Abstract
The KwaZulu‐Natal region of South Africa hosts a large diversity of asclepiads (Apocynaceae: Asclepiadoideae), many of which are endemic to the area. The asclepiads are of particular interest because of their characteristically highly evolved floral morphology. During 3 months of fieldwork (November 2000 to January 2001) the flower visitors and pollinators to an assemblage of nine asclepiads at an upland grassland site were studied. These observations were augmented by laboratory studies of flower morphology (including scanning electron microscopy) and flower colour (using a spectrometer). Two of the specialized pollination systems that were documented are new to the asclepiads: fruit chafer pollination and pompilid wasp pollination. The latter is almost unique in the angiosperms. Taxa possessing these specific pollination systems cluster together in multidimensional phenotype space, suggesting that there has been convergent evolution in response to similar selection to attract identical pollinators. Pollination niche breadth varied from the very specialized species, with only one pollinator, to the more generalized, with up to ten pollinators. Pollinator sharing by the specialized taxa does not appear to have resulted in niche differentiation in terms of the temporal or spatial dimensions, or with regards to placement of pollinaria. Nestedness analysis of the data set showed that there was predictability and structure to the pattern of plant‐pollinator interactions, with generalist insects visiting specialized plants and vice versa. The research has shown that there is still much to be learned about plant–pollinator interactions in areas of high plant diversity such as South Africa.
Key words: Apocynaceae, Asclepiadaceae, plant assemblage, community structure, floral morphology, grassland, mutualism, pollination, nestedness, niche, species interactions, SEM, South Africa
INTRODUCTION
Niche‐partitioning between co‐occurring species of plants may facilitate the development of species‐rich plant communities. Most studies of niche‐breadth and overlap in plant communities have been focused on the physiology and growth form of plants (e.g. Tofts and Silvertown, 2000; Silvertown et al., 2001). Much less attention has been paid to the possibility that high diversity within plant communities is facilitated by niche‐partitioning in the reproductive biology of plants (Parrish and Bazzaz, 1979; Armbruster, 1995). Some attempts were made to ascribe differences in flowering times between plants to niche partitioning (e.g. Robertson, 1895; Heithaus, 1974; Rathcke, 1988), although it is possible that much of the between‐taxa variation in phenology is a result of phylogenetically constrained flowering times (Kochmer and Handel, 1986; Ollerton and Lack, 1992; Johnson, 1993). Recently, there has been renewed interest in partitioning of pollination systems in the context of food‐web theory (Memmott, 1999; Dicks et al., 2002). It is hypothesized that plant species sharing pollinators may experience inter‐specific competition resulting from diminished rates of pollinator visitation, pollen wastage and stigma clogging (Waser, 1978). In general, reproductive interference is expected to be greatest among closely related species pollinated by the same vectors (Armbruster et al., 1994; though see Borba and Semir, 2001).
Studies of plant–pollinator interactions involving assemblages of phylogenetically related, co‐occurring plant species can therefore potentially yield insights into the ecology of niche overlap, competition for resources (pollinators), adaptation and the evolution of convergent suites of floral traits (‘syndromes’) in species sharing common descent (Sakai et al., 1999; Kessler and Krömer, 2000; Borba and Semir, 2001; Johnson et al., 2002). The grasslands of KwaZulu‐Natal offer excellent opportunities for such studies as they are ancient, relatively intact, botanically rich and characterized by high levels of endemism and local radiation of genera (Cowling et al., 1991; O’Connor and Bredenkamp, 2000). Alpha diversity of grassland at the 1000 m2 plot level exceeds all other biomes in southern Africa, including the renowned Cape fynbos, and the biome as a whole contains almost 4000 plant species (Cowling et al., 1991).
In this paper we present the results of a study of the pollination ecology of an assemblage of asclepiads (Apocynaceae subfamily Asclepiadoideae sensu Endress & Bruyns, 2000) co‐occurring and co‐flowering in an upland grassland in Kwazulu‐Natal, South Africa. The asclepiads are of particular interest because of their specialized floral morphology, comprising three highly evolved key features: (1) the gynostegium—congenitally fused and highly synorganized gynoecium and androecium; (2) pollinaria—pollen aggregated into masses (pollinia) with mechanical clips (translators) which attach them to pollinators; and (3) the corona—more or less well‐developed outgrowths of the corolla and/or stamens which serve a number of possible functions (Brown, 1811; Kunze, 1990, 1997; Liede and Kunze, 1993; Fishbein, 2001; Ollerton and Liede, 2003). Although the pollination biology of the asclepiads has received quite a lot of attention (for a review, see Ollerton and Liede, 1997) there are rather few comparative studies of co‐occurring species assemblages (Kephart, 1983, 1987; Vieira and Shepherd, 1999).
South Africa is one of the main centres of diversity and endemism for the asclepiads; there are about 90 genera and 700 species of Apocynaceae in southern Africa (Victor et al., 2000) and the KwaZulu‐Natal grasslands are particularly rich. There are few data on the floristics of grasslands in southern Africa, but Hilliard and Burtt (1987) list the asclepiads with 234 species as the fifth largest plant family in the province of KwaZulu‐Natal. Cowling and Hilton‐Taylor (1997) show that the asclepiads are the eighth largest family in southern Africa, with a remarkable 87% endemism.
Despite this high level of diversity and endemism, there has been a paucity of studies of the pollination ecology of asclepiads in southern Africa, although see Weale (1873), Scott‐Elliot (1891) and Liede and Whitehead (1991). The majority of fieldwork has concentrated on the succulent stapeliads (tribe Ceropegiae; for a review, see Meve and Liede, 1994) but for most asclepiad genera absolutely nothing is known of their pollinator requirements, levels of specialization, breeding systems, etc. Our study addressed the following questions.
(1) How ecologically specialized are these asclepiads in their pollinator requirements?
(2) To what extent do co‐occurring species share pollinators and therefore overlap in their pollination niche? Is the likelihood of pollinator‐sharing linked to phylogenetic relatedness, i.e. the possession of shared floral traits?
(3) Are the patterns of flower visitation random with respect to the overall diversity of flower visiting insects in the community? Or is there filtration of visitors by plants that would lead to predictable structure in the patterns of interaction?
(4) Do non‐related species which share pollinators show evidence of convergent evolution of floral traits, and therefore can specific features of the floral morphology of the studied asclepiads be ascribed to adaptations for particular specialized pollinators?
MATERIALS AND METHODS
The study site was an 8‐ha area of moist montane grassland on the farm Wahroonga (30°08′E, 29°37′S, altitude approx. 1200 m) that is formally protected as a South African Natural Heritage site. The grasslands at this site have never been ploughed and are not grazed by domestic livestock.
Fieldwork was carried out between 15 Nov. 2000 and 16 Jan. 2001. In total, 63 man‐hours were spent observing and collecting flower visitors of nine species of asclepiads. Most of the observation was diurnal, with limited nocturnal surveillance. Samples of insect visitors were returned to the laboratory for analysis of pollinaria loads. Voucher specimens are kept in the university collection of S.D.J.
Flowers of all species were bagged with fine mesh cloth bags to determine nectar volume and concentration following 24 h accumulation. Volume was determined using microcapillary pipettes and sugar concentration (as sucrose equivalents) using Bellingham and Stanley sugar refractometers (for details of these techniques, see Dafni, 1992; Kearns and Inouye, 1993).
Flowers of all species were collected for closer observation in the laboratory, including scanning electron microscopy and reflectance spectrophotometry. Spectral reflectance of flower parts was measured with an Ocean Optics S2000 spectrometer and fibre optic reflection probe (UV/VIS 400 µm) held at 45° to the petal surface. An Ocean Optics DT‐mini deuterium tungsten halogen light source, with an approx. 200–1100 nm spectral range, and an Ocean Optics WS‐1 diffuse reflectance standard to calibrate the spectrometer were used.
In addition, pollinaria removal and pollinia insertion were recorded from the collected flower material (sample range 11–35 flowers per species). Vouchers of all plants are kept in the spirit collection of the Natal University Herbarium (see Table 1 for collection numbers).
Table 1.
Floral traits of the nine species of asclepiads studied at Wahroonga, KwaZulu‐Natal
| Asclepias cucullata (Ollerton 246) | Asclepias woodii (Ollerton 255) | Aspidonepsis diploglossa (Ollerton 228) | Miraglossum pilosum (Ollerton 258) | Miraglossum verticillare (Ollerton 244) | Pachycarpus natalensis (Ollerton 254) | Sisyranthus trichostomus (Ollerton 245) | Xysmalobium gerrardii (Ollerton 251) | Xysmalobium involucratum (Ollerton 253) | |
| Flower diameter | |||||||||
| Mean | 15·1 | 14·6 | 13.0 | 12·0 | 15·1 | 40.0 | 7·9 | 7·9 | 7·7 |
| s.d. | 1·1 | 1·2 | 1.0 | 0·8 | 1·3 | 2·5 | 0·6 | 0·3 | 1·1 |
| Petal length | |||||||||
| Mean | 7·2 | 6·8 | 6.0 | 4·6 | 6·2 | 19.0 | 3·3 | 3·8 | 4·0 |
| s.d. | 1·1 | 0·7 | 1.0 | 0·6 | 0·6 | 1·4 | 0·3 | 0·3 | 0·5 |
| Corona* diameter | |||||||||
| Mean | 8·3 | 7·4 | 5.0 | 4·2 | 5·6 | 15.0 | 2·4 | 3·8 | 3·2 |
| s.d. | 0·8 | 0·5 | 0.0 | 0·4 | 0·5 | 0·5 | 0·3 | 0·4 | 0·2 |
| Flower depth | |||||||||
| Mean | 5·4 | 5·1 | 4.0 | 3·8 | 4·5 | 12.0 | 2·9 | 3·52 | 3·9 |
| s.d. | 0·4 | 0·7 | 1.0 | 0·3 | 0·5 | 0·8 | 0·4 | 0·3 | 0·3 |
| Flowers per plant | |||||||||
| Mean | 11·6 | 43·7 | 6.0 | 12·3 | 9·8 | 13.0 | 25·9 | 510·3 | 15·3 |
| Height of plant | |||||||||
| Mean | 25·0 | 42·3 | 35·0 | 36·2 | 28·9 | 34·0 | 54·6 | 32·8 | 24·0 |
| s.d. | 5·0 | 7·7 | 8·0 | 5·1 | 5·4 | 7·9 | 8·2 | 2·0 | 2·7 |
| Corolla colour | Light creamy‐ brown | Dull white to pale yellow | Bright yellow | Green | Green | Pale lime green | Creamy yellow | Green becoming yellow | Greeny‐white |
| Corona colour | Dull browny‐white | Vivid yellow, rarely paler | Vivid yellow‐orange | Green | Green | Dark purple | Creamy yellow | Green becoming yellow | Dark green, yellow with age |
| Patterning | None | Dark purple edging | Pale green style head | Fine purple spotting | Fine purple spotting | Heavy purple spotting | None | None | Greeny‐white style head |
| Scent | Very faint, sweet | None | None | Faint, sour‐musky | Faint, spicy | None | None | Strong, honey‐like | Strong, fragrant‐fresh |
| Nectar volume | |||||||||
| Mean | 0·4 | 0·7 | 0·1 | 0·2 | 0·3 | 4·2 | 0·2 | 0·9 | 0·2 |
| s.d. | 0·1 | 0·6 | 0·1 | 0·1 | 0·1 | 3·1 | 0·1 | 0·6 | 0·1 |
| Sample size | |||||||||
| Flowers (plants) | 5 (2) | 11 (3) | 5 (4) | 8 (1) | 8 (2) | 9 (4) | 17 (5) | 15 (4) | 11 (2) |
| Nectar concentration | |||||||||
| Mean | 43·6 | 29·0 | ? | 59·7 | 67·8 | 50 | 18·1 | 35·1 | 12·5 |
| s.d. | 3·0 | 9·2 | ? | 5·6 | 2·4 | 11 | 3·6 | 7·3 | 2·8 |
| Sample size | |||||||||
| Flowers (plants) | 4 (2) | 10 (3) | 5 (1) | 5 (2) | 7 (4) | 9 (4) | 14 (3) | 2 (1) | |
| Floral longevity (d) | <5? | 10 | 2 | 5 | 2 | 7 | 4 | 5? | >10 |
All dimensions are in millimetres.
Collection voucher numbers are given in parenthesis.
* Tube opening in Sisyranthus.
On the 16 Dec. 2000, two 1‐h surveys of the insect visitors present on flowers of all plant species encountered within a 2‐m‐wide strip, in two circuits that covered the whole of the extent of the field site, were carried out. These surveys were undertaken simultaneously by two groups of observers and insects were identified to broad taxonomic group as ‘fly’, ‘hoverfly’, ‘bee’, ‘butterfly’, etc., except in those cases in which we were positive of the identity of the insect (usually because they were important visitors to asclepiad flowers).
Statistical analysis
The data collected on floral traits were entered into a species‐trait matrix (see the Appendix) and subjected to multidimensional scaling analysis (e.g. Ollerton and Watts, 2000) This and all other statistical analyses were performed using SPSS 8.0 for Windows (1997, SPSS Inc. Chicago, IL, USA). All means are presented ± 1 standard deviation.
RESULTS
The study species
Our survey of the Wahroonga site during November 2000 to January 2001 yielded over 20 species of asclepiads, although the actual total for the site is probably higher. Many of these species flowered too early or too late, or had too low a population density, to be included in our comparative study of asclepiad pollination biology. This study therefore focused on nine abundant, co‐flowering species. Detailed descriptions of the growth forms and distribution of these species will be presented elsewhere by the authors.
These species varied in their flower size and colour (Figs 1 and 2) and in other floral traits such as morphology, longevity, scent and nectar characteristics (Table 1 and Figs 6–9). The nine species represent six genera belonging to two tribes of the asclepiads, the Ceropegieae [Sisyranthus trichostomus (Harv.) K. Schum.] and the Asclepiadeae [Xysmalobium involucratum Decne., Xysmalobium gerrardii Scott Elliot, Miraglossum verticillare (Schltr.) Kupicha, Miraglossum pilosum (Schltr.) Kupicha, Pachycarpus natalensis N.E. Br., Aspidonepsis diploglossa (Turcz.) A. Nicholas & D.J. Goyder, Asclepias woodii (Schltr.) Schltr., Asclepias cucullata (Schltr.) Schltr.]. They therefore represent different levels of phylogenetic relatedness; however, the exact relationships between these taxa is unclear as a preliminary molecular phylogenetic study has shown that a number of genera (e.g. Xysmalobium and Asclepias) are not monophyletic (D. J. Goyder, A. Nicholas and S. Liede, unpubl. res.). Conclusions that rely on assumptions about the phylogeny of these taxa have therefore been avoided.

Fig. 1. A, The nine species of asclepiad included within this study: (i) Pachycarpus natalensis; (ii) Xysmalobium involucratum Decne; (iii) xysmalobium gerrardii Scott Elliott; (iv) Miraglossum verticillare (Schltr.) Kupicha; (v) Miraglossum pilosum (Schltr.) Kupicha; (vi) Aspidonepsis diploglossa (Turcz.) A. Nicholas; (vii) Sisyranthus trichostomus (Harv.) K. Schum. and D.J. Goyder; (viii) Asclepias woodii (Schltr.) Schltr.; (ix) Asclepias cucullata (Schltr.) Schltr. B, Hemipepsis hilaris (Hymenoptera: Pompilidae) visiting a flower of Pachycarpus natalensis. C, Atrichelaphinis tigrina (Coleoptera: Scarabaeidae: Cetoniini) visiting flowers of Xysmalobium involucratum; note the yellow pollinaria attached to the legs. D, Cyrtothyrea marginalis (Coleoptera: Scarabaeidae: Cetoniini) visiting Sisyranthus trichostomus and feeding through the hairs within the floral tube. E, Atrichelaphinis tigrina visiting Sisyranthus trichostomus; note the pollinaria attached to the mouthparts.
