Abstract
Intact trees of Wollemia nobilis Jones, Hill and Allen (Araucariaceae) routinely develop multiple coppice shoots as well as orthotropic epicormic shoots that become replacement or additional leaders. As these are unusual architectural features for the Araucariaceae, an investigation was made of the axillary meristems of the main stem and their role in the production of epicormic and possibly coppice shoots. Leaf axils, excised from the apex to the base of 2‐m‐high W. nobilis plants (seedling origin, ex situ grown), were examined anatomically. Small, endogenous, undifferentiated (no leaf primordia, no vascular or provascular connections) meristems were found in the axils from near the shoot apex. In the more proximal positions about half the meristems sampled did not differentiate further, but became tangentially elongated to compensate for increases in stem diameter. In the remaining axils the meristems slowly developed into bud primordia, although these buds usually developed few leaf primordia and their apical ‘domes’ were wide and flat. Associated vascular development was generally restricted to provascular dedifferentiation of the cortical parenchyma, with the procambium usually forming a ‘closed loop’ that did not extend back to the secondary vascular tissues. Development of the meristems was very uneven with adjacent axils often at widely differing stages of development into buds. The study shows that, unlike most conifers, W. nobilis possesses long‐lived meristematic potential in most, if not all, leaf axils. Unlike other araucarias that have been investigated, many of the meristems in the orthotropic main stem will slowly develop into bud primordia beneath the bark in intact plants. It appears likely that this slow but continued development provides a ready source of additional or replacement leaders and thus new branches and leaves.
Key words: Wollemi pine, Wollemia nobilis, axillary meristems, epicormic, coppice, branches, resprouting, anatomy, buds
INTRODUCTION
Some 23 architectural models have been recognized in woody plants (Hill, 1997). The Massart model is found in most conifers and is almost universal in the Araucariaceae (Hill, 1997). This model is characterized by a single monopodial trunk and plagiotropic, indeterminate branches. A single iteration is present during the life of conifer species that follow this model, although if the orthotropic leader is lost or damaged it can be replaced by buds or meristems lower on the trunk (traumatic reiteration). Adaptive reiteration (new branches) and reiterative generation (coppice shoots) have also been recorded in the Araucariaceae (Hill, 1997).
Wollemia nobilis W. G. Jones, K. D. Hill, J. M. Allen is one of Australia’s rarest trees with less than 100 adult plants known from a small area of the Wollemi National Park (Offord et al., 1999). In contrast to the other members of the Araucariaceae W. nobilis exhibits two unusual architectural features, the development of coppice (from the base) and epicormic (from the stems and branches) shoots in intact plants (Hill, 1997). Consequently the architecture of W. nobilis is described as a modified Cook model (Hill, 1997). Epicormic shoots and a coppicing habit are characteristic strategies for surviving environmental pressures such as drought and fires (Bellingham and Sparrow, 2000; Bond and Midgley, 2001; Del Tredici, 2001). Numerous coppice shoots have developed at the base of the main trunk in almost all the adult plants (Hill, 1997, fig. 9; Offord et al., 1999, fig. 4) and in some trees over 100 coppice shoots arise, making it difficult to determine what constitutes a single tree.
The branches of W. nobilis trees are unbranched, plagiotropic, short‐lived and shed as single units (Hill, 1997). They are only initiated near the orthotropic shoot apex and form pseudowhorls in all but the youngest plants. Branches produce terminal strobili after several years of growth and these branches are shed by 10–12 years of age (Hill, 1997; P. F. Meagher and C. A. Offord, unpubl. obs.). Young seedlings develop numerous branches, and further branching of these branches rarely occurs unless their tips are damaged (Offord et al., 1999). Although all the branches that form on the primary trunk are shed relatively quickly, older plants clearly display branching in their crowns (Hill, 1997). These branches grow out from orthotropic epicormic buds that apparently originate from the axillary meristems in the main stem (Burrows, 1999). The orthotropic epicormic shoots partially reiterate the architecture of the young adult plant. These epicormic shoots also develop on many of the seedlings grown ex situ (Offord et al., 1999), although fewer develop when grown under high light intensities (C. A. Offord and P. F. Meagher, unpubl. res.). The epicormic shoots arise irregularly on the trunk (Hill, 1997), unlike the pseudowhorls of replacement branches that create the successive nesting crowns of many Araucaria species (Veillon, 1978, 1980).
