Abstract
Nuclear DNA contents for 104 Macaronesian angiosperms, with particular attention on Canary Islands endemics, were analysed using propidium iodide flow cytometry. Prime estimates for more than one‐sixth of the whole Canarian endemic flora (including representatives of 11 endemic genera) were obtained. The resulting 1C DNA values ranged from 0·19 to 7·21 pg for Descurainia bourgeauana and Argyranthemum frutescens, respectively (about 38‐fold difference). The majority of species, however, possessed (very) small genomes, with C‐values <1·6 pg. The tendency towards small nuclear DNA contents and genome sizes was confirmed by comparing average values for Macaronesian and non‐Macaronesian representatives of individual families, genera and major phylogenetic lineages. Our data support the hypothesis that the insular selection pressures in Macaronesia favour small C‐values and genome sizes. Both positive and negative correlations between infrageneric nuclear DNA amount variation and environmental conditions on Tenerife were also found in several genera.
Key words: C‐value, Canary Islands, endemic, flow cytometry, genome size, nuclear DNA content, Macaronesia
INTRODUCTION
Oceanic islands play an important role in maintaining global biodiversity. It has been estimated that they harbour approximately one‐sixth of vascular plant species, a large proportion of which is endangered (World Conservation Monitoring Centre, 1992). The Macaronesian phytogeographic region encompasses five assemblages of volcanic archipelagos in the eastern Atlantic Ocean (the Azores, Madeira, the Salvage Islands, the Canary Islands and the Cape Verde Islands), situated between 15 and 40°N. The flora of this region is diverse and complex. The strongest phytogeographic connections have been proposed to be with the Mediterranean basin and north‐western Africa; apparent links with south‐eastern Africa and neotropical regions can also be traced (Bramwell, 1986; Panero et al., 1999). A distinct feature of indigenous plant species is a high proportion of woody life‐forms in otherwise herbaceous groups, Echium and Sonchus being two prominent examples. Analysis of growth‐form composition also reveals a high percentage of succulents and a low abundance of geophytes and annuals (Shmida and Werger, 1992). The Macaronesian flora has traditionally been suggested to represent the relictual fragment of a subtropical Tertiary plant biota once widespread in Europe and northern Africa (Bramwell, 1976). However, modern phylogenetic analyses of several Macaronesian plant groups (Aeonium, Argyranthemum, Bencomia, Echium) have not provided support for a relict origin, and indicated relatively recent insular diversification from continental ancestors (Emerson, 2002).
Oceanic islands typically show a high degree of endemicity. Macaronesia harbours at least 30 endemic genera (Bramwell, 1976; Kunkel, 1993) and the proportion of Macaronesian endemic species for individual islands has been estimated at between 4 % (Graciosa in the Azores) and 27 % (La Palma in the Canary Islands) (Hobohm, 2000). The main archipelago, the Canary Islands, is by far the richest area, with about 570 endemic species (about 40 % of native plants) (Francisco‐Ortega et al., 2000). Such a high number of endemics could be attributed to the great diversity of habitats on the Canaries where six major ecological zones have developed (Fernandopullé, 1976). A broad range of geological ages (between 0·8 and 21 million years) (Carracedo, 1994), a varying degree of substrate erosion and the proximity of the African continent may also contribute to the species richness. Adaptive radiation (divergent evolution in response to different ecological pressures) and vicariance (divergence due to geographic isolation) seem to be the main processes favouring the diversification of ancestral plant types into several related taxa (Crawford et al., 1987).
The flora of Macaronesia, and of the Canary Islands in particular, has been subjected to numerous karyological studies (e.g. Larsen, 1963; Borgen 1969; Bramwell et al., 1976; Dalgaard, 1991). A very low incidence of polyploidy was repeatedly confirmed. On the basis of 453 species (304 Macaronesian endemics and 149 non‐endemics) described cytologically, Borgen (1974) concluded that only 26·6 % of endemics and 27·8 % of the total flora are polyploids. These percentages may alter to a certain extent as some species traditionally regarded as diploids (e.g. Canarina, Convolvulus, Crambe, Micromeria) might actually be polyploid in their origin. Nevertheless, the frequency of polyploid species, at least amongst the endemic elements, would remain strikingly low.
The extensive karyological literature contrasts with the almost complete lack of information about nuclear DNA amounts of Macaronesian plants. Hitherto, only 12 estimates of C‐value (the DNA amount in the unreplicated haploid nucleus irrespective of the ploidy level of the taxon) ranging from 0·54 pg in Aeonium haworthii to 8·63 pg in Ranunculus cortusifolius have been completed (Cerbah et al., 1999; Bennett and Leitch, 2001; Hanson et al., 2001; Ellul et al., 2002). Nuclear DNA amount is undoubtedly a key character, with many uses in various biological fields. It has been employed as an effective tool for distinguishing taxa in several groups of vascular plants, e.g. in Petunia (Mishiba et al., 2000) and Helleborus (Zonneveld, 2001). Moreover, an analysis of genome size can even reveal new taxa that have so far been neglected (Greilhuber and Speta, 1985). There is increasing demand for C‐values as a phylogenetic markers in evolutionary studies (Leitch et al., 1998). Correlations between nuclear DNA amount and plant phenology (Grime and Mowforth, 1982), life history (Bennett, 1972) or sensitivity to frost (MacGillivray and Grime, 1995) indicate the utilization of C‐values as ecological indicators. Although such information was based on 3500+ estimates of angiosperm C‐values, further targeted work is essential for better representation of taxonomic groups, geographic regions and plant life forms (Bennett et al., 2000). The principal goal of current work was to improve our knowledge of nuclear DNA amount from a geographic point of view. Macaronesia was chosen as an appropriate region because of the high level of endemism that enabled the comparative study of patterns of nuclear DNA amount variation in a phytogeographically distinct area. Moreover, a conspicuous concentration of plant biota at risk is to be found there; it has been estimated that 41 % of Canarian endemic flora is endangered (I.U.C.N., 1983). Therefore, there is an urgent need for various biological data applicable to conservation biology (Bennett et al., 2000).
