Abstract
In the present study, root hydrotropism in an agravitropic mutant of Pisum sativum L. grown in vermiculite with a steep water potential gradient was examined. When wet and dry vermiculite were placed side by side, water diffused from the wet (–0·04 MPa) to the dry (–1·2 MPa) and a steep water potential gradient became apparent in the dry vermiculite close to the boundary between the two. The extent and location of the gradient remained stable between the fourth and sixth day after filling a box with vermiculite, and the steepest gradient (approx. 0·02 MPa mm–1) was found in the initially dry vermiculite between 60 and 80 mm from the boundary. When seedlings with 25–35 mm long roots were planted in the initially dry vermiculite near where the gradient had been established, each of the main roots elongated toward the wet vermiculite, i.e. toward the high water potential. Control roots elongated without curvature in both the wet and the dry vermiculite, in which no water potential gradient was detectable. These results show that pea roots respond to the water potential gradient around them and elongate towards the higher water potential. Therefore, positive hydrotropism occurs in vermiculite just as it does in air. Hydrotropism in soil may be significant when a steep water potential gradient is apparent, such as when drip irrigation is applied.
Key words: Hydrotropism, pea, Pisum sativum L., root elongation, vermiculite
INTRODUCTION
Root hydrotropism, as well as gravitropism, plays an important role in the direction in which roots elongate. This phenomenon has been observed when roots grown in air with a humidity gradient around them bend towards the higher humidity (Jaffe et al., 1985; Takahashi and Suge, 1991). Moreover, when agar blocks with different water potentials were applied to a root tip, the root elongated toward the blocks with the higher water potentials (Takano et al., 1995). Pea roots exhibited hydrotropism when the water potential gradient around them ranged from 0·4–0·5 % relative humidity (RH) mm–1 in air (equivalent to 0·6–0·7 MPa mm–1 at 25 °C) or when the difference in water potential between agar blocks applied to the 1‐mm‐thick root tip was more than 0·5 MPa mm–1 (Takano et al., 1995; Hirasawa et al., 1997). Gradients of less than 0·2 % RH mm–1 in air (equivalent to 0·3 MPa mm–1 at 25 °C) and of less than 0·3 MPa mm–1 in terms of the difference in water potential between agar blocks, do not induce hydrotropism (Takahashi and Scott, 1993; Takano et al., 1995). In contrast, in soil, much smaller water potential gradients of 7 × 10–4 MPa mm–1 and 8 × 10–3 MPa mm–1 have been recorded between a dry surface soil and wet deeper soil (Klepper et al., 1973; Kondo et al., 2000). A ten‐fold steeper gradient has been found at the penetration front of water in irrigated soil (Gardner et al., 1970). The size of the water potential gradient in air, when root hydrotropism has been observed, is much larger than in soil. However, the water potential gradient in soil varies considerably, depending on ambient conditions. Therefore, it is unclear whether hydrotropism occurs in soil as it does in air. Since the cited experiments were performed in air over the course of just a few hours, it is possible that a root might respond to far smaller gradients if such a gradient were applied for a longer time. Moreover, since root gravitropism is stimulated by decreases in the water potential around roots (Oyanagi et al., 1992; Nakamoto, 1993), it is possible that a root might change its direction of elongation in soil in response to the water status of its environment.
In this study, an experimental system with a stable and steep water potential gradient was established in vermiculite, and the responses of roots of an agravitropic mutant of Pisum sativum L. to this gradient were investigated.
MATERIALS AND METHODS
Plant material
Seeds of the agravitropic ageotropum mutant of pea (Pisum sativum L.) were germinated on wet filter paper in a moisture‐saturated Petri dish at 25 °C in the dark. Two‐day‐old seedlings with straight main roots 25–35 mm in length were used in all the experiments.