In the leaf axils of most dicotyledonous trees well‐developed buds are present. If these buds do not develop into branches they usually form dormant epicormic buds located near the bark surface (Fink, 1980, 1983). In most conifers few axillary buds form relative to the large number of leaves. The numerous leaf axils are generally described as ‘blank’, although in some species Fink (1984) found ‘persisting detached meristems’, although most of these meristems were eventually cut off by periderm formation. Consequently most conifers have a limited ability to coppice or to produce epicormic shoots after disturbance (Fink, 1984; Bellingham and Sparrow, 2000; Bond and Midgley, 2001; Del Tredici, 2001). In contrast, species of Agathis, Araucaria and Wollemia (Araucariaceae) possess an apparently unique axillary structure consisting of undifferentiated axillary meristems that have neither leaf primordia nor vascular connections (Fink, 1983; Burrows, 1986, 1987, 1991, 1999). These meristems are initially formed in an exogenous position but are transferred to an endogenous position by the activity of a localized phellogen (Burrows, 1986, 1987) and are consequently not abscissed when widespread bark formation occurs (Burrows, 1986, 1990b). In Araucaria cunninghamii these meristems remain in an undifferentiated state and can remain beneath the bark indefinitely, allowing for increases in stem diameter by infrequent anticlinal cell divisions (Burrows, 1990b). The meristems can develop into shoots when given an appropriate hormonal stimulus (Fink, 1983; Burrows, 1989; Burrows et al., 1988).
Given that the formation of coppice and epicormic shoots in intact plants is both characteristic for W. nobilis and unusual for the Araucariaceae (Hill, 1997) and other conifers, it was considered that an investigation of the ontogeny of the axillary meristems of W. nobilis may shed light on the development of its unusual growth form.
MATERIALS AND METHODS
Leaf axils from three W. nobilis plants were excised. The plants were growing at the Mount Annan Botanic Garden, Mt Annan, Sydney and were grown from seed collected from tree 1, site 1 (RBG accessions 950218AP, 950218BB and 950218BP). The plants were 2 m high, 5 years of age, and growing in 75‐L pots under 70 % shade‐cloth. Two plants were monopodial, while one tree possessed a 60‐cm‐long secondary leader (an orthotropic shoot arising from the main stem) that had developed about 1 m above soil level. In a limited number of axils small stunted or dead buds were present. These buds may have developed into plagiotropic branches or orthotropic epicormic shoots. No coppice shoots were present.
Two leaf axils were collected from each plant at 5–8 cm below the shoot apex, which for the purposes of this paper is termed Position 1. Two axils were then excised from each plant at intervals of about 20 cm down from Position 1, finishing at Position 10, about 10 cm above the level of the potting mix and the cotyledonary node. An additional one or two axils were collected at Position 10 for each plant.
At Position 1 the leaders ranged in diameter from 1·8 to 2·2 cm and the leaf tips and much of the leaf base were uniformly green, although the leaf bases had numerous small horizontal strips of wound periderm (Fig. 1A). At Position 5 the leaf tips were still green while the stem was covered in a thin periderm (Fig. 1B). At Position 10 the stems ranged in diameter from 4 to 5 cm and the axils could only be found by locating the dried, shrivelled remains of the leaves on the bark surface (Fig. 1C).

Fig. 1. Morphology of the orthotropic main stem of 5‐year‐old W. nobilis plants from which the leaf axils were excised. A, Leader apex (Position 1) showing the typical deposition of resin that occurs when an apex is not elongating. The white exudate (arrowed) is resin from where leaf axils had been excised. Stem diameter 2 cm. B, Midway between the apex and base (Position 5). Note the periderm formation on the leaf bases, while the tips are still green. The white square (arrowed) is where an axil had been excised. Stem diameter 3·2 cm. C, Base of plant (Position 10) with remains of the leaves arrowed. Stem diameter 4·5 cm.
Materials were fixed in 50 % formalin/acetic acid/alcohol, dehydrated through a graded ethanol series and infiltrated with Leica HistoResin® under a slight vacuum for a minimum of 2 d. The samples were placed in pharmaceutical gelatin capsules containing HistoResin® and polymerized overnight at 60 °C. They were then sectioned at 4 µm using tungsten‐carbide‐tipped steel blades fitted to a motorized retraction microtome. For each pair of axils one was cut in radial longitudinal section (RLS) and the other in transverse section (TS). Sections were stained with 0·5 % toluidine blue and observed under bright field microscopy.
RESULTS
Meristems or bud primordia were found in 53 of the 64 axils sectioned. Given the relatively small size of the blocks excised and the difficulties associated with determining the location of a minute internal structure based on position relative to variable external indicators, it is considered that the 11 meristems were missed, rather than the axil having no meristematic potential. These 11 apparently blank axils were evenly spread between the three plants and were mainly in the older axils where it was more difficult to determine the median plane of the axil and hence the probable meristem location.
The axils at Positions 1 and 2 possessed a similar anatomy to that previously described for W. nobilis (Burrows, 1999). The main features were small meristems (average dimensions 110 µm deep, 190 µm high, 280 µm wide) that were located, on average, 1·6 mm beneath the general stem surface (Fig. 2A and B). Each meristem had an obvious surface layer that abutted a narrow lacuna, while to the rear the meristem terminated at a curved row of cells with thickened primary walls (the shell zone) (Fig. 2A). The meristems had neither leaf primordia nor vascular or provascular connections (Fig. 2A and B).
