Abstract
Age-related cognitive impairments are particularly prevalent in forms of learning that require a functionally intact hippocampal formation, such as spatial learning and declarative memory. However, there is notable heterogeneity in the cognitive abilities of aged subjects. To date, few studies have determined whether age-related impairments on one learning task relate to impairments on different learning tasks that engage overlapping cognitive processes. Here, we hypothesized that aged animals that were impaired on one hippocampal-dependent behavioral procedure would be impaired on a second hippocampal-dependent procedure. Conversely, aged animals that were unimpaired on one hippocampal-dependent task would be unimpaired with a subsequent hippocampal-dependent form of learning. To test these hypotheses we trained young (2–3m) and aged (28–29m) F344XBN male rats with trace eyeblink conditioning, followed by the Morris water maze. Half of aged rats were impaired during trace conditioning. Nearly half of aged animals were also impaired during water maze probe testing. Performance during trace conditioning correlated with performance during water maze testing in aged animals. Further analyses revealed that, as a group, aged animals that were impaired on one hippocampal-dependent task were impaired on both tasks. Conversely, aged animals that were unimpaired on one task were unimpaired on both tasks. Together, these results suggest that aged-related impairments on one hippocampal-dependent task predict age-related impairments on a second hippocampal-dependent procedure. These results have implications for assigning personalized therapeutics to ameliorate age-related cognitive decline.
Keywords: Aging, Learning, Hippocampus, Eyeblink Conditioning, Water Maze
Introduction
Aging is associated with learning and memory impairments that span numerous cognitive domains. Aged human subjects display deficits in tests of working memory (Park et al., 1996), executive function (Buckner, 2004), processing speed (Salthouse, 1996), and spatial and non-spatial declarative learning and memory (Cheng, Faulkner, Disterhoft, & Desmond, 2010; Foster, 2012; Moffat, Zonderman, & Resnick, 2001). Several animal models of cognitive aging have been established to better understand the neurobiological mechanisms underlying age-related decline. These models typically have strong face validity, as aged rats, rabbits, mice, and dogs display age-related learning and memory impairments (Cotman & Head, 2008; Kaczorowski & Disterhoft, 2009; Knuttinen et al., 2001; Pancani et al., 2013; Thompson et al., 1996; van Praag et al., 2005).
Many forms of learning that are impaired during aging require an intact hippocampal formation. For example, aged human and animal subjects are impaired during acquisition of the trace eyeblink response (Finkbiner & Woodruff-Pak, 1991; Knuttinen et al., 2001), and the hippocampus is necessary for acquisition of this temporal association (Beylin et al., 2001; Tseng, Guan, Disterhoft, & Weiss, 2004; Weiss, Bouwmeester, Power, & Disterhoft, 1999). Aged human and animal subjects also display spatial memory impairments (Foster, 2012; Gallagher, Stocker, & Koh, 2011), and the hippocampus is required for many forms of spatial learning and memory (Morris, Garrud, Rawlins, & O’Keefe, 1982). For example, aged human subjects are impaired during acquisition of a computer-based virtual water maze procedure (Iaria, Palermo, Committeri, & Barton, 2009; Plancher, Gyselinck, Nicolas, & Piolino, 2010), and aged rodents are impaired during acquisition of the hippocampal-dependent hidden platform Morris water maze task (Foster, 2012; Frick, Baxter, Markowska, Olton, & Price, 1995; Gallagher, Burwell, & Burchinal, 1993).
A decline in cognitive ability is often viewed as a natural part of the aging process, however there is a large amount of heterogeneity in the learning abilities of aged human and animal subjects (Thompson et al., 1996). Although many aged individuals display learning and memory impairments, a subset of aged subjects can acquire and remember novel information as well as younger adults (Ardila, 2007; Matzel, Grossman, Light, Townsend, & Kolata, 2008; Thompson et al., 1996). Because there is a large degree of heterogeneity in the cognitive abilities across aged subjects, we hypothesized that this ability would be conserved across tasks, i.e., aged animals that were impaired on one hippocampal-dependent task would also be impaired on a separate hippocampal-dependent task. Conversely, we predicted that aged animals that were unimpaired on one hippocampal-dependent task would be unimpaired on a second hippocampal-dependent task. To test these hypotheses we trained young and aged rats with trace eyeblink conditioning followed by the Morris water maze.
Methods
Subjects
Sixteen young adult (2–3 month) and thirty-six aged (28–29 month) specific pathogen free male F1 hybrid Fischer 344 X Brown Norway (F344XBN) rats were used for the current study. All animals were provided from the National Institute on Aging’s colony at Harlan Laboratories (Indianapolis, IN, USA). Animals were first trained with eyeblink conditioning followed by the Morris water maze (Fig. 1A). Previous research has revealed that training with eyeblink conditioning does not influence subsequent training with the Morris water maze (Kuo, Lee, & Disterhoft, 2006). One young and one aged rat were removed from the analysis due to complications with electrodes used during eyeblink conditioning. An additional one young and four aged rats were removed due to poor performance during visible platform water maze training. Therefore, fourteen young and thirty-one aged rats were used in the final analysis. All animals were fed ad libitum and cared for in an AAALAC approved temperature-controlled clean animal care facility, with a 14:10hr light/dark cycle. They were allowed to acclimate to Northwestern University vivarium for at least one week prior to the onset of the experiment. All rats were group housed to minimize stress. All procedures were approved by the Northwestern University Animal Care and Use Committee, and conformed to NIH standards.
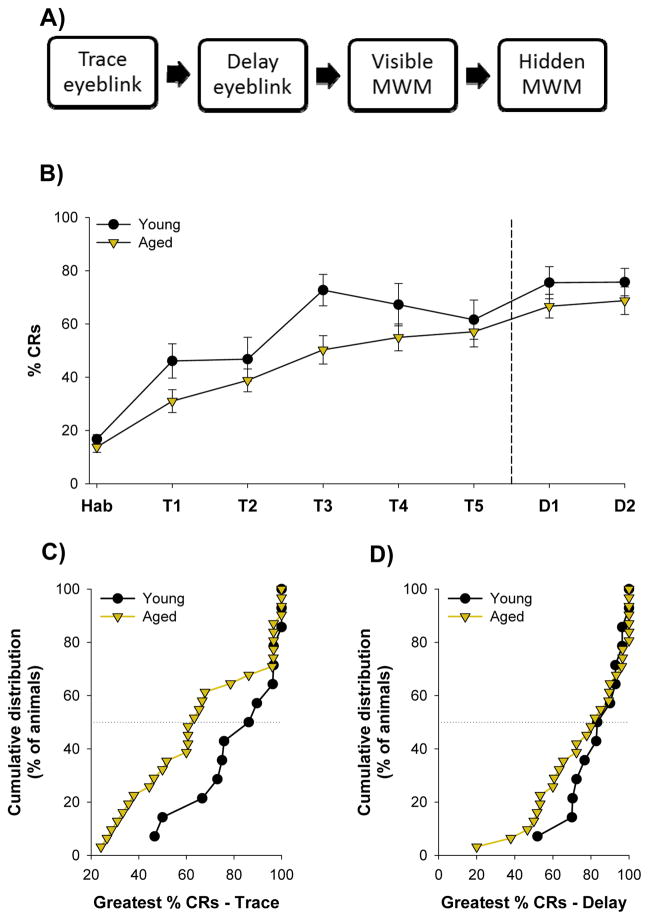
Figure 1.
