Abstract
Objective
In this Phase 2 multicenter study the efficacy and safety of oral abiraterone acetate (1000 mg/once daily) plus prednisolone (5 mg/twice daily) was evaluated in metastatic castration-resistant prostate cancer patients from Japan who had previously received docetaxel-based chemotherapy.
Methods
Men (aged ≥20 years) with metastatic castration-resistant prostate cancer (prostate-specific antigen levels: ≥5 ng/ml), who had received 1 or 2 cytotoxic chemotherapies (with ≥1 regimen being docetaxel) for prostate cancer, were enrolled in this open-label, single-arm study. Primary efficacy endpoint was proportion of patients achieving a ≥50% prostate-specific antigen decline from baseline (prostate-specific antigen response rate) after 12-week treatment. Safety and pharmacokinetics were also assessed.
Results
Confirmed prostate-specific antigen response rate by Week 12 was 28.3% (90% confidence interval: 17.6%; 41.1%) or 13 out of 46 (full analysis set) treated patients. However, total prostate-specific antigen response rate including confirmed and unconfirmed responses was 34.8% (90% confidence interval: 23.2%; 47.9%). Secondary efficacy endpoints and outcomes were: improvement in Eastern Cooperative Oncology Group performance status score by ≥1 unit: 7/16 patients (43.8%); objective radiographic response: complete response, partial response and stable disease in 0, 1/22 (4.5%) and 9/22 (40.9%) patients, respectively; pain palliation response: 9/16 (56.3%) patients. The most common adverse events (>20% patients) were upper respiratory tract infection (13/47, 27.7% patients) and hepatic function abnormal (10/47, 21.3% patients, Grade 3: 8.5%). All mineralocorticoid-related toxicities were Grade 1/2.
Conclusions
Abiraterone acetate plus prednisolone showed favorable efficacy in metastatic castration-resistant prostate cancer Japanese patients who had received chemotherapy. Abiraterone acetate plus prednisolone had an acceptable safety profile.
Clinical trial registration no
Keywords: abiraterone acetate; chemotherapy; confirmed response, metastatic castration-resistant prostate cancer; prostate-specific antigen
INTRODUCTION
The incidence of prostate cancer in Asian population is less common as compared with western countries, and prostate cancer ranks as the sixth highest cause of male cancer mortality in Japan (1). However, demographic aging and westernization of diet are possible contributing factors for gradual increase in prostate cancer incidence and mortality in Japanese population (2–5). Hormonal therapy (medical or surgical castration) remains the main first-line treatment for patients with metastatic prostate cancer (6). However, treatment benefits last for ∼3–5 years when disease transforms into metastatic castration-resistant prostate cancer (mCRPC) (7).
Docetaxel is recommended as a first-line therapy with clinically proven median survival improvement of 2–3 months in mCRPC patients (8,9). However, drug resistance to this therapy, peripheral neurotoxicity and hematopoietic side-effects remain a major cause of concern (10,11). Progression of prostate cancer is associated with androgen-receptor driven increased levels of prostate-specific antigen (PSA), which also increase in patients with progression after treatment with cytotoxic agents (12). Therefore, it is hypothesized that tumors previously treated with cytotoxic agents may be responsive to a potent androgen-receptor signaling inhibitor.
Abiraterone acetate (AA) (Zytiga®, Janssen Biotech), a prodrug for abiraterone, is a first-in-class cytochrome P450 (CYP) 17 (17α-hydroxylase/C17, 20 lyase) inhibitor that selectively inhibits androgen synthesis in testes, adrenal glands and tumor tissues. AA has approval in >70 countries for treatment of chemotherapy-naïve mCRPC patients, and for treating patients in the post-docetaxel setting in over 85 countries. Clinical studies have demonstrated efficacy and safety of AA in non-Japanese mCRPC patients in both the chemotherapy-naïve and post-docetaxel settings (12–14), as well as in chemotherapy-naïve mCRPC patients from Japan (N. Matsubara, H. Uemura, I. Fukui et al., manuscript submitted for publication). Enzalutamide is the only approved drug for treatment of Japanese patients with mCRPC who have received docetaxel-based chemotherapy. Thus, there is a need for other effective treatment options for this patient population in Japan.
This Phase 2 study evaluated the efficacy of 12-week AA therapy (prostate-specific antigen working group [PSAWG] criteria) in Japanese patients with mCRPC who had previously received docetaxel-based chemotherapy. Safety and pharmacokinetics of AA were also evaluated.
PATIENTS AND METHODS
Patients
Inclusion criteria: men aged ≥20 years with histologically/cytologically confirmed prostate adenocarcinoma without neuroendocrine differentiation or small cell histology, either surgically/medically castrated (testosterone level <50 ng/dl) or if treated with a luteinizing-hormone-releasing hormone agonist/antagonist (i.e. no surgical castration), treatment had to be initiated >4 weeks before Cycle 1 Day 1 and continued throughout the study, PSA level ≥5 ng/ml; PSA progression according to PSAWG criteria (PSA level ≥5 ng/ml increased on ≥2 successive occasions, at least 2 weeks apart) or objective progression for patients with measurable disease (target or non-target metastatic lesions) according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria; use of 1 or 2 cytotoxic chemotherapy regimens, 1 of which was docetaxel-containing, for mCRPC; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2.
