-stained for microscopic evaluation (100X) of amastigote infectivity. At least 200 macrophages per cover slip were analyzed, and the concentration that decreased amastigotes by 50% (IC50) was determined (Islamuddin et al., 2014).
The supernatant from control L. donovani infected and treated macrophages (RAW 264.7) were analyzed for nitrite content by the Griess reaction. In brief, equal volume of Griess reagent [1% sulphanilamide and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride in 5% phosphoric acid] was added to the culture supernatant and the plates were incubated for 10 min at room temperature using sodium nitrite as a standard. The absorbance at 550 nm was measured, and the concentration of nitrite was calculated by a linear regression analysis using the standard curve generated with sodium nitrite (Varela et al., 2012). All the reagents including AALEO, were free of lipopolysaccharide (0.2 ng ml-1 endotoxin) as determined by LAL chromogenic endotoxin quantitation kit as per the manufacturer’s instructions (Pierce, Thermo Scientific).
EX VIVO CYTOTOXICITY ASSAY
To evaluate the adverse toxicity of AALEO on mammalian cells, the murine macrophage cell line (RAW 264.7) cultured in RPMI 1640 medium were exposed to increasing concentrations of AALEO (0–200 μg ml-1) at 37°C, 5 % CO2 for 48 h. Pentamidine as the reference drug, and macrophages without any treatment were taken as control. The percentage of cell viability was evaluated by MTT (3-{4,5-dimethylthiazol-2-yl}-2,5-diphenyltetrazolium bromide) assay (Das et al., 2008).
ANALYSIS OF PHOSPHATIDYLSERINE (PS) EXTERNALIZATION
To visualize PS exposure, double staining with Annexin-V and PI was performed with minor modifications. Briefly, promastigotes (2 × 106 cells ml-1) were incubated with AALEO at a concentration of 100 μg ml-1 for 72 h. Untreated and treated parasites were centrifuged at 3000 × g for 10 min, washed twice in cold PBS and the pellet was resuspended in Annexin V-FLUOS labeling solution (100 μl, containing both Annexin-V and PI), as per the manufacturer’s instructions (Roche). After 15 min of incubation in the dark at 26°C, 400 μl of incubation buffer was added, mixed and acquired using a BD LSR II flow cytometer and analyzed using Deva software. Pentamidine served as the reference antileishmanial drug (100 μg ml-1) while parasites without any treatment were taken as control.
IN SITU DETECTION OF DNA FRAGMENTATION BY TERMINAL DEOXYNUCLEOTIDYL TRANSFERASE (TdT) MEDIATED dUTP NICK-END LABELING (TUNEL) ASSAY
In situ detection of DNA fragments within the promastigotes was analyzed by TdT-mediated dUTP nick-end labeling (TUNEL) assay using an in situ cell death detection kit (Roche) according to the manufacturer’s instructions. Briefly, promastigotes (2 × 106 cells ml-1) were incubated at 22°C with AALEO (100 μg ml-1), pentamidine (100 μg ml-1) as the reference drug and with media alone as control. After 72 h, the cells were washed, fixed with 4% paraformaldehyde on ice for 1 h, washed with PBS and incubated with 3% H2O2 in methanol for 10 min at 25°C. This was followed by washing with PBS and the cells were then permeabilized with freshly prepared chilled 0.1% Triton X-100 for 5 min on ice. The cells were washed twice with PBS, after which 50 μl of reaction mixture containing TdT and FLUOS labeled dUTP was added for 1 h at 37°C, washed and finally resuspended in PBS for acquisition in a BD LSR II flow cytometer and analyzed using Deva software.
In a parallel experiment, the stained samples were visualized under a high-resolution fluorescence microscope under 10× objective, and the images captured and processed.
STUDY OF DYSKINETOPLASTIDY BY FLUORESCENCE MICROSCOPY
To detect the changes in kinetoplast DNA (kDNA) of promastigotes, untreated and AALEO (100 μg ml-1) treated exponential-phase promastigotes (2 × 106 cells ml-1) after 72 h were washed twice with PBS, fixed with 80% chilled ethanol and kept at 4°C for 24 h. The cells were then washed with PBS and the pellet was resuspended in 500 μl DNase-free RNase (200 μg ml-1) for 1 h at 37°C. The cells were stained with PI (50 μg ml-1) and incubated in the dark for 20 min at 4°C. An aliquot of 20 μl was taken from each sample, placed on a glass slide and observed directly under 20× objective (without fixation) under a high-resolution fluorescence microscope (Nikon). The images were processed using NIS-Elements imaging software. At least 20 microscopic fields were observed for each sample (Singh et al., 2009).
EFFECT OF AALEO ON CELL CYCLE
Percentage of cells in the different phases of cell cycle was detected through flow cytometry by propidium iodide (PI) staining. Briefly, promastigotes (2 × 106cells ml-1) were treated with AALEO (100 μg ml-1) for 72 h at 22°C, washed twice with PBS, fixed with 80% chilled ethanol and kept at 4°C for 24 h. The cells were then washed with PBS and the pellet resuspended in 500 μl DNase free RNase A (200 μg ml-1) for 1 h at 37°C. The cells were stained with PI (50 μg ml-1) and incubated in the dark for 20 min at 4°C. Data acquisition was carried out using BD FACS Calibur flow cytometer and analyzed using Cell Quest software (Queiroz et al., 2014).
MITOCHONDRIAL MEMBRANE POTENTIAL (ΔΨm) DETERMINATION
The mitochondrial transmembrane potential (ΔΨm) was measured using a fluorogenic cell-permeable cationic dye, JC-1 (5,59,6,69-tetrachloro-1,19,3,39-tetraethylbenzimidazolylcarbo-cyanine iodide). To analyse the changes in ΔΨm, the promastigotes after exposure to AALEO (100 μg ml-1) for 72 h were centrifuged, resuspended in PBS containing JC-1 (10 μg ml-1) and incubated at 37°C for 10 min. The cells were then analyzed by flow cytometry, BD LSR II and DEVA software. The ratio of the red/green fluorescence intensities was considered as the relative ΔΨm value (Roy et al., 2008).
ESTIMATION OF INTRACELLULAR REACTIVE OXYGEN SPECIES (ROS) LEVELS
To monitor the effect of AALEO on the generation of reactive oxygen species (ROS), late log phase promastigotes were treated with AALEO at 22°C for 72 h. The cells were washed twice with PBS and finally resuspended in 500 μl PBS, loaded with a cell permeant probe, 2, 7 dichlorodihydrofluorescein diacetate (H2DCFDA; 10 μM) and incubated for 30 min at 25°C. The cells were analyzed for intracellular ROS by BD LSR II flow cytometer and DEVA software (Misra et al., 2009).
IN VIVO EFFICACY OF AALEO AGAINST L. donovani
Female BALB/c mice (6 to 8-weeks old) were infected with stationary phase promastigotes (2 × 107 per animal) through tail vein. Two months post infection, parasite burden was confirmed in two arbitrarily selected animals; after which, the mice were randomly assigned into four groups. Group-A comprised of control, infected mice without treatment (INF), vehicle control Group-B received normal saline (VC). Group-C was administered AALEO intraperitoneally (i.p.) and further divided into three subgroups (n = 5) that received three doses of AALEO (50, 100, 200 mg/kg body weight {b.w.}) for ten consecutive days. Group-D received the standard antileishmanial drug, AMB (5 mg/kg b.w. for 10 alternate days, intravenously {i.v.}) and served as the positive control. Ten days post treatment, mice were euthanized by CO2 asphyxiation, the liver and spleen parasite burdens were determined from giemsa-stained multiple impression smears and expressed as Leishman-Donovan units (LDU) that was calculated as the number of parasites per 500 nucleated cells × organ weight (mg) (Dutta et al., 2008).
The percent reduction in parasite load (% protection) was calculated using the formula.
Cure or protection correlated with a fall in hepatosplenomegaly and elimination of parasites to negligible levels.