MATERIALS AND METHODS
Plant material
Seeds of most species were collected on the Canary Islands during 2000–2001 (Table 1). The Botanical Garden in Berlin provided seeds of an additional eight species, most of which were also originally sampled in the field. Seeds were germinated on wet filter papers in Petri dishes, and plants were cultivated under the same conditions in a glasshouse at the Experimental Garden of the Institute of Botany in Průhonice, Prague (50°00′N, 14°30′E). Vouchers are kept in the private herbarium of the first author. The great majority of species is currently being grown at the Botanical Garden of the Charles University, Prague.
Table 1.
List of localities of Macaronesian endemics used in the present study
| No. | Locality | Coordinates | Altitude (m) |
| 1 | Tenerife: Cañadas del Teide, rock crevices in Valle de Chiñoque, north of El Sanatorio | 28°14′ 20′′N 16°36′10′′W | 2180 |
| 2 | Tenerife: Cañadas del Teide, rock crevices and volcanic sands east‐south‐east of Parador Nacional de las Cañadas hotel | 28°13′20′′N 16°37′10′′W | 2180 |
| 3 | Tenerife: Cañadas del Teide, rock crevices along hiking trail west of Colmenar hill | 28°15′50′′N 16°33′10′′W | 2060 |
| 4 | Tenerife: Cañadas del Teide, El Portillo, volcanic sands along hiking trail near Montaña de las Arenas Negras hill | 28°17′40′′N 16°33′40′′W | 2000 |
| 5 | Tenerife: Cañadas del Teide, Riscos de la Fortalesa, rock crevices at the top of the mountain | 28°18′50′′N 16°35′50′′W | ∼2150 |
| 6 | Tenerife: Pinus canariensis forest along the road approx. 1 km south of Miradores de la Cumbre, near Montaña Avosa hill | 28°21′50′′N 16°28′00′′W | 1970 |
| 7 | Tenerife: Margarita de Piedra, rock crevices in Barranco de la Zarza | 28°20′30′′N 16°31′30′′W | 1420 |
| 8 | Tenerife: Aquamansa, Fayal‐brezal community along the road approx. 1 km south‐west of the village | 28°21′30′′N 16°30′30′′W | 1160 |
| 9 | Tenerife: Los Pinos, wall next to road in the village | 28°22′50′′N 16°30′50′′W | 520 |
| 10 | Tenerife: Anaga, Casa Carlos, rock crevices in laurel forest along the road near Pico del Ingles hill | 28°31′50′′N 16°15′30′′W | 940 |
| 11 | Tenerife: Anaga, Lomo de Las Bodegas village, laurel forest near Chinobre hill | 28°33′20′′N 16°10′50′′W | 770 |
| 12 | Tenerife: Anaga, Chamorga village, rock crevices in Barranco de Roque Bermejo and laurel forest fragments north‐east of the village, near Montaña Tafada hill | 28°34′N 16°09′W | 0–600 |
| 13 | Tenerife: Anaga, Taganana village, rock crevices in Roque de Enmedio | 28°34′00′′N 16°12′40′′W | 330 |
| 14 | Tenerife: Valle de Güímar, succulent community (cardonal) north of Puerto de Güímar | 28°18′00′′N 16°22′20′′W | 30 |
| 15 | Tenerife: Güímar, Pájara village, rock crevices in Ladera de Güímar | 28°17′30′′N 16°25′30′′W | 840 |
| 16 | Tenerife: Teno, Masca village, rock crevices in Barranco de Masca | 28°17′30′′N 16°51′W | 0–600 |
| 17 | Tenerife: Teno, Las Portelas village, rock crevices along the road near La Tabaiba | 28°19′30′′N 16°51′10′′W | ∼650 |
| 18 | Tenerife: Teno, rock crevices in Roque El Fraile | 28°21′40′′N 16°53′40′′W | 220 |
| 19 | Tenerife: Teno, succulent community (cardonal) near Punta de Teno | 28°20′40′′N 16°55′00′′W | 40 |
| 20 | Tenerife: Vilaflor, Pinus canariensis forest along the road approx. 5 km north of the town | 28°10′40′′N 16°38′40′′W | 1880 |
| 21 | Tenerife: Frontón de San Miguel, succulent community (tabaibal) in valley near the village | 28°06′40′′N 16°36′50′′W | 730 |
| 22 | Tenerife: Adeje, rock crevices in Barranco del Infierno | 28°08′10′′N 16°42′30′′W | 490 |
| 23 | La Palma: Caldera de Taburiente, rock crevices near Mirador de los Andenes | 28°45′40′′N 17°52′00′′W | 2330 |
| 24 | La Palma: forest along the road approx. 1 km north‐west of Tagoja hill (north‐west of Santa Cruz de la Palma city) | 28°43′20′′N 17°47′10′′W | 1110 |
| 25 | La Palma: Caldera de Taburiente, sandy areas approx. 1·5 km north‐north‐west of the Centro de Visitantes | 28°40′00′′N 17°51′20′′W | 950 |
| 26 | La Palma: Caldera de Taburiente, sandy areas along the road approx. 0·5 km south‐east of the Mirador de la Cumbrecita | 28°41′40′′N 17°51′10′′W | 1210 |
| 27 | La Palma: Jedey village, sandy areas along the road SSE of the village | 28°34′50′′N 17°52′40′′W | 700 |
| 28 | La Palma: Caldera de Taburiente, leg. Hanneken (Bot. Garden Berlin) | – | – |
| 29 | Gran Canaria: Ayacata, basalt, leg. Royl (Bot. Garden Berlin) | – | 1330 |
| 30 | Gran Canaria: Moya, Barranco del Laurel, leg. Royl (Bot. Garden Berlin) | – | 600 |
| 31 | Gran Canaria: Montana de Arucas, north‐coast, leg. Royl (Bot. Garden Berlin) | – | 400 |
| 32 | Gran Canaria: Maspalomas, silicate, leg. Royl (Bot. Garden Berlin) | – | – |
| 33 | Fuerteventura (Bot. Garden Berlin, orig. seeds from Bot. Garden Erlangen) | – | – |
| 34 | Lanzarote: Famara, basalt, leg. Royl (Bot. Garden Berlin) | – | 300 |
In total, 104 Macaronesian angiosperms with various distribution patterns were analysed; seven of these were endemic to Macaronesia, 56 endemic to the Canary Islands, 32 endemic to Tenerife, six endemic to La Palma, and the endemics of Lanzarote, Fuerteventura and Gran Canaria were represented by one species. Seven genera (Allago pappus, Dicheranthus, Gesnouinia, Lugoa, Plocama, Tinguarra and Todaroa) were endemic to the Canary Islands, and Argyranthemum, Isoplexis, Pericallis and Schizogyne are the Macaronesian genera‐endemics. The plants were classified into 20 families and 14 orders according to current phylogenetic views (Stevens, 2002), and all but three (Asparagus umbellatus, Dactylis smithii, Dracunculus canariensis) belong to the Eudicots. The vast majority of species was perennial; Senecio teneriffae and Volutaria canariensis being the only two exceptions. Chamaephytes clearly prevailed from a life‐form point of view [the terminology of life‐forms follows the categories adopted in the World Checklist and Bibliography Series database at Royal Botanic Gardens, Kew, UK (www.rbgkew.org.uk/wcb/index.html)]. Considering the ecological profile of the present species set, representatives of five major zones covering an altitudinal range of more than 2300 m were included.