Exclusion criteria: Patients who had received any herbal product known to decrease PSA levels, other hormonal, systemic corticosteroid, started or adjusted bisphosphonate or anti-receptor activator of nuclear factor-κB ligand (RANKL) monoclonal antibody therapies, radiotherapy, chemotherapy, immunotherapy, surgery or local prostatic intervention within 4 weeks before Cycle 1 Day 1; or had received a single fraction of palliative radiotherapy, ketoconazole for prostate cancer, complementary medicines, or any herbal supplements within 2 weeks before Cycle 1 Day 1, any major disease including brain metastasis, active/uncontrolled autoimmune disease requiring corticosteroid therapy, pituitary or adrenal insufficiency or hyperaldosteronism.
The protocol and informed consent documents were reviewed and approved by an Independent Ethics Committee or Institutional Review Board and the study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki and in accordance with ICH Good Clinical Practice guidelines, applicable regulatory requirements, and in compliance with the protocol. All participants provided written informed consent to participate in the study.
Study Design
The data from this Phase 2, open-label, single-arm, multicenter study, which was conducted at 18 sites in Japan, included all patients enrolled from 4 June 2012 to data cut-off date of 28 June 2013 (second interim analysis). The study consisted of three phases: screening (14 days), treatment period (from Cycle 1 Day 1 to documented disease progression [PD] or unacceptable toxicity; each cycle: 28 days), and follow-up (every 3 months up to 5 years). Patients were administered AA (1000 mg, orally, once-daily) at least 1 h before a meal and 2 h after a meal; 5 mg of prednisolone was given concomitantly (orally, twice-daily) to reduce the risk of study drug-related mineralocorticoid adverse events (AE). A 28-day dosing cycle was to be continued until PD or unacceptable toxicity was observed.
Study Endpoints and Evaluations
Efficacy
Primary
The primary efficacy endpoint was the proportion of patients achieving PSA response by 12 weeks of therapy (evaluated at screening and Day 1 of every cycle). PSA response was defined as the first occurrence of PSA decline of ≥50% from baseline; subsequently confirmed by a measurement obtained ≥4 weeks after initial documentation (PSAWG criteria).
Secondary
Secondary endpoints included radiographic objective response rate (ORR) (in accordance with RECIST Version 1.0 criteria), clinical benefit (defined as an observation of ≥1 of the following: PSA response, radiographic response (RR), stable disease (SD) lasting for 6 months, or improvement in ECOG PS score by ≥1 unit), overall survival (OS, assessed every 3 months until 5 years after first dose of study drugs or until approval by Ministry of Health, Labour and Welfare, Japan, whichever was earlier), duration of PSA response, PSA-based progression-free survival, PFS (PSA-PFS), RAD-PFS and modified-PFS (time from the first dose to the first documentation of progressive disease, death or meeting the conditions defined in the statistical analysis plan), Brief-Pain Inventory-Short Form (BPI-SF).
Pharmacokinetics and Pharmacodynamics Evaluations
Venous blood samples (2 ml) were collected to estimate pre-dose and selected post-dose plasma concentrations of abiraterone or its metabolite at each visit. Changes in serum concentration of testosterone were measured for each patient.
Safety
Safety was assessed throughout the study by monitoring AEs, clinical laboratory measurements (hematology, coagulant factors, blood chemistry and urinalysis), 12-lead electrocardiograms, vital sign measurements and body weight. Severity of any serious or non-serious AE was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0.
Statistical Analysis
Sample Size Determination
A threshold response rate of 20% was established, based on previous Phase 2 studies (15,16). Assuming a PSA response rate of 40%, 38 patients were considered sufficient to demonstrate that the lower limit of the two-sided 90% confidence interval (CI) of the response rate would exceed the threshold response rate (20%) with 80% power.
Efficacy Analysis
The primary analysis population for efficacy was the full analysis set (FAS), which was defined as the patients who received treatment with the study drug at least once and had any post-treatment PSA assessment data. For primary endpoint, PSA response rate by 12 weeks and corresponding 90% CI was calculated. Secondary endpoints and other endpoints were summarized descriptively. The Kaplan–Meier method was used to estimate the OS, and event-free time for the time-to-event data along with the corresponding 90% CI.
The primary efficacy endpoint was further analyzed in patients for subgroups defined as: baseline ECOG PS (0, 1 or 2), number of prior chemotherapy regimens (1 or 2), age group (<65, ≥65 or ≥75 years), baseline lactate dehydrogenase (LDH) category (‘low and normal’ or ‘high’), baseline ALP category (‘low and normal’ or ‘high’), baseline hemoglobin category (≤median or >median), baseline PSA category (≤median or >median) and baseline BPI-SF (<4 or ≥4).
Exploratory endpoints included the proportion of patients achieving a PSA decline of ≥30%, ≥75% and ≥90% from baseline.