IN VIVO TOXICITY ASSAY
Hepatic and renal functions of BALB/c mice were evaluated in treated and untreated animals as described previously (Agarwal et al., 2012). Two-weeks post treatment, blood was drawn from the retro-orbital plexus and serum was separated by centrifugation at 5000 × g for 2–3 min. The serum was stored at -80°C until use. The hepatic and renal functions were assessed by measuring the levels of serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), and alkaline phosphatase (ALP), urea and creatinine using commercially available kits (Span Diagnostics Ltd.). The hepatic and renal functions were similarly evaluated in normal BALB/c mice at higher (200 mg/kg b.w.) dose of AALEO.
STATISTICAL ANALYSIS
The in vitro experiments were performed at least in triplicate. A minimum of five mice per group was used for all in vivo experiments. Error bars represent the SEM.
The statistical significance of differences between the groups was determined as described in the figure legends using ANOVA followed by Tukey’s test by Graph Pad Prism 5 software, and a p-value of <0.05 was considered statistically significant.
RESULTS
GC AND GC-MS ANALYSIS OF AALEO
The composition of the essential oil exhibiting leishmanicidal activity was studied by GC and GC-MS. The major chemical constituent was identified as Camphor; 52.06% (Table 1); by comparison of mass spectral data, retention times (RT), and RI. The other constituents present in the essential oil were β-caryophyllene (10.95 %), 1,8-cineole (5.57 %), β-caryophyllene oxide (4.21 %), β-farnesene (3.83 %), α-copaene (2.91 %), β-cyperone (1.93 %), α-selinene (1.54 %), and trans-pinocarveol (1.22 %) as presented in Table 1.
Table 1.
Phytochemical fingerprinting of AALEO by GC-MS.
| Peak | Retention time (RT) | Retention index (RI) | Area | Area% | Compound |
|---|---|---|---|---|---|
| 1 | 3.250 | 876 | 73280 | 0.50 | β-Pinene |
| 2 | 4.549 | 929 | 810646 | 5.57 | 1,8-Cineole |
| 3 | 5.586 | 972 | 167764 | 1.15 | o-Cymol |
| 4 | 5.683 | 976 | 38352 | 0.26 | Isovaleric acid |
| 5 | 9.449 | 1093 | 47296 | 0.33 | 1-Octen-3-ol |
| 6 | 9.990 | 1108 | 41392 | 0.28 | α-Cubebene |
| 7 | 11.028 | 1133 | 422708 | 2.91 | a-Copaene |
| 8 | 11.672 | 1149 | 7573773 | 52.06 | Camphor |
| 9 | 12.269 | 1164 | 47711 | 0.33 | 3-Caren-10-al |
| 10 | 13.772 | 1201 | 1593527 | 10.95 | β -Caryophyllene |
| 11 | 14.451 | 1217 | 38244 | 0.26 | δ-Myrtenal |
| 12 | 15.073 | 1232 | 177125 | 1.22 | (E)-Pinocarveol |
| 13 | 15.308 | 1238 | 54947 | 0.38 | Germacrene B |
| 14 | 15.540 | 1243 | 556838 | 3.83 | β-Farnesene |
| 15 | 15.784 | 1249 | 224008 | 1.54 | α-Selinene |
| 16 | 16.035 | 1255 | 49450 | 0.34 | β-Chamigrene |
| 17 | 16.119 | 1257 | 65761 | 0.45 | g-Muurolene |
| 18 | 16.248 | 1260 | 50529 | 0.35 | α-Terpineol |
| 19 | 16.330 | 1262 | 80643 | 0.55 | Borneol |
| 20 | 16.690 | 1270 | 165303 | 1.14 | α-Amorphene |
| 21 | 16.965 | 1277 | 280737 | 1.93 | β-Cyperone |
| 22 | 17.298 | 1285 | 41837 | 0.29 | S (+)-Carvone |
| 23 | 17.945 | 1300 | 78707 | 0.54 | δ-Cadinene |
| 24 | 18.358 | 1310 | 25951 | 0.18 | (E)-Carveol |
| 25 | 18.636 | 1316 | 32965 | 0.23 | 1,3-Dimethyladamantane |
| 26 | 18.761 | 1319 | 44074 | 0.30 | Myrtenol |
| 27 | 19.907 | 1347 | 75486 | 0.52 | (Z)-Carveol |
| 28 | 20.285 | 1356 | 18366 | 0.13 | p-Cymen-8-ol |
| 29 | 20.807 | 1368 | 41234 | 0.28 | Benzyl valerate |
| 30 | 21.408 | 1383 | 49028 | 0.34 | (2E)-2-Methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butenal |
| 31 | 22.335 | 1405 | 20933 | 0.14 | Vulgarone B |
| 32 | 23.518 | 1434 | 49521 | 0.34 | Viridiflorol |
| 33 | 23.791 | 1441 | 611878 | 4.21 | β -Caryophyllene oxide |
| 34 | 24.383 | 1455 | 54592 | 0.38 | Ethyl chrysanthemate |
| 35 | 24.989 | 1470 | 107844 | 0.74 | Isoaromadendrene epoxide |
| 36 | 26.576 | 1510 | 78911 | 0.54 | Cubenol |
| 37 | 27.128 | 1524 | 143531 | 0.99 | Spathulenol |
| 38 | 28.175 | 1552 | 34667 | 0.24 | β-Acoradiene |
| 39 | 28.495 | 1560 | 28088 | 0.19 | (-)-Globulol |
| 40 | 28.822 | 1568 | 127617 | 0.88 | Longifolenaldehyde |
| 41 | 29.291 | 1580 | 258844 | 1.78 | Diepicedren-1-oxide |
| 42 | 43.679 | 1972 | 63369 | 0.44 | β-Panasinsene |
| 14547477 | 100.00 |
Retention index as determined on a RTX5 MS column using the homologous series of n-alkanes. The major constituents are represented in bold letters.
AALEO MEDIATES DEATH IN PROMASTIGOTES
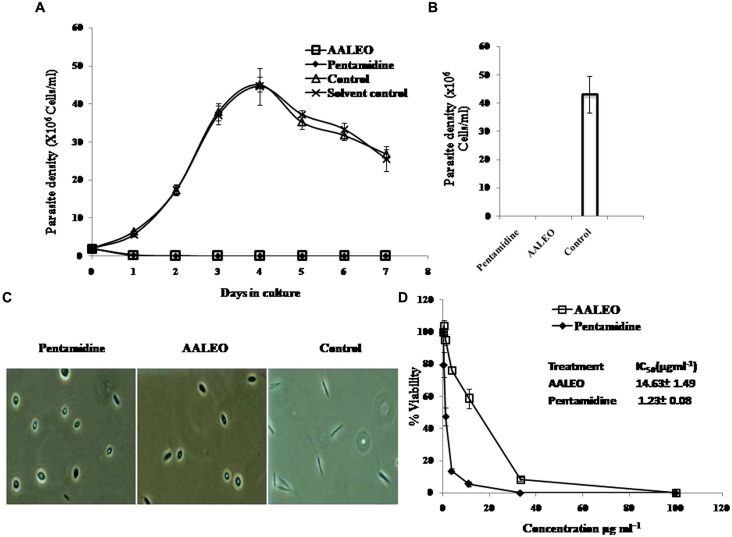
The anti-proliferative effect of AALEO on promastigotes was studied by monitoring the kinetics of cell growth. AALEO exhibited a time-dependent inhibition in the rate of cell proliferation at a concentration of 100 μg ml-1. All the cells appeared dead after 72 h of incubation (Figure 1A). Pentamidine used as a positive control, showed a similar trend in time-dependent parasite killing. Untreated promastigotes (without any drug) proliferated at a normal rate. Solvent control (0.2% DMSO) showed no adverse effect on the promastigote viability.
FIGURE 1.