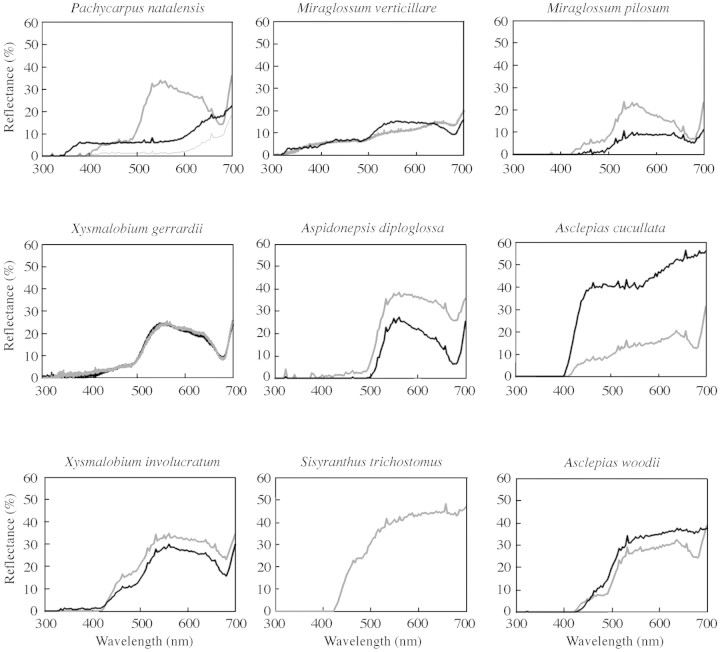
Fig. 2. Reflectance spectra of flowers of the nine asclepiads from Wahroonga. In all cases the dark line is the spectrum of the corona and the light line is the spectrum of the corolla. For Pachycarpus natalensis, the lower, light coloured line is the spectrum for the large purple patches (see Fig. 1A and B). For Sisyranthus trichostomus, the corona is not visible without dissecting the flower.
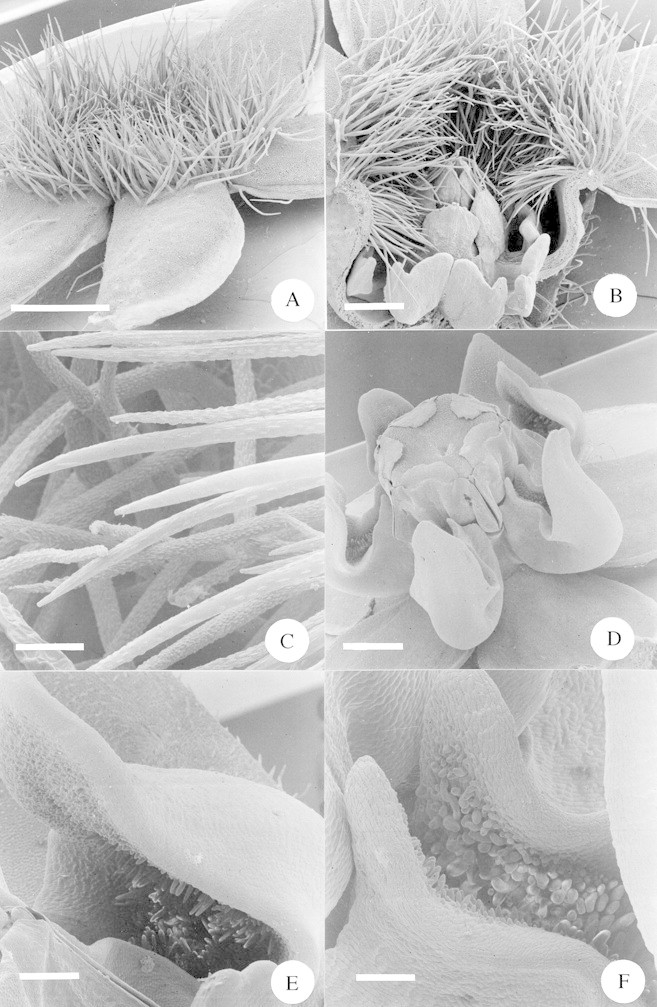
Fig. 6. A, Scanning electron micrograph (SEM) of a whole flower of Sisyranthus trichostomus. Scale bar = 1·0 mm. B, SEM of a half flower of Sisyranthus trichostomus. Note the hairs blocking the corolla tube arching down into the nectar‐holding corona cups. Scale bar = 0·5 mm. C, SEM close‐up of the hairs blocking the corolla tube of Sisyranthus trichostomus, showing the parallel ridges running along the main axis of each hair. Scale bar = 50 µm. D, SEM of a whole flower of Asclepias cucullata. Scale bar = 1·0 mm. E, SEM close‐up of the portion of the nectar cup distal to the gynostegium of Asclepias cucullata. Note the dense papillae within the cup. Scale bar = 250 µm. F, SEM close‐up of the portion of the nectar cup proximal to the gynostegium of Asclepias cucullata. Note the dense papillae within the cup. Scale bar = 150 µm.
Photography using a UV filter indicated that for most species there was very little floral ultraviolet reflectance (not shown) and this was confirmed by spectrophotometric analysis (Fig. 2). Contrast in spectral reflectance between the corona and corolla was evident only in Pachycarpus natalensis and Asclepias cucullata. In general, the spectra of the study species were characterized by lack of spectral purity (chroma), hence the predominance of ‘muddy’ brown, purple and creamy colours to the human observer. This may, in turn, reflect the importance of scent, rather than visual, advertising for the attraction of beetles and wasps (see Pollinators, below).
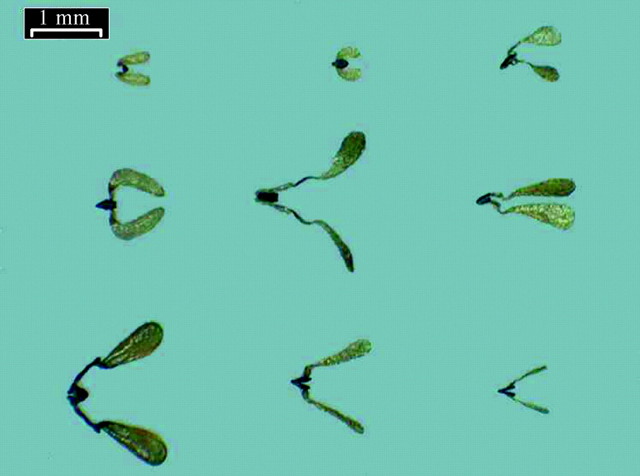
The nine species varied considerably in the size and shape of their pollinaria (Fig. 12).
Fig. 12. Pollinaria of the nine species of asclepiad. Top row, from left to right: Miraglossum pilosum, Sisyranthus trichostomus, Aspidonepsis diploglossa. Middle row: Miraglossum verticilata, Asclepias woodii, Asclepias cucullata. Bottom row: Pachycarpus natalensis, Xysmalobium involucratum, Xysmalobium gerrardii. Scale bar = 1 mm.
Pollinators
In total we recorded over 60 species of insect flower visitor to the nine asclepiads (Table 2; for some examples, see Fig. 1), excluding ants and other very small insects which were considered to be too small to remove pollinaria. The majority of visitors, however, were uncommon and usually did not pick up pollinaria (Table 2). They must therefore be considered as opportunistic nectar thieves rather than legitimate pollinators.
Table 2.
Flower visitors to the nine species of asclepiads
| Asclepias cucullata | Asclepias woodii | Aspidonepsis diploglossa | Miraglossum verticillare | Miraglossum pilosum | Pachycarpus natalensis | Sisyranthus trichostomus | Xysmalobium gerrardii | Xysmalobium involucratum | |
| Hymenoptera | |||||||||
| Hemipepsis hilaris Smith | . | . | . | 18 | 9 | 20 | 2 | 41 | 1 |
| Pompilidae sp. 2 | . | . | . | . | . | . | . | 1 | . |
| Tiphia sp. (Tiphidae) | . | 1 | . | . | . | . | . | . | . |
| Arge sp. (Tenthredinoidea: Argidae) | . | . | . | . | . | . | 1 | . | . |
| Apis mellifera L. | . | . | 1 | . | . | . | 1 | 3 | . |
| Halictidae sp. 1 | . | . | 2 | . | . | . | . | . | . |
| Halictidae sp. 2 | 1 | . | . | . | . | . | . | . | . |
| Other wasps | . | 1 | 1 | . | . | . | . | 1 | 3 |
| Other bees | . | . | . | . | . | . | . | 1 | 1 |
| Other solitary bees | . | 1 | 2 | . | . | 9 | . | . | . |
| Coleoptera | |||||||||
| Atrichelaphinis tigrina (Olivier) | . | 15 | . | 1 | . | . | 35 | 15 | 6 |
| Cyrtothyrea marginalis (Swartz) | . | 8 | . | 1 | . | . | 42 | 6 | . |
| Lycidae sp. | . | . | . | . | . | . | . | 2 | . |
| Cantharidae sp. | . | . | . | . | . | . | . | 2 | . |
| Elateridae sp. | . | . | . | . | . | . | . | . | 4 |
| Chrysomelidae sp. 1 | . | . | . | . | . | 1 | . | . | 1 |
| Chrysomelidae sp. 2 | . | . | . | . | . | . | 1 | 1 | 1 |
| Scarabaeidae: Scarabaeinae sp. 1 | . | . | . | . | . | . | . | 3 | . |
| Scarabaeidae: Scarabaeinae sp. 2 | . | . | . | . | . | . | . | 3 | 1 |
| Scarabaeidae: Scarabaeinae sp. 3 | . | . | . | . | . | . | . | 1 | . |
| Curculionidae sp. 1 | . | . | . | . | . | . | 10 | 4 | 1 |
| Curculionidae sp. 2 | . | 2 | . | . | . | . | . | . | . |
| Coleoptera sp. 3 | . | . | . | . | . | . | . | 2 | . |
| Coleoptera sp. 8 | . | . | . | . | . | . | 1 | . | . |
| Other Coleoptera | . | . | . | . | . | . | . | 4 | 4 |
| Heteroptera | |||||||||
| Aspilocoryphus fasciativentris (Stal) (Lygaeidae: Lygaeoidea) | 1 | . | . | 1 | . | 4 | 1 | 139 | 1 |
| Lygaeidae sp. 2 | . | . | . | 1 | . | 1 | . | 8 | 2 |
| Coreidae sp. | . | . | . | . | . | . | . | 1 | . |
| Spilostethus rivularis (F.) (Lygaeidae) | . | . | . | . | . | 1 | . | . | . |
| Homoecerus annulatus Thunberg. (Lygaeidae) | . | . | . | . | . | 1 | . | . | . |
| Pentatomidae: Pentatomoidea sp. | . | . | . | . | . | . | . | 1 | . |
| Other Heteroptera | . | . | . | . | . | . | . | 1 | . |
| Diptera | |||||||||
| Calliphoridae genus 1 | . | . | . | . | . | . | . | 1 | . |
| Calliphoridae genus 2 | . | . | . | . | . | . | 2 | 6 | . |
| Calliphoridae genus 3 | . | . | . | . | . | . | . | 1 | . |
| Sarcophaga sp. (Sarcophagidae) | . | 1 | . | 6 | . | 11 | . | 53 | 1 |
| Musca domestica L. (Muscidae) | . | . | 2 | . | . | . | . | 3 | . |
| Muscidae genus 2 | . | . | 1 | . | . | . | . | . | . |
| Empididae sp. 1 | 2 | . | . | . | . | 1 | . | . | . |
| Empididae sp. 2 | . | . | . | . | . | . | . | 1 | . |
| Chloropidae | . | . | 1 | . | . | . | . | 1 | . |
| Microphthalma sp. 1 (Tachinidae) | . | . | . | . | . | 1 | . | . | . |
| Microphthalma sp. 2 (Tachinidae) | . | . | . | . | . | . | . | 1 | . |
| Tachinidae subfamily Goniinae | . | . | . | . | . | . | . | 1 | . |
| Tachinidae genus 2 | . | . | . | . | . | . | . | 1 | . |
| Actea sp. (Tachinidae) | . | . | . | . | . | . | . | 1 | . |
| Sepsidae sp. 1 | . | . | . | . | . | . | . | 3 | 1 |
| Sepsidae sp. 2 | . | . | . | . | . | . | . | . | 1 |
| Sepsidae sp. 3 | . | . | . | 1 | . | . | . | . | . |
| Dacus sp. (Tephritidae) | . | . | . | . | . | 1 | . | . | . |
| Bibionidae | . | . | . | . | . | . | . | 1 | . |
| Diptera sp. 3 | . | . | . | . | . | . | 1 | . | . |
| Diptera sp. 22 | . | . | . | . | . | 1 | . | . | . |
| Other Diptera | . | 1 | . | 1 | . | 1 | . | 15 | . |
| Lepidoptera | |||||||||
| Unidentified butterfly | . | . | . | . | . | . | 1 | . | . |
| Unidentified micromoth | 2 | . | . | . | . | . | . | . | . |
Figures are total numbers of individual insects observed to be actively foraging on the flowers. For each plant species, those insect species in bold are the ones which carried pollinaria of that plant and which are therefore assumed to be legitimate pollinators.
The numbers of pollinators recorded for the nine asclepiads ranged from 1 to 10 (mean = 3·1 ± 3·1, median = 2·0, n = 7 asclepiad species, excluding the two species for which pollinators were not determined exactly—see below). There was a strong correlation between number of flower visitors and number of pollinators per plant species (Pearson’s r = 0·93, d.f. = 5, P = 0·003; once again excluding the two species for which pollinators were inferred but not determined). The mean ratio of flower visitors to pollinators was 6·0 (± 3·4, range 2·7–12·0).
The species Pachycarpus natalensis and Miraglossum verticillare were both pollinated exclusively by male and female individuals of Hemipepsis hilaris (Hymenoptera: Pompilidae) (Fig. 1B). Interestingly, these were also the only two species that have flower parts that reflect ultraviolet light, i.e. wavelengths below about 400 nm (Fig. 2). This spider‐hunting wasp was the sole visitor to the less common species Miraglossum pilosum and, whilst none of the pompilids collected on M. pilosum carried pollinaria, it is thought that this is the legitimate pollinator, and that the absence of pollinaria‐carrying individuals of Hemipepsis hilaris is due to the low sample size (Table 2). Unlike the other two pompilid wasp‐pollinated taxa, the flowers of M. pilosum do not reflect in the ultraviolet, and neither do the other three asclepiads for which H. hilaris is a minor pollinator or flower visitor (Table 2). This suggests that UV reflectance per se is not an important attraction trait for flowers visited by this spider‐hunting wasp, but its role in the pollination biology of P. natalensis and M. verticillare remains unclear and requires further study.
The pollinators of Asclepias woodii and Sisyranthus trichostomus were chafers (Coleoptera: Scarabaeidae: Cetoniini) belonging to two species, Atrichelaphinis tigrina and Cyrtothyrea marginalis. The single record of Apis mellifera carrying pollinaria of S. trichostomus must be considered unusual in relation to the large number of visits by the chafers. Atrichelaphinis tigrina appears also to be the main pollinator of Xysmalobium involucratum, although visitation rates to this asclepiad were rather low, and H. hilaris was also captured carrying pollinaria on one occasion.