A) Experimental design. All animals were trained with trace eyeblink conditioning followed by delay conditioning. They were then trained with the visible platform version of the Morris water maze, followed by the hidden platform version of the maze. Probe trials were conducted following the third, fourth, and fifth sessions of hidden platform training. B) Young (N=14) and aged (N=31) animals acquired the trace and then delay eyeblink responses. C) Cumulative distributions of the greatest %CRs emitted by each young and aged rat during any one session of trace conditioning. D) Cumulative distributions of the greatest %CRs emitted during any one session of delay conditioning. Vertical dashed line indicates transition from trace to delay conditioning. Horizontal dotted lines indicate median of cumulative distributions. Error bars represent standard error of the mean.
Eyeblink conditioning
Animals were secured in a stereotaxic device for implantation of a headstage and four periorbital electrodes which were used during eyeblink conditioning. During surgery animals were anesthetized with isoflurane gas, supplemented with Buprenex (0.03mg/kg, subcutaneous). Two Teflon coated stainless steel wire periorbital electrodes (0.003in. diameter) were inserted into the right orbicularis oculi to measure blink related activity. Two wires were inserted into the periorbital region lateral to the eye to deliver the unconditioned stimulus (US), and one stainless steel ground wire (0.005in. diameter) was connected to two screws threaded into the skull.
After recovering from surgery animals were habituated to the conditioning chamber for twenty minutes, while spontaneous blink activity was recorded. Three hours after the habituation session animals began training with thirty trials of trace eyeblink conditioning per day, for five days. During training the conditioned stimulus (CS) was a 250ms 85dB 8kHz tone (5ms rise/fall time). The CS was followed by a 500ms stimulus-free trace period, which was followed by a 100ms unconditioned stimulation (US) to the periorbital region (6 pairs of biphasic 1ms pulses). The intensity of the stimulation was calibrated for each animal, such that the minimum amount of current necessary to elicit an unconditioned eyeblink reflex was used. The intertrial interval was 45±15sec. The occurrence of a conditioned eyeblink response was determined from integrated EMG recordings of the orbicularis oculi. During each trial a blink occurred when there was integrated EMG activity that was greater than the average EMG activity during the 250ms preCS baseline period plus four standard deviations, with a duration of at least 15ms. Conditioned responses (CRs) were blinks that were present at least 50ms after the onset of the CS, but before the onset of the US.
Three days after the last session of trace conditioning all rats began training with delay eyeblink conditioning in order to assay the functional integrity of brainstem and cerebellar circuitry related to eyeblink conditioning, and to ensure that there were no sensory or motor deficits which prevented the animal from hearing or responding to the conditioned stimulus. Two thirty-trial sessions of delay conditioning were performed per day. Animals were trained with at least two sessions of delay conditioning. Delay conditioning was conducted exactly as trace conditioning, except that the duration of the CS was extended so that it remained on for the full 750ms time window between CS and US onset.
Morris water maze
Following eyeblink conditioning we assessed spatial learning and memory with the Morris water maze. Water maze training took place in a circular pool (180cm diameter) filled with 25±1C° water. The water was made opaque with the addition of non-toxic white paint. All animals were first trained with the visible platform version of the task, to ensure that they were capable of locating, navigating, and climbing on to the escape platform. During visible platform training the escape platform (20.3cm×25.4cm) was placed in one of three different start locations, and there were no distinctive cues surrounding the pool. The platform was raised 2mm above the water level, and several visual cues were attached to the platform. Six trials of visible platform training were performed per session. After every two trials the escape platform was moved to a different quadrant of the pool. During each trial the animal was placed approximately 75mm from the edge of the pool, in the center of one of three quadrants that did not contain the hidden escape platform. Animals began each trial by facing the edge of the pool. Each animal was given 60sec to locate the visible platform. If the platform was not located after 60sec the animal was guided to the platform by the experimenter. All animals were trained with at least two, but no more than three, sessions of visible platform training. If an animal’s average path length during the last three trials of visible platform training was greater than 250cm that animal was classified as sensory/motor impaired and removed from the study. We chose this criterion as the vast majority of young and aged rats were able to locate the visible platform with an average path length of less than 250cm by the end of training.
Following visible platform training all animals were trained with the hidden platform procedure. During hidden platform training a distinctive unique cue was in place on each of the four walls surrounding the pool. The escape platform was placed in a novel location and submerged approximately 1cm below the water level. The location of the platform did not change during hidden platform training. Animals were trained with six trials per day for five days. During each trial an animal was placed in the center of one of the three quadrants that did not contain the hidden platform. Each animal was given 60sec to locate the hidden platform. If the platform was not located after 60sec the animal was guided to the platform by the experimenter. All animals were allowed to remain on the platform for 20sec at the end of each trial.
Several studies have revealed that young animals with hippocampal lesions, or inactivation of the hippocampus, can perform various spatial tasks, including the cross maze and the hidden platform water maze, through the use of non-spatial search strategies (Packard & McGaugh, 1996; Pouzet, Zhang, Feldon, & Rawlins, 2002). However, animals with hippocampal lesions show no or little preference for the platform location during water maze probe testing, indicating impaired spatial memory in these animals (Broadbent, Squire, & Clark, 2006). Therefore, we used probe trials to assess hippocampal-dependent spatial memory during water maze training. One probe trial was conducted at the end of the third, fourth, and fifth days of hidden platform training. During each probe trial the escape platform was removed from the pool and each animal was allowed to swim for 60sec. Average proximity from the center of the location of the pool that previously contained the escape platform was used as a measure of memory for the platform location, as this measure is particularly sensitive to detecting age-related spatial impairments (Gallagher et al., 1993; Maei, Zaslavsky, Teixeira, & Frankland, 2009). Computerized tracking software was used during training and subsequent offline analysis (WaterMaze, Actimetrics, Wilmette, IL).
Statistics
Performance during trace conditioning and water maze training was analyzed with repeated measures ANOVA, with Greenhouse-Geisser corrections applied when Mauchley’s test indicated that the assumption of sphericity had been violated. Levene’s test for equality of variance, univariate ANOVA with post-hoc Tukey comparisons, and unpaired t-tests were also used as appropriate. Group data are presented as cumulative distributions, and as means with the standard error of the mean.