Anti-promastigote activity of Artemisia annua leaves (AALEO). Log phase Leishmania donovani promastigotes were incubated with AALEO (100 μg ml-1) for 7 days at 22°C as described in “Materials and Methods.” (A) Viable parasites enumerated microscopically every day (B) promastigote growth reversibility analysis after 4 days of culture, post AALEO withdrawal (C) morphological analysis of promastigotes by light microscopy (40×) at 72 h post-treatment and (D)% viability ascertained upon incubation of promastigotes for 72 h with increasing concentrations of AALEO (0–100 μg ml-1) for IC50 determination (inset). Each point or bar corresponds to the mean ± SE of triplicate samples and is representative of one of three independent experiments.
LEISHMANICIDAL ASSAY OF THE TREATED PROMASTIGOTES
To corroborate the lethal effects of AALEO on promastigotes, treated and untreated parasites after 4 days were washed and re-suspended in fresh media and their viability was determined under phase contrast microscope. After an additional 4 days in culture, the parasites did not revert back to their normal morphology and still appeared dead in case of prior incubation with AALEO, as also observed in positive control (pentamidine), confirming their leishmanicidal effects. Untreated control promastigotes reverted to late log phase (Figure 1B).
AALEO CAUSES ALTERATIONS IN CELLULAR MORPHOLOGY OF PROMASTIGOTES
Microscopic inspection of promastigotes upon treatment with AALEO (100 μg ml-1) revealed that the parasites altered to an ovoid shape with cell shrinkage, loss of flagella, cytoplasmic condensation, resulting in complete circularization of the promastigotes and substantial reduction in size compared to the untreated control. These changes, typical of programmed cell death (PCD; Figure 1C), were also observed in pentamidine treated parasites.
EVALUATION OF IC50 OF AALEO AGAINST L. donovani PROMASTIGOTES
Artemisia annua leaves (0–100 μg ml-1) treatment demonstrated a dose-dependent killing of the promastigotes and a 50% inhibitory concentration was achieved at 14.63 ± 1.49 μg ml-1 (Figure 1D). The established antileishmanial drug pentamidine, used as a positive control, showed a similar trend in parasite killing. Parasite viability was not affected by DMSO (0.2%, data not shown) used as solvent control.
ANTI-AMASTIGOTE ACTIVITY OF AALEO
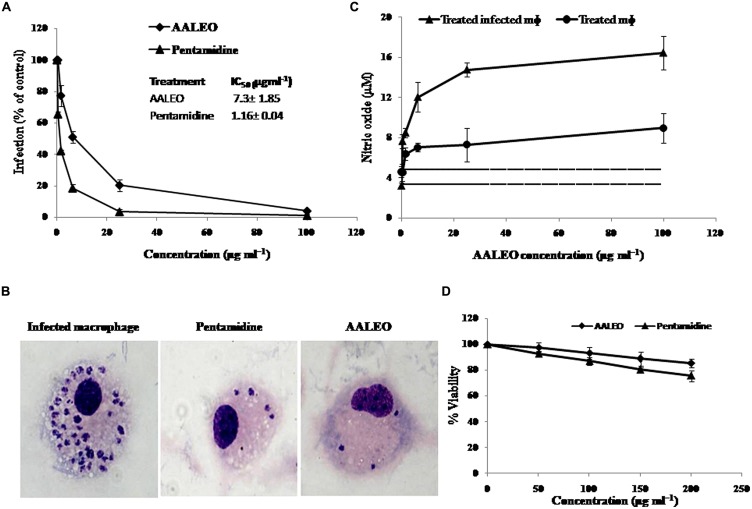
The activity of AALEO on phagocytosed amastigotes within the macrophages was analyzed by microscopic observation of giemsa-stained cells. Macrophages entry by promastigotes involves the formation of membrane bound parasitophorous vacuoles, where they transform into non-motile amastigote form. The parasites survival within the parasitophorous vacuoles determines the pathogenesis. Therefore, it was important to test the activity of AALEO toward the macrophage-resident amastigotes. Treatment with AALEO (0–100 μg ml-1) demonstrated a dose-dependent inhibition of amastigote infectivity with IC50 of 7.3 ± 1.85 μg ml-1 (Figure 2A). Giemsa-stained micrographs of infected macrophages showed almost total clearance of the intracellular amastigotes at the highest dose (100 μg ml-1); similar effect was observed with pentamidine (Figure 2B).
FIGURE 2.
Effect of AALEO on L. donovani intracellular amastigote forms. Promastigote-infested RAW 264.7 macrophages were incubated at 37°C in a CO2 incubator with serial fourfold dilutions of AALEO (starting at 100 μg ml-1) for 48 h. (A)% infectivity estimated for determination of IC50 on amastigotes (inset) (B) Micrographs of giemsa-stained infected macrophages upon treatment (100×) (C) NO generation by uninfected (filled circle) or infected (filled triangle) macrophages post-incubation with AALEO. The dotted and bold dotted lines represent the basal levels of NO produced by uninfected and infected macrophages, respectively. (D) Cytotoxicity of AALEO to RAW 264.7 macrophages ascertained as % viability 48 h post-incubation with increasing concentrations of AALEO or pentamidine (0–200 mg ml-1). Each point corresponds to the mean ± SE of triplicate samples and is representative of one of three independent experiments.
AALEO INDUCES NITRIC OXIDE PRODUCTION IN EX VIVO MODEL
Estimation of nitric oxide (NO) in the culture supernatants of murine non-parasitized and parasitized macrophages (RAW 264.7) indicated low basal levels of NO in the parasitized macrophages corroborating with disease progression. Following addition of AALEO (0–100 μg ml-1) for 48 h, a dose-dependent increase in NO production occurred in the infected macrophages (Figure 2C) that was distinctly higher than that released by normal macrophages. AALEO at 100 μg ml-1 induced 8.96 ± 1.45 and 16.43 ± 1.68 μM of NO in normal and infected macrophages, respectively.
CYTOTOXICITY OF AALEO ON RAW 264.7 MACROPHAGES CELL LINE
In vitro cytotoxicity assay was done with the murine macrophage cell line RAW 264.7 to check the adverse effects of AALEO, using pentamidine as a reference drug. The toxicity assay revealed that AALEO had no adverse effects on the viability and morphology of the macrophages (Figure 2D).
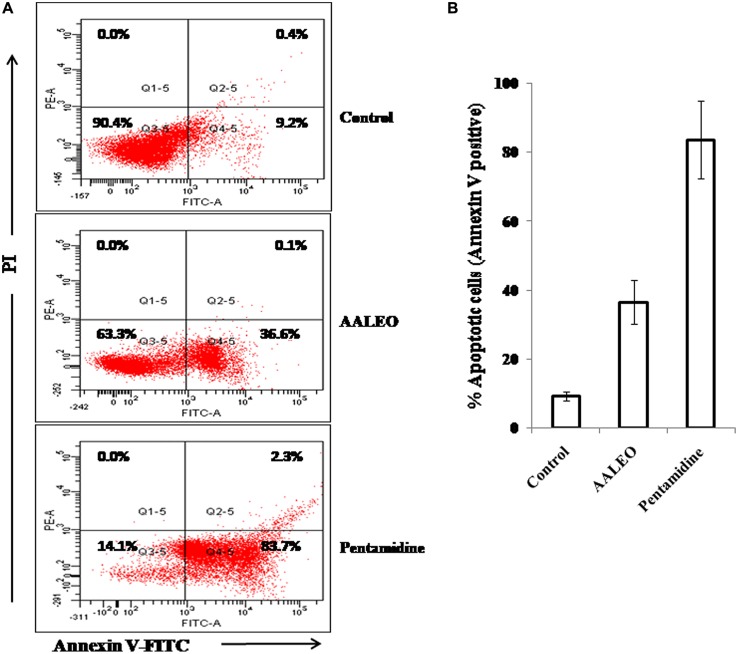
AALEO INDUCES PS EXTERNALIZATION IN L. donovani PROMASTIGOTES
During apoptosis in unicellular parasitic cells, PS is translocated from the inner side to the outer leaflet of the plasma membrane. Annexin V, a Ca2+-dependent phospholipid binding protein with an affinity for PS, is routinely used to label externalization of PS. Since annexin V can also label necrotic cells, PI was used to differentiate among apoptotic cells (annexin V +ve and PI –ve), necrotic cells (annexin V –ve and PI +ve), late apoptotic cells (annexin V +ve and PI +ve) and live cells (annexin V –ve and PI –ve). In control late log phase promastigotes, the basal binding of annexin V was 9.2%. Whereas, with AALEO, the annexin V binding (a correlate of apoptotic cells) increased to 36.6% and, to 83.7% with pentamidine (Figures 3A,B). Taken together, our data indicate that AALEO caused apoptosis in promastigotes with externalization of PS.