Of the remaining three species, Xysmalobium gerrardii appears to be the most generalist, with H. hilaris, Atrichelaphinis tigrina, Aspilocoryphus fasciativentris (Heteroptera: Lygaeidae: Lygaeoidea) and Sarcophaga sp. (Diptera: Sarcophagidae) all recorded as major pollinators, in addition to a range of less common pollinators. The lygaeid bug Aspilocoryphus fasciativentris was a very common and distinctive component of the pollinator fauna of this species and individuals regularly carried pollinaria on the tarsi of one or more legs. These lygaeids were very active within plants, but their flying abilities and the extent of movement between plants is unknown.
Low visitation rates make it difficult to be certain, but Aspidonepsis diploglossa appears to be primarily bee pollinated, with occasional visits by Diptera which also do carry pollinaria. The final species, Asclepias cucullata, is infrequently visited by Diptera, bees and other insects (Table 2). None of these visitors carried pollinaria, and so it can only tentatively be classified as possibly mainly bee pollinated.
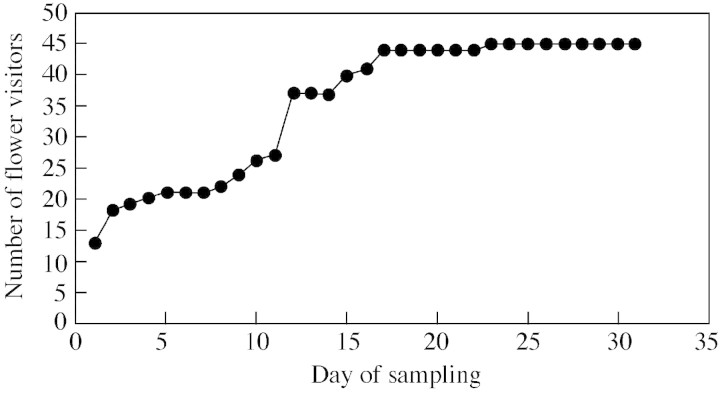
An analysis of the cumulative observation of new flower visitors over time (Fig. 3) indicates that, by day 23, an asymptote had reached, beyond which no new flower visitors were recorded. This masks a great deal of individual variation between the asclepiad species, with some very specialized species reaching an asymptote following as little as 2–5 d of sampling (data not shown). However, these results suggest that, in this assemblage at this site during this flowering season, the majority of the flower visitor diversity for the asclepiads had been sampled.
Fig. 3. The relationship between sampling effort and number of recorded flower visitors of the nine asclepiads. The curve is the cumulative number of insect species recorded as flower visitors over the period of observation.
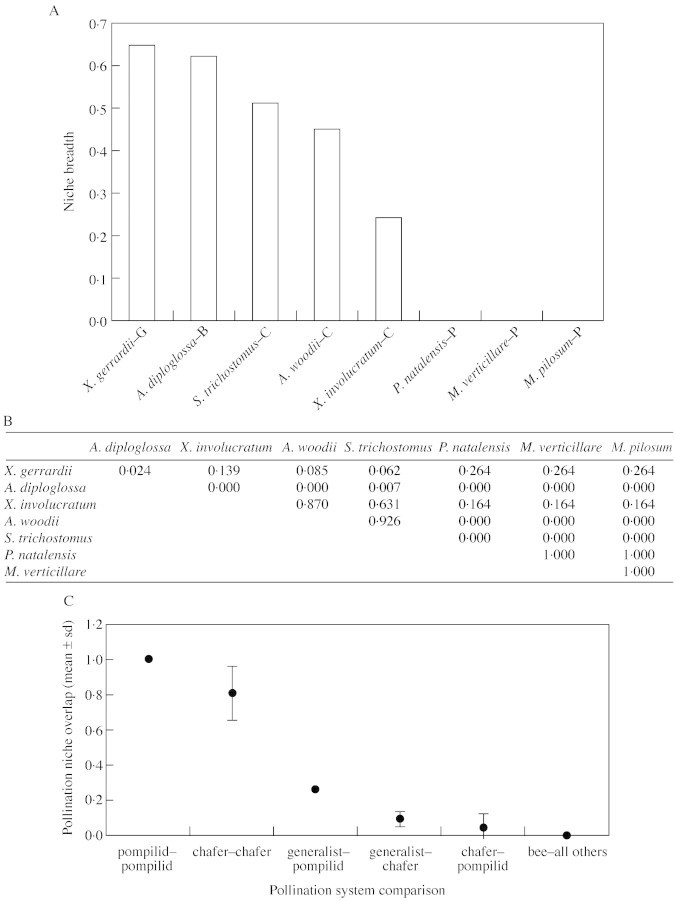
Pollination niche breadth and overlap
Pollination niche breadth was calculated for each of the eight asclepiads for which pollinators were identified, following the approach of Levins (1968), as presented in Waite (2000). With reference to Fig. 11A, niche breadth of the generalist X. gerrardii and the bee (?)‐pollinated A. diploglossa were the highest of the eight species, with the three chafer‐pollinated species having a rather narrower niche breadth. The three pompilid wasp‐pollinated taxa each had the smallest possible niche breadth (0·0), as only one species of pollinator was identified. It appears that the chafer‐pollinated species, although exhibiting a number of specific adaptations to pollination by cetoniid beetles (see below), are rather less specialized than the pompilid wasp‐pollinated taxa.
Fig. 11. Niche breadth and overlap statistics. A, Pollination niche breadths of the eight species of asclepiads for which pollinators were identified. The single letter suffixes to the names of taxa indicate the pollination system of that species (G, generalist; B, bee (?); C, chafer; P, pompilid wasp). This index starts at 0·0, indicating complete specialization on a single pollinator. B, Pollination niche overlap between all pairs of asclepiads. The index of overlap scales between 0·0 (no overlap in pollinator use) to 1·0 (complete overlap in pollinator use). C, Mean pollination niche overlap comparisons between taxa having the same and different pollination systems. Data points are mean values ± s.d. (from Fig. 11B). Note that, for three sets of comparisons, s.d. = 0.
Pollination niche overlap was calculated for all pairs of species, again following the approach by Levins (1968), as presented in Waite (2000) (Fig. 11B). Comparison of mean pollination niche overlap between taxa with different pollination systems reveals some interesting patterns (Fig. 11C). The pompilid wasp‐pollinated taxa show complete pollination niche overlap, whilst the chafer‐pollinated taxa show rather less overlap, though the average is still high. Niche overlap between the generalist X. gerrardii and the pompilid wasp‐pollinated taxa is higher than that with the chafer‐pollinated taxa. Overlap between the chafer‐ and pompilid wasp‐pollinated taxa is low, though not zero (because the pompilid wasp Hemipepsis hilaris was an occasional visitor to the otherwise chafer‐pollinated X. involucratum). The bee(?)‐pollinated A. diploglossa showed almost zero overlap with the other species, though Apis mellifera was an occasional visitor to both it and the otherwise chafer‐pollinated S. trichostomus.
Assemblage structure of the flower visitors and pollinators
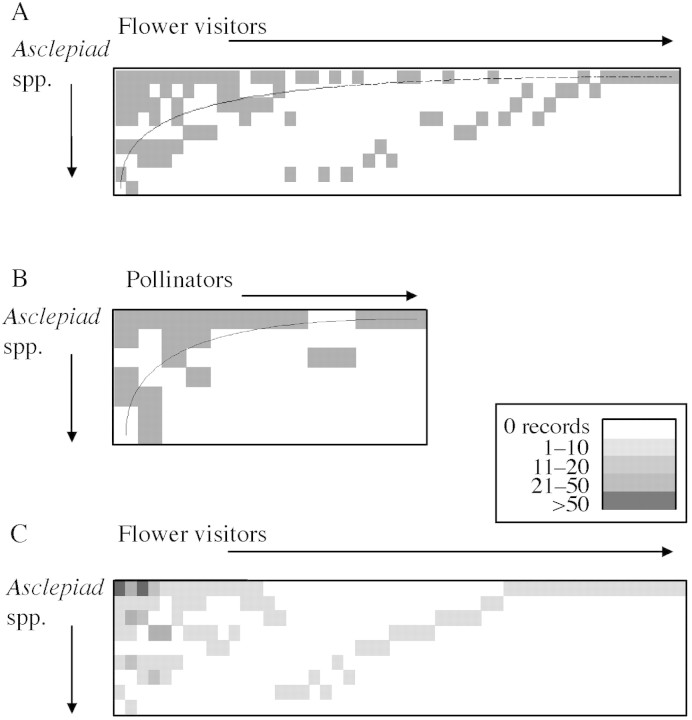
The flower visitor and pollinator data sets were subjected to nestedness analysis using the AICS Research Inc. software (http://www.aics‐research.com/research/index.html) (Atmar and Patterson, 1993). The degree of nestedness within a data set refers to the extent to which ‘smaller’ samples are subsets of ‘larger’ samples. This type of analysis has classically been performed for island biotas, to calculate the extent to which species present on small islands comprise a subset of those on larger islands (Patterson, 1990).
In the present analysis, the approach of Bascompte et al. (2003) and Dupont et al. (2003) is followed. The asclepiad species are analogous to islands, ranked by degree of specialization (number of flower visitors or pollinators) rather than ‘island size’. The flower visitors/pollinators are in turn ranked according to the number of plant species that they visit. The data can be considered as a matrix of presence–absence values for interactions between each plant and each insect (Fig. 4A and B). A perfectly nested data set would fill the matrix with a triangle of positive data running bottom left to top right of the matrix. In both the flower visitor matrix (Fig. 4A) and the pollinator matrix (Fig. 4B) that triangular pattern is strongly suggested, though not perfectly realized. The descriptive statistic used to describe the degree of fit to the nested ideal is the matrix ‘temperature’, defined as the ‘heat of disorder’, i.e. the degree of order or disorder in the matrix, relative to the idealized, perfectly nested pattern (Atmar and Patterson, 1993). A matrix temperature of 0° indicates a perfectly nested pattern, whilst one of 100° indicates a completely random pattern (Atmar and Patterson, 1993). The probability that this pattern, and the resultant matrix temperature, are non‐random can be calculated using Monte‐Carlo simulations of the original data set. The flower visitor and the pollinator data sets both show a significantly nested pattern of insect use amongst these asclepiads: flower visitor matrix temperature = 18·02°, P = 0·00007 based on 1000 Monte‐Carlo iterations. Pollinator matrix temperature = 14·34°, P = 0·05 based on 1000 Monte‐Carlo iterations.
Fig. 4. Nestedness analyses of A, the flower visitor–plant interactions matrix; B, the pollinator–plant interaction matrix; C, the flower visitor–plant interactions matrix with abundance of insect species overlain. Note the legend. The matrix is identical to that in Fig. 4A except that the order of some insect species is different.
As a refinement of this approach we have overlain insect abundance values (from Table 2) on the nestedness matrix for flower visitors (Fig. 4C). This shows that those insect species which visit a wide range of asclepiad species (i.e. those which cluster in the upper left of the matrix) are also those species that are in greatest abundance. This suggests that insect population size may be one factor determining the relative breadth of generalization of flower visiting insects, in terms of numbers of plant species visited.
Community flower visitor survey
The combined total for the two 1‐h surveys was 381 individual insects in 14 broad taxonomic groups (Table 3). The purpose of this survey was to ascertain if insects were visiting flowers of the asclepiad species in proportion to their abundance at the site or, conversely, whether the asclepiad flower visitors were a selected subset of the flower‐visiting insects in the community. The ideal statistical test for such data would be a test of frequencies (such as the χ2‐ or G‐tests). However, the large number of zero observations for many of our categories of insect flower visitor renders these tests invalid. As an alternative approach, the Spearman rank correlation of proportional abundance of each visitor group in the community survey was calculated against the proportion of that insect group on each asclepiad species. In all cases there was no statistically significant relationship; the asclepiad flower visitors do not visit those flowers in proportion to their overall abundance (Table 3). Clearly, the specific floral traits of the asclepiads filter particular flower visitors. Some groups of flower visitors are notably rare in the community (e.g. Hemipepsis hilaris, Cyrtothyrea marginalis and Heteroptera) but common as pollinators of asclepiads. Conversely, there are some insect groups which are rare as asclepiad flower visitors in relation to their community abundance (Apis mellifera and butterflies). All of the asclepiads that were studied overlapped broadly in their flowering phenologies, although they did not necessarily coincide in their peak flowering times. This survey was conducted almost exactly mid‐way through the study period. Hence the difference found between asclepiad pollinator abundance and the abundances of flower visitors in the community is not simply a reflection of a seasonally changing insect fauna, but is a consequence of the filtering effect that specific sets of floral traits have on the attraction of insects.
Table 3.
Comparison of abundances of flower visitors in the Wahroonga community with abundances on the nine species of asclepiad
| Community | Xysmalobium gerrardii | Xysmalobium involucratum | Asclepias woodii | Asclepias cucullata | Aspidonepsis diploglossa | Pachycarpus natalensis | Sisyranthus trichostomus | Miraglossum verticillare | Miraglossum pilosum | |
| Syrphidae | 2·1 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 |
| Other Diptera | 22·0 | 27·1 | 10·3 | 6·5 | 33·3 | 40·0 | 30·8 | 3·1 | 26·7 | 0·0 |
| Hemipepsis hilaris | 0·8 | 12·5 | 3·4 | 0·0 | 0·0 | 0·0 | 38·5 | 2·0 | 60·0 | 100·0 |
| Other wasps | 1·6 | 0·6 | 10·3 | 6·5 | 0·0 | 10·0 | 0·0 | 1·0 | 0·0 | 0·0 |
| Apis mellifera L. | 9·2 | 0·9 | 0·0 | 0·0 | 0·0 | 10·0 | 0·0 | 1·0 | 0·0 | 0·0 |
| Other bees | 14·7 | 0·3 | 3·4 | 3·2 | 16·7 | 40·0 | 17·3 | 0·0 | 0·0 | 0·0 |
| Atrichelaphinis tigrina | 10·2 | 4·6 | 20·7 | 48·4 | 0·0 | 0·0 | 0·0 | 35·7 | 3·3 | 0·0 |
| Cyrtothyrea marginalis | 2·1 | 1·8 | 0·0 | 25·8 | 0·0 | 0·0 | 0·0 | 42·9 | 3·3 | 0·0 |
| Weevils | 1·3 | 1·2 | 3·4 | 9·7 | 0·0 | 0·0 | 0·0 | 10·2 | 0·0 | 0·0 |
| Other beetles | 26·5 | 5·5 | 37·9 | 0·0 | 0·0 | 0·0 | 1·9 | 2·0 | 0·0 | 0·0 |
| Heteroptera | 2·6 | 45·6 | 10·3 | 0·0 | 16·7 | 0·0 | 11·5 | 1·0 | 6·7 | 0·0 |
| Butterflies | 4·7 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 1·0 | 0·0 | 0·0 |
| Moth | 1·0 | 0·0 | 0·0 | 0·0 | 33·3 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 |
| Miscellaneous and unidentified | 1·0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 |
| Total number of insects observed | 381 | 329 | 29 | 31 | 6 | 10 | 52 | 98 | 30 | 9 |
| Spearman rank correlation | rs = 0·32 | rs = 0·48 | rs = 0·18 | rs = 0·19 | rs = 0·44 | rs = 0·27 | rs = 0·21 | rs = 0·01 | rs = –0·45 | |
| P = 0·27 | P = 0·09 | P = 0·54 | P = 0·52 | P = 0·11 | P = 0·35 | P = 0·47 | P = 0·96 | P = 0·11 |
Numbers are percentages of each flower visitor group recorded. Spearman rank correlation coefficients are given for rank abundance of flower visitors in the community survey vs. each of the asclepiads.