Sensitivity analysis of the primary efficacy endpoint was performed on the evaluable set. The evaluable set included patients who received treatment with AA at least once and met the following two criteria: (1) had tumor assessments or PSA measurements made at baseline and at least once post-baseline; and (2) received a minimum of three cycles of study drug.
Safety Analysis
Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) Version 15.0. All reported AEs with onset during the treatment period (i.e. treatment-emergent AEs including AEs that worsened post-baseline) were included in the analysis. For each AE, the proportion of patients who experienced at least one occurrence of the given event was calculated.
RESULTS
Patient Disposition and Baseline Characteristics
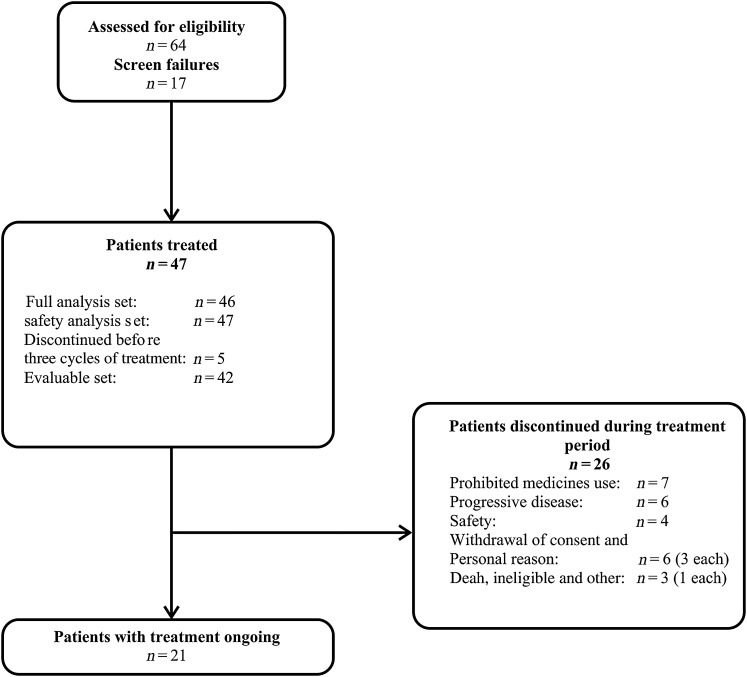
A total of 47 patients were enrolled and 21 (44.7%) patients were still receiving the study treatment at the time of data cut-off (Fig. 1). Of these 47 patients, 1 was excluded from the FAS, because the patient had no post-baseline PSA measurements. The median age of the patients was 72.0 years, with 27.7% (n = 13/47) of patients ≥75 years of age (Table 1).
Figure 1.
Patients’ disposition.
Table 1.
Demographic and baseline disease characteristics (safety analysis set)
| Baseline characteristicsa | Patients (N = 47) |
|---|---|
| Age (years) | 72.0 (51; 83) |
| Weight (kg) | 62.1 (47.8; 89.5) |
| Height (cm) | 164.5 (148.2; 177.0) |
| Body mass index (kg/m2) | 23.6 (18.2; 32.8) |
| Gleason score at initial diagnosis, N (%) | |
| 7 | 8 (17.0) |
| ≥8 | 37 (78.7) |
| Unknown | 2 (4.3) |
| Duration of disease (years) | 4.4 (1.6; 15.3) |
| Stage at initial diagnosis, N (%) | |
| Stage II | 3 (6.4) |
| Stage III | 3 (6.4) |
| Stage IV | 39 (83.0) |
| Incomplete reporting | 2 (4.3) |
| Evidence of disease progression, N (%) | |
| PSA only | 34 (72.3) |
| Radiographic progression with or without PSA progression | 13 (27.7) |
| Extent of disease, N (%) | |
| Bone | 44 (93.6) |
| Hepatic | 2 (4.3) |
| Lymphatic | 17 (36.2) |
| Pulmonary | 5 (10.6) |
| Other | 3 (6.4) |
| Time from initiating LH–RH to first dose (months) | |
| N | 45 |
| Median, range | 41.23 (4.4; 182.8) |
| Previous prostate cancer therapy, N (%) | |
| Radiotherapy | 20 (42.6) |
| Surgery | 5 (10.6) |
| Chemotherapy | 47 (100.0) |
| Hormone | 47 (100.0) |
| Others | 45 (95.7) |
| Number of anti-androgenic therapy regimen, N (%) | |
| 1 | 6 (12.8) |
| 2 | 23 (48.9) |
| 3 | 11 (23.4) |
| 4 | 5 (10.6) |
| 5 | 2 (4.3) |
| ECOG performance status, N (%) | |
| 0 | 25 (53.2) |
| 1 | 16 (34.0) |
| 2 | 6 (12.8) |
| BPI-SF worst pain score | 2.5 (0; 8) |
| Pain, N (%) | |
| Absent | 30 (63.8) |
| Present | 16 (34.0) |
| Baseline PSA (ng/ml) | 143.0 (7.2; 1450.0) |
BPI-SF, Brief-Pain Inventory-Short Form; ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen.
aData are presented as median (range) or otherwise stated.