FIGURE 3.
Detection of apoptosis in L. donovani promastigotes by annexin V-FITC and PI co-staining. Parasites were incubated with AALEO or pentamidine (100 μg ml-1) for 72 h, and analyzed by flow cytometry as described in the Methods. (A) Dual parametric dot plots combining Annexin V-FITC and PI fluorescence, (B) % apoptotic cell population. This is a representative profile of three independent experiments.
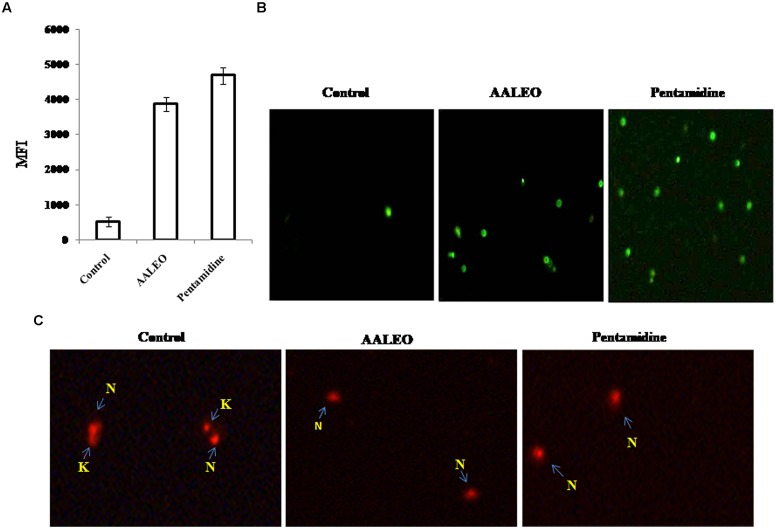
OLIGONUCLEOSOMAL DNA FRAGMENTATION IN AALEO TREATED PROMASTIGOTES
One of the important biochemical hallmarks of eukaryotic apoptotic cell death is the fragmentation of nuclear DNA into nucleosomal units. DNA nicking following treatment with AALEO was detected using a TUNEL assay in which the proportion of DNA nicks was quantified by measuring the binding of dUTP–FLUOS to the nicked ends via TdT. Thus, the proportion of DNA nicks was directly proportional to the fluorescence obtained from dUTP–FLUOS. Promastigotes treated with 100 μg ml-1 of AALEO for 72 h showed an increase in nuclear DNA fragmentation as evidenced from dUTP–FLUOS binding. The mean fluorescence intensity (MFI) of untreated promastigotes (522) increased to 4694 and 3876 upon treatment with AALEO and pentamidine, respectively (Figure 4A). The results clearly indicate that AALEO caused DNA nicking in promastigotes.
FIGURE 4.
Artemisia annua leaves treated promastigotes represent TdT-mediated dUTP nick-end labeling (TUNEL) positivity and dyskinetoplastidy. Exponential phase promastigotes were incubated with AALEO or pentamidine (100 μg ml-1) for 72 h. Cells were fixed and stained with dUTP–FITC in the presence of TdT. (A) Bar graphs representing MFI of TUNEL expression as analyzed by flow cytometry (B) Image acquisition via a fluorescence microscope under 10× objective. (C) Treated and untreated parasites were probed with PI for assessment of dyskinetoplastidy and images taken under 20× objective as described in Section “Materials and Methods.” N and K represent nuclear and kinetoplast DNA, respectively. Each bar corresponds to the mean ± SE of triplicate samples and is a representative profile of three independent experiments. Images are representative of one of three similar results.
Fluorescence microscopic observation showed the appearance of green fluorescence in treated samples, representing incorporation of dUTP labeled with FLUOS (Figure 4B). Thus, the results further confirm AALEO-mediated induction of apoptosis in L. donovani promastigotes.
AALEO-INDUCED CYTOTOXICITY CAUSES DYSKINETOPLASTIDY IN PROMASTIGOTES
The kDNA has been shown to be susceptible to elimination (dyskinetoplastidy) by certain DNA intercalating drugs for example, acriXavine and berenil. We observed that AALEO induced genomic DNA fragmentation in L. donovani promastigotes. Based on this, we investigated whether AALEO caused any damage to kDNA. Untreated and promastigotes treated with AALEO (100 μg ml-1) were permeabilized and stained with PI that binds with DNA of the promastigotes, i.e., kDNA and nuclear DNA. The loss of mitochondrial kDNA was detected at 72 h of treatment with approximately all the cells showing dyskinetoplastic feature (Figure 4C) against the intact kDNA structure in untreated cells.
AALEO-INDUCED APOPTOSIS CAUSES CELL-CYCLE ARREST IN L. donovani PROMASTIGOTES
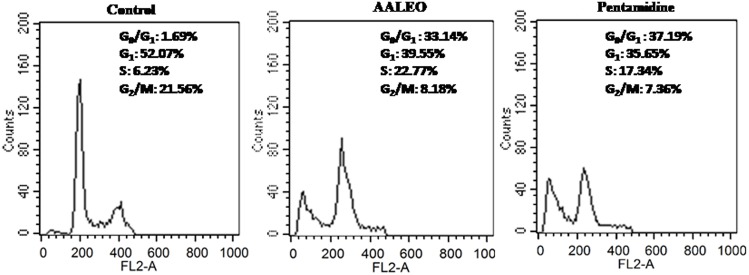
The changes in cell cycle progression in L. donovani promastigotes were examined by flow cytometric analysis of permeabilized and PI stained cells after treatment with AALEO (100 μg ml-1) for 72 h. In a given cell, the amount of PI correlates with the DNA content, and accordingly, DNA fragmentation in an apoptotic cell translates into a sub G0–G1 peak (Sarkar et al., 2008). Promastigotes treated with AALEO caused cell cycle arrest at sub G0–G1 phase (Figure 5). The proportion of cells in the sub G0–G1 phase was 1.69% in the untreated control, which increased to 33.14 and 37.19%, respectively, in the promastigotes treated with AALEO and pentamidine. Thus, the data clearly show that AALEO induced cell cycle arrest at sub G0–G1 phase.
FIGURE 5.
Artemisia annua leaves induces cell cycle arrest in L. donovani promastigotes at sub-G0–G1 phase. After treatment with AALEO and pentamidine (100 μg ml-1) for 72 h, the promastigotes were fixed in chilled methanol, probed with PI and the cells acquired using a BD LSR-II flow cytometer for subsequent analysis using Cell Quest software as described in Section “Materials and Methods.” Inset shows the cell cycle distribution. This is the representative profile of three independent experiments.