Results
Half of aged rats were impaired during acquisition of trace eyeblink conditioning
Prior to trace eyeblink conditioning all rats were habituated to the conditioning chambers. The percent of spontaneous blinks emitted during habituation did not differ between young and aged rats (t(43)=0.36, p≥0.05; FIG. 1B). Across all five sessions of trace conditioning young animals tended to emit more CRs (58% ± 5%) than aged animals (46% ± 4%), however this comparison did not reach significance (F(1, 43) = 3.23, p≥0.05; FIG. 1B) Following trace conditioning all animals were trained with delay conditioning. The percent CRs emitted during delay conditioning did not differ between young (76% ± 5%) and aged (68% ± 4%) rats (F(1, 43) = 1.25, p≥0.05 FIG. 1B).
To quantify peak performance during training we examined the greatest %CRs emitted by each rat during any session of training. During trace conditioning the average greatest %CRs emitted by young rats (82 ± 5%) was greater than that of aged rats (66 ± 5%; t(43) = 2.04, p<0.05; FIG. 1C), confirming that aged rats were impaired during trace eyeblink conditioning. There was also a trend towards increased variance in the greatest %CRs emitted during any session of trace conditioning in aged rats, as compared to young rats, although this comparison did not reach significance (F = 3.42, p=0.07; FIG. 1C). During delay conditioning there was no difference in the greatest %CRs emitted by young (84 ± 4%) and aged rats (77 ± 4%; t(43) = 1.09, p≥0.05; Fig. 1D), confirming that both groups could acquire the delay eyeblink response. However, there was significantly more variance in aged animals (F = 4.97, p<0.05; Fig. 1D). Therefore, although some aged rats performed poorly during eyeblink conditioning others performed at young-like levels.
Previous research has revealed that approximately 50% of aged (27–29mo) F344XBN rats fail to learn the trace eyeblink response (Knuttinen et al., 2001). To determine whether a similar proportion of aged rats were impaired with trace conditioning in the current study we separated aged animals into impaired or unimpaired. To do so we established a criterion, defined as the average of the greatest %CRs emitted by each young animal during any one session of trace conditioning, minus one standard deviation. This was equal to 64% CRs. Aged rats that never emitted at least 64% CRs during at least one session of trace conditioning were considered impaired (AItEBC). The remaining aged animals were classified as unimpaired (AUtEBC). 52% (N=16) of aged rats failed to reach criterion, and were impaired during trace conditioning. The remaining 48% (N=15) of rats were unimpaired during trace conditioning. For comparison, fewer than 15% (N=2) of young rats failed to achieve criterion-level performance. As would be expected, aged rats that were impaired during trace conditioning emitted significantly fewer CRs than either young rats (F(1,28) = 26.45, p<0.05) or aged-unimpaired rats (F(1,29) = 56.73, p<0.05). Importantly, these aged-impaired animals displayed no increase in conditioned responding across the five sessions of trace conditioning, confirming that they did not acquire the trace eyeblink response (F(4,60) = 2.38, p≥0.05). However, there was a significant increase in conditioned responding in aged-impaired animals between the last session of trace conditioning (34% ± 3%) and the first session of delay conditioning (57% ± 5%), indicating that these animals could process and respond to the conditioned stimuli (F(1,15) = 23.04, p<0.05; Fig. 1B). Together, these results confirm that half of the aged rats were impaired during acquisition of trace eyeblink conditioning.
Half of aged animals possessed impaired spatial memory
Animals were first trained with the visible platform procedure, to ensure that they could locate and navigate to the escape platform. Since young rats (31 ± 2cm/sec) swam significantly faster than aged rats (36 ± 1cm/sec; t(50) = 6.59, p<0.01) we used path length to the escape platform as our index of performance during training. A significant decline in path length during trials 1–12 indicated that both young (F(2.47,32.13) = 6.96, p<0.05) and aged (F(5.82, 174.49) = 7.14, p<0.05) animals acquired the location of the visible platform (Fig. 2A inset). There was no decline in path length during the last three trials of visible platform training for young (F(2, 26) = 1.28, p≥0.05) or aged (F(2,60) = 0.29, p≥0.05) rats, and path length during the last three trials did not differ between these groups (F(1,43) = 0.04, p≥0.05; Fig. 2A inset), indicating that both young and aged rats attained comparable plateau performance by the end of visible platform training.
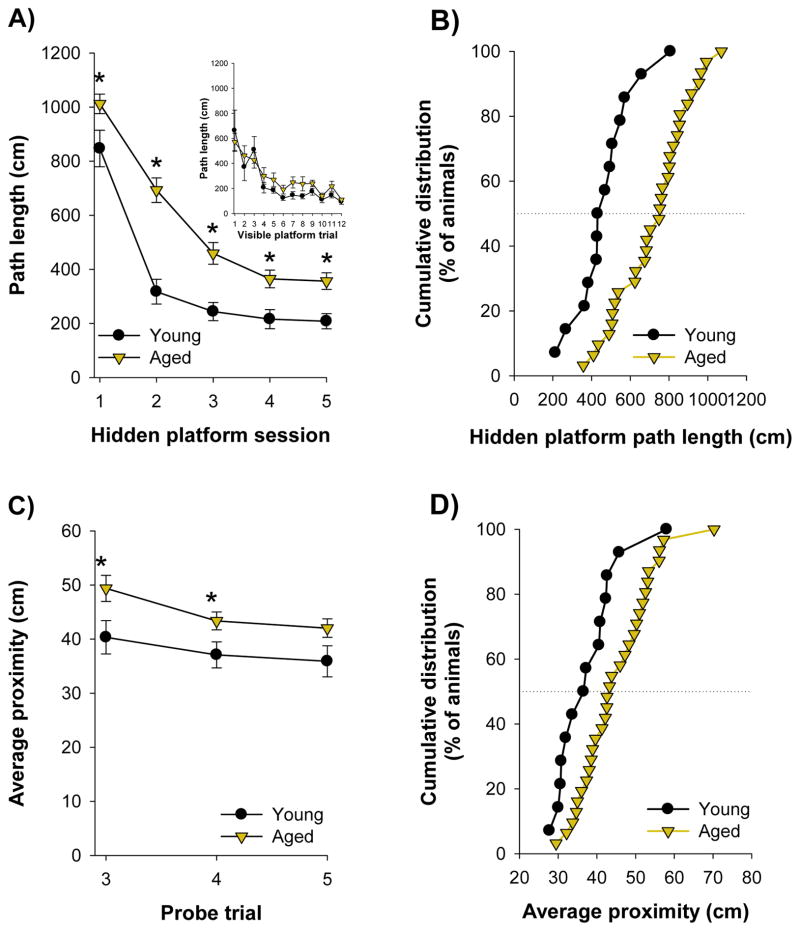
Figure 2.
A) Both young and aged rats decreased their path length over the course of hidden platform training. However, young rats consistently had shorter path lengths than aged rats. Inset: Young and aged animals had comparable path lengths during visible platform training. B) Cumulative distributions of mean path length during the last three sessions of hidden platform training. C) A probe trial was conducted at the end of the third, fourth, and fifth session of hidden platform training. Aged animals consistently had larger average proximities than young animals, indicating that aged rats possessed impaired spatial memory for the platform location. D) Distributions of the average proximity across the three probe sessions. Asterisks (*) indicate significantly different from young (p<0.05). Dotted lines indicate median of cumulative distributions. Error bars represent standard error of the mean.