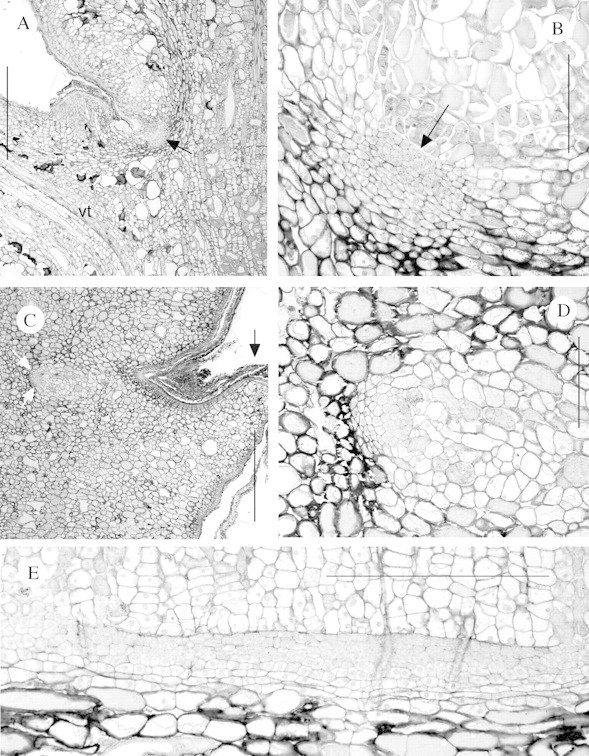
Fig. 2. Axillary meristems of W. nobilis where development into buds or bud primordia had not occurred. A, Longitudinal section of an axil at Position 2. Note the axillary meristem (arrowed) and one of the vascular traces (vt) to the axillant leaf. Bar = 1000 µm. B, Axillary meristem from Position 1 cut in longitudinal section. Note the presence of a lacuna (arrowed) abutting the surface layer of the meristem, the shell zone (the arc of thicker‐walled cells to the rear of the meristem) and the absence of leaf primordia or vascular connections. Bar = 200 µm. C, Longitudinal section of an axil at Position 10. Note the axillary meristem (white arrows). Note also that compared with A the leaf tip (black arrow) is only a small stub and periderm formation is greater. Bar = 1000 µm. D, Detail of C showing the meristem. Bar = 200 µm. E, Transverse section of an axillary meristem at Position 10, with the outside of the stem to the top of the figure. Note that the meristem is tangentially elongated. Bar = 500 µm.
At position 1 the axillary meristems were located several cell layers in from the stem surface (Fig. 2A and B). The presence of a lacuna, small phellogen and its phellem products in the axil were an indication that the meristems had been transferred from an exogenous to an endogenous position, in the same manner as other species in the family (Burrows, 1986, 1987). In the lower positions, where the main periderm had been initiated, the meristems had not been abscissed (Fig. 2C), indicating that they should be long‐lived.
As noted, all Position 1 axils possessed essentially undifferentiated meristems. Undifferentiated meristems were found in a further 17 axils, found in approximately equal numbers in all three plants. While the majority were from distal positions, they were also found at Positions 6, 8 and 10. The meristems found at Positions 2–8 were, on average, 140 µm deep, 215 µm high and 450 µm wide, while the four undifferentiated meristems located at Position 10 were, on average, 95 µm deep, 110 µm high and over 1500 µm wide (Fig. 2C–E). These measurements and infrequently observed division figures indicate that the cells undergo infrequent anticlinal divisions to allow for increase in stem diameter and thus the meristems become increasingly tangentially elongated.
The meristems in the other 30 axils had begun to differentiate into bud primordia, which were associated with various degrees of dedifferentiation of the adjacent cortical cells to form provascular or vascular tissues. While undifferentiated meristems were generally located in the distal positions and the most developed buds in the proximal, in all three plants exceptions were found, e.g. undifferentiated meristems at Position 10 (Fig. 2C–E) and one plant with a well‐developed bud primordium at Position 2. Axil pairs from a single plant could show substantially different stages of development.
Four main stages or types of bud development were observed:
(1) An increase in depth of the axillary meristem and slight development of an apical dome, but no associated leaf primordia or provascular development (Fig. 3A–D).