Pollinaria removal and pollinia insertion, and reproductive success of the asclepiads
The removal and insertion of pollinia were scored in a random sample of flowers from all asclepiad taxa. Each of the nine species of asclepiad was found to have had pollinaria removed from flowers, and all except Xysmalobium involucratum had pollinia inserted between the guide rails, indicating successful pollination (Table 4). In almost all cases there is evidence that the pollinators which were carrying pollinaria were also carrying out pollination (see also Pollinaria placement on pollinators, below). Later in the season, fruit‐set was apparent on most of the asclepiads that were studied, although this was not quantified. Insertion rates were generally one or two orders of magnitude smaller than removal rates, which is commonly documented in asclepiads (Wyatt, 1978; Ali and Ali, 1989) despite the two‐fold greater opportunity for insertion (i.e. the paired pollinia must be removed together [as a single pollinarium], but may be inserted separately).
Table 4.
Rates of pollinaria removal and pollinia insertion
| Mean no. of removals per flower | Mean no. of insertions per flower | Ratio of I: R | Sample size (no. of flowers) | |
| Xysmalobium gerrardii | 1·3 | 0·13 | 0·10 | 23 |
| Xysmalobium involucratum | 1·8 | 0·00 | 0·00 | 35 |
| Miraglossum pilosum | 1·2 | 0·20 | 0·17 | 20 |
| Miraglossum verticillare | 1·2 | 0·94 | 0·80 | 35 |
| Sisyranthus trichostomus | 2·3 | 0·45 | 0·20 | 11 |
| Pachycarpus natalensis | 0·5 | 0·03 | 0·06 | 32 |
| Asclepias woodii | 3·5 | 0·03 | 0·01 | 35 |
| Aspidonepsis diploglossa | 3·3 | 0·20 | 0·06 | 25 |
| Asclepias cucullata | 1·0 | 0·05 | 0·05 | 19 |
What is the relationship between site of pollinaria placement on an insect’s body and the likelihood of pollination occurring? It may be expected that those asclepiads with ‘messy’ pollination systems (e.g. chafer‐pollinated Xysmalobium involucratum, which places pollinaria all over the bodies of the pollinators) would have a lower number of pollinia insertions relative to pollinaria removals, compared with taxa which place pollinaria on specific body areas (e.g. pompilid wasp‐pollinated Pachycarpus natalensis). However, examination of the mean ratio of insertions to removals in Table 4 suggests that there is no such relationship. The probability of pollination is more likely to be governed by other factors such as the rate of insect visitation.
Relationship between floral traits and pollination system
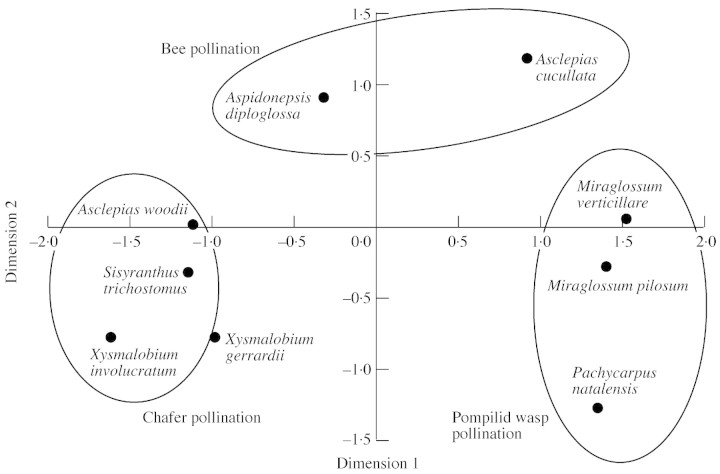
The multidimensional scaling analysis of floral traits (see the Appendix) indicates that species possessing the same specialized pollination systems (pompilid wasp and chafer) cluster closely in phenotype space (Fig. 5). Traits shared in common by the chafer‐pollinated species include dense, mechanically strong inflorescences, yellow or cream petal and corona coloration, and moderate to concentrated nectar in small to moderate quantities. The generalist‐pollinated Xysmalobium gerrardi clusters nearby and shares a number of traits in common with the chafer‐pollinated group. This shows that floral traits alone may not always be a good predictor of pollinators and may instead reflect phylogenetic relatedness, in this case of the two Xysmalobium species (although see comments in the Introduction). Taxa pollinated by the pompilid wasp shared the following traits: medium to large flowers in sparse inflorescences, with fewer than 15 flowers open per plant at any one time, a green corolla with purple spotting, quite drab in appearance, and possessing concentrated nectar The two species which are inferred to be bee‐pollinated (Aspidonepsis diploglossa and Asclepias cucullata) form a separate, although mutually distant cluster, sharing traits such as medium‐sized, open flowers of short life‐span, arranged in sparse inflorescences, with fewer than 15 flowers open at any one time, and a discrete nectar cup containing small amounts of moderately concentrated nectar.
Fig. 5. Multidimensional scaling analysis of the floral traits of the nine asclepiads (see the Appendix). Pollination systems are mapped onto the clusters of species.
Pollinator behaviour in relation to flower morphology
The scanning electron microscope (SEM) study of the floral morphology of these nine asclepiads revealed a number of specific adaptations for pollination which can be directly correlated with insect behaviour on the flowers. This is particularly true of the shape, size and function of the corona elements, and is detailed below for each taxon.
Sisyranthus trichostomus was the only species of asclepiad with conspicuously hairy floral parts (Fig. 6A). The dense frill of hairs originates around the rim of the mouth of the short, wide corolla tube and effectively obscures the gynostegium (Fig. 6B). The hairs are multicellular and tapered; along their length are many short, raised ridges, each orientated parallel with the long axis of the hair (Fig. 6C). The innermost ranks of hairs point downwards and into the (presumably nectar‐filled) cups formed by the corona (Fig. 6B). The body and legs of chafers feeding at these flowers are excluded from the gynostegium by the short corolla tube and the corolla hairs (Fig. 1D). All pollinaria placement was therefore on the mouthparts of the chafers (Fig. 1E and Table 5) as would be expected from this combination of flower morphology and beetle behaviour. The exact function of these hairs is obscure, but it is hypothesized that they may be multifunctional and: (a) exclude small nectar‐robbing insects such as ants; (b) act as a wick to draw up nectar from the nectar cups, encouraging the chafers to probe more deeply with their mouthparts; (c) serve as a visual contrast, highlighting the presence of the opening of the corolla tube (note the darker colouration of the hairs, compared with the corolla, Fig. 1A). An alternative hypothesis is that the hairs may function as food rewards for the beetles, as occurs in some cetoniid‐pollinated South African orchids (S. D. Johnson, unpub. res.), although no evidence (i.e. damaged, eaten hairs) was found that would support this.
Table 5.
Pollinaria placement on flower visiting insects to nine asclepiad species
| Asclepias cucullata | Asclepias woodii | Aspidonepsis diploglossa | Miraglossum pilosum | Miraglossum verticillare | Pachycarpus natalensis | Sisyranthus trichostomus | Xysmalobium gerrardii | Xysmalobium involucratum | |
| Mouthparts | 0 | 5 | 0 | 0 | 6 | 12 | 111 | 29 | 6 |
| % inserted | – | 40 | – | – | 100 | 66 | 25 | 59 | 50 |
| Leg 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 67 | 19 |
| % inserted | – | – | 66 | – | – | – | – | 33 | 58 |
| Leg 2 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 15 | 45 |
| % inserted | – | 0 | 100 | – | – | – | – | 67 | 42 |
| Leg 3 | 0 | 18 | 1 | 0 | 0 | 0 | 0 | 21 | 36 |
| % inserted | – | 33 | 100 | – | – | – | – | 33 | 50 |
| Body | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 10 |
| % inserted | – | 50 | – | – | – | – | – | 100 | 50 |
| Sample size of insects with pollinaria (total insects examined) | 0 (6) | 10 (20) | 3 (7) | 0 (1) | 4 (15) | 6 (23) | 30 (74) | 58 (136) | 6 (17) |
The plain numbers are the number of pollinaria recorded from the sample of insects specified in the final row of each column. The ‘% inserted’ rows in bold indicate the proportion of the pollinaria which had one or more pollinia missing, indicating that those pollinia had been trapped by the guide rails of flowers and pollination had been carried out.
Kunze (1991) investigated the functional morphology of an undetermined Sisyranthus sp. and concluded that the dense hairs blocking the corolla tube served to guide the proboscis of a foraging insect towards the nectary and guide rails. That particular Sisyranthus sp. possesses a much deeper tubular corolla and Kunze’s interpretation is reasonable. It is felt that this is a less likely interpretation for S. trichostomus, however, given its much shallower tube. This illustrates the fact that homologous structures in flowers, even when they appear to serve the same purpose, can in fact be fulfilling entirely different functions, or may even have no function at all if they are present only because of common descent.
Asclepias woodii is also a specialized chafer‐pollinated species. The fat, fleshy corona lobes are prominent and clearly have an attractive function (Figs 1A and 9A). They also function as nectar secretion and storage organs; the inside surfaces of these ‘cups’ are strikingly papillate, probably indicating the site of nectar secretion (Fig. 9B). The opening to these cups is unusually narrow for the genus Asclepias and its relatives; e.g. the more usual form taken by Asclepias cucullata shown in Fig. 6D–F. The odd nectar cup morphology becomes understandable when one observes the feeding behaviour of the chafers Atrichelaphinis tigrina and Cyrtothyrea marginalis: their mouthparts are exactly the right proportions to penetrate the partially closed cups and access the nectar. To do so they must move radially around the flower, at a tangent to the circumference, accessing each cup in turn. It follows, therefore, that most pollinaria placement was on the legs of the chafers (Table 5).
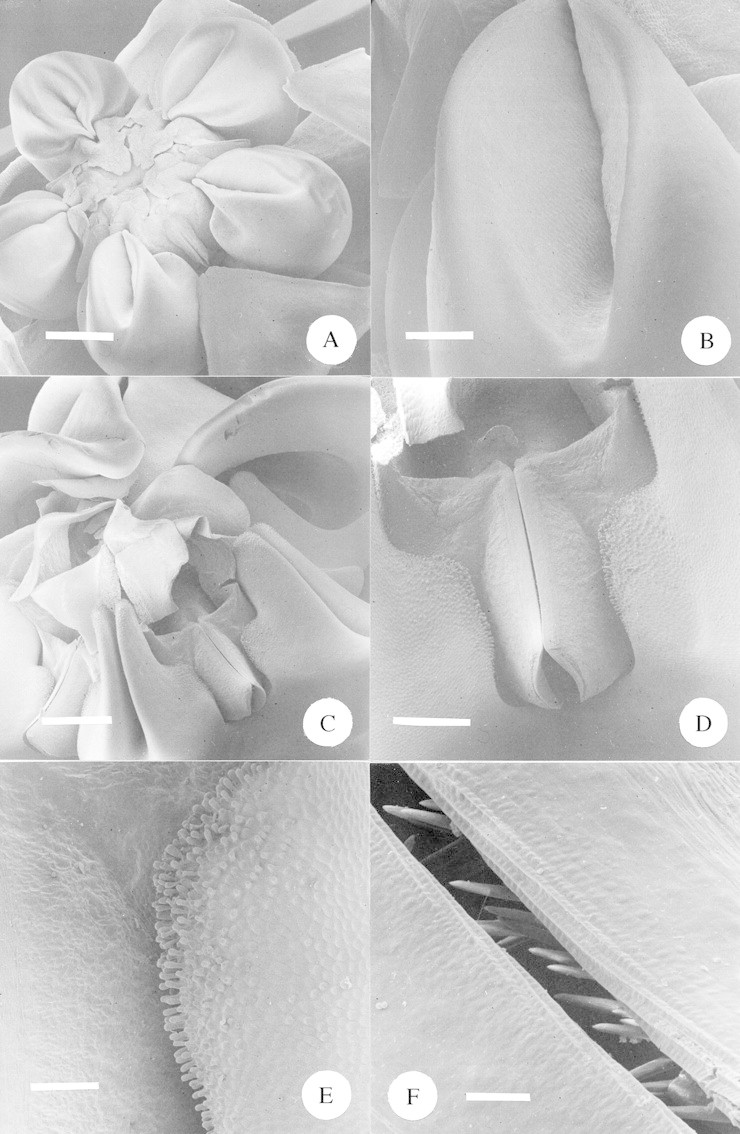
Fig. 9. A, SEM of a whole flower of Asclepias woodii. Scale bar = 1·0 mm. B, SEM close‐up of the inner surface of the nectar ‘cup’ of Asclepias woodii. Scale bar = 0·25 mm. C, SEM of the central portion of the large flower of Pachycarpus natalensis. The over‐arching portions of the three closest corona lobes have been removed to facilitate a view of the gynostegium. Scale bar = 1·0 mm. D, SEM close‐up of guide rails of Pachycarpus natalensis. The adjacent corona lobes fuse with the gynostegium at either side. Scale bar = 0·5 mm. E, SEM close‐up of Pachycarpus natalensis, showing the point at which the gynostegium (to the left) meets with the corona (to the right). Note the densely papillate epidermis of the corona. Scale bar = 150 µm. F, SEM close‐up of the guide rails of Pachycarpus natalensis. Note the upward‐pointing ‘teeth’ within the guide rails. Scale bar = 20 µm.
Xysmalobium involucratum is the final specialized chafer‐pollinated species, but the fine structure of flower morphology does not appear to show any particular adaptations to chafer pollination, and indeed a range of other insect species do visit the flowers, and even occasionally pollinate them in the case of Hemipepsis hilaris (Table 2). Nectar is secreted by the inside surface of the corona lobe, where it presses against the gynostegium (Fig. 7A). The long, narrow cleft at the tip of the corona lobe (Fig. 7B) is superficially similar (although smaller) to the opening of the nectar cup in Asclepias woodii (Fig. 9A and B) but we did not observe chafers feeding specifically at this cleft; rather, they appeared to be feeding all over the sides of the gynostegium and corona. The small flowers of this species meant that chafers feeding on one flower were being supported by other flowers and picking up pollinaria from them (Fig. 1C). Consequently, pollinaria were placed all over the chafers’ bodies, although concentrated on their legs (Table 5), as they scrambled over the densely packed inflorescences.
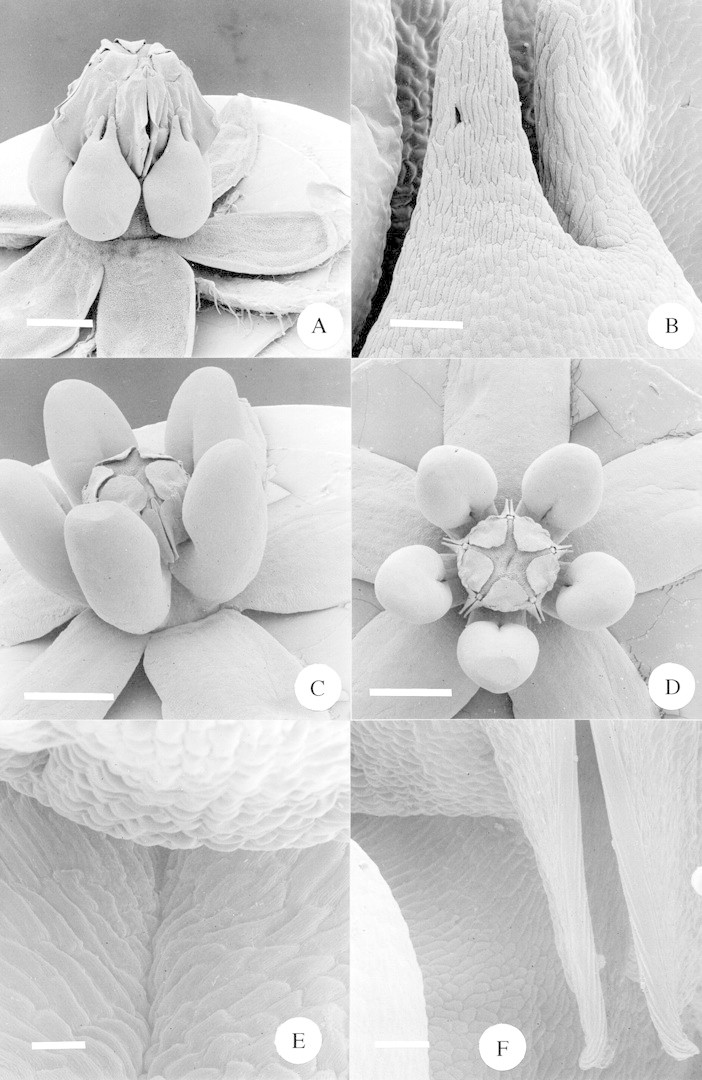
Fig. 7. A, SEM of whole flower of Xysmalobium involucratum. Scale bar = 1·0 mm. B, SEM close‐up of the top portion of the corona of Xysmalobium involucratum. Scale bar = 100 µm. C, SEM of whole flower of Xysmalobium gerrardii. Scale bar = 1·0 mm. D, SEM top view of whole flower of Xysmalobium gerrardii. Scale bar = 1·0 mm. E, SEM close‐up of Xysmalobium gerrardii, showing the space between the gynostegium (top of picture) and the inner surface of the corona (bottom of picture). Scale bar = 25 µm. F, SEM close‐up of Xysmalobium gerrardii, showing the region where the gynostegium and the corona meet, with the lower portion of the guide rails to the right. Scale bar = 50 µm.