All rats were then trained with five daily sessions of the hidden platform procedure. Both young (F(2.08, 26.98) = 57.73, p<0.05) and aged (F(2.99, 89.77) = 97.70, p<0.05) animals decreased their path length across the five sessions. However, aged animals consistently had longer path lengths (F(1,43) = 20.99, p<0.05 ; Fig. 2A). Analysis of the last three sessions revealed that path length was larger in aged animals (t(43) = 4.47, p<0.05; Fig. 2A, B), confirming that aged rats were impaired during hidden platform training. Although there was increased variance in aged animals during eyeblink conditioning we observed no difference in variance of path length between young and aged rats during hidden platform training (F = 1.55, p≥0.05; Fig. 2B). A probe trial was conducted after the third, fourth, and fifth sessions of hidden platform training. Average proximity from the region of the pool that previously contained the escape platform was used as a measure of spatial memory. To ensure that we used a reliable measure of spatial memory we computed the mean average proximity score for each animal across all three probe tests. Aged rats had greater average proximity (45 ± 2cm) than young rats (38 ± 2cm), revealing that, as a group, aged rats had impaired memory for the platform location (t(43) = 2.54, p<0.05; Fig. 2C, 2D).
To characterize aged rats as impaired or unimpaired during probe testing we established a criterion based on performance of the young animals. This criterion was equal to the average proximity of young rats across the three probe tests, plus one standard deviation of the mean. This was equal to 46cm. Aged rats with average proximity across the three probe tests of greater than 46cm were classified as spatially impaired. Those with proximities equal to or less than 46cm were classified as unimpaired. 45% of aged rats were impaired with the water maze (AIMWM; N=14), whereas 55% were unimpaired (AUMWM; N=17). Only 7% of young rats failed to reach this criterion (N=1). Path length during the last three trials of visible platform training did not differ between aged animals that were impaired (125 ± 12cm) or unimpaired (104 ± 11cm) during probe testing (t(29) = 1.28, ≥0.05). Univariate ANOVA revealed that average proximity across the three probe trials differed between young, AUMWM, and AIMWM rats (F(2,42) = 28.89, p<0.05). As expected, AIMWM rats had greater proximity measures (53 ± 2cm) than young (38 ± 2cm) and AUMWM animals (38 ±1cm; p’s<0.05). Proximity scores were not different between young and AUMWM rats (p≥0.05), confirming that the aged-unimpaired animals were able to perform this procedure at a young-like level. Together, these results confirm that approximately half of aged animals were impaired during water maze probe testing.
Aged-related spatial memory impairments correlated with impairments during trace conditioning
To determine whether age-related impairments during eyeblink conditioning were related to age-related impairments during water maze testing we examined correlations between hippocampal-dependent and independent measures of performance during training with these two tasks. Hippocampal-dependent measures included the greatest %CRs emitted during any session of trace conditioning, and average proximity during the three water maze probe tests. Measures of hippocampal-independent function included the greatest %CRs emitted during any one session of delay conditioning, path length during the last three trials of cued platform training, path length during the last three sessions of hidden platform training, and swim speed (Table 1). We included path length during hidden-platform training as a hippocampal-independent measure since young animals with hippocampal lesions can learn the location of the hidden platform through the use of non-spatial search strategies (Pouzet et al., 2002). In young rats few significant correlations were observed. Path length during hidden platform training correlated with average proximity during probe tests (r = 0.76, p<0.05), and swim speed correlated with %CRs during delay conditioning (r = −0.59, p<0.05). However, these were the only significant correlations observed in young animals, likely because the majority of young rats mastered both the trace conditioning and water maze procedures.
Table 1.
Correlations of hippocampal-dependent and hippocampal-independent measures of performance during eyeblink conditioning and water maze testing in young and aged rats. Measures included the greatest % CRs emitted during any one session of trace conditioning (Trace) or delay conditioning (Delay), path length during the last three trials of visible platform training (Cued), path length during the last three sessions of hidden platform training (Hidden Platform), average proximity across the three probe tests (MWM Probe), and swim speed (Swim Speed). Asterisks indicate p<0.05.
| Young | ||||||
|---|---|---|---|---|---|---|
| Trace | MWM Probe | Delay | Hidden Platform | Cued Platform | Swim Speed | |
| Trace | −.328 | .151 | −.255 | −.111 | .222 | |
| MWM Probe | −.328 | −.240 | .761* | .067 | −.203 | |
| Delay | .151 | −.240 | −.137 | .120 | −.591* | |
| Hidden Platform | −.255 | .761* | −.137 | .425 | −.293 | |
| Cued Platform | −.111 | .067 | .120 | .425 | −.475 | |
| Swim Speed | .222 | −.203 | −.591* | −.293 | −.475 | |
| Aged | ||||||
|---|---|---|---|---|---|---|
| Trace | MWM Probe | Delay | Hidden Platform | Cued Platform | Swim Speed | |
| Trace | −.405* | .503* | −.060 | −.067 | .095 | |
| MWM Probe | −.405* | −.273 | .515* | .250 | −.253 | |
| Delay | .503* | −.273 | .046 | −.068 | .058 | |
| Hidden Platform | −.060 | .515* | .046 | .095 | −.219 | |
| Cued Platform | −.067 | .250 | −.068 | .095 | −.291 | |
| Swim Speed | .095 | −.253 | .058 | −.219 | −.291 | |
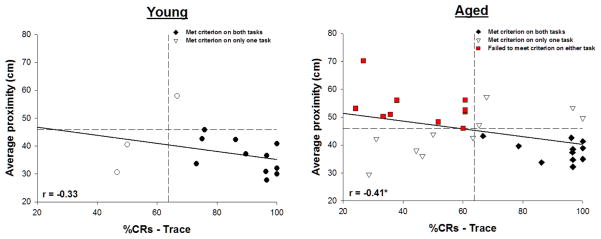
In aged animals path length during hidden platform training correlated with proximity during probe trials (r = 0.52, p<0.05), and performance during trace conditioning correlated with performance during delay conditioning (r = 0.50, p≥0.05). These within task correlations were likely the result of transfer effects. Importantly, performance during trace conditioning also correlated with average proximity during water maze probe testing, i.e. aged rats that emitted more conditioned responses during trace conditioning also did well during water maze testing, having the smallest average proximities during probe tests (r = −0.41, p<0.05; Fig. 3). This relationship between spatial memory and trace conditioning was unique to measures of hippocampal-dependent function, as no other correlations were observed between performance during eyeblink conditioning and water maze training in aged animals. For example, swim speed did not correlate with %CRs during trace conditioning (r = 0.10, p≥0.05), nor did path length during visible platform training (r = −0.07, p≥0.05). Likewise, the greatest %CRs emitted during delay conditioning did not correlate with average proximity during probe trials (r = −0.27, p≥0.05; Table 1). Together, these results reveal that aged animals that were impaired on one-hippocampal-dependent task were likely to be impaired on both hippocampal-dependent procedures.