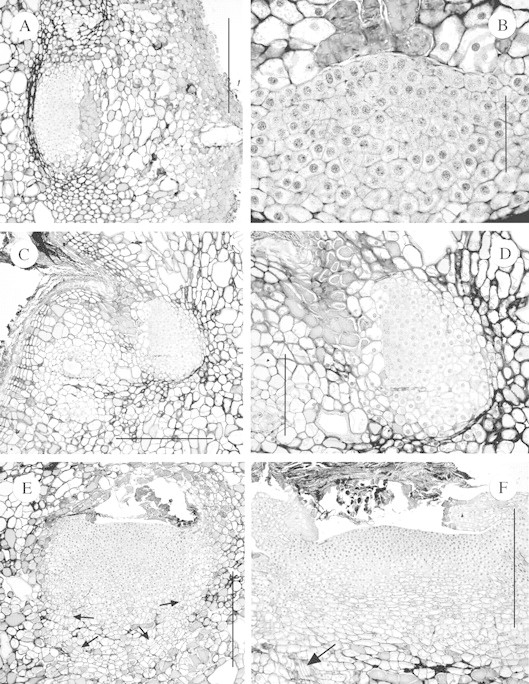
Fig. 3. Sections of axillary meristems of W. nobilis that had commenced development into bud primordia beneath the stem surface. A, Transverse section of a meristem at Position 2 showing increase in meristem size but no vascular or leaf primordia development. Bar = 500 µm. B, Detail of a section close to that shown in A. Bar = 100 µm. C, Similar to A except a longitudinal section of a developing meristem at Position 3. Bar = 500 µm. D, Detail of C. Bar = 200 µm. E, Transverse section of a large meristem at Position 4. Note the development of a procambium (arrowed), a very wide apical ‘dome’ and the absence of leaf primordia. Bar = 500 µm. F, As per E but at Position 10. Note the very wide apical dome, the procambial cells (arrowed) and the somewhat distorted leaf primordia. Bar = 500 µm.
(2) Many primordia had a wide (400–800 µm) and flat apical ‘dome’ with a small number of leaf primordia on the flanks (Fig. 3E and F). This type of bud primordium was wider (average 850 µm) than high (average 510 µm), presumably as a result of developing from a tangentially elongated meristem, within a confined and constricting space.
(3) Buds with an apical dome of typical shape and size, but with no or only a small number of leaf primordia (Fig. 4A–D).
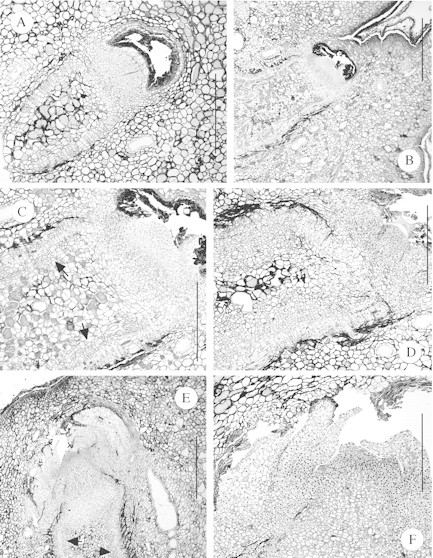
Fig. 4. Axillary meristems of W. nobilis that had commenced development into bud primordia beneath the stem surface. A–D are longitudinal sections, while E and F are transverse sections. A, Bud primordium at Position 8 with well‐developed apical dome and associated provascular tissues, but no leaf primordia. Bar = 500 µm. B, Similar to A but from Position 9 and with greater vascular development, both in extent and degree of differentiation. Bar = 1000 µm. C, Detail of B showing the well‐developed apical dome and secondary xylem (arrowed). Bar = 500 µm. D, Similar to B but with even greater vascular differentiation. Bar = 500 µm. E, Relatively large bud primordium with extensive vascular development (arrowed) (see Fig. 5F) and several distorted leaf primordia. Bar = 1000 µm. F, Detail of a section immediately adjacent to that shown in E, showing detail of the leaf primordia and zonation in the apical dome. Bar = 500 µm.
(4) Buds with numerous but somewhat distorted leaf primordia, forming a relatively typical bud primordium (Figs 4E and F and 5A and B).
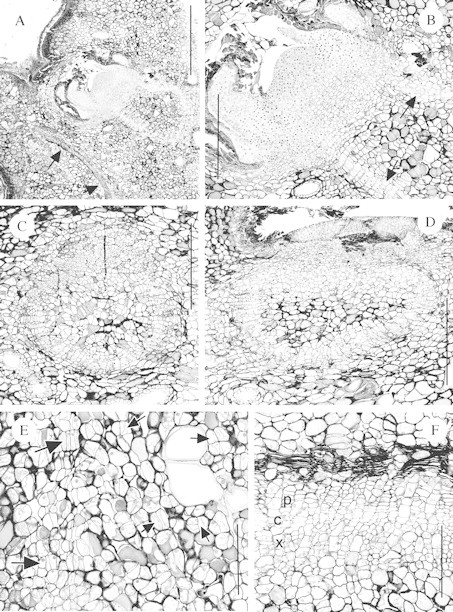
Fig. 5. Sections of endogenous bud primordia and associated vascular development in W. nobilis. A, Longitudinal section of a bud primordium at Position 10. Note the leaf vascular trace (arrowed) and that the provascular tissue of the bud primordium forms a ‘closed‐loop’. Bar = 1000 µm. B, Detail of A showing the apical dome, leaf primordia and provascular tissues (arrowed). Bar = 500 µm. C, Transverse section of the tissues immediately beneath a bud primordium at Position 7. The outside of the stem is to the top of the photo. Note that the procambium forms almost a complete circle. Bar = 500 µm. D, Similar to C except the axil is at Position 9 and the procambium forms an ellipse. Bar = 500 µm. E, Similar to C except a longitudinal section of an axil at Position 4 and vascular development is less advanced. Note the initial procambial divisions (large arrows) and other divisions leading to the dedifferentiation of the cortical parenchyma (small arrows). Bar = 200 µm. F, Detail of the vascular tissue of the bud primordium shown in Fig. 4E. Note the presence of the xylem (x), cambium (c) and phloem (p). Bar = 200 µm.