Flow cytometry
To reveal potential intrapopulation variability in nuclear DNA amount, three to six plants from each species were measured simultaneously in the first step. As one narrow peak with unimodal distribution was always obtained, one individual per species was randomly selected and analysed further. Each plant was measured at least three times on different days by the same operator, and a different leaf was used for each analysis.
Nuclear samples were prepared from a young fresh intact leaf. Approximately 50 mm2 of leaf tissue was co‐chopped with an appropriate volume of internal standard in 1 ml of ice‐cold Otto I Buffer (0·1 m citric acid, 0·5 % Tween 20) using a new razor blade (Otto, 1990). The following internal standards were employed: Raphanus sativus L. ‘Saxa’ (2C = 1·11 pg), Lycopersicum esculentum Mill. ‘Stupnické polní tyčkové rané’ (2C = 1·96 pg), Glycine max (L.) Merrill ‘Polanka’ (2C = 2·5 pg), Zea mays L. ‘CE‐777’ (2C = 5·43 pg) and Pisum sativum L. ‘Ctirad’ (2C = 9·09 pg) (Dolez̆el et al., 1992, 1994; Lysák and Dolez̆el, 1998). Selection of a suitable standard followed these criteria: (1) the smallest ratio between the 2C‐values of an analysed plant and the internal standard to minimize the potential non‐linearity of flow‐cytometer measurements; (2) at least 12 % difference in 2C‐values of the internal standard and sample to exclude bias due to very close or overlapping peaks; and (3) the same internal standard was used for all species belonging to one genus. The crude suspension was filtered through a 42‐µm nylon filter and centrifuged at 150 g for 5 min. The pellet was resuspended in 100 µl fresh Otto I buffer and samples were incubated for 30 min at room temperature. Subsequently, 1 ml of staining solution Otto II (0·4 m Na2HPO4.12H2O) supplemented with propidium iodide and RNAse (both at 50 µg ml–1) was added and, after incubation for 30–45 min at room temperature, fluorescence intensity of isolated nuclei was measured using a Partec PA II flow cytometer (Partec GmbH, Germany) equipped with an argon ion laser (488 nm). The flow rate did not exceed 50 fluorescent events per second in a huge majority of species, and the fluorescence of at least 5000 particles was recorded. Two histograms for each sample were recorded and only when both peaks were symmetrical and of approximately equal height were analyses taken into account when calculating the C‐value of an analysed plant. When converting picogram values to base pairs, 980 megabase pairs (Mbp) were assumed to be equivalent to 1 picogram of DNA (Bennett et al., 2000).
Chromosome counts
Observations were made on root tip cells of germinated seedlings. Samples were pre‐treated with a saturated solution of p‐dichlorbenzene or 1‐monobromnaphtalene for 3 h at room temperature, fixed in 3 : 1 ethanol : acetic acid overnight at 4 °C and kept in 70 % ethanol at the same temperature. After maceration in 1 : 1 hydrochloric acid : ethanol for 60 s, the root tip cuttings were squashed in lacto‐propionic orceine. As a rule, at least three mitoses per plant and two individuals per species were counted.
Statistical analyses
DNA amount data were analysed with the SAS 8.1 statistical package using ANOVA, CORR, GLM and UNIVARIATE procedures (SAS Institute, Cary, NC, USA). Differences in DNA content between species within a genus were tested by ANOVA, and Tukey’s procedure was applied to compare mean values. Differences in C‐values between major angiosperm lineages of Macaronesian plants were analysed using the GLM procedure because of an unbalanced design. Differences in C‐values and genome sizes between Macaronesian vs. non‐Macaronesian representatives were tested using the signed rank test on paired data to avoid problems of data non‐linearity. The Spearman‐rank correlation coefficient was employed in testing whether infrageneric DNA amount variation (mean values for individual species) correlated with the altitude and environmental characteristics (climatic data were taken from Fernandopullé, 1976).
RESULTS
Chromosome counts
Table 2 gives a chromosome count or an estimated number for 35 Macaronesian angiosperms; eight of these represent new species records. Special attention was paid to taxa from the supracanarian zone (generally above 2000 m altitude). The chromosome counts for 63 other taxa were taken from the literature, and the number of chromosomes for six species is apparently unknown.