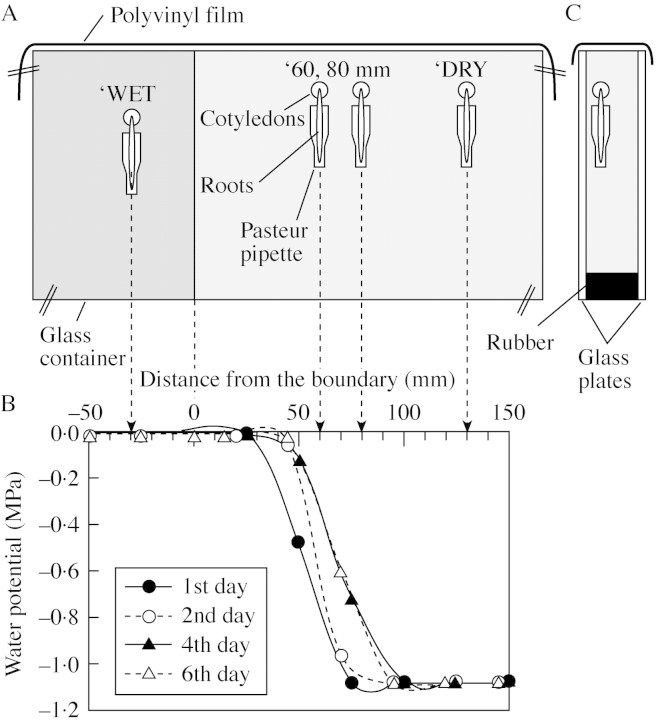
Experimental system
Wet and dry vermiculite were placed side by side to establish a steep water potential gradient in the dry vermiculite. Two glass plates (500 × 500 mm) with a rubber rod (25 mm thick) as a base and sides between them were used to make a container. One‐third of the container was filled with wet vermiculite with a water content (on the basis of dry weight) of 170 % (wet vermiculite; WV), the remainder being filled with dry vermiculite with a water content of 10 % (dry vermiculite; DV) (Fig. 1A). All the vermiculite had previously been sifted through a sieve with a 2‐mm mesh. The WV was prepared by soaking vermiculite in a 0·1 mm solution of CaCl2 and then allowing it to drain overnight. The DV was prepared by air‐drying vermiculite overnight at 90 °C then spraying it with the CaCl2 solution and mixing in a plastic container. The water potential of the vermiculite was calculated from the water content by reference to the relationship between water content and water potential determined with an isopiestic psychrometer (Boyer and Knipling, 1965; Boyer, 1995). Vermiculite was sampled using a cork borer of 20 mm diameter, the samples being placed in a Vaseline‐coated chamber of the psychrometer. The water potentials of the WV and the DV were –0·04 MPa and –1·2 MPa, respectively. As water diffused from the WV to the DV, the water potential of the DV near the boundary between the two changed considerably; a steep water potential gradient being apparent (Fig. 1B). A stable gradient was present between 25 and 65 mm from the boundary 1 d after the container had been filled, and between 40 and 70 mm from the boundary on the second day. The steepest gradient slope was between 60 and 80 mm from the boundary, reaching approx. 0·02 MPa mm–1 by the sixth day, similar to that reached by the fourth day. No gradient was detected either in the DV at greater distances from the boundary or in the WV. Therefore, the effects of the gradient between four and six days after the container had been filled with vermiculite were investigated.
Fig. 1. A schematic representation of the glass container (500 × 500 × 25 mm) filled with vermiculite, showing seedlings in Pasteur pipettes (A and C) and the water potential in the vermiculite at various distances from the boundary between the wet and the dry vermiculite (B). A, About one‐third of the volume of the container was filled with wet vermiculite (dark shading), the rest with dry vermiculite (light shading). The top of the container was sealed with polyvinyl film to prevent evaporation. The original axis of each root was parallel to the boundary between the wet and the dry vermiculite. B, Positive and negative values for the distance from the boundary indicate the measurements in the dry and the wet vermiculite, respectively. A 20‐mm‐diameter cork borer was used to collect samples of vermiculite to determine water content. The water potential was calculated from the relationship between the water potential and the water content of the vermiculite, as noted in the text. C, A side view of the container. Seedlings were placed close to one of the glass plates for easy observation.