AALEO INDUCES DEPOLARIZATION OF MITOCHONDRIAL MEMBRANE (Ψm)
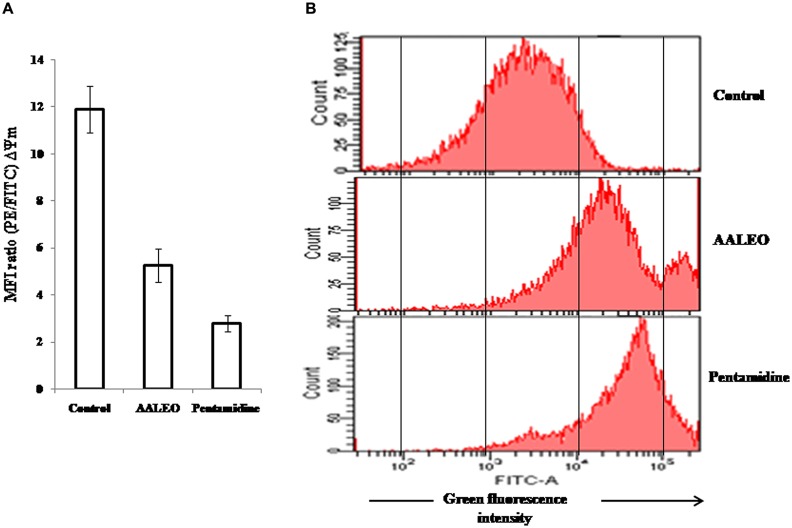
Alteration of mitochondrial membrane potential (ΔΨm) is a characteristic feature of metazoan apoptosis and has been show to play a key role in drug-induced death in the unicellular protozoan parasites (Sen et al., 2007). The loss of ΔΨm was determined using a mito-sensor dye, JC-1. JC-1 is a cationic lipophilic fluorescent dye that freely permeates the mitochondrial membranes and forms J-aggregates in healthy cells, exhibiting pronounced red fluorescence. An apoptotic stimulus induces a decrease in the ΔΨm and JC-1 fails to enter the mitochondria, remaining as cytosolic monomers and emits green fluorescence. Therefore, the ratio of J-aggregates/monomers serve as an effective indicator of the mitochondrial energy state of the parasites, allowing apoptotic cells to be easily distinguished from their non-apoptotic counterparts (Verma et al., 2007). In control promastigotes, the red/green fluorescence ratio was 11.9. Treatment with AALEO (100 μg ml-1) and pentamidine for 72 h, induced a significant decrease in ΔΨm, resulting in a predominance of JC-1 monomers that fluoresced green, thus, translating into a decrease in the red/green fluorescence ratio to 5.27 and 2.79, respectively (Figure 6A).
FIGURE 6.
Collapse of mitochondrial membrane potential and ROS generation in L. donovani promastigotes by AALEO. Exponential phase promastigotes were incubated with AALEO and pentamidine (100 μg ml-1) for 72 h and (A) probed with JC-1; bar diagram represents the ratio of red/green fluorescence intensity (MFI) obtained from histogram statistics. (B) Histograms depict shift in green fluorescence intensity (FL-1) from left, depicting increased production of ROS in treated promastigotes loaded with H2DCFDA. Each bar corresponds to the mean ± SE of triplicate samples and histograms are representative profiles of three independent experiments.
AALEO INDUCES PRO-OXIDANT ACTIVITY IN L. donovani PROMASTIGOTES
To evaluate the effect of AALEO on the oxidative status of promastigotes, H2DCFDA, a lipid soluble, membrane permeable compound was used that following cleavage by cellular non-specific esterases, forms an impermeable H2DCF, which is subsequently oxidized by intracellular ROS to produce a fluorescent compound, DCF. Therefore, the resultant fluorescence (green) is directly proportional to the quantum of ROS generated. AALEO (100 μg ml-1) treatment of promastigotes for 72 h led to significantly enhanced ROS levels with increase in the green fluorescence intensity (FI-1), in comparison to the control untreated parasites as observed by flow cytometry (Figure 6B).
AALEO ACTIVITY AGAINST L. donovani IN VIVO
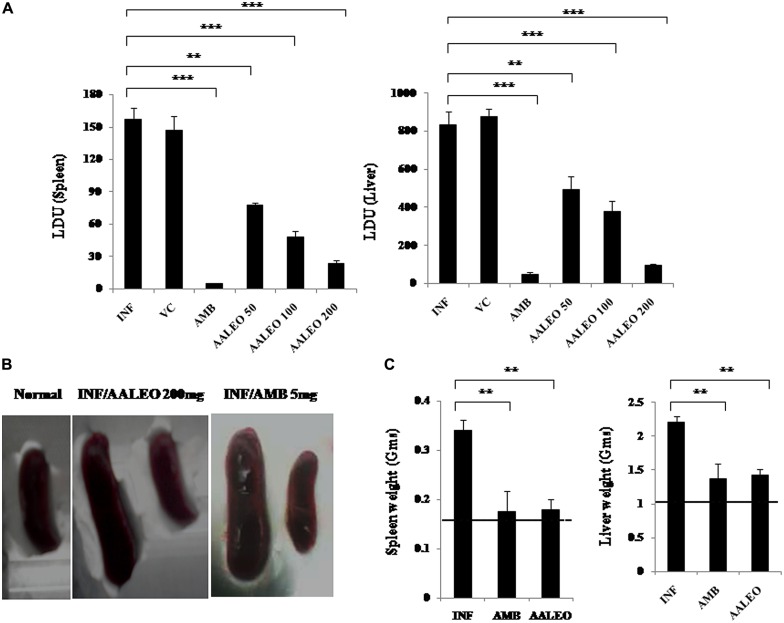
Treatment of L. donovani infected BALB/c mice with AALEO at 50, 100, and 200 mg/kg b.w. resulted in 45.26 ± 8.95, 63.23 ± 7.28, and 88.68 ± 5.52 % parasite reduction, respectively, in the liver, while decrease in the splenic parasite load was more evident, being 72.48 ± 3.88, 80.72 ± 6.61, and 91.66 ± 3.07 %, respectively (Figure 7A). With AMB, parasite elimination in the liver and spleen was 94.02 ± 1.81 and 98.09 ± 2.44 %, respectively. Compared to the infected controls, a significant reduction in spleen and liver weight was also observed with AALEO (200 mg/kg b.w.) and AMB (Figure 7C). Morphological observation of spleen in the treated groups also demonstrated reduction in size that was comparable to the naïve group (Figure 7B).
FIGURE 7.
Antileishmanial efficacy of AALEO on established L. donovani infection. Ten weeks infected BALB/c mice were left untreated (INF) or treated for 10 days with PBS (VC), AALEO (50, 100, or 200 mg/kg bw, daily, i.p.) or amphotericin B (AMB; 5 mg/kg bw, alternate days, i.v.) as elaborated in Section “Materials and Methods.” Ten days post-treatment (A) splenic and hepatic parasite burden (LDU) were determined by stamp smear method, (B) morphology of spleen, and (C) weights of liver and spleen were also determined. The dotted lines indicate the respective organ weights of naïve mice. Data represent the mean ± SE for five animals per group. Data were tested by ANOVA. Differences between means were assessed for statistical significance by Tukey’s test (**P≤ 0.01; ***P≤ 0.001). Results are from one of three representative experiments.
IN VIVO TOXICITY STUDIES
Estimation of serum SGOT, SGPT, and ALP for liver dysfunction and urea and creatinine for renal dysfunction, 10 days post injection of AALEO in normal BALB/c mice as well as infected and treated mice demonstrated normal levels of the serum enzymes. AALEO (up to 200 mg/kg b.w.) proved to be non-toxic in BALB/c mice used in antileishmanial screening (Tables 2A,B) indicating no in vivo toxicity.
Table 2.