At the time of data cut-off, the median number of AA treatment cycles was 9.0 (range: 1–13), with 35 (74.5%) patients having started ≥6 cycles of AA therapy. The median duration of AA treatment was 8.3 (range: 0.2 to 12.0) months. The majority of patients (44/ 47, 93.6%) showed treatment compliance with AA and >95% compliance with prednisolone.
Efficacy
Primary
In the full analysis set, 13/46 patients (28.3% [90% CI: 17.6%, 41.1%]) had a confirmed PSA response. The lower limit of the two-sided 90% CI (17.6%) was lower than the threshold response rate (20%) for confirmed PSA response. Total PSA response rate (confirmed and unconfirmed) was observed in 16/46 patients (34.8%; 90% CI: 23.2%, 47.9%) (Table 2).
Table 2.
PSA response rate by Week 12 according to PSAWG criteria (full analysis set)
| PSA response, N (%) [90% CI] |
|||
|---|---|---|---|
| Confirmed | Unconfirmed | Total (N = 46) | |
| 50% Reduction in PSA | 13 (28.3) [17.6; 41.1] | 3 (6.5) [1.8; 16.0] | 16 (34.8) [23.2; 47.9] |
| 30% Reduction in PSA | 15 (32.6) [21.3; 45.7] | 6 (13.0) [5.8; 24.1] | 21 (45.7) [33.0; 58.7] |
| 75% Reduction in PSA | 9 (19.6) [10.6; 31.7] | 2 (4.3) [0.8; 13.1] | 11 (23.9) [14.0; 36.5] |
| 90% Reduction in PSA | 2 (4.3) [0.8; 13.1] | 3 (6.5) [1.8; 16.0] | 5 (10.9) [4.4; 21.5] |
CI, confidence interval; PSAWG, prostate-specific antigen working group.
Secondary
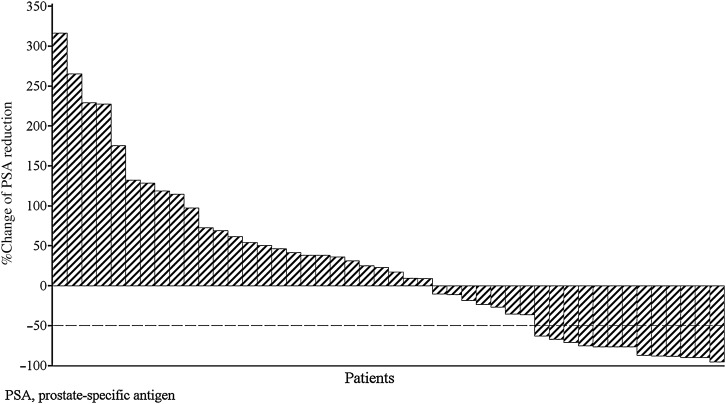
The PSA response rate during the treatment period was 28.3% [90% CI: 17.6%, 41.1%]. The total PSA response rate was 34.8% (16/46 patients; 90% CI: 23.2%, 47.9%). These results were the same as those achieved by Week 12. The median duration of PSA response was estimated to be 142.0 days (90% CI: 85.0, not estimable) ranging from 64 to >309 days. Of the 13 patients with a confirmed PSA response during the treatment period, 8 (61.5%) had subsequent PSA progression by the cut-off date. In accordance with PCWG2 criteria, the median percent change in PSA level at Week 12 from baseline was 20.05% (range: −95.4%, 316.1%) (Fig. 2).
Figure 2.
Waterfall plot of percent change in PSA at Week 12 from baseline (full analysis set).
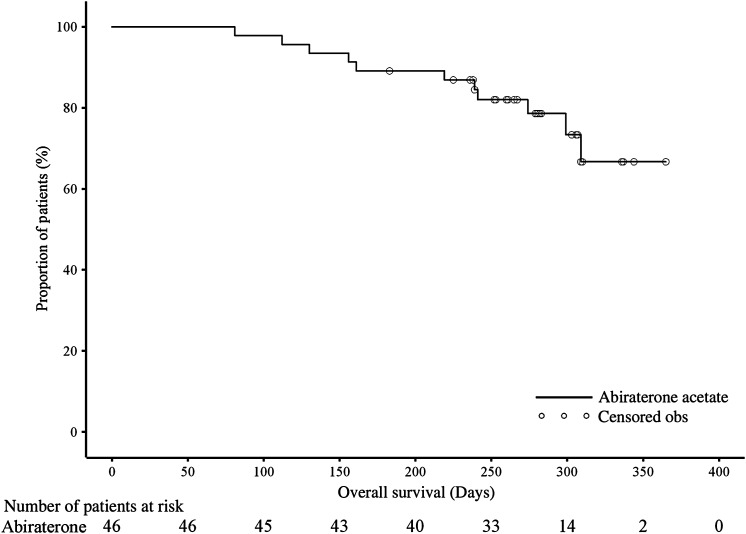
Based on the RECIST criteria, radiographic objective partial response was observed in 1 of 22 (4.5%) patients with measurable lesions at baseline, none achieved complete response; 9 (40.9%) patients attained SD and 12 (54.5%) patients had PD. The clinical benefit demonstrated for 16/46 patients (34.8%) was as follows—PSA response: 13 patients (81.3%), radiographic RR: 1 patient (6.3%); SD lasting for 6 months: 1 patient (6.3%), improvement in ECOG PS score by ≥1 unit: 7 patients (43.8%). By data cut-off date, 11 (23.9%) patients had died (Fig. 3). The median OS was not reached, 6-month survival rate was estimated to be 89.1% (90% CI: 78.6, 94.6%) and patients who met the criteria for PSA-, radiographic- and modified PFS were 80.4%, 84.4% and 52.2%, respectively (Table 3).