Figure 3.
Performance during trace conditioning correlated with spatial memory in aged, but not young, rats. In young rats the greatest percentage of conditioned responses (CRs) emitted by each animal during any session of trace conditioning did not significantly correlate with that animal’s average proximity across the three probe tests (r = −0.33, p≥0.05). However, performance during trace conditioning and water maze testing did significantly correlate in aged animals (r = −0.41, p<0.05). Vertical dashed line indicates criterion for trace conditioning. Horizontal dashed line indicates criterion for water maze testing. Black circles indicate young rats that achieved criterion on both tasks, whereas white circles indicate young rats that achieved criterion on only one task. Diamonds indicate aged rats that achieved criterion on both tasks, triangles indicate aged rats that achieved criterion on only one task, and squares indicate aged rats that failed to achieve criterion on both tasks. A much greater degree of behavioral heterogeneity was observed in aged animals. Asterisk (*) indicates p<0.05.
Performance during trace conditioning predicted performance during water maze testing in aged animals
To determine whether age-related impairments during trace conditioning were predictive of impairments during water maze testing we separated aged rats into those that were unimpaired and those that were impaired during trace conditioning. We then determined how well those groups performed during water maze training. Swim speed was comparable between aged animals that were unimpaired (AUtEBC) and those that were impaired during trace conditioning (AItEBC; t(29) = 0.57, p≥0.05). Likewise, path length during the last three trials of visible platform training was not different between young, AUtEBC, and AItEBC rats (F(2,45) = 0.10, p≥0.05; Fig. 4A inset). Therefore, aged animals that were impaired or unimpaired with trace conditioning could acquire the visible platform procedure.
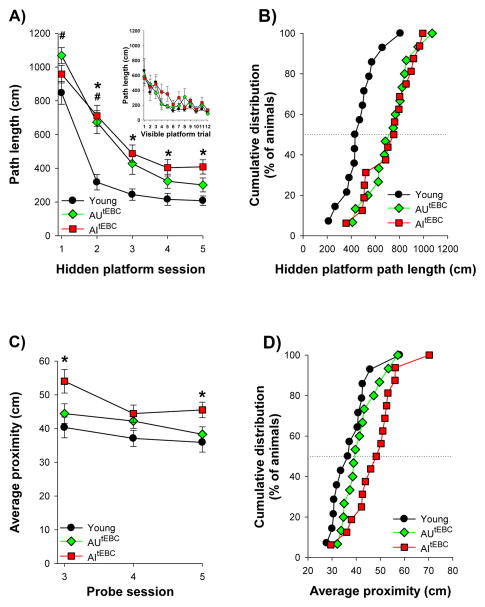
Figure 4.
Aged rats were separated into those that were unimpaired (AUtEBC; N=15) or impaired during trace conditioning (AItEBC; N=16). A) Both AUtEBC and AItEBC animals decreased their path length across the five sessions of hidden platform training, whereas young rats consistently had shorter path lengths than either of these groups. Inset: All three groups displayed comparable path lengths during visible platform training. B) Cumulative distributions of average path length during the last three sessions of hidden platform training. C) Average proximity across the three probe tests. There was no difference in average proximity between aged rats that were unimpaired during trace conditioning and young rats. However, aged rats that were impaired during trace conditioning had greater proximity measures than young animals, confirming that these rats were also impaired during water maze probe testing. D) Cumulative distributions of average proximity across the three probe tests. Together, these results reveal that aged rats that were impaired during trace conditioning were also impaired during water maze probe testing. Aged animals that were unimpaired during trace conditioning were unimpaired during probe testing. Dotted lines indicate median of cumulative distributions. Error bars represent standard error of the mean.
Across the five sessions of hidden platform training path length decreased for both aged animals that were unimpaired during trace conditioning (F(2.50, 35.05) = 68.80, p<0.05) and those that were impaired during trace conditioning (F(4,60) = 37.32, p<0.05; Fig. 4A). However, average path length during the last three sessions of hidden platform training differed between young, AUtEBC and AItEBC rats (F(2,45) = 9.76 p<0.05; Fig. 4B). Post-hoc Tukey comparisons revealed that young rats had shorter path lengths than AUtEBC and AItEBC animals (all p values <0.05). Path length during hidden platform training did not differ between aged rats that were unimpaired or impaired with trace conditioning (p≥0.05). Average proximity during the three probe tests differed between young, AUtEBC, and AItEBC animals (F(2,42) = 5.66, p<0.05). Post-hoc comparisons revealed that aged rats that were impaired during trace conditioning had proximity scores that were significantly greater than those of young rats (p<0.05; Fig. 4C, 4D). However, proximity scores from aged rats that were unimpaired during trace conditioning were comparable to those of young animals (p≥0.05; Fig. 4C, 4D). Together, these results reveal that aged animals that were impaired during trace conditioning were also impaired during probe trial memory tests. Likewise, those that were unimpaired during trace conditioning were unimpaired during water maze probe testing.