Table 2.
2C nuclear DNA content with standard error, 1C nuclear DNA content in picograms and megabase pairs (1 pg = 980 Mbp), chromosome number (2n), ploidy level, unreplicated genome size, life‐form, internal standard used, coefficients of variance of internal standard and the sample, species distribution patterns and original locality for 104 Macaronesian species from 20 families
| Taxon | Family | 2C DNA amount ± s.e. (pg) | 1C DNA amount (pg)¶ | 1C DNA amount (Mbp) | 2n | Ploidy level (x)† | Genome size (2C DNA amount/ploidy level) (pg)† | Life form** | Internal standard‡ | CV of internal standard (%) | CV of sample (%) | Distribution pattern§ | Locality |
| Allagopappus dichotomus (L. fil.) Cass. | Asteraceae | 1·75 ± 0·01 | 0·88 | 862 | 20 | 2 | 0·88 | C | G | 2·60–3·57 | 3·71–4·88 | C | 14 |
| Argyranthemum adauctum (Link) Humphr. ssp. adauctum | Asteraceae | 13·69 ± 0·03 | 6·84 A | 6703 | 18* | 2 | 6·84 | C | P | 2·21–2·66 | 2·28–2·67 | T | 7 |
| Argyranthemum adauctum (Link) Humphr. ssp. dugourii (Bolle) Humphr. | Asteraceae | 13·77 ± 0·05 | 6·89 A | 6752 | 18* | 2 | 6·89 | C | P | 1·67–2·92 | 1·89–3·02 | T | 20 |
| Argyranthemum broussonetii (Pers.) Humphr. ssp. broussonetii | Asteraceae | 14·14 ± 0·06 | 7·07 B | 6929 | 18 | 2 | 7·07 | C | P | 1·99–2·81 | 2·26–3·18 | T | 10 |
| Argyranthemum foeniculaceum (Willd.) Webb ex Sch. Bip. | Asteraceae | 14·28 ± 0·05 | 7·14 B | 6997 | 18 | 2 | 7·14 | C | P | 1·54–2·48 | 1·84–2·60 | T | 17 |
| Argyranthemum frutescens (L.) Sch. Bip. ssp. frutescens | Asteraceae | 14·41 ± 0·04 | 7·21 C | 7066 | 18 | 2 | 7·21 | C | P | 1·83–2·65 | 1·84–2·82 | C | 12 |
| Argyranthemum gracile Sch. Bip. | Asteraceae | 14·18 ± 0·06 | 7·09 B | 6948 | 18 | 2 | 7·09 | C | P | 1·74–2·54 | 1·86–2·39 | T | 21 |
| Argyranthemum haouarytheum Humphr. & Bramw. | Asteraceae | 13·39 ± 0·08 | 6·69 D | 6556 | 18* | 2 | 6·69 | C | P | 1·27–2·23 | 1·54–2·64 | P | 26 |
| Argyranthemum teneriffae Humphr. | Asteraceae | 13·94 ± 0·05 | 6·97 E | 6831 | 18* | 2 | 6·97 | H/C | P | 1·65–2·25 | 1·82–2·39 | T | 2 |
| Artemisia thuscula Cav. | Asteraceae | 11·43 ± 0·06 | 5·71 | 5596 | 18 | 2 | 5·71 | C | P | 2·01–2·76 | 2·02–3·29 | C | 12 |
| Asparagus umbellatus Link | Asparagaceae | 2·56 ± 0·03 | 1·28 | 1254 | 20 | 2 | 1·28 | Cl NP | L | 2·52–3·56 | 2·54–3·67 | C | 16 |
| Bupleurum salicifolium R. Br. in Buch ssp. aciphyllum (Webb ex Parl.) Sund. & Kunk. | Apiaceae | 1·58 ± 0·004 | 0·79 | 774 | 32 | 4 | 0·40 | C | L | 1·84–2·24 | 2·50–2·97 | C | 12 |
| Canarina canariensis (L.) Vatke | Campanulaceae | 5·71 ± 0·02 | 2·85 | 2793 | 34 | 4 | 1·43 | Cl G | P | 1·92–2·86 | 1·79–2·83 | C | 11 |
| Carlina xeranthemoides L. fil. | Asteraceae | 7·11 ± 0·06 | 3·56 | 3489 | 20* | 2 | 3·56 | C | Z | 1·61–2·94 | 2·05–3·10 | T | 2 |
| Ceropegia dichotoma Haw. | Apocynaceae (incl. Asclepiadaceae) | 0·89 ± 0·01 | 0·44 A | 431 | 22 | 2 | 0·44 | S H | R | 2·79–3·56 | 4·06–4·53 | C | 12 |
| Ceropegia fusca Bolle | Apocynaceae (incl. Asclepiadaceae) | 0·86 ± 0·01 | 0·43 B | 421 | 22 | 2 | 0·43 | S H | R | 3·38–4·08 | 3·49–4·74 | C | 14 |
| Cheirolophus teydis (Chr. Sm. in Buch) G. López | Asteraceae | 1·43 ± 0·02 | 0·71 | 696 | ∼30* | ≥2 | ≤0·71 | C | L | 2·01–2·99 | 1·88–3·08 | C | 3 |
| Convolvulus floridus L. fil. | Convolvulaceae | 2·12 ± 0·01 | 1·06 A | 1039 | 30 | 6 | 0·35 | NP | G | 2·82–3·38 | 3·94–4·41 | C | 19 |
| Convolvulus perraudieri Coss. | Convolvulaceae | 2·13 ± 0·01 | 1·06 A | 1039 | 30!* | 6 | 0·35 | Cl NP | G | 2·38–3·21 | 2·23–2·96 | C | 16 |
| Crambe arborea Webb ex Christ var. indivisa Svent. | Brassicaceae | 1·86 ± 0·01 | 0·93 A | 911 | 30 | 6 | 0·31 | C | G | 2·90–3·43 | 4·11–4·49 | T | 15 |