Figure 3.
Kaplan–Meier plot of overall survival (full analysis set).
Table 3.
Overall survival and PFS (full analysis set)
| Overall survival (N = 46) | PSA-based PFSa (N = 46) | RAD-PFSb (N = 45) | Modified PFSc (N = 46) | |
|---|---|---|---|---|
| Event, n (%) | 11 (23.9) | 37 (80.4) | 38 (84.4) | 24 (52.2) |
| Censored, n (%) | 35 (76.1) | 9 (19.6) | 7 (15.6) | 22 (47.8) |
| Survival (days), (90% CI) | ||||
| 25th percentilec | 299.0 (239.0, NE) | 57.0 (29.0, 59.0) | 82.0 (80.0, 85.0) | 161.0 (106.0, 225.0) |
| Mediand | – | 108.5 (85.0, 114.0) | 106.0 (85.0, 169.0) | 281.0 (239.0, NE) |
| Range (min; max) | 81; 365+ | 28; 337+ | 43; 337 | 45; 365+ |
| Six-month progression-free rate | 0.891 (0.786, 0.946) | 0.217 (0.127, 0.323) | 0.378 (0.261, 0.494) | 0.674 (0.546, 0.773) |
CI, confidence interval; NE, not estimable; PFS, progression-free survival; RAD, radiographic; RECIST, response evaluation criteria in solid tumors
aPSA-based PFS evaluated in accordance with PSAWG criteria.
bRAD-PFS was evaluated in accordance with RECIST criteria
cModified-PFS is defined as the time from the first dose to the first documented progressive disease by death or the meeting conditions defined in the statistical analysis plan.
dKaplan–Meier estimate.
The pain palliation response, evaluated using the BPI-SF, was observed in 9 of 16 (56.3%) patients with a baseline pain score of ≥4. On the basis of BPI-SF, the median time to pain progression was not reached. Of 16 patients with a baseline pain score of ≥4.3 (18.8%) had pain progression. The 6-month pain progression-free rate was estimated to be 0.795 (90% CI: 0.548, 0.916).
Apparent differences in the PSA response rate by week 12 were observed in some subgroups except those categorized by baseline age, LDH and hemoglobin (Table 4).
Table 4.
Subgroup analysis: PSA response rate by Week 12 according to PSAWG criteria (full analysis set)
| Abiraterone acetate |
||
|---|---|---|
| Patients evaluable for PSA response | PSA response (confirmed) N (%) [90% CI] | |
| Total number of subjects | 46 | |
| Baseline ECOG PS | ||
| 0 | 24 | 8 (33.3%) [17.8%; 52.1%] |
| 1 | 16 | 4 (25.0%) [9.0%; 48.4%] |
| 2 | 6 | 1 (16.7%) [0.9%; 58.2%] |
| Number of prior chemotherapy regimens | ||
| 1 | 18 | 7 (38.9%) [19.9%; 60.8%] |
| 2 | 28 | 6 (21.4%) [9.8%; 38.0%] |
| Age groups | ||
| <65 | 13 | 2 (15.4%) [2.8%; 41.0%] |
| ≥65 | 33 | 11(33.3%) [19.9%; 49.1%] |
| ≥75 | 12 | 3 (25.0%) [7.2%; 52.7%] |
| Baseline PSA category | ||
| ≤Median | 23 | 9 (39.1%) [22.2%; 58.3%] |
| >Median | 23 | 4 (17.4%) [6.2%; 35.5%] |
| Baseline LDH category | ||
| Low and normal | 24 | 6 (25.0%) [11.5%; 43.5%] |
| High | 22 | 7 (31.8%) [16.0%; 51.5%] |
| Baseline ALP category | ||
| Low and normal | 28 | 10 (35.7%) [20.8%; 53.0%] |
| High | 18 | 3 (16.7%) [4.7%; 37.7%] |
| Baseline hemoglobin category | ||
| ≤Median | 26 | 6 (23.1%) [10.6%; 40.5%] |
| >Median | 20 | 7 (35.0%) [17.7%; 55.8%] |
| Baseline BPI-SF | ||
| <4 | 29 | 10 (34.5%) [20.0%; 51.4%] |
| ≥4 | 16 | 3 (18.8%) [5.3%; 41.7%] |
ALP, alkaline phosphatase; LDH, lactate dehydrogenase.
Exploratory analysis—confirmed PSA decline from baseline: ≥30%: 32.6% (90% CI: 21.3%, 45.7%) patients, ≥75%: 19.6% (90% CI: 10.6%, 31.7%) patients and ≥90%: 4.3% (90% CI: 0.8%, 13.1%) patients. Sensitivity analysis: confirmed PSA response rate was 31.0% (90% CI: 19.4%, 44.6%).