A seedling root was inserted into a glass tube and placed in the vermiculite after one of the glass plates was removed. The plate was then replaced. The tube was, therefore, located close to one of the glass walls and at a depth of more than 30 mm from the top of the container. The root axis was parallel to the boundary between the WV and the DV. The tube was a 40‐mm portion of a Pasteur pipette (IK‐PAS‐9P; Asahi Technoglass Co., Chiba, Japan) close to the point where it becomes narrower (Fig. 1A). The root tip was placed about 15 mm from the narrower aperture so that the root was forced to elongate without bending for a while after transplanting. The root tip entered the vermiculite 1 d after transplanting. The root’s length and curvature were measured 2 d after transplanting. All experiments were performed in a room in which the air temperature was maintained at 25 °C.
RESULTS
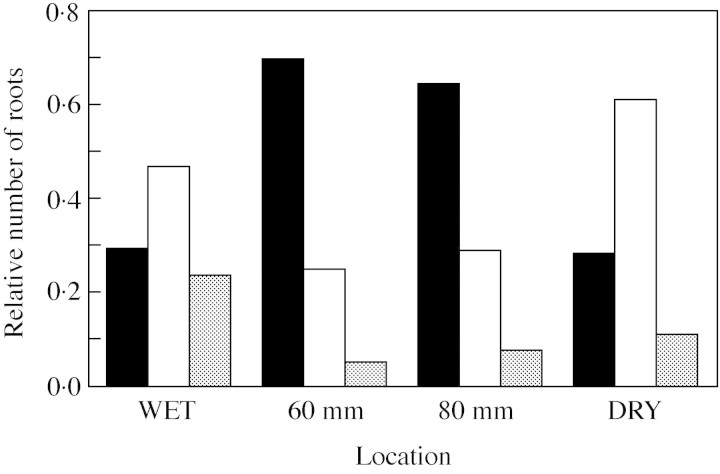
In seedlings transplanted to the DV 60 mm from the boundary between the WV and the DV, where there was a steep water potential gradient, 74 % of their roots bent toward the WV, i.e. towards the higher water potential (Fig. 2). In seedlings transplanted to the DV 80 mm from the boundary, where a gradient was also present, 64 % of their roots bent toward the WV. In seedlings transplanted to the WV, where there was no water potential gradient, half their roots elongated without bending. The rest did bend, but the number of roots bending toward the DV was equal to the number bending in the opposite direction. In seedlings transplanted to the DV more than 100 mm from the boundary, where there was no gradient, 60 % of their roots elongated without bending (Fig. 2).
Fig. 2. Directions of elongation of roots in vermiculite at various water potentials 2 d after transplanting. Transplanted seedlings were located in both the wet and the dry vermiculite at sites where no water potential gradient was detected (WET and DRY, respectively), and in the dry vermiculite at 60 and 80 mm from the boundary between the wet and the dry vermiculite where there was a steep water potential gradient (60 and 80 mm, respectively). Bars show the relative numbers of roots in each test group that elongated in each direction [towards the wet vermiculite, left (black bars); towards the dry vermiculite, right (grey bars); no bending, middle (open bars)]. The vertical axis shows the relative numbers of roots that curved in each direction. See also Table 1.
Mean curvatures at 60 and 80 mm from the boundary, where there was a steep water potential gradient around the seedlings, were greater than those within the DV and the WV at sites where no gradient was detected (Table 1). Elongation rates tended to decrease as the distance from the WV increased, i.e. as the water potential of the vermiculite decreased.
Table 1.