(A) Effect of AALEO on hepatic and renal functions of normal BALB/c mice. (B) Effect of AALEO on hepatic and renal functions of infected BALB/c mice post-treatment.
| (A) | |||||
| Group (n = 5) | SGOT (U/L) | SGPT (U/L) | ALP (U/L) | Urea (mg/dL) | Creatinine (mg/dL) |
| Control | 52.40 ± 6.18 | 33.09 ± 10.06 | 86.14 ± 1.59 | 18.12 ± 3.17 | 0.96 ± 0.15 |
| Essential oils (EO) 200 mg/kg (i.p) | 53.16 ± 4.56 | 33.18 ± 4.36 | 87.18 ± 7.96 | 16.79 ± 4.91 | 0.84 ± 0.08 |
| Mice (n = 5) received EO daily for 10 consecutive days. Enzymatic estimations (mean ± SE) were done using commercial kits. | |||||
| (B) | |||||
| Group (n = 5) | SGOT (U/L) | SGPT (U/L) | ALP (U/L) | Urea (mg/dL) | Creatinine (mg/dL) |
| Infected control | 48.40 ± 5.58 | 29.78 ± 3.77 | 89.42 ± 3.8 | 15.86 ± 2.60 | 1.16 ± 0.39 |
| AmB 5 mg/kg (i.v) | 53.93 ± 2.78 | 38.10 ± 1.16 | 85.36 ± 1.7 | 18.19 ± 1.90 | 0.99 ± 0.14 |
| EO 50 mg/kg (i.p) | 45.60 ± 5.39 | 29.99 ± 5.62 | 83.19 ± 2.9 | 16.24 ± 0.96 | 0.89 ± 0.11 |
| EO 100 mg/kg (i.p) | 42.43 ± 4.29 | 33.21 ± 3.07 | 87.31 ± 11.6 | 18.96 ± 1.93 | 1.00 ± 0.16 |
| EO 200 mg/kg (i.p) | 51.60 ± 0.56 | 31.78 ± 3.36 | 84.18 ± 5.9 | 17.49 ± 1.69 | 1.09 ± 0.10 |
Mice (n = 5) received EO daily for 10 consecutive days. Enzyme estimations (mean ± SE) were done using commercial kits.
DISCUSSION
Natural products, especially EO from the aromatic herbs, have shown a wide spectrum of biological activities including antifungal (Kordali et al., 2008) antibacterial (Silva et al., 2010) and antiprotozoal (Machado et al., 2010) among others. Plant EO and active components can be used as alternatives or adjunct to current antiparasitic therapies. Leishmanicidal activity of EO has recently been documented (Rodrigues et al., 2013; Sanchez-Suarez et al., 2013) such as linalool-rich essential oil from leaves of Croton cajucara (Rosa et al., 2003), eugenol-rich essential oil from Ocimum gratissimum (Ueda-Nakamura et al., 2006) and sesquiterpenes-rich essential oil from Eugenia uniflora (Rodrigues et al., 2013) that have been shown to be cytotoxic against L. amazonensis parasites. EO from A. herba-alba and A. annua have been reported to exhibit antifungal, and antibacterial activities (Juteaua et al., 2002). In the present study, we reported antileishmanial activity of the essential oil from AALEO. The essential oil of A. absinthium, having oxygenated monoterpene camphor as the major constituent has been reported to exhibit activity against the promastigotes and amastigote forms of L. aethiopica and L. donovani (Tariko et al., 2011). Our findings are in agreement with these studies as the GC-MS analysis of AALEO revealed significantly higher content of camphor (52.06%) followed by β-caryophyllene (10.95%).
The camphor-rich essential oil of A. annua exhibited a dose-dependent significant antileishmanial effect against the promastigotes as well as the intracellular amastigotes of L. donovani. The effect was leishmanicidal as evidenced by growth reversibility analysis and alterations in cellular morphology including shrinkage in the promastigotes that became round in shape with disrupted flagella and had no motility.
The leishmanicidal activities of the plant secondary metabolites such as curcumin (Das et al., 2008), racemoside A (Dutta et al., 2007), artemisinin (Sen et al., 2007), Aloe vera leaf exudates (Dutta et al., 2008), and ethanolic extract of leaves of Piper betle (Sarkar et al., 2008) have been reported to be mediated by apoptosis. Leishmania parasites can undergo PCD in response to natural compounds and EO (Dutta et al., 2007; Sen et al., 2007; Das et al., 2008; Misra et al., 2009; Islamuddin et al., 2014). The mode of cell-death caused by AALEO was further investigated, since to the best of our knowledge, no such study on AALEO or camphor has been reported earlier.
During PCD in unicellular parasites, PS is externalized from the inner to the outer surface of the plasma membrane. In our studies, AALEO-treated L. donovani promastigotes also caused PS exposures at a significant rate as evidenced by the increased annexin V binding. The changes occurring in the nuclear material during PCD were further characterized. In situ TUNEL of nicked DNA was observed with AALEO thus, strongly substantiating apoptosis in L. donovani promastigotes. This has also been evidenced with essential oil from Piper betle and Syzygium aromaticum (Misra et al., 2009; Islamuddin et al., 2014).
DNA fragmentation in an apoptotic cell translates into a sub G0–G1 peak. Cell cycle analysis with PI showed a significant increase in the proportion of cells in the sub G0–G1 phase when treated with AALEO. This confirmed that the apoptotic cell was arrested at the sub G0–G1 phase. Similar observation has been reported with other natural antileishmanial molecules such as curcumin, artemisinin and berberine chloride, wherein, a substantial population of cells have been identified in the sub G0–G1 phase (Sen et al., 2007; Das et al., 2008; Saha et al., 2009; Islamuddin et al., 2014).
The mitochondria of the parasites seem to be central to the cytotoxic action of different natural and synthetic molecules such as pentamidine, miltefosine, and essential oil of S. aromaticum that have caused dyskinetoplastidy in L. donovani promastigotes (Verma et al., 2007; Islamuddin et al., 2014). In the present study, we found that the AALEO-induced leishmanicidal effect was accompanied by dyskinetoplastidy as evidenced by PI staining, corroborating the earlier studies.
Permeabilization of the outer and inner mitochondrial membranes can lead to cell death by apoptosis or necrosis. The loss of mitochondrial membrane potential (ΔΨm) is generally an early change associated with apoptosis as the dissipation of ΔΨm following permeabilization of the inner mitochondrial membrane triggers the release of several apoptotic factors. Artemisinin, berberine chloride, racemoside A, Piper betle, and essential oil of S. aromaticum have been reported to demonstrate leishmanicidal activity that was associated with a loss in ΔΨm (Dutta et al., 2007; Sen et al., 2007; Misra et al., 2009; Saha et al., 2009). In our studies, AALEO too caused mitochondrial membrane depolarization.
In multicellular and unicellular organisms, the mitochondria serve as an important cellular source for generation of ROS and reactive nitrogen species (RNS), critical for induction of apoptosis. The production of ROS during early phase of apoptosis usually follows an imbalance in the cellular redox homeostasis. Withaferin A, curcumin, ethanolic extracts of Piper betle and eugenol-rich essential oil from S. aromaticum (Dutta et al., 2007; Das et al., 2008; Misra et al., 2009; Islamuddin et al., 2014) have been reported to induce apoptosis by generating ROS in promastigotes. This prompted us to evaluate whether AALEO could elicit oxidative stress in L. donovani. In our study, we found that AALEO triggered the production of ROS within the promastigotes that might have contributed to their apoptotic death. NO intermediates have earlier been reported to intercede antileishmanial effect by Kalanchoe pinnata (Da-Silva et al., 1999). NO has also been reported to cause extensive fragmentation of nuclear DNA in both axenic and intracellular amastigotes of L. amazonensis (Holzmuller et al., 2002). Our studies are in agreement with the previous reports as a significant increase in NO was observed after exposure of amastigote-infected macrophages to AALEO. Generation of NO following drug treatment in infected macrophages further indicated the involvement of RNS in amastigote death. EO from Ocimum gratissimum (Ueda-Nakamura et al., 2006) and Croton cajucara (Do Socorro et al., 2003) have shown similar NO enhancing activities.
We further explored the therapeutic efficacy of AALEO against VL using L. donovani infected BALB/c mice. The major findings emerging from this study are that AALEO (200 mg/kg b.w) resulted in maximum clearance of parasites (88–91%) from the liver and spleen of mice with established L. donovani infection as compared to untreated infected controls. Significant reduction in spleen and liver weight was also observed with AALEO. Similar therapeutic effect has been reported with the essential oil of Chenopodium ambrosioides (Monzote et al., 2006) and Bixa orellana (Monzote et al., 2013).