Aged rats with impaired spatial memory were also impaired during trace conditioning
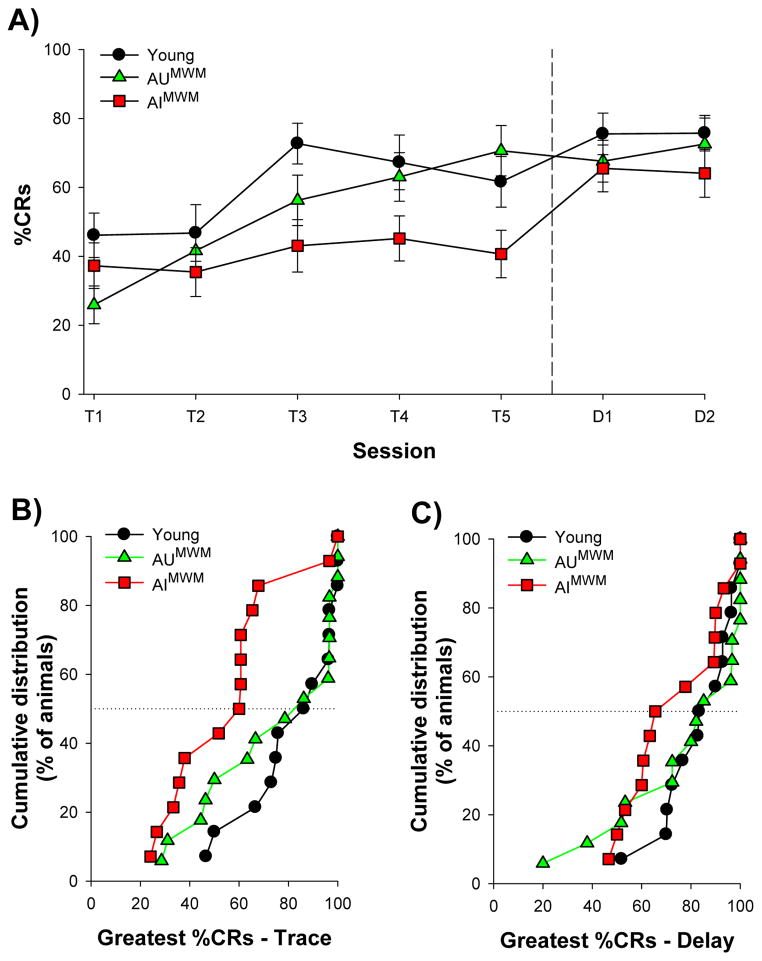
To determine whether aged animals that performed poorly during water maze testing also performed poorly during eyeblink conditioning we separated aged rats into those that were impaired (AIMWM) or unimpaired (AUMWM) during water maze probe testing, and examined their performance during eyeblink conditioning. During habituation young, AUMWM, and AIMWM animals emitted a comparable percentage of spontaneous blinks (F(2,42) = 0.69, p≥0.05). Repeated measures ANOVA of %CRs emitted across the five sessions of trace conditioning, with session as the repeated measure, revealed a significant increase in conditioned responding in young (F(4,52) = 5.19, p<0.05) and aged rats that were unimpaired during water maze testing (F(2.24, 35.77) = 14.74, p<0.05). However, aged rats that were impaired during water maze testing displayed no increase in %CRs during trace conditioning (F(4, 52) = 1.05, p≥0.05; Fig. 5A). Moreover, these AIMWM rats emitted significantly fewer conditioned responses than young rats (F(1,26) = 5.23, p<0.05; Fig. 5A). The greatest %CRs emitted during any session of trace conditioning also differed among these three groups (F(2,42) = 4.99, p<0.05). Post-hoc comparisons revealed that aged rats that were impaired during water maze testing emitted significantly fewer CRs than young rats (p<0.05; Fig. 5B), whereas the greatest %CRs emitted by aged animals that were unimpaired during water maze testing was comparable to that of young animals (p≥0.05; Fig. 5B).
Figure 5.
Aged rats were separated into those that were unimpaired (AUMWM; N=17) or impaired (AIMWM; N=14) during water maze probe testing. A) Young rats and aged rats that were unimpaired during probe testing increased the percent of CRs emitted across the five sessions of trace conditioning. Aged rats that were impaired during water maze testing did not increase the percent of CRs emitted during trace conditioning. All three groups emitted a similar percent of CRs during delay conditioning. B) Cumulative distributions of the greatest percent CRs emitted during any session of trace conditioning. The greatest percent CRs emitted by AUMWM rats was comparable to that of young rats. However, AIMWM rats emitted significantly fewer CRs than young animals during trace conditioning. C) Cumulative distributions of the greatest percent CRs emitted during any session of delay conditioning. The greatest percent CRs emitted during delay conditioning did not differ among young, AUMWM, and AIMWM rats. Vertical dashed line indicates transition from trace to delay conditioning. Horizontal dotted lines indicate median of cumulative distributions. Error bars represent standard error of the mean.
Repeated measures ANOVA of performance during delay conditioning revealed that the average %CRs emitted during delay conditioning did not differ among young (72% ± 5%), AUMWM (65% ± 6%) and AIMWM (65% ± 6%) rats (F(1,42)=0.12, p 0.05). Likewise, the greatest %CRs emitted during any one session of delay conditioning did not differ among these groups (F(2,42) = 0.81, p≥0.05; Fig. 5C). Importantly, a significant increase in conditioned responding was observed between the fifth session of trace conditioning and the first session of delay conditioning in aged animals that were impaired during water maze testing (F(1,13) = 14.36, p<0.05), confirming that these animals were able to process and respond to the conditioned stimuli. Together, these results reveal that the aged animals that were impaired during water maze testing were also impaired during trace eyeblink conditioning, whereas aged animals that were unimpaired during water maze testing were able to acquire the trace eyeblink response at a “young-like” level. All groups, whether impaired or unimpaired on the hippocampal-dependent components of the two tasks, were able to learn the non-hippocampal aspects of both tasks equally well.
Discussion
The present results confirm that a subset of aged F344XBN rats are impaired during acquisition of the hippocampal-dependent trace eyeblink conditioning and Morris water maze procedures (Foster, 2012; Gallagher, Burwell, & Burchinal, 1993; Knuttinen et al., 2001; Thompson et al., 1996). However, not every aged animal was impaired. Approximately half of aged rats were impaired during acquisition of the trace eyeblink response, whereas the remaining aged animals performed trace conditioning at “young-like” levels. Furthermore, only half of aged rats possessed impaired spatial memory, as assessed with water maze probe trials. The age-related impairments during trace conditioning were likely not the result of impaired cerebellar/brainstem circuitry, or sensory processing, as both aged-unimpaired and aged-impaired animals were able to acquire the hippocampal-independent delay eyeblink response. Likewise, the age-related impairments during water maze testing were not likely the result of overall sensory or motor impairments, as young, aged-unimpaired, and aged-impaired animals performed similarly at the end of visible platform training (prior to the hidden platform training). Although several studies have revealed that training with one behavioral task can influence acquisition of a subsequent behavioral procedure (Curlik & Shors, 2013; Nokia, Sisti, Choksi, & Shors, 2012), previous findings have revealed that training with eyeblink conditioning does not influence water maze performance when the two procedures are performed consecutively in young adult rats (Kuo, Lee, & Disterhoft, 2006). Therefore, it is highly likely that eyeblink conditioning did not influence spatial learning or memory in our current study. Overall these findings confirm that there are robust learning and memory impairments in aged F344xBN rats. However, like aged human subjects, aged rats exhibited heterogeneous performance on tests of learning and memory.
To determine whether age-related impairments with one hippocampal-dependent procedure were related to impairments on a second hippocampal-dependent procedure we correlated performance during eyeblink conditioning and water maze training. In young rats few significant correlations were observed, and performance during eyeblink conditioning was not a reliable predictor of performance during water maze probe testing, likely due to a ceiling effect in young animals. However, in aged animals performance during trace eyeblink conditioning significantly correlated with performance during water maze probe testing, such that aged rats that were impaired during trace conditioning were likely to be impaired during water maze testing, and vice versa. Importantly, we did not observe correlations between measures of hippocampal-independent function during eyeblink conditioning and water maze training in aged rats. For example, swim speed and path length during visible platform training did not significantly correlate with performance during trace conditioning. Likewise, performance of the hippocampal-independent delay eyeblink response did not correlate with average proximity during water maze probe tests.
As a group, aged animals that failed to reach criterion on one task were impaired during the second task, and vice versa. These results are consistent with previous findings revealing that age-related impairments during acquisition of the MWM procedure correlate with acquisition impairments when animals are retrained with a novel platform location (Guidi, Kumar, Rani, & Foster, 2014). They are also consistent with results revealing that age-related spatial learning impairments correlate with age-related impairments during an odor discrimination task (LaSarge et al., 2007), and those demonstrating that age-related impairments during water maze testing correlate with recollection deficits during an olfactory discrimination task (Robitsek, Fortin, Koh, Gallagher, & Eichenbaum, 2008). However, our results are the first to reveal that age-related impairments on the water maze procedure correlate with impairments during trace eyeblink conditioning, a commonly used and well understood test of hippocampal-dependent learning in human subjects and laboratory animals. Together, these results suggest that a decline in hippocampal function in a subset of aged animals results in cognitive deficits that can be observed across multiple tests of hippocampal function.