The corona elements of the putatively bee‐pollinated Asclepias cucullata are shallow and laterally compressed, with slightly arching, raised hoods (Fig. 6D). They too function as nectar cups and the inner epidermis of the cups is presumably the site of nectar secretion, judging by its densely papillate appearance (Fig. 6E and F). The corona of the bee‐pollinated Aspidonepsis diploglossa also functions to present nectar to pollinating insects (Fig. 8E). In this case, there is no evidence of morphologically distinct nectar‐secreting tissue (Fig. 8F), although it is possible that the nectaries are more deeply situated in the nectar cup and therefore are not immediately apparent from SEM views. The tongue of tissue within the nectar cup is curious and is found in a number of species of Aspidonepsis (Nicholas and Goyder, 1992). If it has any function it may be to effectively raise the nectar level (and therefore its appearance and/or accessibility to pollinators?) without incurring the greater resource cost of actually producing more nectar. Prof. Sigrid Liede (University of Bayreuth) has suggested that the tongue may also increase brilliance effects (pers. comm., April 2003).
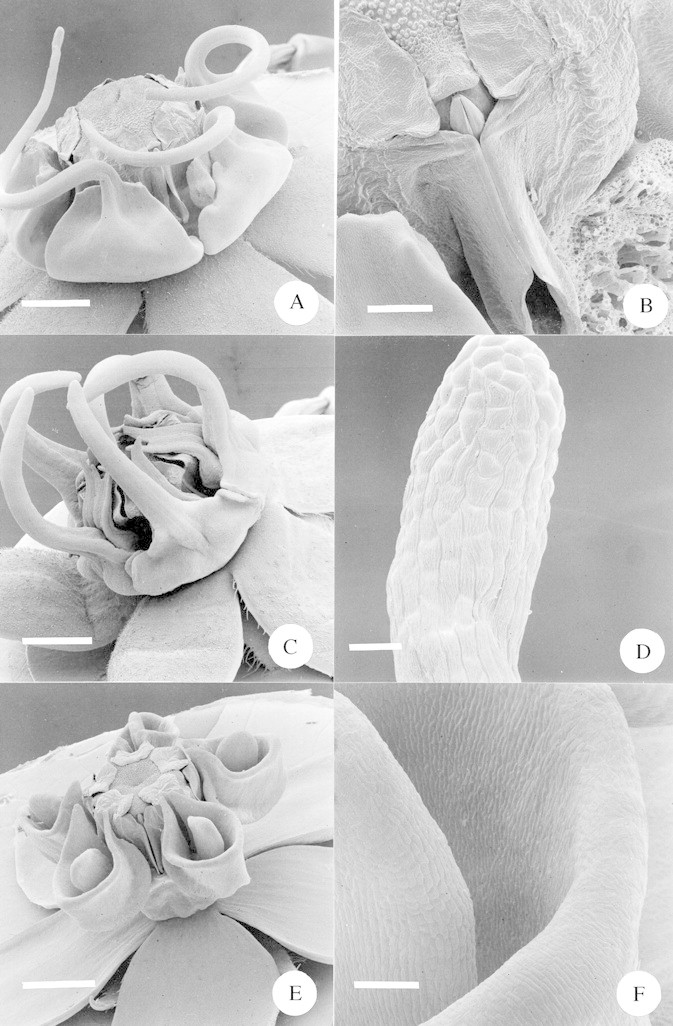
Fig. 8. A, SEM of whole flower of Miraglossum verticillare. Note that the spiral arms of the two distant corona elements were lost during specimen processing. Scale bar = 1·0 mm. B, SEM close‐up of the guide rails of Miraglossum verticillare, with the pollinarium in situ. To the left is the edge of an adjacent corona element; the one to the right has been removed. Scale bar = 250 µm. C, SEM of whole flower of Miraglossum pilosum. Note that the filiform corona elements are usually straight and were deformed during specimen processing. Scale bar = 1·0 mm. D, SEM close‐up of the terminal end of a filiform corona element of Miraglossum pilosum. Scale bar = 50 µm. E, SEM of whole flower of Aspidonepsis diploglossa. Scale bar = 1 mm. F, SEM close‐up of the inner wall of the nectar cup of Aspidonepsis diploglossa. To the left is the ‘tongue’ situated within the cup. Scale bar = 100 µm.
The specialized pompilid wasp‐pollinated taxa each show rather different adaptations to pollination by these insects, even between the closely related Miraglossum spp. The elongated corona lobes of M. verticillare are laterally bent through 90° relative to the main floral axis before turning through a second 90° angle to turn upwards (Fig. 8A). Nectar is secreted at the base of the guide rails, in the space between the broad proximal lobes of the corona (Fig. 8B). A pompilid wasp has its head braced downwards by the lateral coronal ‘bar’ as it approaches the position of nectar secretion. This presumably encourages the wasp to force its mouthparts deeper into the nectar chamber, increasing the likelihood of pollinarium removal or pollinium deposition. This contrasts with the situation in M. pilosum where the elongated corona lobes extend straight upwards (Fig. 8C, note that the re‐curving of the corona lobes to form a cage is an artefact of the SEM preparation, cf. Fig. 1A). In M. pilosum nectar is accessed from the ‘back’ of the nectar chamber, rather than the front as in M. verticillare. A wasp approaching the flower braces its front legs against two adjacent corona lobes and accesses the nectar in the chamber diametrically opposite, not the one in the space between its braced legs. This distinction between pollination mode of the two Miraglossum spp. may give a clue as to how pollinia from the two species do not become lodged in the ‘wrong’ species. In M. verticillare, pollinaria will mainly become attached to the top of the mouthparts, whereas in M. pilosum, pollinaria should attach to the underside of the mouthparts. It is in fact rather difficult to observe this distinction in pinned specimens.
Our original idea, that the extended corona lobes of these Miraglossum spp. may be acting as osmophores (specialized scent‐producing organs) appears to be unfounded; a close examination of the epidermis of the corona lobes (Fig. 8D) revealed that there were none of the papillae or dense stomata that one might expect to find associated with osmophore tissue (e.g. Vogel, 1990; Curry et al., 1991; Stpiczyñska, 1993).
The third specialized pompilid wasp‐pollinated species was Pachycarpus natalensis. In this species the overall flower dimensions are much larger than in the Miraglossum spp. (Fig. 1A and Table 1) and the corona lobes are large, flat structures which over‐arch the whole of the gynostegium (Fig. 9C). Nectar is secreted by the corona lobes where they fold together to form a groove (Fig. 9C) and at the point where the corona folds against the gynostegium (Fig. 9D). Copious nectar runs either side of the guide rails and the epidermis morphology of the inner surface of each corona is consistent with its function as a nectary (Fig. 9D and E). Within the guide rails there are very distinct upward‐pointing teeth half‐hidden behind the outermost flap of the rails (Fig. 9F). The function of these teeth is probably to prevent the pollinator from backing out of the guide rails and the pollinium from being pulled backwards following insertion. Variations on this feature are common amongst asclepiads (e.g. Kunze, 1991; Dr Ulrich Meve, University of Bayreuth, pers. comm. 2001). Pompilid wasps forage around the circumference of the corona lobes (Fig. 1B), feeding on the nectar at the bases of the lobes and picking up pollinaria on their mouthparts (Table 5).
Xysmalobium gerrardii is the most ecologically generalized of the nine species under consideration. It possesses the rather globular corona lobes characteristic of this genus (Fig. 7C and D, cf. X. involucratum, Fig. 7A) as taxonomically circumscribed. Nectar appears to be secreted by the inner surface of the lobes where they press against the gynostegium. Close examination of this area has revealed no evidence of morphologically specialized nectary tissue (Fig. 7E and F).
Pollinaria placement on pollinators
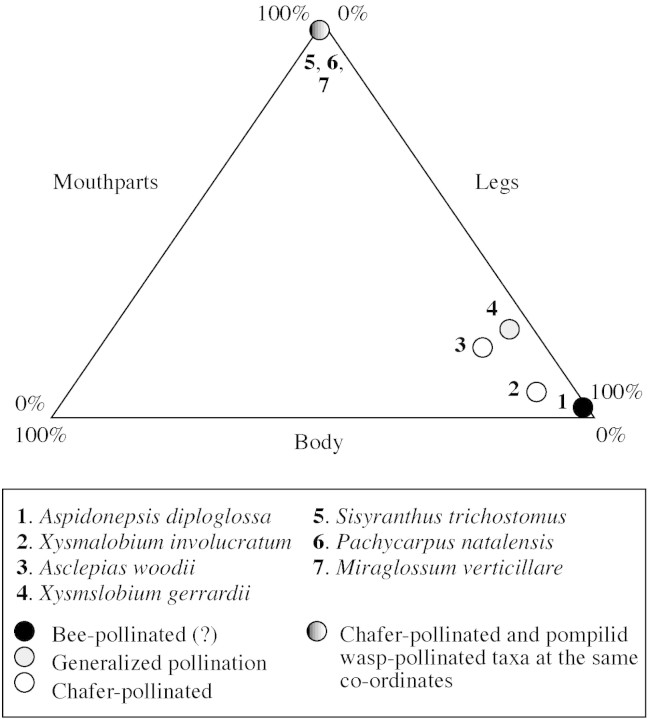
Pollinaria size and shape varied considerably between taxa (Fig. 12). Insects visiting the nine species of asclepiads picked up pollinaria on their mouthparts, each of the three pairs of legs and/or the underside of the body (Table 5 and Fig. 10). However, distinct patterns of pollinaria placement were apparent, which in part related to the pollination system and in part to the phylogenetic identity of the plant. The data in Table 5 have been converted to proportions of pollinaria attached to either mouthparts, legs or body. These data have been plotted onto a triangular graph (Fig. 13). Each data point represents a species of Wahroonga asclepiad and plots the proportion of pollinaria that were attached to one of the three areas of the insect. For example, of the chafer‐pollinated guild, Sisyranthus trichostomus placed pollinaria only on the mouthparts of the beetles, while Xysmalobium involucratum and Asclepias woodii placed pollinaria on more or less all of the possible positions (Table 4 and Fig. 13). Conversely, the wasp‐pollinated taxa placed pollinaria only on the mouthparts of the insects.
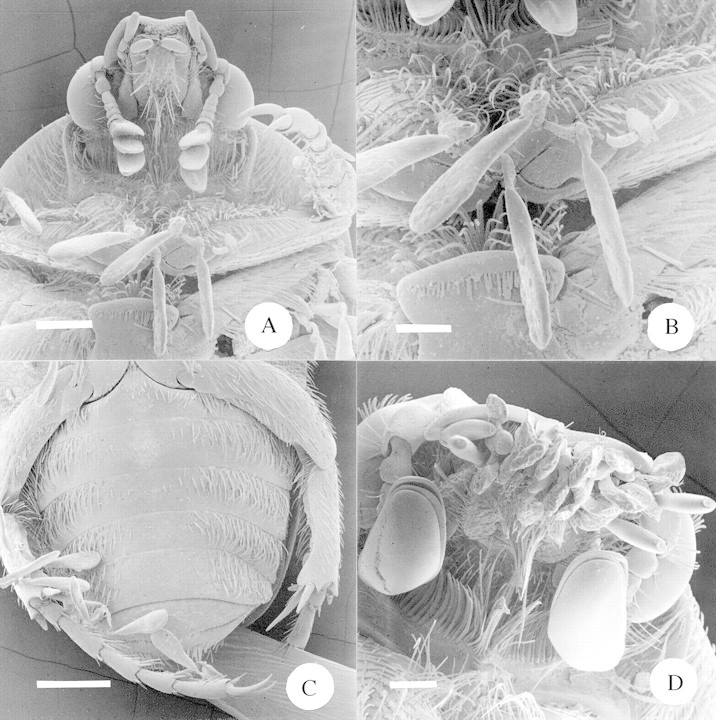
Fig. 10. A, SEM of the underside of the head and thorax of Atrichelaphinis tigrina, showing the attachment of pollinaria of Xysmalobium involucratum to the hairs of the thorax (cf. Fig. 1C). Scale bar = 0·5 mm. B, SEM close‐up view of Fig. 10A, showing the attachment of pollinaria to the hairs of the thorax. The pollinarium to the right has had both of its pollinia removed, presumably during pollination. Scale bar = 250 µm. C, SEM of the underside of the abdomen of Atrichelaphinis tigrina, showing the attachment of pollinaria of Xysmalobium involucratum to the hairs on the rear legs. Scale bar = 1 mm. D, SEM of the underside of the head of Cyrtothyrea marginalis, showing the attachment of pollinaria of Sisyranthus trichostomus to the mouthparts (cf. Fig. 1E). Scale bar = 250 µm.
Fig. 13. Placement of pollinaria onto the bodies of the pollinators of the nine asclepiads, plotted onto a triangular graph. Each data point represents the proportion of pollinaria (as a percentage of the total) found on the legs, body and mouthparts of the pollinating insects of the seven species of asclepiad for which pollinators were confirmed.
The advantage of using a triangular graph to represent these data is that pollination systems can be overlain onto the graph to assess whether pollinator identity determines the site of pollinaria placement, or whether other factors such as insect body size and the phylogenetic identity of the plant also have an influence (see Discussion).