Note that even the largest buds (Figs 4E and 5A) were entirely beneath the bark and had presumably taken several months or years to reach this relatively limited stage of development. The meristems or primordia were located between 0·6 and 2·2 mm (average 1·3 mm) below the general level of the stem surface. There was no statistical difference (Student’s t‐test, P = 0·05) in the depth of the meristems and/or primordia in Positions 1–5 and Positions 6–10.
Vascular or provascular development was generally further advanced than bud primordium development, as several bud primordia with no or only a few leaf primordia had associated vascular development (Fig. 4A), which was relatively extensive in some axils (Figs 4B–D and 5F). Buds were never found without provascular or vascular tissues (e.g. Figs 4E and 5A). The vascular development usually formed a ‘closed‐loop’ or cup‐shaped structure to the rear and base of the bud primordium. The vascular connections were ‘U‐shaped’ in RLS (Figs 4A and 5A) and circular or elliptical in TS (Fig. 5C and D). In most axils only procambial development was present (e.g. Figs 3E and 5B–E), with secondary xylem and phloem observed in only a few axils (Figs 4C and D and 5F). Dedifferentiation of the cortical cells to form the procambium could be observed, with thin, newly formed walls within existing cells (Fig. 5E). Where relatively extensive secondary xylem formation had occurred it appeared that this resulted in the crushing of some cells, perhaps the secondary phloem (Fig. 5F).
In one axil at Position 10 a dead bud primordium was found. The bud had produced numerous leaf primordia but the tips of these only just protruded into the base of the leaf axil and thus externally it was impossible to tell that a bud had formed.
DISCUSSION
The main findings of this study are that: (1) W. nobilis has long‐lived, bud‐forming potential in the leaf axils of the orthotropic main stem; and (2) many of the axillary meristems slowly develop into bud primordia beneath the stem surface. These axillary meristems or buds are responsible for the formation of orthotropic secondary leaders. These secondary leaders and their branches replace the leaves lost when the primary branches are abscissed.
Resprouting in conifers
Conifers are generally considered to have limited or non‐existent powers of vegetative regeneration (Bellingham and Sparrow, 2000; Bond and Midgley, 2001; Del Tredici, 2001). For example, Jeník (1994) notes that the formation of epicormic buds in gymnosperms is rare, while natural root sprouting or suckering appears unknown or very rare among gymnosperms (Groff and Kaplan, 1988; Jeník, 1994; Peterson and Jones, 1997). Araucaria and Agathis provide several exceptions to these generalizations with various species able to form root shoots (Araucaria cunninghamii, Burrows, 1990a; Araucaria araucana, Burns, 1993; Veblen 1982; Agathis robusta, Haley, 1957), coppice and epicormic shoots from the stem (Araucaria cunninghamii, Burrows, 1990b) and coppice shoots after severe injury (Araucaria humboldtensis, Veillon, 1978). Other southern conifers, from a range of families, also provide a wide range of exceptions (Enright and Hill, 1995). However, most of these exceptions occur only after traumatic damage and only a few species of trees will produce secondary trunks when undisturbed (Bellingham and Sparrow, 2000; Del Tredici, 2001).
Secondary branches in the Araucariaceae
In many species of Araucaria there is a form of reiteration that results in the formation of successive nesting crowns (Veillon, 1978). For example, in Araucaria bidwillii the first order branches are long‐lived and may reach over 7 m in length (G. E. Burrows, unpubl. obs.). These branches are eventually lost by self‐pruning but new branches may develop at the site where the original branches had broken off. It appears that the bases of these long‐lived branches are overgrown by the main stem and thus some plagiotropic axillary meristems could become buried in the orthotropic stem. New first order branches could then develop from the outermost of these partially overgrown plagiotropic meristems.
The primary branches of W. nobilis are relatively short‐lived, unbranched and shed as a single unit (Hill, 1997). If these branches were not replaced adult trees would have the appearance of a tall pole with the foliage at the apex. The primary branches are sylleptic, while the other main stem axils possess proleptic, endogenous, orthotropic buds or meristems. Thus, to replace the primary branches on the lower trunk the orthotropic meristems must be activated, leading to the formation of secondary leaders and a complete reiteration of the tree’s architecture. As the need to replace branches is an on‐going process and is usually a non‐traumatic event, perhaps this promotes the slow development of some axillary meristems into buds and bud primordia. While apical dominance is usually associated with the terminal bud, perhaps the abscission of primary branches also alters hormone levels at a localized level in the trunk, which leads to some of the buried bud primordia beginning rapid growth.