| Crambe laevigata DC. ex Christ | Brassicaceae | 1·90 ± 0·01 | 0·95 B | 931 | ∼30!* | 6 | 0·32 | C | G | 2·55–3·70 | 3·57–3·85 | T | 16 |
| Crambe scaberrima Webb ex Bramw. | Brassicaceae | 1·85 ± 0·01 | 0·92 A | 902 | ∼30* | 6 | 0·31 | C | G | 2·49–3·39 | 2·76–3·73 | T | 18 |
| Crambe strigosa L.′ Hér. | Brassicaceae | 1·98 ± 0·02 | 0·99 C | 970 | 30 | 6 | 0·33 | C | G | 2·70–3·76 | 2·88–4·53 | C | 8 |
| Dactylis smithii Link ssp. smithii | Poaceae | 4·35 ± 0·02 | 2·18 | 2136 | 14 | 2 | 2·18 | H | Z | 1·95–2·52 | 2·08–3·37 | C | 12 |
| Descurainia bourgeauana (Fourn.) O. E. Schulz | Brassicaceae | 0·38 ± 0·01 | 0·19 A | 186 | 14* | 2 | 0·19 | C | R | 2·56–3·52 | 5·72–8·57 | T | 1 |
| Descurainia gilva Svent. | Brassicaceae | 0·45 ± 0·01 | 0·22 BC | 216 | 14!* | 2 | 0·22 | C | R | 2·41–3·32 | 5·64–7·67 | P | 23 |
| Descurainia gonzalesii Svent. | Brassicaceae | 0·46 ± 0·01 | 0·23 B | 225 | 14!* | 2 | 0·23 | C | R | 3·23–4·34 | 5·43–7·68 | T | 3 |
| Descurainia lemsii Bramw. | Brassicaceae | 0·45 ± 0·01 | 0·22 BC | 216 | 14 | 2 | 0·22 | C | R | 2·63–2·99 | 4·99–6·56 | T | 6 |
| Descurainia millefolia (Jacq.) Webb & Berth. | Brassicaceae | 0·44 ± 0·01 | 0·22 C | 216 | 14* | 2 | 0·22 | C | R | 2·68–3·45 | 6·07–7·79 | C | 12 |
| Dicheranthus plocamoides Webb | Caryophyllaceae | 1·49 ± 0·003 | 0·75 | 735 | 16 | 2 | 0·75 | C | L | 2·84–3·65 | 3·60–4·18 | C | 16 |
| Dorycnium eriophtalmum Webb & Berth. | Fabaceae | 2·24 ± 0·01 | 1·12 | 1098 | 14 | 2 | 1·12 | C | G | 2·85–3·28 | 2·89–3·65 | C | 22 |
| Dracunculus canariensis Kunth | Araceae | 7·90 ± 0·09 | 3·95 | 3871 | 28 | 4 | 1·98 | G | P | 1·84–2·85 | 2·10–3·27 | C | 12 |
| Erigeron calderae Hans. | Asteraceae | 3·10 ± 0·01 | 1·55 | 1519 | 18* | 2 | 1·55 | H | G | 3·27–4·12 | 3·49–4·44 | T | 4 |
| Erysimum bicolor (Hornem.) DC. | Brassicaceae | 1·16 ± 0·01 | 0·58 A | 568 | 28 | 4 | 0·29 | C | L | 2·11–3·01 | 3·55–4·87 | M | 29 |
| Erysimum scoparium (Brouss. ex Willd.) Wettst. | Brassicaceae | 1·08 ± 0·02 | 0·54 B | 529 | 28* | 4 | 0·27 | C | L | 2·01–3·17 | 3·41–4·99 | C | 2 |
| Forsskaolea angustifolia Retz. | Urticaceae | 0·64 ± 0·01 | 0·32 | 314 | 22 | 2 | 0·32 | C | R | 2·56–3·03 | 3·81–4·87 | C | 27 |
| Gesnouinia arborea (L. fil.) Gaud. | Urticaceae | 1·02 ± 0·01 | 0·51 | 500 | 20 | 2 | 0·51 | NP | L | 2·70–3·45 | 5·54–6·91 | C | 11 |
| Hypericum canariense L. | Clusiaceae | 1·01 ± 0·01 | 0·51 A | 500 | 40 | 4 | 0·25 | NP | R | 2·62–3·38 | 3·24–4·08 | M | 30 |
| Hypericum grandifolium Choisy | Clusiaceae | 0·81 ± 0·01 | 0·41 B | 402 | 40 | 4 | 0·20 | C | R | 2·98–3·48 | 3·85–4·84 | M | 8 |
| Hypochoeris oligocephala (Svent. & Bramw.) Lack | Asteraceae | 2·28 ± 0·02 | 1·14 | 1117 | 6 | 2 | 1·14 | H | L | 2·31–3·16 | 2·99–3·55 | T | 18 |
| Isoplexis canariensis (L.) Loud. | Plantaginaceae | 1·99 ± 0·01 | 1·00 | 980 | 56 | 8 | 0·25 | C | G | 2·43–3·01 | 3·85–4·05 | C | 11 |
| Lactuca palmensis Bolle | Asteraceae | 2·05 ± 0·01 | 1·03 | 1009 | ? | (2) | (1·03) | H | G | 2·29–3·20 | 3·23–4·01 | P | 23 |
| Lavandula buchii Webb var. buchii | Lamiaceae | 1·01 ± 0·01 | 0·51 A | 500 | 22 | 2 | 0·51 | C | L | 2·12–3·54 | 3·81–6·76 | T | 12 |
| Lavandula multifida L. ssp. canariensis (Mill.) Pit. & Pr. | Lamiaceae | 1·02 ± 0·01 | 0·51 A | 500 | 22 | 2 | 0·51 | C | L | 2·28–3·08 | 5·08–5·42 | M | 31 |
| Limonium macrophyllum (Brouss.) O. Kuntze | Plumbaginaceae | 10·90 ± 0·06 | 5·45 A | 5341 | 14* | 2 | 5·45 | H | P | 1·97–2·87 | 1·78–2·56 | T | 12 |
| Limonium pectinatum (Ait.) O. Kuntze var. pectinatum | Plumbaginaceae | 5·23 ± 0·01 | 2·62 B | 2568 | 12 | 2 | 2·62 | H | P | 1·59–2·27 | 2·01–3·02 | C | 12 |