Pollinaria number and pollinia size in relation to pollinator size
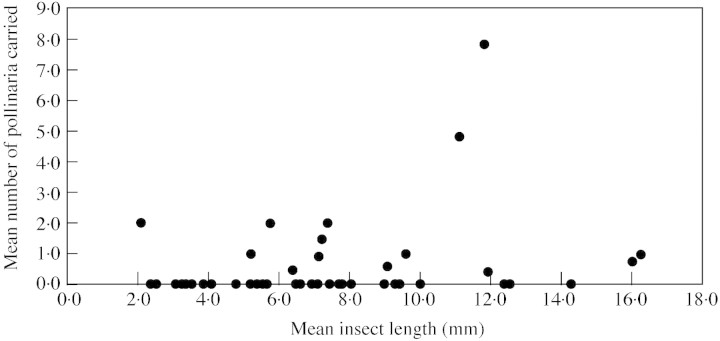
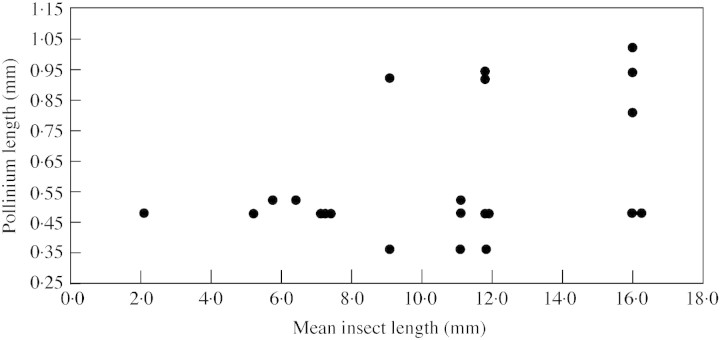
Pollinator size ought to affect two aspects of the biomechanics of pollinaria dispersal: (1) the probability of pollinaria being removed, because an insect must be large and strong enough to pull the pollinaria out of the flower; and (2) the number of pollinaria carried by the insect, because larger insects have more area over which pollinaria can attach. Using insect length as a measure of insect ‘size’, there is a weak, marginally statistically significant positive relationship between the mean length of the flower visitor and the mean number of pollinaria carried by those insects (Fig. 14; Spearman rank correlation, rs = 0·26, n = 46, P = 0·085). However, this relationship disappears when flower visitors which carried no pollinaria are removed from the analysis (Pearson product moment correlation, r = 0·11, d.f. = 12, P = 0·70). This suggests that there is not a simple linear relationship between insect size and pollinaria load; below a certain size threshold, most insects will not be large enough to remove pollinaria from flowers, but above that threshold there is no relationship between insect size and number of pollinaria carried. With reference to Fig. 14, this threshold appears to be about 5 mm. However, we note that this threshold is not absolute because one very small insect (Coleoptera sp. 3, 2·1 mm in length) is able to remove pollinaria of Xysmalobium gerrardii. Other aspects of insect biology, such as behaviour at the flowers and specificity of pollinaria placement, are likely to be much more important in determining pollinaria loads. For example, chafers visiting inflorescences of Xysmalobium involucratum are large in relation to the size of the flowers and may be simultaneously picking up pollinaria from a number of different flowers (Fig. 1C). There is no relationship between pollinarium size and the size of the insect bearing it (Fig. 15; Spearman rank correlation, rs = 0·24, n = 22, P = 0·27). However, the triangular shape of the distribution of data points in Fig. 15 suggests that the relationship between pollinarium size and insect size is not random: large insects can transport both large and small pollinaria, but small insects are only strong enough to remove the smaller pollinaria.
Fig. 14. The relationship between insect body length and number of pollinaria carried per insect.
Fig. 15. The relationship between insect body length and pollinium length.
DISCUSSION
Specialization and generalization amongst the Wahroonga asclepiads
The average number of pollinators per asclepiad (mean = 3·1, median = 2·0) is low compared with the averages for the family as a whole (mean = 11·8, median = 3·0) which were published by Ollerton and Liede (1997). As far as can be ascertained from the data presented here, given that they relate to only one site in one season, the Wahroonga asclepiads are on average rather more ecologically specialized (sensu Waser et al., 1996) than most other species of asclepiad. This may be evidence to support the assertion of Johnson and Steiner (2000) that areas of high plant diversity such as South Africa accommodate many more highly specialized plant–pollinator interactions compared with areas of lower diversity. This is certainly true on a case‐by‐case basis (there are a large number of specialized interactions in the South African flora), but whether the plants in South Africa are, on average, more ecologically specialized is unknown [though see Ollerton and Cranmer (2002) in contrast to Olesen and Jordano (2002)].
Three very distinct pollination systems can be recognized within this assemblage of nine asclepiad species. (1) Specialized pompilid wasp pollination: Miraglossum pilosum, Miraglossum verticillare, Pachycarpus natalensis; (2) specialized fruit chafer pollination: Asclepias woodii, Sisyranthus trichostomus, Xysmalobium involucratum; and (3) generalized insect pollination: Xysmalobium gerrardii. In addition, it is suspected that Asclepias cucullata and Aspidonepsis diploglossa may be mainly bee pollinated, but this requires further observation.
Pollination by pompilid wasps has been documented for a number of North American Asclepias species (e.g. Robertson, 1928; Kephart, 1979) and South American Oxypetalum species (Vieira and Shepherd, 1999) but only as a component of (sometimes extremely) generalized pollination systems, never as a specialized interaction. A Pepsis sp. was recorded as a pollinator of Morrenia odorata and Philibertia gilliesii in Argentina (Sigrid Liede, University of Bayreuth, unpub. res., see the ASCLEPOL online database of plant–pollinator interactions in the asclepiads at: http://www.uni‐bayreuth.de/departments/planta2/research_wgl/pollina/as_pol_t.html) but it is not known if these asclepiads possess specialized pompilid wasp pollination systems. In South Africa, flowers of Pachycarpus asperifolius are pollinated by a large number of wasps, including Sphex sp. (Sphecidae) and Hemipepsis dedjas (Pompilidae), but this appears to be an example of a ‘general’ wasp‐pollinated system (Craig Peter, University of Natal, unpub. res.). Specialized vespid wasp (Hymenoptera: Vespidae) pollination is known in South American Oxypetalum (Vieira and Shepherd, 1999; J. Ollerton, unpubl. res.) and Blepharodon nitidum (J. Ollerton, unpubl. res.), as well as in South African Gomphocarpus (S. D. Johnson, unpub. res.). These results have therefore confirmed that pompilid wasp pollination joins vespid wasp pollination as another specialized pollination system in the asclepiads. Floral traits shared in common by these specialized wasp‐pollinated taxa include green flowers, often with purple colouration or spotting, and sometimes a spicy‐sour odour.
Specialized pompilid wasp pollination appears to be virtually unknown in the rest of the flowering plants. The only other known case is pollination of a South African orchid, Disa bivalvata, by precisely the same pompilid wasp, Hemipepsis hilaris (Steiner et al., 1994). The Disa system is based on the sexual deception of male wasps by the orchid, and so differs to that of the asclepiads under consideration in this work, which is probably a more straightforward attraction–reward situation, as wasps of both sexes were attracted and all of the asclepiad taxa produce nectar. However, the flower coloration of D. bivalvata is rather similar to that of Pachycarpus natalensis (Fig. 1), with dark purple patterning on a light green background, suggesting that both species are employing similar visual attraction cues. The same pompilid wasp has also been observed visiting flowers of the related Pachycarpus appendiculatus (another species with green and purple flowers) elsewhere in South Africa (Dr Peter Bruyns, University of Cape Town, pers. comm.). Adult pompilid wasps are commonly known to visit flowers to feed on nectar, so it should not be surprising if other examples of specialized pompilid wasp pollination are discovered in the future.
Specialized beetle pollination is rare in the asclepiads (Ollerton and Liede, 1997) and, once again, most records are of beetles as occasional pollinators of highly generalized North American Asclepias species (e.g. Robertson, 1928; Willson and Price, 1977; Queller, 1985). Forster (1989) documented what may be specialized beetle pollination in the asclepiad Marsdenia fraseri, involving the lycid Metriorrhynchus lateralis (Coleoptera: Lycidae) in Australia, though this was based on a single day of observations and therefore it is unknown whether other insects may be involved in pollination of this species. The only other record of cetoniid chafers visiting asclepiad flowers are those of Woodell (1979), who recorded Mausoleopsis aldabrensis as a common visitor to three asclepiads on Aldabra but did not record their status as pollinators. Interestingly, these chafers were also important flower visitors to other plants on the island, which has intriguing parallels with our findings in KwaZulu‐Natal (see below).
Ollerton and Liede (1997) predicted that specialized beetle pollination systems may prove to be more common as research progresses, and that certainly has been shown to be the situation in KwaZulu‐Natal. The three species of chafer‐pollinated asclepiads share a range of floral traits in common, despite their diverse phylogenetic histories, suggesting that convergent evolution towards a specialized chafer pollination system has occurred.
The two species of chafers found at Wahroonga were common flower visitors to a wide range of other plants in grasslands around the Natal Midlands area during our fieldwork period (J. Ollerton et al., pers. obs.; S. D. Johnson and P. Neal, unpubl. res.; C. Peter, pers. comm.). Bees are relatively uncommon in these grasslands and the chafers appear to be fulfilling the role of the large bees (e.g. Bombus spp.) which would commonly be found in, for instance, European grasslands. These chafers are agile, fast flying, hairy and appear to mainly feed on nectar. Importantly, they do not damage flowers when they are feeding, which other beetles often do (Proctor et al., 1996). At Wahroonga and other sites, the chafer‐pollinated asclepiads appear to form a guild with plants from other families, such as Helichrysum spp. and Berkheya setifera (Asteraceae) and Zantedeschia bimaculata (Araceae), although this needs confirmation (J. Ollerton et al., unpubl. res.). Holm and Marais (1992) note that many cetoniids can be collected from flowers, as well as the fruit which gives them their vernacular name of ‘fruit chafers’. Whitehead et al. (1987) point out the importance of cetoniid beetles (and other Scarabaeidae) as pollinators of plants in the Cape flora and the same may well be true in other parts of South Africa. In addition, Liede (1989) and Struck (1995) have shown that pollination by Scarabaeidae is important in some South African genera of Mesembryanthemaceae. Chafer pollination may therefore be much more common than previously realized and, on the basis of the observations made during summer 2000–2001, fruit chafers may prove to be one of the dominant pollinators in KwaZulu‐Natal grasslands, functionally replacing large bees. A similar argument has been made by Steiner (1998) regarding the importance of monkey beetles (Scarabaeidae: Hopliini) as dominant pollinators in the south‐western Cape region of South Africa.
Heteropteran bugs frequently visit flowers with easily accessible nectar or pollen, but are usually not considered to be efficient pollinators (Proctor et al., 1996). There are no known examples of specialized heteropteran‐ pollinated plants (reviewed by Ollerton, 1999). The most abundant pollinator of the generalist Xysmalobium gerrardii was the lygaeid bug Aspilocoryphus fasciativentris, which carried abundant pollinaria and was a very active insect. Heteroptera are known to visit flowers and carry pollinaria of North American Asclepias species, and are probably minor pollinators within these generalist pollination systems (Robertson, 1928; Willson and Bertin, 1979; Fishbein and Venable, 1996). More work is required to understand the importance of this lygaeid in the pollination ecology of X. gerrardi (e.g. quantifying bug movements between plants) but it is suspected that it may prove to be a significant, and possibly the major, pollinator of this species.
In a theoretical analysis of classical pollination syndromes by Ollerton and Watts (2000) and J. Ollerton and R. Alarcon (unpub. res.), pollination by wasps and beetles falls relatively close to one another in phenotype space. This suggests that transitions between these pollination systems during speciation events are more likely than, for instance, transitions between wasp and moth pollination or beetle and bee pollination. Moderately large genera of southern African asclepiads such as Pachycarpus (approx. 30 species) and Xysmalobium (approx. 40 species) would provide an excellent test of these theoretical expectations, by combining phylogenetic analyses with field surveys of pollination systems. It is hoped this will be made a focus of future work on the pollination biology of southern African asclepiads.
Pollinator and flower visitor diversity
Species accumulation curves are rarely presented in studies of flower–visitor interactions (Ollerton and Cranmer, 2002; though see Petanidou and Ellis, 1993; Minckley et al., 1999) yet they are a useful tool for determining how comprehensive the sampling of a pollinator or flower‐visitor fauna has been. In this study, species accumulation curves were used to gauge how accurate was the assessment of flower‐visitor diversity for these species. There was considerable variation between asclepiad species: for the specialists, with only one or two pollinators, the full diversity of flower visitors had been sampled within 2 d. For the more generalist species, new species were still being added after 20 (non‐consecutive) field days. For the combined assemblage, an asymptote was reached after 23 d. There are few published data with which to compare our results but, clearly, highly generalist taxa such as many Asteraceae and Apiaceae (or even some North American Asclepias spp.) are going to show a rather different pattern, with the cumulative curve possibly never reaching an asymptote within practical sampling time. Similarly, studies that extend over multiple seasons may reach a temporary asymptote within each season, only to accumulate further species in the subsequent season, as Petanidou and Ellis (1993) found. It would be very useful if data were presented in this way more often by pollination ecologists.
Floral traits and pollinator identity
Multidimensional analysis of floral traits showed that the functionally specialized taxa largely grouped together, but that there may also be some phylogenetic conservatism with regard to floral traits which may result in a species grouping with the ‘wrong’ taxa, i.e. the generalist Xysmalobium gerrardii clustering with the chafer‐pollinated species, and specifically it’s congener, X. involucratum, on the basis of floral characteristics that may be phylogenetically conservative. Clearly, multivariate floral trait analysis can be a useful tool for exploring the relationship between flower phenotype and pollination system, and is starting to become widely used (Ollerton and Dafni, 2003) but the results should be treated cautiously, particularly in the absence of information regarding the identity of a plant’s pollinator(s) or an explicit phylogeny. Recent examples confirm this: Borba et al. (2002) analysed floral traits in populations of five species of fly‐pollinated Pleurothallis (Orchidaceae). Principal component analysis separated out the populations into three groups, depending upon the exact identity of the pollinating flies. However, the floral phenotypes associated with two of the pollination systems (involving the fly genera Tricimba and Megaselia) were in close proximity in phenotype space, suggesting that it is not always possible to predict the precise pollinator purely from the observed floral traits. Similarly, research on the relationship between pollinator identity and floral traits in Bornean gingers by Sakai et al. (1999) resolved 44 species of Zingiberaceae and Costaceae into three pollination guilds. The three guilds were moderately discrete in their relative positions in phenotype space, but some species did not cluster within the main groupings and defining the groupings themselves was a little arbitrary (see their Fig. 2). Of the 29 species for which pollinators were known, two were positioned in the ‘wrong’ area for their pollinator. Finally, in a multivariate analysis of ten species of Labiatae in Greece, there was no correspondence between the pattern of clustering using floral traits and that obtained using the identities of the principal bee pollinators (Petanidou and Vokou, 1993). Apparently floral traits could not distinguish between the exact type of bee pollinator at this fine, guild scale. It is re‐emphasized, therefore, that conclusions regarding the identity of pollinators based on the multivariate analysis of floral traits should always be confirmed by field observations of actual pollinators. This mirrors the recent arguments regarding the ‘ground truthing’ of conclusions concerning pollinator identity that are drawn only from an impression of a plant’s pollination syndrome (Ollerton, 1998); see also the argument of Johnson et al. (2001) that pollination syndromes should be primarily used to generate hypotheses to be tested by field observations.
Nectar volume and concentration has long been considered to be a useful predictor of pollinator identity, although there are numerous caveats to the argument that there is a simple relationship between the energetic content of a nectar source and the energy requirements of its pollinators (Cruden et al., 1983). Is there an association between nectar volume and concentration and pollinator identity for the specialized Wahroonga asclepiads? No pattern is apparent from the nectar volume data. However, the nectar concentration data show that the three species with (on average) the most dilute nectar are chafer pollinated (range 12·5–29·0%), whilst the three wasp‐pollinated taxa are characterized by more concentrated nectar (range 50·0–67·8 %). It is tempting to speculate that the higher nectar concentration reflects the active, predatory habits of female pompilid wasps, but further data on pompilid wasp‐pollinated species are required to confirm the generality of this observation.