Agathis australis possesses self‐pruning branches (Wilson et al., 1998). This capability persists for the life of the tree and occurs in all branch types. Abscission zone diameter was significantly greater than mid‐point branch diameter in all branch types, with abscission in some branch types resulting in smooth separation surfaces. Initial observations are that all branches of W. nobilis have enlarged abscission zones with smooth separation faces (G. E. Burrows, unpubl. obs.). This is another indication that the shedding of the short‐lived branches followed by their replacement by branches borne on orthotropic epicormic shoots is a fundamental component of the species growth.
Axillary meristems in large diameter stems
The anatomy of araucarian axillary meristems has been examined in 14 species of Araucaria (Fink, 1983; Burrows, 1986, 1987), six species of Agathis (Burrows, 1987) and W. nobilis (Burrows, 1999), but usually only from axils near the shoot apex. Prior to the present study the structure of meristems in larger diameter stems had only been described in two species (Araucaria angustifolia and Araucaria cunninghamii) and details of these are given below.
For Araucaria angustifolia Fink (1983) presented photomicrographs of axillary meristems from near the shoot apex of a tree of unspecified age and from 15‐ and 30‐year‐old trees, although location on these trees was not given. The meristems in the shoot tip were about 50 µm deep by 50 µm high (Fink, 1983, figs 29–31), with the width not shown or specified. In the 15‐year‐old tree the meristems were approx. 100 µm deep, 170 µm high and at least 1900 µm wide (Fink, 1983, figs 32 and 33). Fink (1983) noted that the meristems stretch tangentially as stem diameter increases but normally do not differentiate further, i.e. no leaf primordia or vascular connections. He also noted that the meristems could develop into buds if a tree is defoliated but are eventually cut off by rhytidome formation.
Burrows (1990b) measured and illustrated the radial, vertical and tangential dimensions of the axillary meristems of Araucaria cunninghamii at the base of 2‐month‐old, 2‐ and 4–5‐year‐old plants, with basal diameters of 1·5, 10 and 50 mm, respectively. Small increases were found in the radial and vertical dimensions with the increase in age and diameter and a large increase in tangential extent as the meristem cells divide anticlinally to compensate for girth increases. Like Fink (1983), Burrows (1989) found that the meristems usually did not differentiate further but could develop into buds upon release from apical dominance (Burrows, 1989, 1990b). Unlike Fink (1983), Burrows (1990b) presented evidence that the axillary meristems of hoop pine were probably never abscissed by periderm formation.
In Araucaria angustifolia (Fink, 1983), Araucaria cunninghamii (Burrows, 1990b) and also in Agathis robusta and Araucaria bidwillii (G. E. Burrows, unpub. res.) the axillary meristems remain indefinitely in an undifferentiated state, unless the tree is damaged. In contrast, in W. nobilis many of the axillary meristems slowly develop into buds or bud primordia beneath the bark. It seems reasonable to assume that these slowly developing buds are more likely to develop into epicormic shoots in intact plants than the quiescent axillary meristems observed in other araucarian species.
Coppice shoots in Wollemi pine
It is possible that the meristems and buds in the lowermost axillary positions may be the progenitors of the coppice shoots of W. nobilis. As the trees are located near the base of deep, narrow canyons it is possible that their bases might be covered by soils washed down from above and also by the distinctive and dense litter layer (Hill, 1997; Offord and Meagher, 2001). This partial burial may stimulate the bud primordia to develop into coppice shoots. However, recent observations (C. A. Offord and P. F. Meagher, unpubl. obs.) of ex situ grown plants indicate that the coppice shoots probably originate from adventitious buds developing in the hypocotyl region.
Vascular development
In terms of vascular development the axillary meristems of the Araucariaceae have some characteristics of adventitious buds. When the axillary buds of most species form near the shoot apex, their vascular system is integrated with that of the main shoot and the resulting branches or dormant buds have an established connection (the bud trace) to the central vascular tissues. In contrast, araucarian axillary meristems must establish a vascular connection through dedifferentiation of cortical tissues (Burrows, 1989), as do exogenous adventitious buds. The cup‐shaped vascular systems of W. nobilis (Figs 4A and 5A) are an unusual development as the vascular development does not extend across to the secondary vascular tissues. Interestingly, a similar development occurs within the epicormic strands of the eucalypts (Burrows, 2000, fig. 11; Burrows, 2002, figs 8b and 10a), where the meristem strips share some structural similarities of araucarian axillary meristems. Cup‐shaped vascular systems are also associated with the inhibited buds of Taxus baccata and Sequoiadendron giganteum (Fink, 1984, figs 6 and 19).