Many experiments examining therapeutics for age-related cognitive decline are designed so that the therapeutic is administered prior to any behavioral characterization. In these cases, the large amount of variance in the cognitive abilities of aged subjects indicates that these treatments will be administered to both aged-impaired and unimpaired subjects. Our experiments revealed that approximately half of aged rats are impaired during trace conditioning and/or water maze testing. Therefore, treatments designed at ameliorating age-related impairments in trace eyeblink conditioning and/or the water maze will likely have no, or minimal, effect in at least one half of aged F344XBN rats, as these animals already perform at “young-like” levels. Because therapeutics designed at ameliorating age-related learning and memory impairments will likely have no, or little, benefit on the cognitive abilities of aged-unimpaired subjects, combining the data from aged-impaired and unimpaired populations will likely result in increased variance, and a decreased signal-to-noise ratio. Separating aged animals into those that are cognitively impaired or unimpaired prior to the administration of experimental therapeutics should decrease this variance. Furthermore, by characterizing aged animals as impaired or unimpaired prior to administration of experimental therapeutics, one can determine the differential effects of those therapeutics on the aged-impaired and unimpaired populations (Haberman, Colantuoni, Koh, & Gallagher, 2013). Our current results provide one method by which age-related learning and memory impairments can be characterized, by training aged animals with one hippocampal-dependent learning procedure, and separating those animals into impaired or unimpaired groups based on their performance. Those groups can then receive experimental treatments before being trained with a second behavioral procedure that relies on overlapping regions of the brain.
We chose trace eyeblink conditioning and the Morris water maze as acquisition of both of these procedures requires several overlapping brain regions, including the hippocampus and cerebellum (Beylin et al., 2001; Lalonde, 1994; Morris et al., 1982; Thompson & Steinmetz, 2009; Weiss et al., 1999). Although age-related impairments have been reported during the initial sessions of delay EBC (Weiss & Thompson, 1992) in otherwise naïve F1 hybrid rats, that result may be due more to their advanced age (i.e., 36 months old) and age-related degeneration of cerebellar Purkinje neurons (Woodruff-Pak, Cronholm, & Sheffield, 1990) than to deficits in hippocampal mechanisms. Although the exact mechanisms underlying age-related associative and spatial learning and memory impairments are unknown, there are numerous age-related changes that occur to the hippocampal formation which may contribute to these impairments. For example, the “calcium hypothesis of aging”, predicts that a disruption of calcium homeostasis underlies age-related cognitive impairments (Disterhoft, Moyer, & Thompson, 1994; Gibson & Peterson, 1987; Landfield, 1987, Oh, Oliveira, Waters, & Disterhoft, 2013). Others have hypothesized that age-related spatial memory impairments result from an increase in the strength of the auto-associative network of area CA3 (Wilson, Gallagher, Eichenbaum, & Tanila, 2006; Wilson, Ikonen, Gallagher, Eichenbaum, & Tanila, 2005). Of course, myriad changes occur to the brain with aging, including morphological changes, synaptic changes, metabolic changes, and connectivity changes that are not discussed here (Nicholson, Yoshida, Berry, Gallagher, & Geinisman, 2004). It is highly unlikely that any one change, or changes to any one structure, are solely responsible for the age-related cognitive impairments observed in the current study. However, the tasks we chose to examine are highly dependent on hippocampal function, and it is likely that age-related changes to the hippocampus are at least partially responsible for the age-related cognitive impairments we observed.
Approximately 12% percent of the world’s population is over the age of 60. This percentage is expected to almost double, with 21% of the world’s population over age 60 by 2050 (World Population Ageing 2013). This increased longevity presents several potential social and medical issues, and it has led to a plethora of research investigating methods of preventing, or reversing, age-related cognitive decline. One key challenge of studying age-related cognitive impairments is the large amount of heterogeneity that exists in cognitive performance of aged subjects. Although some aged subjects display cognitive impairments, others do not (Matzel et al., 2008; Rogalski et al., 2013). When developing treatments for age-related learning and memory impairments researchers will benefit from the ability to these predict these impairments prior to the administration of experimental treatments designed at reversing them. Our current findings provide one method by which investigators can characterize age-related cognitive impairments before administration of experimental therapeutics. Although we only examined learning and memory during normal aging, it is possible that these findings will also translate to learning impairments observed in disease states such as Alzheimer’s Disease.
Acknowledgments
The authors thank John Linardakis for assistance with behavioral characterization of the animals, Dr. Marcia D. Antion for assistance with data analysis, and Dr. M. Matthew Oh for his insightful comments on the manuscript. We thank the Northwestern University Behavioral Phenotyping Core for their help. This work was supported by NIH R37 AG008796 and R01 AG017139 to J.F.D, R01 AG047073 to D.A.N., and T32 AG20506 and P30 AG13854 to D.M.C.