Pharmacokinetics and Pharmacodynamics
There was not much variation in mean plasma pre-dose abiraterone concentrations during multiple administrations of AA (1000 mg), regardless of the visit (mean [SD], 10.6 ng/ml [8.9] to 14.3 ng/ml[18.9]). However, individual plasma abiraterone post-dose concentrations showed large variability as samples were collected during the absorption phase.
Mean serum testosterone concentrations declined from baseline (Cycle 1 Day 1) following multiple administrations of AA. Although serum testosterone concentrations showed variability following multiple administrations of AA, most serum testosterone concentrations and median serum testosterone concentrations were below the quantification limit.
Safety
Of the 47 patients who received the treatment, 44 (93.6%) reported ≥1 AE. The AEs reported by at least 10% of the patients included upper respiratory tract infection (27.7%), hepatic function abnormal (21.3%), constipation (19.1%) and weight decreased (12.8%) (Table 5). The expected AEs with AA (≥1) were reported in 20/47 (42.6%) patients; most common were hepatic function abnormal (10.6%) and hypertension (6.4%).
Table 5.
Adverse events reported ≥5% of patients (Safety analysis set)
| AE | Patients (N = 47) N (%) |
|---|---|
| Total number of patients with AEs | 44 (93.6) |
| Upper respiratory tract infection | 13 (27.7) |
| Hepatic function abnormal | 10 (21.3) |
| Constipation | 9 (19.1) |
| Weight decreased | 6 (12.8) |
| Urinary tract infection | 4 (8.5) |
| Nausea | 4 (8.5) |
| Vomiting | 4 (8.5) |
| Disease progression | 4 (8.5) |
| Decubitus ulcer | 4 (8.5) |
| Diabetes mellitus | 4 (8.5) |
| Hyperkalemia | 4 (8.5) |
| Hypokalemia | 4 (8.5) |
| Hypophosphatemia | 4 (8.5) |
| Rash | 3 (6.4) |
| Back pain | 3 (6.4) |
| Edema | 3 (6.4) |
| Weight increased | 3 (6.4) |
| Hypercholesterolemia | 3 (6.4) |
| Hypermagnesemia | 3 (6.4) |
| Hypocalcemia | 3 (6.4) |
| Insomnia | 3 (6.4) |
| Dizziness | 3 (6.4) |
| Hypertension | 3 (6.4) |
| Hot flush | 3 (6.4) |
| Herpes zoster | 3 (6.4) |
| Anemia | 3 (6.4) |
| Lymphopenia | 3 (6.4) |
AE, adverse event.
Nineteen patients (40.4%) reported AEs of Grade >2. Grade 3 AEs were reported for 17 (36.2%) patients; the most frequent (≥2 patients) were hepatic function abnormal (n = 4; 8.5%), hypermagnesemia (n = 3; 6.4%), pneumonia, urinary tract infection, anemia and disease progression (each, n = 2; 4.3%). Two patients (4.3%) experienced Grade 4 AEs (cerebral infarction, subarachnoid hemorrhage and disease progression) and there was one report of a patient who experienced a Grade 5 fatal disease progression (prostate cancer).
A total of 16 (34.0%) patients reported serious AEs, of which the most frequent were disease progression (n = 4; 8.5%), pneumonia, urinary tract infection and dehydration (each, n = 2, 4.3%). Drug-related SAEs and AEs leading to treatment discontinuation were noted in 6 (12.8%) patients. The AEs that led to dose modification or interruption of the study drug were noted in 11/47 (23.4%) patients.
At the time of data cut-off, 4 (8.5%) patients had died of AEs within 30 days of the last dose of study drug (disease progression: n = 3, cerebral infarction and subarachnoid hemorrhage: n = 1). One additional patient died of disease progression within 30 days of the last dose, after study discontinuation (withdrawal of consent).
The most common (≥5% patients) AEs of special interest for AA were hepatic function abnormal (21.3%), hypokalemia (8.5%), anemia, edema and hypertension (each 6.4%). Most of these AEs were of Grade 1/2 except Grade 3 AEs of hepatic function abnormal (four patients, 8.5%), anemia (two patients, 4.3%) and hyperbilirubinemia (one patient, 2.1%). None of these events led to study drug discontinuation. Most laboratory abnormalities for hematology, serum chemistry and urinalysis were ≤Grade 2. Mean changes from baseline in vital signs were considered to be not clinically relevant. Most patients started the study with ECOG PS score of 0 or 1, and none had a score of 3 or 4 at baseline. During the post-baseline treatment period, five (10.6%) patients had a worst ECOG PS score of 3, and 2 (4.3%) had a score of 4.
The most common potentially prednisolone related AEs were diabetes mellitus (8.5%), hypertension (6.4%) and increase in weight (6.4%) (Table 6).
Table 6.