Bud shape and number
As noted, many of the developing buds were asymmetrical (wider than high), presumably because the buds develop from meristems that are wider than high. Likewise the bud primordia initially may develop very wide (400–800 µm), flat ‘faces’ or ‘domes’ (Fig. 3E and F) as they develop from tangentially elongated meristems. By comparison the width or diameter of apical domes of typical buds from the leaders or branches of the Araucariaceae have been recorded as between 200 and 400 µm across, when measured between the youngest leaf primordia (Sterling, 1958; Burrows, 1985). In W. nobilis a meristem that may be 1000 µm wide (e.g. Fig. 2E) probably forms a single, somewhat distorted, bud. In contrast, in the eucalypts, from similar elongated meristems several small buds could be formed (Cremer, 1972, fig. 22; Burrows, 2000, fig. 20). Burrows (1990b) hypothesized that once an araucarian axillary meristem was several thousand microns in tangential extent it may initiate several buds and this may explain why several buds may develop in close proximity at the base of decapitated hoop pines.
Fire protection
The most deeply buried meristems of W. nobilis were only 2·2 mm (average 1·3 mm) below the surface of stems with a diameter of 5 cm. Jones et al. (1995) indicated that the bark of W. nobilis forms a layer up to 2 cm deep. In the context of the taxonomic description it appears that ‘bark’ refers to the phellem, not all tissues external to the cambium. While a 2‐cm phellem thickness should offer good thermal protection to the inner tissues, W. nobilis possesses a ‘bubble bark’ with some of the nodules or tubercles up to 1·5 cm in length (Jones et al., 1995; Burrows and Bullock, 1999). While Wollemia trees have survived fires (National Parks and Wildlife Service, 1998; Offord et al., 1999) it appears that the meristems probably remain relatively close to the stem surface and may be killed by the heat of a fire, even if the vascular cambium and the tree survives. Various eucalypt species are found on the fire‐prone ridges surrounding the canyons where the Wollemi Pine has been located. In contrast to Wollemia the epicormic meristems of the eucalypts are best developed in the inner bark. This provides excellent insulation for the meristems and results in a pronounced ability to form epicormic shoots after fire (Burrows, 2002). In Araucaria araucana the bark is 10–15 cm thick (Veblen, 1982) and epicormic shoots may be produced after fire (Burns, 1993). There is evidence that trunks of W. nobilis have been killed by fire (C. A. Offord, unpubl. obs.) although the trees persist, through the growth of coppice stems. This, in part, accounts for the survival of this relictual species.
Concluding comments
This study was largely based on ex situ shade‐house‐grown 2‐m‐high seed‐derived plants. Information from this material has been used to interpret the growth and form of in situ adult trees. Some caution should be used with the extrapolations that have been made but they are currently our best way of understanding this species, given that excision of buds and meristems from the trunks of the in situ trees will never be permitted.
ACKNOWLEDGEMENTS
We thank Jenni Horsnell for assistance in constructing the digital photographic plates and acknowledge the financial support of an Australian Research Council Small Grant.
Supplementary Material
Received: 6 June 2003; Returned for revision: 5 August 2003; Accepted: 10 September 2003
References
- BellinghamPJ, Sparrow AD.2000. Resprouting as a life history strategy in woody plant communities. Oikos 89: 409–416. [Google Scholar]
- BondWJ, Midgley JJ.2001. Ecology of sprouting in woody plants: the persistence niche. Trends in Ecology and Evolution 16: 45–51. [DOI] [PubMed] [Google Scholar]
- BurnsBR.1993. Fire‐induced dynamics of Araucaria araucana–Nothofagus antarctica forest in the southern Andes. Journal of Biogeography 20: 669–685. [Google Scholar]
- BurrowsGE.1985.Axillary meristem ontogeny in the Araucariaceae. PhD Thesis, University of Queensland, Australia. [Google Scholar]
- BurrowsGE.1986. Axillary meristem ontogeny in Araucaria cunninghamii Aiton ex D. Don. Australian Journal of Botany 34: 357–375. [Google Scholar]
- BurrowsGE.1987. Leaf axil anatomy in the Araucariaceae. Australian Journal of Botany 35: 631–640. [Google Scholar]
- BurrowsGE.1989. Developmental anatomy of axillary meristems of Araucaria cunninghamii released from apical dominance following shoot apex decapitation in vitro and in vivo Botanical Gazette 150: 369–377. [Google Scholar]
- BurrowsGE.1990a. Anatomical aspects of root bud development in hoop pine (Araucaria cunninghamii). Australian Journal of Botany 38: 73–78. [Google Scholar]