Curvature and elongation of roots transplanted to vermiculite at various water potentials
| Location | Mean curvature (°) | Elongation rate (µm s–1) | n |
| Wet | 2·4 ± 3·7 a | 0·36 ± 0·02 a | 17 |
| 60 mm | 24·8 ± 5·8 b | 0·37 ± 0·02 a | 20 |
| 80 mm | 15·0 ± 7·3 b | 0·30 ± 0·02 b | 14 |
| Dry | 5·0 ± 3·7 a | 0·25 ± 0·01 b | 18 |
Seedlings were transplanted to four different locations in vermiculite and grown for 2 d.
Wet and Dry, seedlings transplanted to wet and dry vermiculite with no detectable water potential gradient around them; 60 mm and 80 mm, seedlings transplanted to dry vermiculite at these distances from the boundary between the wet and dry vermiculite; curvature, the angle between the root axis and the direction of elongation of the root tip, positive and negative values indicating elongation toward the wet and dry vermiculite, respectively.
Values given are means ± standard errors with the number of seedlings tested (n); same following letters indicate no significant difference (P = 0·05, ANOVA).
DISCUSSION
An experimental system with a stable and steep water potential gradient in originally dry vermiculite was established by arranging two samples of vermiculite with water potentials of –0·04 MPa and –1·2 MPa side by side (Fig. 1). When roots showed obvious hydrotropism, the water potential gradient around them was approx. 0·02 MPa mm–1. However, do similar gradients exist in soil under natural conditions? It is estimated that a gradient of 0·1 MPa mm–1 might exist within 10 mm of a root surface in soil when its water potential decreases to –1·5 MPa and its hydraulic permeability is very low (Gardner, 1960), although it is unlikely that a difference in water potential exists between the sides of an individual root under such conditions. In crop fields, a gradient of 7 × 10–4 MPa mm–1 between dry soil at a depth of 530 mm and wet soil at a depth of 1430 mm (cotton field in summer in New York; Klepper et al., 1973) and a gradient of 8 × 10–3 MPa mm–1 between depths of 100 and 300 mm (upland rice field during the dry season in the Philippines; Kondo et al., 2000) have been recorded. These gradients are far smaller than those established in the present study. Crop plants grown in the field, where the surface soil is drying out, increase their root length density in deep soil compared with plants grown in wet soil (Hida et al., 1995; Hirasawa et al., 1998). When the water potential gradients in the soil are considered, as described above, it seems likely that root hydrotropism might not contribute significantly to the fact that roots in deeper soil develop at a higher density. However, root hydrotropism might be important in establishing the direction of root elongation when a steep water potential gradient is established between near the penetration front of irrigation water and dry soil, when only a restricted rooting zone is watered, such as during the application of drip irrigation. A water potential gradient of more than 0·02 MPa mm–1 can easily be established at the penetration front of irrigation water, even several days after irrigation (Gardner et al., 1970). Root hydrotropism might contribute to limiting root growth into irrigated soil and would be important for the efficient use of irrigation water in such a system.
Interactions between tropisms, such as thigmotropism and gravitropism (Massa and Gilroy, 2003) and phototropism and gravitropism (Correll and Kiss, 2002), have been established. Hydrotropism is affected also by gravitropism (Takahashi and Scott, 1991, 1993; Oyanagi et al., 1995). Roots show a greater response to gravitropism than to hydrotropism when the intensity of gravistimulation is high. The direction of lateral root elongation is less sensitive to gravity (Ransom and Moore, 1983). Therefore, hydrotropism may be more significant for lateral roots than for main roots. The mechanism responsible for lateral root hydrotropism is not yet understood even though root systems consist mainly of lateral roots. Further studies of the actual development of root systems in irrigated soil are necessary to plan for the efficient use of water in the irrigation of crop plants.
ACKNOWLEDGEMENTS
This work was carried out under the auspices of the Joint Research Program of the Institute of Genetic Ecology, Tohoku University (grant no. 963005), and was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 05404008).