| Lobularia canariensis (Webb) Borgen ssp. palmensis (Christ) Borgen | Brassicaceae | 1·13 ± 0·01 | 0·56 | 549 | 22 | 2 | 0·56 | C | L | 2·30–3·09 | 2·78–4·16 | C | 26 |
| Lotus dumetorum Webb ex Murr. | Fabaceae | 1·22 ± 0·003 | 0·61 A | 598 | 14* | 2 | 0·61 | H | L | 2·28–2·89 | 3·60–4·24 | T | 12 |
| Lotus campylocladus (Webb & Berth.) | Fabaceae | 1·24 ± 0·01 | 0·62 B | 608 | 14* | 2 | 0·62 | H | L | 2·25–3·02 | 2·21–3·42 | T | 6 |
| Lotus glaucus Ait. | Fabaceae | 2·48 ± 0·02 | 1·24 C | 1215 | 28* | 4 | 0·62 | H | L | 2·03–2·82 | 2·09–2·90 | M | 10 |
| Lugoa revoluta (Chr. Sm. in Buch) DC. | Asteraceae | 11·94 ± 0·01 | 5·97 | 5851 | 18* | 2 | 5·97 | H/C | P | 2·12–2·95 | 2·12–2·87 | T | 12 |
| Micromeria glomerata Pérez | Lamiaceae | 0·88 ± 0·01 | 0·44 A | 431 | ? | ? | ? | C | R | 3·65–4·59 | 5·18–6·11 | T | 13 |
| Micromeria herpyllomorpha Webb & Berth. | Lamiaceae | 0·76 ± 0·01 | 0·38 B | 372 | ? | (≥2) | (≤0·38) | C | R | 3·29–3·99 | 5·11–6·35 | P | 25 |
| Micromeria hyssopifolia Webb & Berth. var. hyssopifolia | Lamiaceae | 0·72 ± 0·01 | 0·36 C | 353 | ? | (≥2) | (≤0·36) | C | R | 3·19–4·12 | 4·32–5·77 | C | 8 |
| Micromeria lachnophylla Webb & Berth. | Lamiaceae | 0·74 ± 0·01 | 0·37 CD | 363 | 30* | ≥2 | ≤0·37 | C | R | 3·20–4·08 | 4·48–5·84 | T | 5 |
| Micromeria varia Bentham ssp. varia | Lamiaceae | 0·75 ± 0·01 | 0·37 BD | 363 | 30 | ≥2 | ≤0·37 | C | R | 3·20–4·21 | 4·43–5·90 | C | 12 |
| Nauplius sericeus (L. fil.) Cass. | Asteraceae | 1·72 ± 0·01 | 0·86 | 843 | 14* | 2 | 0·86 | C | G | 2·52–3·34 | 2·98–3·54 | F | 33 |
| Nepeta teydea Webb. & Berth. | Lamiaceae | 0·55 ± 0·01 | 0·27 | 265 | 16 | 2 | 0·27 | H | R | 2·65–3·49 | 3·67–4·87 | C | 1 |
| Paronychia canariensis (L. fil.) Juss. | Caryophyllaceae | 2·68 ± 0·02 | 1·34 | 1313 | 32 | 4 | 0·67 | C | L | 2·45–2·87 | 2·27–2·91 | C | 12 |
| Pericallis appendiculata (L. fil.) B. Nord. | Asteraceae | 1·09 ± 0·01 | 0·55 A | 539 | 60 | 6 | 0·18 | C | L | 2·82–4·41 | 4·89–7·13 | C | 11 |
| Pericallis cruenta (L.′ Hér.) Bolle | Asteraceae | 1·36 ± 0·01 | 0·68 B | 666 | 60 | 6 | 0·23 | H | L | 2·40–3·25 | 4·12–4·94 | C | 7 |
| Pericallis echinata (L. fil.) B. Nord. | Asteraceae | 1·46 ± 0·02 | 0·73 C | 715 | 60 | 6 | 0·24 | H | L | 2·24–3·25 | 3·97–4·52 | T | 9 |
| Pericallis lanata (L.′ Hér.) B. Nord. | Asteraceae | 1·15 ± 0·004 | 0·57 D | 559 | 60 | 6 | 0·19 | C | L | 2·32–2·67 | 3·00–4·85 | T | 16 |
| Pericallis papyracea (DC.) B. Nord. | Asteraceae | 1·20 ± 0·01 | 0·60 E | 588 | 60 | 6 | 0·20 | H | L | 2·46–3·00 | 3·93–5·36 | P | 24 |
| Pericallis webbii (Sch. Bip.) Bolle | Asteraceae | 1·14 ± 0·01 | 0·57 D | 559 | 60 | 6 | 0·19 | H | L | 2·42–3·13 | 4·53–5·51 | G | 31 |
| Periploca laevigata Ait. | Apocynaceae (incl. Asclepiadaceae) | 1·00 ± 0·01 | 0·50 | 490 | 22 | 2 | 0·50 | Cl NP | L | 2·51–2·94 | 4·72–5·57 | C | 14 |
| Phagnalon umbelliforme DC. | Asteraceae | 2·19 ± 0·03 | 1·09 | 1068 | 18 | 2 | 1·09 | C | G | 2·49–3·28 | 2·76–3·49 | C | 14 |
| Pimpinella cumbrae Link | Apiaceae | 4·60 ± 0·05 | 2·30 | 2254 | 20 | 2 | 2·30 | H | Z | 1·87–3·13 | 2·45–3·51 | C | 3 |
| Plantago arborescens Poir. ssp. arborescens var. arborescens | Plantaginaceae | 0·97 ± 0·01 | 0·48 A | 470 | 12 | 2 | 0·48 | C | L | 2·18–2·85 | 3·73–4·96 | C | 10 |
| Plantago famarae Svent. | Plantaginaceae | 1·00 ± 0·01 | 0·50 B | 490 | 12 | 2 | 0·50 | C | L | 2·29–2·99 | 3·86–4·94 | L | 34 |
| Plantago webbii Barn. | Plantaginaceae | 1·11 ± 0·01 | 0·56 C | 549 | 12* | 2 | 0·56 | C | L | 2·26–2·99 | 3·32–5·00 | C | 23 |
| Plocama pendula Ait. | Rubiaceae | 2·79 ± 0·01 | 1·39 | 1362 | 44 | 4 | 0·70 | NP | G | 2·53–2·91 | 2·38–3·39 | C | 19 |