Prednisolone related adverse events reported ≥4% of patients (safety analysis set)
| AE | Patients (N = 47) N (%) |
|---|---|
| Total number of subjects with AEs | 23 (48.9) |
| Pneumonia | 2 (4.3) |
| Diabetes mellitus | 4 (8.5) |
| Hypercholesterolemia | 2 (4.3) |
| Hypertension | 3 (6.4) |
| Hepatic function abnormal | 2 (4.3) |
| Weight increased | 3 (6.4) |
DISCUSSION
In this Phase 2, open-label study in Japanese patients with mCRPC who had received prior docetaxel-based chemotherapy, the confirmed PSA response rate as observed by Week 12 (28.3%) demonstrated efficacy of AA, with an acceptable safety profile distinct from cytotoxic chemotherapy.
Although this result was suspected to be due to Japanese specific medical practice for prostate cancer (e.g. first generation androgen-receptor antagonists are used more frequently and the course of docetaxel treatment is much longer), the subgroup analysis did not reveal these relationships. But it was suggested that five factors (Stage, Gleason score, ECOG-PS, baseline ALP and number of regimen of chemotherapy) might influence PSA response. However, the PSA response rate at Week 12 was also similar to results (29%) from a placebo-controlled trial in western population (12).
The 1000 mg once-daily dose of AA was as recommended in previous studies conducted in Japanese (K. Inoue, A. Shishido, N. Vaccaro et al., manuscript in preparation) and non-Japanese populations (12). Also, previous studies described the utility of using concomitant administration of oral prednisolone to reduce the risk of study drug-related AEs like hypertension, swelling, hypokalemia and increase in mineralocorticoid level (12).
The improvements in primary and secondary efficacy outcomes in these Japanese mCRPC patients receiving AA were considerably reduced compared with the improvements observed in the chemotherapy-naïve mCRPC Japanese (N. Matsubara, H. Uemura, T. Satoh et al., manuscript in preparation) and western patient populations (17). The pain palliation rate as observed by BPI-SF and clinical endpoint ECOG PS score showed clinically meaningful response. However, concomitant treatment of prednisolone could have favorably affected these parameters.
Individual plasma abiraterone concentrations in post-dosing samples collected during the absorption phase showed large variability which was consistent with previous pharmacokinetic studies (18,19). Also, in this study mean serum DHEA-S concentrations declined during AA treatment in combination with prednisolone as expected from the CYP17 inhibitory activity of the AA (20,21).
Overall, the safety findings were consistent with those of other studies in men with mCRPC who had received docetaxel-chemotherapy (12). The incidence of Grade 3/4 AEs in this study was similar to the chemotherapy-naïve Japanese patients (∼40%) (N. Matsubara, H. Uemura, T. Satoh et al., manuscript in preparation) but lower in comparison with chemotherapy-naïve non-Japanese patients (48–49%) (17,22). Although the incidence of hepatotoxicity was much greater (23.4%) than in chemotherapy-naïve Japanese patients (10%) (N. Matsubara, H. Uemura, T. Satoh et al., manuscript in preparation) and the global Phase 3 study, it was well manageable with pre-specified rules for dose reduction or interruption of AA treatment. The AA-associated AEs related to mineralocorticoid excess and cardiac toxicities were easily treatable, did not lead to AA dose reduction or discontinuation and were similar to those in other studies (14,17).
This open-label non-comparative study has limitations inherent with the study design. Also, results from subgroup analysis of the PSA response rate need to be interpreted with caution due to small sample size of some subgroups. However, results obtained from this study were consistent with placebo-controlled trial conducted in western population (12).
Treatment with hormonal agents is generally not considered as a treatment option in patients who have received chemotherapy. However, AA is a valuable second-line hormonal treatment choice in the post-chemotherapy mCRPC patients. Enzalutamide, a recently approved oral hormonal treatment in Japan has similar clinically benefits as it prolongs survival and has demonstrated appreciable declines in PSA levels in this patient population (23), though caution should be exercised in its administration to patients with a history of seizures. With the growing treatment armamentarium for mCRPC, further studies are warranted to gain important insights into the additive effects of AA use concurrently or subsequent to other effective therapies for better outcomes in this patient population.
To summarize, treatment with AA plus prednisolone resulted in favorable efficacy outcomes in Japanese patients with mCRPC who had received prior docetaxel-based chemotherapy, albeit lower than the threshold PSA response rate. Treatment with AA plus prednisolone demonstrated an acceptable safety profile. Thus, AA plus prednisolone can be an important post-chemotherapy treatment option for mCRPC patients in Japan.
Acknowledgements
The authors thank the study patients, without whom this study would never have been accomplished, and the following investigators from Japan: Nobuo Shinohara, Hokkaido University Graduate School of Medicine, Hokkaido; Kazuhiro Suzuki, Gunma University Graduate School of Medicine, Gunma; Jyoji Yuasa, Kuki General Hospital, Saitama; Hiroomi Nakatsu, Asahi General Hospital, Chiba; Hiroyoshi Suzuki, Toho University Sakura Medical Center, Chiba; Takatsugu Okegawa, Kyorin University School of Medicine, Tokyo; Sumio Noguchi, Yokosuka Kyosai Hospital, Kanagawa; Takao Nakashima, Ishikawa Prefectural Central Hospital, Ishikawa; Hirotsugu Uemura, Kinki University Faculty of Medicine, Osaka; Yoshiyuki Kakehi, Kagawa University Faculty of Medicine, Kagawa; Katsuyoshi Hashine, National Hospital Organization Shikoku Cancer Center, Ehime. The authors also thank the following IDMC members for their participation in this study: Shiro Hinotsu, Okayama University, Okayama; Michio Imawari, Shin-Yurigaoka General Hospital, Kanagawa; Rishabh Pandey (SIRO Clinpharm Pvt Ltd.) provided writing assistance for this manuscript and Dr Namit Ghildyal (Janssen Research & Development, LLC) provided editorial support for the development of this manuscript.