The hepatic and renal toxicities in normal and L. donovani infected BALB/c mice upon subsequent administration of AALEO (upto 200 mg/kg b.w.) were evaluated by monitoring the serum levels of SGOT, SGPT, ALP, urea, and creatinine. The enzymatic levels were in the normal range, indicating preclusion of hepato- and nephro-toxicity. In ex vivo studies also, AALEO was not found to compromise the macrophage viability.
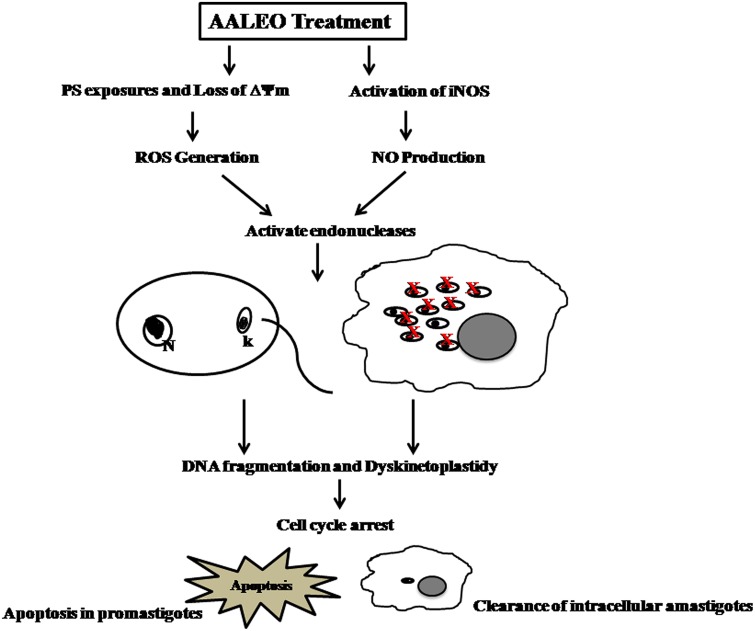
Thus, we conclusively demonstrate that camphor-rich oil of AALEO exhibited antileishmanial efficacy against the promastigotes and intracellular amastigotes. The leishmanicidal activity was further confirmed in L. donovani infected BALB/c mice where ≥90% inhibition of parasite burden was observed. The leishmanicidal effect was mediated by PCD as evidenced by PS externalization, in situ DNA nicking, dyskinetoplastidy, cell cycle arrest at sub- G0–G1 phase, reduction of ΔΨm, ROS, and RNI generation (Figure 8). Moreover, no cytotoxic effect was observed on the mammalian macrophages and there was no impairment of liver and kidney functions of BALB/c mice treated with AALEO. Our study widens the scope for future designing and strengthening of the chemotherapeutic strategies for better management of this debilitating disease.
FIGURE 8.
Proposed model for AALEO-induced PCD in L. donovani promastigotes and amastigotes.
AUTHOR CONTRIBUTIONS
MI, FA conceived and designed the experiments. MI, FA, GC performed the experiments. MW assisted in ex vivo experiments while MT, DS, and MA assisted in extraction of oil. FA, MI analyzed the data and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to Dr. Mohd. Aslam for proficient assistance in the initial stages of this study. We are highly grateful to Dr. Nahid Ali (Indian Institute of Chemical Biology, Kolkata, India) for providing the WHO strain of L. donovani. We are appreciative to Dr. Ajai Kumar, Senior Technical Assistant, Advanced Instrumentation Research Facility, Jawaharlal Nehru University (New Delhi) for GC and GC-MS analysis. We are thankful to Fateh Mohammad, Laboratory Technician for his assistance throughout this work. This work was supported by research grants to FA from the Department of Biotechnology (DBT), Department of Science and Technology, Indian Council of Medical Research (ICMR), Central Council for Research in Unani Medicine, New Delhi, India. Taibah University, Kingdom of Saudi Arabia is duly acknowledged for meeting the publication cost. Mohammad Islamuddin was formerly the recipient of fellowship from the DBT project and is presently Senior Research Fellow of ICMR, Government of India.
REFERENCES
- Afrin F., Dey T., Anam K., Ali N. (2001). Leishmanicidal activity of stearylamine-bearing liposomes in vitro. J. Parasitol. 87 188–193. 10.1645/0022-3395(2001)087[0188:LAOSBL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Agarwal V., Sharma A. K., Upadhyay A., Singh G., Gupta R. (2012). Hypoglycemic effects of Citrullus colocynthis roots. Acta. Pol. Pharm. 69 75–79. [PubMed] [Google Scholar]
- Das R., Roy A., Dutta N., Majumder H. K. (2008). Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis 13 867–882. 10.1007/s10495-008-0224-7 [DOI] [PubMed] [Google Scholar]
- Da-Silva S. A., Costa S. S., Rossi-Bergmann B. (1999). The anti-leishmanial effect of Kalanchoe is mediated by nitric oxide intermediates. Parasitology 118 575–582. 10.1017/S0031182099004357 [DOI] [PubMed] [Google Scholar]
- Do Socorro S., Rosa Mdo S., Mendonça-Filho R. R., Bizzo H. R., de Almeida Rodrigues I., Soares R. M. (2003). Antileishmanial activity of a linalool-rich essential oil from Croton cajucara. Antimicrob. Agents Chemother. 47 1895–1901. 10.1128/AAC.47.6.1895-1901.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., Ghoshal A., Mandal D., Mondal N. B., Banerjee S. (2007). Racemoside a, an anti-leishmanial, water soluble, natural steroidal saponin, induces programmed cell death in Leishmania donovani. J. Med. Microbiol. 56 1196–1204. 10.1099/jmm.0.47114-0 [DOI] [PubMed] [Google Scholar]
- Dutta A., Sarkar D., Gurib-Fakim A., Mandal C., Chatterjee M. (2008). In vitro and in vivo activity of Aloe vera leaf exudates in experimental visceral leishmaniasis. Parasitol. Res. 102 1235–1242. 10.1007/s00436-008-0899-2 [DOI] [PubMed] [Google Scholar]
- Fyfe L., Armstrong F., Stewart J. (1997). Inhibition of Listeria monocytogenes and Salmonella enteritidis by combinations of plant oils and derivatives of benzoic acid: the development of synergistic antimicrobial combinations. Int. J. Antimicrob. Agents 9 195–199. 10.1016/S0924-8579(97)00051-4 [DOI] [PubMed] [Google Scholar]
- Holzmuller P., Sereno D., Cavaleyra M., Mangot I., Daulouede S. (2002). Nitric oxide-mediated proteasome- dependent oligonucleosomal DNA fragmentation in Leishmania amazonensis amastigotes. Infect. Immun. 70 3727–3735. 10.1128/IAI.70.7.3727-3735.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islamuddin M., Sahal D., Afrin F. (2014). Apoptosis-like death in Leishmania donovani promastigotes induced by eugenol-rich oil of Syzygium aromaticum. J. Med. Microbiol. 63 74–85. 10.1099/jmm.0.064709-0 [DOI] [PubMed] [Google Scholar]
- Juteaua F., Masottia V., Bessiéreb J. M., Dherbomezc M., Vianoa J. (2002). Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia 71 532–535. 10.1016/S0367-326X(02)00175-2 [DOI] [PubMed] [Google Scholar]
- Kordali S., Cakir A., Ozer H., Cakmakci R., Kesdek M. (2008). Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Biores. Technol. 99 8788–8795. 10.1016/j.biortech.2008.04.048 [DOI] [PubMed] [Google Scholar]
- Machado M., Dinis A. M., Salgueiro L., Cavaleiro C., Custódio J. B. A. (2010). Anti-Giardia activity of phenolic-rich essential oils: effects of Thymbra capitata, Origanum virens, Thymus zygis subsp. sylvestris, and Lippia graveolens on trophozoites growth, viability, adherence, and ultrastructure. Parasitol. Res. 106 1205–1215. 10.1007/s00436-010-1800-7 [DOI] [PubMed] [Google Scholar]
- Mdoe F. P., Cheng S. S., Msangi S., Nkwengulila G., Chang S. T. (2014). Activity of Cinnamomum osmophloeum leaf essential oil against Anopheles gambiae. Parasites Vectors 7 209–214 10.1186/1756-3305-7-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra P., Kumar A., Gupta S., Khare P., Kumar N. (2009). Pro-apoptotic effect of landrace Bangla Mahoma of Piper betle on Leishmania donovani may be due to high content of eugenol. J. Med. Microbiol. 58 1058–1066. 10.1099/jmm.0.009290-0 [DOI] [PubMed] [Google Scholar]