| Polycarpaea aristata (Ait.) DC. | Caryophyllaceae | 0·93 ± 0·01 | 0·47 A | 461 | ? | (2) | (0·47) | H/C | R | 2·76–3·33 | 2·94–3·56 | C | 8 |
| Polycarpaea latifolia Willd. | Caryophyllaceae | 0·89 ± 0·003 | 0·44 B | 431 | 18 | 2 | 0·44 | H | R | 2·77–3·19 | 2·68–3·03 | C | 10 |
| Polycarpaea smithii Link | Caryophyllaceae | 1·09 ± 0·01 | 0·54 C | 529 | ? | ? | ? | H | L | 2·55–3·68 | 3·03–5·11 | C | 28 |
| Polycarpaea tenuis Webb ex Christ | Caryophyllaceae | 0·91 ± 0·003 | 0·45 D | 441 | 18!* | 2 | 0·45 | H/C | R | 2·78–3·20 | 2·67–3·38 | C | 5 |
| Pterocephalus dumetorum (Brouss. ex Willd.) Coult. | Dipsacaceae | 3·56 ± 0·01 | 1·78 | 1744 | 18* | 2 | 1·78 | C | G | 2·34–3·32 | 2·44–2·68 | C | 15 |
| Reichardia ligulata (Vent.) Kunk. & Sund. | Asteraceae | 3·78 ± 0·01 | 1·89 | 1852 | 16* | 2 | 1·89 | H | G | 2·06–3·08 | 1·75–2·66 | C | 12 |
| Rumex lunaria L. | Polygonaceae | 12·47 ± 0·09 | 6·23 A | 6105 | 36 | 4 | 3·12 | C | P | 2·92–3·84 | 2·17–3·94 | C | 12 |
| Rumex maderensis Lowe | Polygonaceae | 1·38 ± 0·01 | 0·69 B | 676 | 20 | 2 | 0·69 | H | L | 2·49–3·05 | 3·45–4·69 | M | 7 |
| Salvia broussonetii Bentham | Lamiaceae | 0·86 ± 0·01 | 0·43 | 421 | 22 | 2 | 0·43 | C | R | 3·07–4·02 | 3·55–5·55 | T | 16 |
| Scrophularia glabrata Ait. | Scrophulariaceae | 2·06 ± 0·01 | 1·03 A | 1009 | 56 | 8 | 0·26 | H | G | 3·10–4·02 | 4·10–4·62 | C | 2 |
| Scrophularia smithii Hornem. ssp. smithii | Scrophulariaceae | 2·08 ± 0·01 | 1·04 B | 1019 | 58 | 8 | 0·26 | H | G | 2·80–3·42 | 2·91–3·69 | T | 10 |
| Senecio palmensis (Chr. Sm. in Buch) Link | Asteraceae | 1·96 ± 0·01 | 0·98 A | 960 | 20* | 2 | 0·98 | C | G | 2·50–3·03 | 2·80–3·23 | C | 23 |
| Senecio teneriffae Schultz Bip. | Asteraceae | 5·26 ± 0·02 | 2·63 B | 2577 | 60 | 6 | 0·88 | T | P | 1·64–2·37 | 2·83–3·46 | C | 7 |
| Seseli webbii Coss. | Apiaceae | 3·79 ± 0·02 | 1·89 | 1852 | 22 | 2 | 1·89 | H | G | 2·86–3·25 | 2·56–2·93 | C | 12 |
| Schizogyne glaberrima DC. | Asteraceae | 2·05 ± 0·01 | 1·02 A | 1000 | 18 | 2 | 1·02 | C | G | 2·54–3·00 | 2·98–3·69 | C | 32 |
| Schizogyne sericea (L. fil.) DC. | Asteraceae | 2·05 ± 0·01 | 1·02 A | 1000 | 18 | 2 | 1·02 | C | G | 2·52–3·23 | 3·09–3·74 | M | 12 |
| Sideritis canariensis L. | Lamiaceae | 3·56 ± 0·01 | 1·78 A | 1744 | 44 | 4 | 0·89 | C | G | 3·25–4·07 | 2·45–3·03 | C | 26 |
| Sideritis macrostachysPoir. | Lamiaceae | 4·03 ± 0·03 | 2·01 B | 1970 | ∼36* | 4 | 1·01 | C | G | 2·98–4·78 | 2·80–4·40 | T | 12 |
| Sideritis oroteneriffaeNegrín & Pérez var. oroteneriffae | Lamiaceae | 3·65 ± 0·01 | 1·82 C | 1784 | 44 | 4 | 0·91 | C | G | 2·59–3·73 | 2·29–3·88 | T | 3 |
| Silene berthelotiana Webb | Caryophyllaceae | 5·11 ± 0·04 | 2·55 A | 2499 | 24!* | 2 | 2·55 | H | P | 1·77–2·84 | 2·04–3·44 | C | 6 |
| Silene lagunensis Chr. Sm. ex Christ | Caryophyllaceae | 5·19 ± 0·01 | 2·59 BC | 2538 | 24* | 2 | 2·59 | H | P | 1·75–2·37 | 2·04–3·30 | T | 12 |
| Silene nocteolens Webb & Berth. | Caryophyllaceae | 5·16 ± 0·01 | 2·58 C | 2528 | 24* | 2 | 2·58 | H | P | 1·86–2·80 | 2·11–3·40 | T | 5 |
| Silene pogonocalyx (Svent.) Bramw. | Caryophyllaceae | 5·23 ± 0·01 | 2·61 B | 2558 | 24!* | 2 | 2·61 | H | P | 2·15–3·12 | 2·73–3·84 | P | 23 |
| Sonchus acaulis Dum.‐Cours. | Asteraceae | 2·86 ± 0·03 | 1·43 A | 1401 | 18 | 2 | 1·43 | H | L | 2·90–3·65 | 2·59–3·14 | C | 8 |
| Sonchus congestus Willd. | Asteraceae | 2·87 ± 0·02 | 1·44 B | 1411 | 18 | 2 | 1·44 | C | L | 2·80–3·09 | 2·57–3·11 | C | 9 |
| Sonchus radicatus Ait. | Asteraceae | 2·62 ± 0·02 | 1·31 C | 1284 | 18* | 2 | 1·31 | H | L | 2·24–3·04 | 2·33–3·06 | T | 12 |