Pollinia placement on insect bodies
Specificity of pollinaria placement does not seem to be related to pollinator specificity: all of the specialized wasp‐pollinated species place pollinaria on mouthparts, but then so does the specialized chafer species Sisyranthus trichostomus. However, the two Xysmalobium species both place their pollinaria predominantly on the legs, and occasionally the mouthparts and body, despite their very different pollination systems. The small, open, rather densely packed flowers of the two Xysmalobium species facilitate pollinaria placement on any part of the body as the medium‐ to large‐bodied insects crawl over the crowded inflorescences. Therefore pollinaria placement and, more importantly, effectiveness of pollinia insertion, may be as much a phylogenetic trait as a function of specialization of pollination system. For example, Sisyranthus trichostomus is a phylogenetically basal member of the tribe Ceropegieae (Meve and Liede, 2001). All members of this tribe so far investigated place their pollinaria on the mouthparts of the pollinating insects, be they large flies, microdiptera, moths, bees or beetles (Meve and Liede, 1994; Chaturvedi and Pant, 1986; Nel, 1995; J. Ollerton, unpubl. res.). Pollinaria placement within the very diverse tribe Ceropegieae is clearly phylogenetically conservative, probably because of the position of the nectar‐holding corona cups (or their analogues, Dr Peter Bruyns, University of Cape Town, pers. comm.) directly below the guide rails, which occurs in most taxa, even those basal within the tribe such as Sisyranthus (see Fig. 6B: also illustrations in, for example, Meve, 1995, 1998).
Randomness and pattern in the attraction of flower visitors
The nine asclepiad species at Wahroonga attract a non‐random mix of flower visitors, a small proportion of which are of the necessary size and show the necessary behaviour to act as pollinators. This subset of flower visitors and pollinators is in turn non‐randomly distributed between the asclepiads. The highly specialized asclepiads share their flower visitors with one another and with the less specialized asclepiads. Similarly, many of the flower visitors to the generalist Xysmalobium gerrardii are also to be found visiting the more specialized asclepiads. On average, only one in every six flower visitors functioned as a pollinator, although the range around this average was from approximately one in every three to one in every 12 flower visitors.
The nestedness analysis shows that the visitors to the most specialized asclepiads are a subset of the visitors to the less specialized species. This is commonly found in traditional analyses of nestedness (using reserve or island size) but has only recently been documented with plant–pollinator and animal–seed dispersal interactions (Bascompte et al., 2003; Dupont et al., 2003). The significance of this finding is that specialized plants are specializing on common, ubiquitous flower‐visiting insects. Conversely, the most specialized insects are visiting plants with abundant, easily accessible nectar rewards that are attracting a wide range of other insects. Mapping insect abundance onto the flower visitor matrix confirms this conclusion, and Dupont et al. (2003) also found that the most abundant species in a community tended to be the ones which interacted with many other species. This makes evolutionary sense—over long time scales, one‐to‐one interactions in which a plant and its pollinator are mutually dependent double the likelihood of either species becoming extinct. Such interactions are likely to be filtered out of communities, perhaps suggesting one reason why mutually obligate plant–pollinator relationships are so rare.
Recent research by Dicks et al. (2002) has indicated that some plant–pollinator interaction webs may possess a degree of compartmentalization, whereby ‘compartments’ within the web are characterized by particular plant–pollinator interaction combinations that are more strongly connected within compartments than between compartments. Although our data set is limited to only nine plant species, these species are nonetheless apparently compartmentalized in their interactions with pollinators, in that chafer‐pollinated taxa and pompilid wasp‐pollinated taxa form almost mutually exclusive compartments within the web. In turn, the guild of specialized chafer‐pollinated species may nest within a guild of plants (from all families) at Wahroonga that form a compartment.
Geographic and temporal variation in plant–pollinator interactions and the importance of detailed morphological study
It is known that the taxonomic identity and relative importance of different pollinator taxa can vary for plants between populations and from year to year within the same population (Kephart, 1983; Herrera, 1988; Fishbein and Venable, 1996; Ollerton, 1996; Lamborn and Ollerton, 2000). Field‐based pollination biology research is therefore often faced with the dilemma of whether to study a limited number of populations in detail or a large number of populations more superficially. As this research constitutes the first concerted attempt to study the pollination biology of asclepiads in South Africa, the former approach was adopted, in full knowledge that some of the findings may not apply to other populations of these asclepiads in KwaZulu‐Natal, or to Wahroonga in other years. However, unpublished observations by S.D.J. in the years subsequent to this study have shown that the specialized species at Wahroonga are visited by the same insect species in every year. This suggests some degree of temporal stability to these interactions. In addition, it was observed that cetoniid chafers pollinated plants of Sisyranthus trichostomus in a second population at Impendle Nature Reserve, some 40 km from Wahroonga (J. Ollerton, unpubl. res.), and Langley (1980) described seeing pollinaria‐carrying ‘beetles’ on plants of Xysmalobium involucratum on Kirkenberg Mountain, Orange Free State, approx. 200 km from Wahroonga. These limited observations therefore hint that there is also some geographical stability within the pollination systems of at least two of the specialized chafer‐pollinated taxa.
Pollination niche overlap
Amongst a limited subset (estimated at about one‐third) of asclepiads at one KwaZulu‐Natal locality, significant pollination niche overlap was discovered in two‐thirds of the species studied. This niche overlap was most apparent amongst the pompilid wasp‐pollinated taxa, all three of which shared the same species of pollinator and which placed pollinaria on the mouthparts of the wasps. However, it was only slightly less apparent amongst the chafer‐pollinated species, where both of two species of cetoniid beetle pollinated two of the asclepiads, and one of those was also the pollinator of a third species. A similar result was obtained by Sakai et al. (1999), in a study of pollination guilds in gingers (Zingiberaceae and Costaceae) in Borneo. Pollinators were identified for 29 species of ginger and each species could be assigned more or less specifically to one of three guilds (two bee‐pollinated and one bird‐pollinated). A great deal of pollinator sharing between ginger species was observed, leading the authors to suggest that ‘. . . many species of rare understory plants have evolved without segregating pollinators in each pollination guild’. In contrast, Armbruster et al. (1994) found that sympatric sets of Stylidium species (Stylidiaceae) in Western Australia had segregated pollination niches, either because they used different pollinators or because different species placed pollen on separate parts of the insect. In an earlier study of assemblage structure in co‐occurring Dalechampia (Euphorbiaceae), in which actual assemblages were tested against null models, Armbruster (1986) had found that there was limited pollinator sharing amongst sympatric species and suggested that the most likely explanation invoked a mix of evolution by character displacement and ecological sorting of competing species. However, the results obtained were sensitive to the assumptions of the null models, and it was indicated that further work was required to confirm these conclusions. Petanidou and Vokou (1993) also found that the structure of plant–pollinator interactions amongst Mediterranean Lamiaceae was not characterized by niche overlap, and that ‘. . . the high degree of floral resemblance . . . [among species] . . . did not result in a significant level of bee pollinator sharing . . .’.
Kephart (1983) examined niche breadth and overlap in three species of Asclepias in mixed experimental and natural assemblages. The results for the natural assemblages are of particular interest to the present study: Levins’ niche breadth values for the three species ranged from 0·09 to 0·55, depending upon the population. Within species, niche breadth varied to some extent: A. incarnata, range = 0·16–0·38, mean = 0·30, s.d. = 0·09; A. verticillata, range = 0·27–0·55, mean = 0·39, s.d. = 0·11; A. syriaca, range = 0·09–0·27, mean = 0·20, s.d. = 0·06. In comparison, the Wahroonga asclepiads had a much broader range of niche breadths, from 0·0 (i.e. complete specialization on one pollinator) to 0·65 (mean = 0·31, s.d. = 0·28). The Wahroonga data set therefore included species that were both much more generalized (e.g. Xysmalobium gerrardii) and much more specialized (i.e. the three species which were pollinated by pompilid wasps) than the North American Asclepias species studied by Kephart (1983). This is perhaps not surprising given the greater number of species included in this study, and their broader taxonomic diversity. Kephart’s study also found significant pollination niche overlap between co‐occurring Asclepias species at her natural sites. She found some evidence of pollination niche differentiation between A. syriaca and the other two species, but none between A. incarnata and A. verticillata. Spatial and temporal variation appeared to be much more important in determining the identity of the main pollinators of these species, rather than specialization per se.
In an insightful analysis of the theoretical expectations of niche partitioning due to pollinator sharing, Stone et al. (1998) identified five major resource axes along which sympatric plant populations could diverge to minimize pollen limitation due to competition for pollinators and/or reproductive disadvantage cause by heterospecific pollen transfer. These axes have been evaluated in relation to pollinator partitioning in the data set presented here; for each axis the evidence is assessed for its importance in structuring the observed patterns of plant–pollinator interaction amongst the Wahroonga asclepiads:
Axis 1: variation in the identity of pollinators. Clearly this has not occurred in the two sets of three taxa which share largely identical pollinators at the species‐specific level.
Axis 2: spatial separation of populations of different species utilizing the same pollinators. By definition, this has not occurred as the asclepiad assemblage was sympatric.
Axis 3: seasonal separation of flowering times of species sharing the same pollinators. Although the flowering phenologies of the nine asclepiads were not specifically recorded, it was clear during the time spent in the field that there was significant overlap between the flowering times of all taxa, including those which shared pollinators. However, the possibility that there is subtle temporal partitioning of pollinators for some taxa cannot be ruled out.
Axis 4: placement of pollen on different parts of the pollinator’s body by plant taxa sharing pollinators. There is strong evidence that divergence along this axis is not important. Amongst the chafer‐pollinated taxa, Xysmalobium involucratum and Asclepias woodii place pollinaria on the mouthparts, legs and bodies of insects, while Sisyranthus trichostomus places pollinaria only on the mouthparts, probably for reasons of phylogenetic conservatism, as discussed above. All of the species of pompilid wasp‐pollinated asclepiads place pollinaria on the mouthparts only. It was not uncommon to find individual insects carrying pollinaria of more than one asclepiad on the same body part (data not presented).
Axis 5: daily partitioning of pollinator use by divergence in flower opening times and/or pollen presentation. Casual observations of flower opening times in these asclepiads does not lead us to believe that this is an important factor within this assemblage. Indeed, almost all members of the Asclepiadoideae have flowers which are open day and night, the rare exceptions being night‐blooming taxa such as Pergularia (S. Liede, University of Bayreuth, pers. comm.). In addition, the persistence of pollinaria on individual insects (between 0·26 and 1·10 d, even in a heavily visited Asclepias syriaca population; Morse, 1982), and particularly the inability of insects to groom the pollinaria from their bodies, suggests that daily partitioning of pollinators is not occurring.
The five axes of Stone et al. (1998) may well be important in some plant assemblages (and in fact Axis 5 was shown to be significant in their study of sympatric Acacia species) but none of these appear to us to be acting to reduce competition for pollinators in this assemblage of species. It may be that the pollinators servicing the Wahroonga asclepiad assemblage are abundant enough for there to be no competition between species. However, abundant pollinators could still result in pollen limitation of reproductive success if individuals are being disadvantaged by heterospecific pollen transfer, due to stigma clogging and/or loss of male gametes (Waser, 1978). Heterospecific pollen disadvantage might be expected to be particularly acute in the asclepiads, as each pollinium inserted into the guide rails of a different species represents a loss of potentially 10 % of a flower’s pollen, whilst each heterospecific pollinium which clogs the guide rails and monopolizes a stigmatic chamber reduces the probability of successful pollination by one‐fifth. Of the 235 flowers that were examined, no examples were found where more than one pollinium had been inserted into the same guide rails, and only one example where it was suspected that the wrong pollinium had been inserted, despite high visitation rates to individual plants.
It is clear from the above examples that it is not possible to generalize about the level of pollinator sharing and niche overlap between co‐occurring assemblages of phylogenetically related plants, which may be evidence that plant–pollinator interactions are not always an important determinant of plant community structure (though see Parrish and Bazzaz, 1979).
In conclusion, this study of floral traits, pollinator diversity and assemblage structure in these nine species has revealed some novel pollination systems, evidence of intricate relationships between flower structure and pollinator behaviour and complex patterns of interactions. Clearly much is yet to be discovered about the ecology of plant–pollinator interactions in the asclepiads of South Africa.
ACKNOWLEDGEMENTS
We thank, in particular, Dr Jason Londt and Dr David Barraclough (Natal Museum), Dr Denis Brothers (University of Natal) and Dr Mick Day for insect identifications; Dr Ashley Nicholas (University of Durban, Westville) for the asclepiad identifications; the ever‐helpful staff of the Centre for Electron Microscopy, University of Natal, Pietermaritzburg; Dr Ulrich Meve (University of Bayreuth) and Dr Peter Bruyns (University of Cape Town) for discussion; and Dr Ashley Nicholas, Dr David Goyder (Royal Botanic Gardens, Kew) and Professor Sigrid Liede (University of Bayreuth) for sharing unpublished information. Dr Paul Neal, Dr Ronny Alexandersson and Craig Peter provided useful debate, insights and companionship in South Africa. Invaluable comments on the manuscript were provided by Professor Sigrid Liede, Professor Don Levin (University of Texas), Dr Peter Bruyns and Dr Yoko Dupont (University of Aarhus). J.O. would specifically like to thank Ellen, Patrick, James and Susie for support in South Africa and the UK, and Kathy Johnson for being a great help on many occasions. We are greatly indebted to Martin Kunhardt for permission to work at Wahroonga. This research was carried out under permit no. 4296/2000 from the KwaZulu‐Natal Nature Conservation Service. The authors thank The Royal Society and The Leverhulme Trust for funding.
APPENDIX
Table 1A.