- BurrowsGE.1990b. The role of axillary meristems in coppice and epicormic bud initiation in Araucaria cunninghamii. Botanical Gazette 151: 293–301. [Google Scholar]
- BurrowsGE.1991. A scanning and transmission electron microscope study of leaf axil structure in Araucaria cunninghamii. Australian Journal of Botany 39: 67–76. [Google Scholar]
- BurrowsGE.1999. Wollemi pine (Wollemia nobilis, Araucariaceae) possesses the same unusual leaf axil anatomy as the other investigated members of the family. Australian Journal of Botany 47: 61–68. [Google Scholar]
- BurrowsGE.2000. An anatomical study of epicormic bud strand structure in Eucalyptus cladocalyx (Myrtaceae). Australian Journal of Botany 48: 233–245. [Google Scholar]
- BurrowsGE.2002. Epicormic strand structure in Angophora, Eucalyptus and Lophostemon (Myrtaceae) – implications for fire resistance and recovery. New Phytologist 153: 111–131. [Google Scholar]
- BurrowsGE, Bullock S.1999. Leaf anatomy of Wollemi pine (Wollemia nobilis, Araucariaceae). Australian Journal of Botany 47: 795–806. [Google Scholar]
- BurrowsGE, Doley DD, Haines RJ, Nikles DG.1988.In vitro propagation of Araucaria cunninghamii and other species of the Araucariaceae via axillary meristems. Australian Journal of Botany 36: 665–676. [Google Scholar]
- CremerKW.1972. Morphology and development of the primary and accessory buds of Eucalyptus regnans. Australian Journal of Botany 20: 175–195. [Google Scholar]
- Del TrediciP.2001. Sprouting in temperate trees: a morphological and ecological review. Botanical Review 67: 121–140. [Google Scholar]
- EnrightNJ, Hill RS (eds).1995.Ecology of the southern conifers. Melbourne: Melbourne University Press. [Google Scholar]
- FinkS.1980. Anatomische untersuchungen über das vorkommen von spross‐ und wurzelanlagen im stammbereich von laub‐ und nadelbäumen. 1. Proventive anlagen. Allgemeine Forst‐ und Jagdzeitung 151: 160–180. [Google Scholar]
- FinkS.1983. The occurrence of adventitious and preventitious buds within the bark of some temperate and tropical trees. American Journal of Botany 70: 532–542. [Google Scholar]
- FinkS.1984. Some cases of delayed or induced development of axillary buds from persisting detached meristems in conifers. American Journal of Botany 71: 44–51. [Google Scholar]
- GroffPA, Kaplan DR.1988. The relation of root systems to shoot systems in vascular plants. Botanical Review 54: 387–422. [Google Scholar]
- HaleyC.1957. The present status of tree breeding work in Queensland. Seventh British Commonwealth Forestry Conference, Australia and New Zealand. Department of Forestry, Brisbane, Australia. [Google Scholar]
- HillKD.1997. Architecture of the Wollemi pine (Wollemia nobilis, Araucariaceae), a unique combination of model and reiteration. Australian Journal of Botany 45: 817–826. [Google Scholar]
- JeníkJ.1994. Clonal growth in woody plants: a review. Folia Geobotanica and Phytotaxonomica 29: 291–306. [Google Scholar]
- JonesWG, Hill KD, Allen JM.1995.Wollemia nobilis, a new living Australian genus and species in the Araucariaceae. Telopea 6: 173–176. [Google Scholar]
- National Parks and Wildlife Service.1998.Wollemi pine recovery plan NSW National Parks and Wildlife Service, Sydney (http://www.nationalparks.nsw.gov.au/PDFs/wollemi.pdf). [Google Scholar]
- OffordCA, Meagher PF.2001. Effects of temperature, light and stratification on seed germination of Wollemi pine (Wollemia nobilis, Araucariaceae). Australian Journal of Botany 49: 699–704. [Google Scholar]
- OffordCA, Porter CL, Meagher PF, Errington G.1999. Sexual reproduction and early plant growth of the Wollemi pine (Wollemia nobilis), a rare and threatened Australian conifer. Annals of Botany 84: 1–9. [Google Scholar]
- PetersonCJ, Jones RH 1997. Clonality in woody plants: a review and comparison with clonal herbs. In: de Kroon H, van Groenendael J, eds. The ecology and evolution of clonal plants Leiden: Backhuys Publishers, 263–289. [Google Scholar]
- SterlingC.1958. Dormant apical bud of Agathis lanceolata Botanical Gazette 120: 49–53. [Google Scholar]
- VeblenTT.1982. Regeneration patterns in Araucaria araucana forests in Chile. Journal of Biogeography 9: 11–28. [Google Scholar]
- VeillonJ‐M.1978. Architecture of the New Caledonian species of Araucaria In: Tomlinson PB, Zimmerman MH, eds. Tropical trees as living systems Cambridge: Cambridge University Press, 233–245. [Google Scholar]
- VeillonJ‐M.1980. Architecture des espèces néo‐calédoniennes du genre Araucaria Candollea 35: 609–640. [Google Scholar]
- WilsonVR, Gould KS, Lovell PH, Aitken‐Christie, J.1998. Branch morphology and abscission in kauri, Agathis australis (Araucariaceae). New Zealand Journal of Botany 36: 135–140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