Supplementary Material
Received: 31 March 2003; Returned for revision: 3 June 2003; Accepted: 22 August 2003 Published electronically: 8 October 2003
References
- BoyerJS.1995.Measuring the water status of plants and soils. San Diego: Academic Press. [Google Scholar]
- BoyerJS, Knipling EB.1965. Isopiestic technique for measuring leaf water potentials with a thermocouple psychrometer. Proceedings of the National Academy of Sciences of the USA 54: 1044–1051. [PMC free article] [PubMed] [Google Scholar]
- CorrellMJ, Kiss JZ.2002. Interactions between gravitropism and phototropism in plants. Journal of Plant Growth Regulation 21: 89–101. [DOI] [PubMed] [Google Scholar]
- GardnerWR.1960. Dynamic aspects of water availability to plants. Soil Science 89: 63–73. [Google Scholar]
- GardnerWR, Hillel D, Benyamini Y.1970. Post‐irrigation movement of soil water. 1. Redistribution. Water Resources Research 6: 851–861. [Google Scholar]
- HidaY, Hirasawa T, Ishihara K.1995. Differences in dry matter production and root system development between soybean cultivars under deficient soil moisture conditions. Japanese Journal of Crop Science 64: 573–580 [in Japanese with English abstract]. [Google Scholar]
- HirasawaT, Takahashi H, Suge H, Ishihara K.1997. Water potential, turgor and cell wall properties in elongating tissue of the hydrotropically bending roots of pea (Pisum sativum L.). Plant, Cell & Environment 20: 381–386. [Google Scholar]
- HirasawaT, Nakahara M, Izumi T, Iwamoto Y, Ishihara K.1998. Effects of pre‐flowering soil moisture deficits on dry matter production and ecophysiological characteristics in soybean plants under well irrigated conditions during grain filling. Plant Production Science 1: 8–17. [Google Scholar]
- JaffeMJ, Takahashi H, Biro RL.1985. A pea mutant for the study of hydrotropism in roots. Science 230: 445–447. [DOI] [PubMed] [Google Scholar]
- KlepperB, Taylor HM, Huck MG, Fiscus EL.1973. Water relations and growth of cotton in drying soil. Agronomy Journal 65: 307–310. [Google Scholar]
- KondoM, Murty MVR, Aragones DV.2000. Characteristics of root growth and water uptake from soil in upland rice and maize under water stress. Soil Science and Plant Nutrition 46: 721–732. [Google Scholar]
- MassaGD, Gilroy S.2003. Touch modulates gravity sensing to regulate the growth of primary roots of Arabidopsis thaliana Plant Journal 33: 435–445. [DOI] [PubMed] [Google Scholar]
- NakamotoT.1993. Effect of soil water content on the gravitropic behavior of nodal roots in maize. Plant and Soil 152: 261–267. [Google Scholar]
- OyanagiA, Sato A, Wada M.1992. Effect of water potential of culture medium on geotropic response of primary seminal root in Japanese wheat cultivars. Japanese Journal of Crop Science 61: 119–123. [Google Scholar]
- OyanagiA, Takahashi H, Suge H.1995. Interactions between hydrotropism and gravitropism in the primary seminal roots of Triticum aestivum L. Annals of Botany 75: 229–235. [Google Scholar]
- RansomJS, Moore R.1983. Geoperception in primary and lateral roots of Phaseolus vulgaris (Fabaceae). I. Structure of columella cells. American Journal of Botany 70: 1048–1056. [PubMed] [Google Scholar]
- TakahashiH, Scott TK.1991. Hydrotropism and its interaction with gravitropism in maize roots. Plant Physiology 96: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TakahashiH, Scott TK.1993. Intensity of hydrostimulation for the induction of root hydrotropism and its sensing by the root cap. Plant, Cell & Environment 16: 99–103. [DOI] [PubMed] [Google Scholar]
- TakahashiH, Suge H.1991. Root hydrotropism of an agravitropic pea mutant, ageotropum Physiologia Plantarum 82: 24–31. [Google Scholar]
- TakanoM, Takahashi H, Hirasawa T, Suge H.1995. Hydrotropism in roots: sensing of gradient in water potential by the root cap. Planta 197: 410–413. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.