- Mohamed A. H. H., El-Sayed M. A., Hegazy M. E., Helaly S. E., Esmail A. M. (2010). Chemical constituents and biological activities of Artemisia herba-alba. Rec. Nat. Prod. 4 1–25. [Google Scholar]
- Monzote L., García M., Scull R., Cuellar A., Setzer W. N. (2013). Antileishmanial activity of the essential oil from Bixa orellana. Phytother. Res. 5 753–758. 10.1002/ptr.5055 [DOI] [PubMed] [Google Scholar]
- Monzote L., Montalvo A. M., Almanonni S., Scull R., Miranda M. (2006). Activity of the essential oil from Chenopodium ambrosioides grown in cuba against Leishmania amazonensis. Chemotherapy 52 130–136. 10.1159/000092858 [DOI] [PubMed] [Google Scholar]
- Murray H. W. (2004). Treatment of visceral leishmaniasis in 2004. Am. J. Trop. Med. Hyg. 71 787–794. [PubMed] [Google Scholar]
- Paris C., Loiseau P. M., Bories C., Breard J. (2004). Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 48 852–859. 10.1128/AAC.48.3.852-859.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirali-Kheirabadi K. H., Teixeira da Silva J. (2011). In vitro assessment of the acaricidal properties of Artemisia annua and Zataria multiflora essential Oils to control cattle ticks. Iran. J. Parasitol. 6 58–65. [PMC free article] [PubMed] [Google Scholar]
- Queiroz E. A., Puukila S., Eichler R., Sampaio S. C., Forsyth H. L. (2014). Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS ONE 23:e98207 10.1371/journal.pone.0098207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues K. A. F., Amorim L. V., Oliveira J. M. G., Dias C. N., Moraes D. F. C. (2013). Eugenia uniflora L. essential oil as a potential anti-Leishmania agent: effects on Leishmania amazonensis and possible mechanisms of action. Comp. Alter. Med. 279726 10 10.1155/2013/279726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M. S. S., Mendonça-Filho R. R., Bizzo H. R., Rodrigues I. A., Maria R. A. (2003). Antileishmanial activity of a linalool-rich essential oil from croton cajucara. Antimicrob. Agents Chemother. 47 1895–1901. 10.1128/AAC.47.6.1895-1901.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Ganguly A., Dasgupta S. B., Das B. B., Pal C., Jaisankar P. (2008). Mitochondria dependent reactive oxygen species mediated programmed cell death induced by 3, 3’- diindilylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol. Pharmacol. 74 1292–1307. 10.1124/mol.108.050161 [DOI] [PubMed] [Google Scholar]
- Saha P., Sen R., Hariharan C., Kumar D., Das P. (2009). Berberine chloride causes a caspase-independent, apoptotic-like death in Leishmania donovani promamastigotes. Free. Radical. Res. 43 1101–1110. 10.1080/10715760903186124 [DOI] [PubMed] [Google Scholar]
- Sanchez-Suarez J. F., Riveros I., Delgado G. (2013). Evaluation of the Leishmanicidal and cytotoxic potential of essential oils derived from ten colombian Plants. Iran. J. Parasitol. 8 129–136. [PMC free article] [PubMed] [Google Scholar]
- Santoro G. F., Cardoso M. G., Guimarães L. G., Mendonça L. Z., Soares M. J. (2007). Trypanosoma cruzi: activity of essential oils from Achillea millefolium L., Syzygium aromaticum L. and Ocimum basilicum L. on epimastigotes and trypomastigotes. Exp. Parasitol. 116 283–290. 10.1016/j.exppara.2007.01.018 [DOI] [PubMed] [Google Scholar]
- Sarkar A., Sen R., Saha P., Ganguly S., Mandal G. (2008). An ethanolic extract of leaves of Piper betle (Paan) Linn mediates its antileishmanial activity via apoptosis. Parasitol. Res. 102 1249–1255. 10.1007/s00436-008-0902-y [DOI] [PubMed] [Google Scholar]
- Schelz Z., Hohmann J., Molnar J. (2010). “Recent advances in research of antimicrobial effects of essential oils and plant derived compounds on bacteria,” in Ethnomedicine: A Source of Complementary Therapeutics, ed. Chattopadhyay D. (Kerala: Research Signpost; ), 281–304. [Google Scholar]
- Sen R., Bandyopadhyay S., Dutta A., Mandal G., Ganguly S. (2007). Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. J. Med. Microbiol. 56 1213–1218. 10.1099/jmm.0.47364-0 [DOI] [PubMed] [Google Scholar]
- Silva C. J., Barbosa L. C. A., Demuner A. J., Montanari R. M., Peinheiro A. L. (2010). Chemical composition and antibacterial activities from the essential oils of myrtaceae species planted in Brazil. Quim. Nova 33 104–108. 10.1590/S0100-40422010000100019 [DOI] [Google Scholar]
- Singh N., Kaur J., Kumar P., Gupta S., Singh N. (2009). An orally effective dihydropyrimidone (DHPM) analogue induces apoptosis-like cell death in clinical isolates of Leishmania donovani overexpressing pteridine reductase. Parasitol. Res. 105 1317–1325. 10.1007/s00436-009-1557-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S. (2003). Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 6 849–854. 10.1046/j.1365-3156.2001.00778.x [DOI] [PubMed] [Google Scholar]
- Tariko Y., Hymete A., Hailu A., Rohloff J. (2010). Essential-oil composition, antileishmanial, and toxicity study of Artemisia abyssinica and Satureja punctata ssp. punctata from Ethiopia. Chem. Biodivers. 7 1009–1018 10.1002/cbdv.200900375 [DOI] [PubMed] [Google Scholar]
- Tariko Y., Hymete A., Hailu A., Rohloff J. (2011). In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chem. Biodivers. 8 614–623. 10.1002/cbdv.201000331 [DOI] [PubMed] [Google Scholar]
- Tzenkova R., Kamenarska Z., Draganov A., Atanassov A. (2010). Composition of Artemisia annua essential oil obtained from species growing wild in Bulgaria. Biotechnol. Biotechnol. Eq. 24 1833–1835. 10.2478/V10133-010-0030-6 [DOI] [Google Scholar]
- Ueda-Nakamura T. R., Mendonc F., Morgado-Díaz J. A., Maza P. K., Filho B. P. D. (2006). Antileishmanial activity of eugenol-rich essential oil from Ocimum gratissimum. Parasitol. Int. 55 99–105. 10.1016/j.parint.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Varela-M R. E., Villa-Pulgarin J. A., Yepes E., Muller I., Modolell M. (2012). In vitro and in vivo efficacy of ether lipid edelfosine against Leishmania spp. and SbV-resistant parasites. PLoS. Negl. Trop. Dis. 6:e1612 10.1371/journal.pntd.0001612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N. K., Singh G., Dey C. S. (2007). Miltefosine induces apoptosis in arsenite-resistant Leishmania donovani promastigotes through mitochondrial dysfunction. Exp. Parasitol. 116 1–13. 10.1016/j.exppara.2006.10.007 [DOI] [PubMed] [Google Scholar]