Authors’ contributions
All authors have contributed to conception, design and interpretation of data. H.U., K.T., T.S., T.N., A.T., A.Y., and T.N. were the principal investigators of the study. K.I. was the medical expert and clinical responsible physician and H.A. was the medical advisor. S.O. was Chief of Independent Data Monitoring Committee Members.
Funding
This study was supported by Janssen Pharmaceutical K.K. Funding to pay the Open Access publication charges for this article was provided by Janssen Pharmaceutical K.K. (Japan).
Conflict of interest statement
Dr Keiichiro Imanaka is an employee of Janssen Pharmaceutical K.K., Tokyo, Japan. Dr Hideyuki Akaza has received honoraria from Janssen, Astellas Pharma Inc., GlaxoSmithKline K.K., Takeda pharmaceutical company Ltd and Sanofi K.K. Dr Kazunari Tanabe has received honoraria from Astellas Pharma Inc. and Pfizer. Dr Seiichiro Ozono was Chief of Independent Data Monitoring Committee Members for this study.
References
- 1. Prostate cancer estimated incidence: mortality and prevalence worldwide in 2012. IARC. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. (3 May 2014)
- 2.Ito H. Everything about prostate cancer: from basics to clinical practices. Medical View Co. Ltd 2004 (in Japanese)
- 3.Kawahara T, Miyoshi Y, Sekiguchi Z, et al. Risk factors for metastatic castration-resistant prostate cancer (CRPC) predict long-term treatment with docetaxel. PLoS One. 2012;7:e48186. doi: 10.1371/journal.pone.0048186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura K, Nonomura N, Hashine K, et al. Prolonged treatment with three-weekly docetaxel plus daily prednisolone for metastatic castration-resistant prostate cancer: a multicenter, phase II, open-label, non-comparative, extension study in Japan. Int J Clin Oncol. 2013;18:306–13. doi: 10.1007/s10147-012-0380-1. [DOI] [PubMed] [Google Scholar]
- 5. Cancer Statistics 2013 in Japan. http://ganjoho.jp/pro/statistics/en/backnumber/2005_en.html .
- 6.Akaza H. Future prospects for luteinizing hormone-releasing hormone analogues in prostate cancer treatment. Pharmacology. 2010;85:110–12. doi: 10.1159/000274486. [DOI] [PubMed] [Google Scholar]
- 7.Yamada T, Nakayama M, Shimizu T, et al. Genetic polymorphisms of CYP17A1 in steroidogenesis pathway are associated with risk of progression to castration-resistant prostate cancer in Japanese men receiving androgen deprivation therapy. Int J Clin Oncol. 2013;18:711–17. doi: 10.1007/s10147-012-0430-8. [DOI] [PubMed] [Google Scholar]
- 8.Galsky MD, Vogelzang NJ. Docetaxel-based combination therapy for castration-resistant prostate cancer. Ann Oncol. 2010;21:2135–44. doi: 10.1093/annonc/mdq050. [DOI] [PubMed] [Google Scholar]
- 9.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Eng J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 10.Maggioni D, Nicolini G, Chiorazzi A, Meregalli C, Cavaletti G, Tredici G. Different effects of erythropoietin in cisplatin- and docetaxel-induced neurotoxicity: an in vitro study. J Neurosci Res. 2010;1:3171–79. doi: 10.1002/jnr.22465. [DOI] [PubMed] [Google Scholar]
- 11.Vainas O, Ariad S, Amir O, et al. Personalising docetaxel and G-CSF schedules in cancer patients by a clinically validated computational model. Br J Cancer. 2012;21:814–22. doi: 10.1038/bjc.2012.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Eng J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–95. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. Eng J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fosså SD, Slee PH, Brausi M, et al. Flutamide versus prednisone in patients with prostate cancer symptomatically progressing after androgen-ablative therapy: a phase III study of the European organization for research and treatment of cancer genitourinary group. J Clin Oncol. 2001;19:62–71. doi: 10.1200/JCO.2001.19.1.62. [DOI] [PubMed] [Google Scholar]
- 16.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 17.Ryan CJ, Smith MR, de Bono JS, et al. Randomized phase 3 trial of abiraterone acetate in men with metastatic castration-resistant prostate cancer and no prior chemotherapy. N Engl J Med. 2013;368:138–48. [Google Scholar]
- 18.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 19.Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–88. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30:637–43. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302) Eur Urol. 2014 doi: 10.1016/j.eururo.2014.02.056. doi:10.1016/j.eururo.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]