Works cited
- Ardila A. Normal aging increases cognitive heterogeneity: Analysis of dispersion in WAIS-III scores across age. Archives of Clinical Neuropsychology. 2007;22(8):1003–1011. doi: 10.1016/j.acn.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiology of Learning and Memory. 2001;76(3):447–61. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. European Journal of Neuroscience. 2003;18(1):215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Reversible hippocampal lesions disrupt water maze performance during both recent and remote memory tests. Learning & Memory. 2006;13(2):187–91. doi: 10.1101/lm.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Memory and Executive Function in Aging and AD. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Faulkner ML, Disterhoft JF, Desmond JE. The effects of aging in delay and trace human eyeblink conditioning. Psychology and Aging. 2010;25(3):684–90. doi: 10.1037/a0017978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. Journal of Alzheimer’s Disease : JAD. 2008;15(4):685–707. doi: 10.3233/jad-2008-15413. [DOI] [PubMed] [Google Scholar]
- Curlik DM, Shors TJ. Training your brain: Do mental and physical (MAP) training enhance cognition through the process of neurogenesis in the hippocampus? Neuropharmacology. 2013;64:506–514. doi: 10.1016/j.neuropharm.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989;243(4892):809–11. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Moyer JR, Thompson LT. The calcium rationale in aging and Alzheimer’s disease. Evidence from an animal model of normal aging. Annals of the New York Academy of Sciences. 1994;747:382–406. doi: 10.1111/j.1749-6632.1994.tb44424.x. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14385–90. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbiner RG, Woodruff-Pak DS. Classical eyeblink conditioning in adulthood: effects of age and interstimulus interval on acquisition in the trace paradigm. Psychology and Aging. 1991;6(1):109–17. doi: 10.1037//0882-7974.6.1.109. [DOI] [PubMed] [Google Scholar]
- Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Progress in Neurobiology. 2012;96(3):283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiology of Aging. 1995;16(2):149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal MR. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behavioral Neuroscience. 1993;107(4):618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Stocker AM, Koh MT. Mindspan: lessons from rat models of neurocognitive aging. ILAR Journal/National Research Council, Institute of Laboratory Animal Resources. 2011;52(1):32–40. doi: 10.1093/ilar.52.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Peterson C. Calcium and the aging nervous system. Neurobiology of Aging. 1987;8(4):329–43. doi: 10.1016/0197-4580(87)90072-8. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Brocardo PS, Choquette W, Gothard R, Simpson JM, Christie BR. Hippocampal Neurogenesis Levels Predict WATERMAZE Search Strategies in the Aging Brain. PloS One. 2013;8(9):e75125. doi: 10.1371/journal.pone.0075125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi M, Kumar A, Rani A, Foster TC. Assessing the emergence and reliability of cognitive decline over the life span in Fisher 344 rats using the spatial water maze. Frontiers in Aging Neuroscience. 2014;6:2. doi: 10.3389/fnagi.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, Colantuoni C, Koh MT, Gallagher M. Behaviorally activated mRNA expression profiles produce signatures of learning and enhanced inhibition in aged rats with preserved memory. PloS One. 2013;8(12):e83674. doi: 10.1371/journal.pone.0083674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Palermo L, Committeri G, Barton JJS. Age differences in the formation and use of cognitive maps. Behavioural Brain Research. 2009;196(2):187–91. doi: 10.1016/j.bbr.2008.08.040. [DOI] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft JF. Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learning & Memory. 2009;16(6):362–366. doi: 10.1101/lm.1365609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuttinen MG, Gamelli AE, Weiss C, Power JM, Disterhoft JF. Age-related effects on eyeblink conditioning in the F344 x BN F1 hybrid rat. Neurobiology of Aging. 2001;22(1):1–8. doi: 10.1016/s0197-4580(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Enhanced long-term potentiation during aging is masked by processes involving intracellular calcium stores. Journal of Neurophysiology. 2004;91(6):2437–44. doi: 10.1152/jn.01148.2003. [DOI] [PubMed] [Google Scholar]
- Kuo AG, Lee G, Disterhoft JF. Simultaneous training on two hippocampus-dependent tasks facilitates acquisition of trace eyeblink conditioning. Learning & Memory. 2006;13(2):201–7. doi: 10.1101/lm.98406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R. Cerebellar contributions to instrumental learning. Neuroscience & Biobehavioral Reviews. 1994;18(2):161–170. doi: 10.1016/0149-7634(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Landfield PW. Increased calcium-current” hypothesis of brain aging. Neurobiology of Aging. 1987;8(4):346–347. doi: 10.1016/0197-4580(87)90074-1. [DOI] [PubMed] [Google Scholar]
- LaSarge CL, Montgomery KS, Tucker C, Slaton GS, Griffith WH, Setlow B, Bizon JL. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiology of Aging. 2007;28(6):928–36. doi: 10.1016/j.neurobiolaging.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Maei HR, Zaslavsky K, Teixeira CM, Frankland PW. What is the Most Sensitive Measure of Water Maze Probe Test Performance? Frontiers in Integrative Neuroscience. 2009;3:4. doi: 10.3389/neuro.07.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Grossman H, Light K, Townsend D, Kolata S. Age-related declines in general cognitive abilities of Balb/C mice are associated with disparities in working memory, body weight, and general activity. Learning & Memory. 2008;15(10):733–46. doi: 10.1101/lm.954808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Resnick SM. Age differences in spatial memory in a virtual environment navigation task. Neurobiology of Aging. 2001;22(5):787–796. doi: 10.1016/s0197-4580(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. The Journal of Neuroscience. 2004;24(35):7648–53. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokia MS, Sisti HM, Choksi MR, Shors TJ. Learning to Learn: Theta Oscillations Predict New Learning, which Enhances Related Learning and Neurogenesis. PloS One. 2012;7(2):e31375. doi: 10.1371/journal.pone.0031375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Oliveira FA, Waters J, Disterhoft JF. Altered Calcium Metabolism in Aging CA1 Hippocampal Pyramidal Neurons. Journal of Neuroscience. 2013;33(18):7905–7911. doi: 10.1523/JNEUROSCI.5457-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Pancani T, Anderson KL, Brewer LD, Kadish I, DeMoll C, Landfield PW, Blalock EM, Porter NM, Thibault O. Effect of high-fat diet on metabolic indices, cognition, and neuronal physiology in aging F344 rats. Neurobiology of Aging. 2013;34(8):1977–87. doi: 10.1016/j.neurobiolaging.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Smith AD, Lautenschlager G, Earles JL, Frieske D, Zwahr M, Gaines CL. Mediators of long-term memory performance across the life span. Psychology and Aging. 1996;11(4):621–37. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- Plancher G, Gyselinck V, Nicolas S, Piolino P. Age effect on components of episodic memory and feature binding: A virtual reality study. Neuropsychology. 2010;24(3):379–90. doi: 10.1037/a0018680. [DOI] [PubMed] [Google Scholar]
- Pouzet B, Zhang WN, Feldon J, Rawlins JNP. Hippocampal lesioned rats are able to learn a spatial position using non-spatial strategies. Behavioural Brain Research. 2002;133(2):279–291. doi: 10.1016/s0166-4328(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. The Journal of Neuroscience. 2008;28(36):8945–54. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski EJ, Gefen T, Shi J, Samimi M, Bigio E, Weintraub S, Geula C, Mesulam MM. Youthful memory capacity in old brains: anatomic and genetic clues from the Northwestern SuperAging Project. Journal of Cognitive Neuroscience. 2013;25(1):29–36. doi: 10.1162/jocn_a_00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272(5264):1017–20. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Disterhoft JF. Trace eyeblink conditioning in rabbits demonstrates heterogeneity of learning ability both between and within age groups. Neurobiology of Aging. 1996;17(4):619–629. doi: 10.1016/0197-4580(96)00026-7. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162(3):732–755. doi: 10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14(1):58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- Unived Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2013. 2013. ST/ESA/SER.A/348. [Google Scholar]
- Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of Neuroscience. 2005;25(38):8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behavioural Brain Research. 1999;99(2):123–32. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Weiss C, Thompson RF. Delayed acquisition of eyeblink conditioning in aged F1 hybrid (Fischer-344 x Brown Norway) rats. Neurobiology of Aging. n.d;13(2):319–23. doi: 10.1016/0197-4580(92)90045-y. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends in Neurosciences. 2006;29(12):662–70. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. The Journal of Neuroscience. 2005;25(29):6877–86. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Cronholm JF, Sheffield JB. Purkinje cell number related to rate of classical conditioning. Neuroreport. 1990;1(2):165–8. doi: 10.1097/00001756-199010000-00020. [DOI] [PubMed] [Google Scholar]