Matrix for multidimensional scaling analysis
| Flower size | Inflorescence | No. of flowers per plant | Inflorescence height | Corolla colour | Corona colour | Flower shape | Nectar cup | Nectar concentration | Nectar volume | Scent | Flower longevity | |||||||||||||||||||||||||||||
| Asclepias cucullata | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| Aspidonepsis diploglossa | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Asclepias woodii | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pachycarpus natalensis | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Sisyranthus trichostomus | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Miraglossum verticillare | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| Miraglossum pilosum | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 |
| Xysmalobium gerrardii | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Xysmalobium involucratum | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
Supplementary Material
Received: 14 March 2003; Returned for revision: 17 July 2003; Accepted: 10 September 2003
References
- AliT, Ali S I.1989. Pollination biology of Calotropis procera subsp. hamiltonii (Asclepiadaceae). Phyton 29: 175–188. [Google Scholar]
- ArmbrusterWS.1986. Reproductive interactions between sympatric Dalechampia species – are natural assemblages random or organized? Ecology 67: 522–533. [Google Scholar]
- ArmbrusterWS.1995. The origins and detection of plant community structure: reproductive versus vegetative processes. Folia Geo botanica Phytotaxonomica 30: 483–497. [Google Scholar]
- ArmbrusterWS, Edwards ME, Debevec EM.1994. Floral character displacement generates assemblage structure of Western‐Australian triggerplants (Stylidium). Ecology 75: 315–329. [Google Scholar]
- AtmarW, Patterson BD.1993. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 96: 373–382. [DOI] [PubMed] [Google Scholar]
- BascompteJ, Jordano, P, Melián CJ, Olesen JM.2003. The nested assembly of plant‐animal mutualistic networks. Proceedings of the National Academy of Sciences USA 100: 9383–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BorbaEL, Semir J.2001. Pollinator specificity and convergence in fly‐pollinated Pleurothallis (Orchidaceae) species: a multiple population approach. Annals of Botany 88: 75–88. [Google Scholar]
- BorbaEL, Sheperd GJ, van den Berg C, Semir J.2002. Floral and vegetative morphometrics of five Pleurothallis (Orchidaceae) species: correlation with taxonomy, phylogeny, genetic variability and pollination systems. Annals of Botany 90: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BrownR.1811. On the Asclepiadeae, a natural order of plants separated from the Apocineae of Jussieu. Memoires of the Wernerian Natural History Society 1: 12–78. [Google Scholar]
- ChaturvediSK, Pant DD.1986. Further studies in the pollination of some Indian asclepiads. Bulletin of the Botanical Survey of India 28: 23–30. [Google Scholar]
- CowlingRM, Hilton‐Taylor C.1997. Phytogeography, flora and endemism. In: Cowling RM, Richardson DM, Pierce, SM, eds. Vegetation of southern Africa Cambridge, Cambridge University Press, 43–61. [Google Scholar]
- CowlingRM, Gibbs Russell GE, Hoffman MT, Hilton‐Taylor C.1991. Patterns of plants species diversity in southern Africa. In: Huntley BJ, ed. Biotic diversity in southern Africa: concepts and conservation Cape Town: Oxford University Press, 19–50. [Google Scholar]
- CrudenRW, Hermann SM, Peterson S.1983. Patterns of nectar production and plant‐pollinator coevolution. In: Bentley B, Elias, T. eds. The biology of nectaries New York, Columbia University Press, 80–125. [Google Scholar]
- CurryKJ, McDowell LM, Judd WS, Stern WL.1991. Osmophores, floral features, and systematics of Stanhopea (Orchidaceae). American Journal of Botany 78: 610–623. [Google Scholar]
- DafniA.1992.Pollination ecology – a practical approach. Oxford, IRL Press. [Google Scholar]
- DicksLV, Corbet SA, Pywell RF.2002. Compartmentalization in plant‐insect flower visitor webs. Journal of Animal Ecology 71: 32–43. [Google Scholar]
- DupontYL, Hansen DM, Olesen JM.2003. Structure of a plant‐pollinator network in the high altitude sub‐alpine desert of Tenerife, Canary Islands. Ecography 26: 301–310. [Google Scholar]
- EndressME, Bruyns PV.2000. A revised classification of the Apocynaceae s.l. Botanical Review 66: 1–56. [Google Scholar]
- FishbeinM.2001. Evolutionary innovation and diversification in the flowers of the Asclepiadaceae. Annals of the Missouri Botanical Garden 88: 603–623. [Google Scholar]
- FishbeinM, Venable DL.1996. Diversity and temporal change in the effective pollinators of Asclepias tuberosa Ecology 77: 1061–1073. [Google Scholar]
- ForsterPI.1989. Pollination of Marsdenia fraseri (Asclepiadaceae) by Metriorrhynchus lateralis (Coleoptera: Lycidae). Coleopterists Bulletin 43: 311–312. [Google Scholar]
- HeithausER.1974. The role of plant–pollinator interactions in determining community structure. Annals of the Missouri Botanical Garden 61: 675–691. [Google Scholar]
- HerreraCM.1988. Variation in mutualisms: the spatio‐temporal mosaic of a pollinator assemblage. Biological Journal of the Linnean Society 35: 95–125. [Google Scholar]
- HilliardO, Burtt B.1987. The botany of the southern Natal Drakensberg. Annals of the Kirstenbosch Botanical Gardens 15: 1–253. [Google Scholar]
- HolmE, Marais E.1992.Fruit chafers of southern Africa (Scarabaeidae: Cetoniini). Hartebeespoorte, Ekogilde. [Google Scholar]
- JohnsonSD.1993. Climatic and phylogenetic determinants of flowering seasonality in the Cape Flora. Journal of Ecology 81: 567–572. [Google Scholar]
- JohnsonSD, Steiner KE.2000. Generalization versus specialization in plant pollination systems. Trends in Ecology and Evolution 15: 190–193. [DOI] [PubMed] [Google Scholar]
- JohnsonSD, Edwards TJ, Carbutt C, Potgieter C.2002. Specialization for hawkmoth and long‐proboscid fly pollination in Zaluzianskya section Nycterinia (Scrophulariaceae). Botanical Journal of the Linnean Society 138: 17–27. [Google Scholar]
- JohnsonSD, Pauw A, Midgley, J.2001. Rodent pollination in the African lily Massonia depressa (Hyacinthaceae) American Journal of Botany 88: 1768–1773. [PubMed] [Google Scholar]
- JordanoP.1987. Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence asymmetries, and coevolution. American Naturalist 129: 657–677. [Google Scholar]
- KearnsCA, Inouye, DW.1993.Techniques for pollination biologists. Niwot, University Press of Colorado. [Google Scholar]
- KephartSR.1979.The floral ecology and reproductive isolation of three sympatric species of Asclepias. PhD Thesis, Indiana University. Bloomington. [Google Scholar]
- KephartSR.1983. The partitioning of pollinators among 3 species of Asclepias Ecology 64: 120–133. [Google Scholar]
- KephartSR.1987. Phenological variation in flowering and fruiting of Asclepias American Midland Naturalist 118: 64–76. [Google Scholar]
- KesslerM, Krömer T.2000. Patterns and ecological correlates of pollination modes among bromeliad communities of Andean forests in Bolivia. Plant Species Biology 2: 659–669. [Google Scholar]
- KochmerJP, Handel SN.1986. Constraints and competition in the evolution of flowering phenology. Ecological Monographs 56: 303–325. [Google Scholar]
- KunzeH.1990. Morphology and evolution of the corona in Asclepiadaceae and related families. Tropische und Subtropische Pflanzenwelt 76: 7–51. [Google Scholar]
- KunzeH.1991. Structure and function in asclepiad pollination. Plant Systematics and Evolution 176: 227–253. [Google Scholar]
- KunzeH.1997. Corona and nectar system in Asclepiadinae (Asclepiadaceae). Flora 192: 175–183. [Google Scholar]
- LambornE, Ollerton J.2000. Experimental assessment of the functional morphology of inflorescences of Daucus carota (Apiaceae): testing the “fly catcher effect”. Functional Ecology 14: 445–454. [Google Scholar]
- LangleyRW 1980.Taxonomic studies in the Asclepiadeae with particular reference to Xysmalobium R.Br. in southern Africa. MSc Thesis, University of Natal, Republic of South Africa. [Google Scholar]
- LevinsR.1968.Evolution in changing environments. Princeton, Princeton University Press. [Google Scholar]
- LiedeS.1989. Untersuchungen zur Ökologie der Gattung Erepsia Beitrage zur Biologie der Pflanzen 65: 1–98. [Google Scholar]
- LiedeS, Kunze H.1993. A descriptive system for corona analysis in Asclepiadaceae and Periplocaceae. Plant Systematics and Evolution 186: 275–284. [Google Scholar]
- LiedeS, Whitehead V.1991. Studies in the pollination biology of Sarcostemma viminale R.BR. sensu lato South African Journal of Botany 57: 115–122. [Google Scholar]
- MemmottJ 1999. The structure of a plant–pollinator food web. Ecology Letters 2: 276–280. [DOI] [PubMed] [Google Scholar]
- MeveU.1995.Neoschumannia (including Swynnertonia), a primitive genus of the Asclepiadaceae‐Stapelieae. Plant Systematics and Evolution 197: 233–242. [Google Scholar]
- MeveU.1998.Emplectanthus N.E.Br.: a close relative of Riocreuxia Decne. in the Asclepiadaceae‐Stapelieae. Botanische Jahrbuche für Systematik 120: 123–130. [Google Scholar]
- MeveU, Liede S.1994. Floral biology and pollination in stapeliads – new results and a literature review. Plant Systematics and Evolution 192: 99–116. [Google Scholar]
- MeveU, Liede S.2001. Inclusion of Tenaris and Macropetalum in Brachystelma (Apocynaceae‐Asclepiadeae‐Ceropegieae) inferred from non‐coding nuclear and chloroplast DNA sequences. Plant Systematics and Evolution 228: 89–105. [Google Scholar]
- MinckleyRL, Cane JH, Kervin L, Roulston TH.1999. Spatial predictability and resource specialization of bees (Hymenoptera: Apoidea) at a superabundant, widespread resource. Biological Journal of the Linnean Society 67: 119–147. [Google Scholar]
- MorseDH.1982. The turnover of milkweed pollinia on bumblebees, and implications for outcrossing. Oecologia 53: 187–196. [DOI] [PubMed] [Google Scholar]
- NicholasA, Goyder DJ.1992. Aspidonepsis (Asclepiadaceae), a new Southern African genus. Bothalia 22: 23–35. [Google Scholar]
- NelM.1995. Rare and interesting plants of the Namib Desert. Part 2. Three desert plants. Veld and Flora 81: 14–15. [Google Scholar]
- O’ConnorTG, Bredenkamp, GJ.2000. Grasslands. In: Cowling RM, Richardson DM, Pierce SM, eds. Vegetation of southern Africa Cambridge: Cambridge University Press, 215–257. [Google Scholar]
- OlesenJM, Jordano P.2002. Geographic patterns in plant–pollinator mutualistic networks. Ecology 83: 2416–2424. [Google Scholar]
- OllertonJ.1996. Reconciling ecological processes with phylogenetic patterns: the apparent paradox of plant–pollinator systems. Journal of Ecology 84: 767–769. [Google Scholar]
- OllertonJ.1998. Sunbird surprise for syndromes. Nature 394: 726–727. [Google Scholar]
- OllertonJ.1999. The evolution of pollinator–plant relationships within the arthropods. In: Melic A, DeHaro JJ, Mendez M. Ribera I, eds. Evolution and phylogeny of the Arthropoda Zaragoza: Ento mological Society of Aragon, 741–758. [Google Scholar]
- OllertonJ, Cranmer L.2002. Latitudinal trends in plant–pollinator interactions: are tropical plants more specialized? Oikos 98: 340–350. [Google Scholar]
- OllertonJ, Dafni A.2003. Functional floral morphology and phenology. In: Dafni A, Kevan P, eds. Manual of field methods in pollination ecology. Ontario, Enivoquest. [Google Scholar]
- OllertonJ, Lack AJ.1992. Flowering phenology: an example of relaxation of natural selection? Trends in Ecology and Evolution 7: 274–276. [DOI] [PubMed] [Google Scholar]
- OllertonJ, Liede S.1997. Pollination systems in the Asclepiadaceae: a survey and preliminary analysis. Biological Journal of the Linnean Society 62: 593–610. [Google Scholar]
- OllertonJ, Liede S.2003. Corona structure in Cynanchum: linking morphology to function. Ecotropica in press. [Google Scholar]
- OllertonJ, Watts S.2000. Phenotype space and floral typology: towards an objective assessment of pollination syndromes. Det Norske Videnskaps‐Akademi I. Matematisk‐Naturvitenskapelig Klasse, Avhandlinger, Ny Serie 39: 149–159. [Google Scholar]
- ParrishJAD, Bazzaz FA.1979. Differences in pollination niche relationships in early and late successional communities. Ecology 60: 597–610. [Google Scholar]
- PattersonBD.1990. On the temporal development of nested subset patterns of species composition. Oikos 59: 330–342. [Google Scholar]
- PetanidouT, Ellis WE.1993. Pollinating fauna of a phryganic ecosystem: composition and diversity. Biodiversity Letters 1: 9–22. [Google Scholar]
- PetanidouT, Vokou D.1993. Pollination ecology of Labiatae in a phryganic (East Mediterranean) ecosystem. American Journal of Botany 80: 892–899. [Google Scholar]
- ProctorM, Yeo P, Lack A.1996.The natural history of pollination. London, HarperCollins. [Google Scholar]
- QuellerDC.1985. Proximate and ultimate causes of low fruit production in Asclepias exaltata Oikos 44: 373–381. [Google Scholar]
- RathckeB.1988. Flowering phenologies in a shrub community – competition and constraints. Journal of Ecology 76: 975–994. [Google Scholar]
- RobertsonC.1895. Flowers and insects XIII. Botanical Gazette 20: 104–110. [Google Scholar]
- RobertsonC.1928.Flowers and insects: lists of visitors of four hundred and fifty‐three flowers. Carlinville, IL: privately published. [Google Scholar]
- SakaiS, Kato M, Inoue T.1999. Three pollination guilds and variation in floral characteristics of Bornean gingers (Zingiberaceae and Costaceae). American Journal of Botany 86: 646–658. [PubMed] [Google Scholar]
- Scott‐ElliotGF.1891. Notes on the fertilisation of South Africa and Madagascar flowering plants. Annals of Botany 5: 333–405. [Google Scholar]
- SilvertownJ, Dodd M, Gowing D.2001. Phylogeny and the niche structure of meadow plant communities. Journal of Ecology 89: 428–435. [Google Scholar]
- SteinerKE.1998. Beetle pollination of peacock moraeas (Iridaceae) in South African orchids. Plant Systematics and Evolution 209: 47–65. [Google Scholar]
- SteinerKE, Whitehead VB, Johnson SD.1994. Floral and pollinator divergence in 2 sexually deceptive South African orchids. American Journal of Botany 81: 185–194. [Google Scholar]
- StoneGN, Willmer P, Rowe JA.1998. Partitioning of pollinators during flowering in an African Acacia community. Ecology 79: 2808–2827. [Google Scholar]
- StpiczyñskaM.1993. Anatomy and ultrastructure of osmophores of Cymbidium tracyanum Rolfe (Orchidaceae). Acta Societatis Botanicorum Poloniae 62: 5–9. [Google Scholar]
- StruckM.1995. Land of blooming pebbles. Aloe 32: 56–65. [Google Scholar]
- ToftsR, Silvertown J.2000. Niche differences and their relation to species’ traits in Cirsium vulgare and Cirsium eriophorum Folia Geobotanica 35: 231–240. [Google Scholar]
- VictorGE, Bredenkamp CL, Venter HJT, Bruyns PV, Nicholas, A.2000. Apocynaceae. In: Leistner OA, ed. Seed plants of southern Africa: families and genera Pretoria: National Botanical Institute, 71–98. [Google Scholar]
- VieiraMF, Shepherd GJ.1999. Pollinators of Oxypetalum (Asclepiadaceae) in Southeastern Brazil. Revista Brasileira de Biologia 59: 693–704. [DOI] [PubMed] [Google Scholar]
- VogelS.1990.The role of scent glands in pollination. Rotterdam: A.A. Balkema. [Google Scholar]
- WaiteS.2000.Statistical ecology in practice. Harlow: Prentice Hall. [Google Scholar]
- WaserNM.1978. Interspecific pollen transfer and competition between co‐occurring plant species. Oecologia 36: 223–236. [DOI] [PubMed] [Google Scholar]
- WaserNM, Chittka L, Price MV, Williams N, Ollerton J.1996. Generalization in pollination systems, and why it matters. Ecology 77: 1043–1060. [Google Scholar]
- WealeJPM.1873. Observations on the mode in which certain species of Asclepiadæ are fertilized. Linnean Journal – Botany 13: 48–58. [Google Scholar]
- WhiteheadVB, Giliomee JH, Rebelo AG.1987. Insect pollination in the Cape flora. In: Rebelo AG, ed. A preliminary synthesis of pollination biology in the Cape flora Pretoria: South African National Scientific Programmes report no. 141, 52–82. [Google Scholar]
- WillsonMF, Bertin RI.1979. Flower visitors, nectar production, and inflorescence size of Asclepias syriaca Canadian Journal of Botany 57: 1380–1388. [Google Scholar]
- WillsonMF, Price PW.1977. The evolution of inflorescence size in Asclepias (Asclepiadaceae). Evolution 31: 495–511. [DOI] [PubMed] [Google Scholar]
- WoodellSRJ.1979. The role of unspecialized pollinators in the reproductive success of Aldabran plants. Philosophical Transactions of the Royal Society, Series B 286: 99–108. [Google Scholar]
- WyattR.1978. Experimental evidence concerning the role of the corpusculum in Asclepias pollination. Systematic Botany 3: 313–321. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.