| Teline canariensis (L.) Webb. & Berth. | Fabaceae | 3·00 ± 0·03 | 1·50 | 1470 | 48!* | 4 | 0·75 | NP | G | 3·32–4·26 | 4·21–4·63 | C | 12 |
| Tinguarra montana (Webb ex Christ) A. Hans. & Kunk. | Apiaceae | 2·45 ± 0·02 | 1·22 | 1196 | 22 | 2 | 1·22 | H | L | 2·88–3·81 | 2·54–3·89 | C | 6 |
| Todaroa aurea Parl. | Apiaceae | 2·87 ± 0·01 | 1·44 | 1411 | 22 | 2 | 1·44 | H | L | 2·72–3·63 | 2·08–2·71 | C | 27 |
| Tolpis laciniata (Sch. Bip. ex Webb & Berth.) Webb | Asteraceae | 2·66 ± 0·03 | 1·33 A | 1303 | 18 | 2 | 1·33 | H | L | 2·50–3·08 | 1·86–2·46 | C | 25 |
| Tolpis webbii Sch. Bip. ex Webb & Berth. | Asteraceae | 2·75 ± 0·01 | 1·37 B | 1343 | 18* | 2 | 1·37 | H | L | 2·55–3·07 | 2·42–3·11 | T | 1 |
| Volutaria canariensis Wagenitz | Asteraceae | 1·60 ± 0·01 | 0·80 | 784 | 32 | 4 | 0·40 | T | L | 2·81–3·13 | 2·62–3·62 | C | 14 |
* Chromosome numbers determined in the present work; !, new species record. All other counts were taken from literature.
† An assumed ploidy level and genome size given in parentheses.
‡ G, Glycine max ‘Polanka’ (2C = 2·5 pg); L, Lycopersicum esculentum ‘Stupnické polní tyčkové rané’ (2C = 1·96 pg); P, Pisum sativum ‘Ctirad’ (2C = 9·09 pg); R, Raphanus sativus ‘Saxa’ (2C = 1·11 pg); Z, Zea mays ‘CE‐777’ (2C = 5·43 pg).
§ C, Canary Islands; F, Fuerteventura; G, Gran Canaria; L, Lanzarote; M, Macaronesia; P, La Palma; T, Tenerife.
¶ Letters indicate group of taxa within the same genus that are not significantly different at α = 0·05.
** C, Chamaephyte; Cl G, climbing geophyte; Cl NP, climbing nanophanerophyte; G, geophyte; H, hemicryptophyte; NP, nanophanerophyte; S H, succulent hemicryptophyte; T, therophyte.
An analysis of ploidy level revealed the following: diploids, 63 taxa; tetraploids, 16 taxa; hexaploids, 13 taxa; and octoploids, three taxa. The proportion of polyploid plants was therefore 33·7 %. Despite usually being regarded as diploids, Crambe species were included amongst the hexaploids on the basis of comparative genome mapping results (Leitch and Bennett, 1997). Three taxa with a basic chromosome number higher than 13 were excluded from the comparison as it was uncertain whether they were diploid or polyploid (Grant, 1971), and the omission also applied to six species which lacked an exact chromosome count.
Nuclear DNA amounts
Table 2 shows 2C DNA content (pg) and standard error, 1C DNA content expressed in picograms and megabase pairs, number of chromosomes and ploidy level, genome size (calculated as 2C DNA value/ploidy level), life‐form, internal standard used, coefficients of variance (CV) of both the internal standard and plant analysed, species distribution pattern and original locality for 104 taxa from 20 families. Prime C‐values represent 98 % of the estimates in the present species set; only Hypochoeris oligocephala and Plocama pendula have been analysed previously.
Flow‐cytometric measurements yielded histograms with CV values ranging from 1·27 to 4·78 % (mean 2·85 %) for internal standards, and 1·54 to 8·57 % (mean 3·57 %) for analysed plants, depending on the taxon, DNA amount and the quality of sample preparation. A CV value not exceeding the arbitrary level of 3 % was achieved in 69 and 47 % of internal standard and analysed plant acquisitions, respectively. The standard error of the mean described the difference in 2C‐values between individual runs of the same species caused by cytometer instability, non‐identical sample preparation, etc. Values smaller than 1 % of the plant 2C DNA amount were attained in 82 % of taxa; the limit of 2 % was never been exceeded. Therefore, the data on nuclear DNA content can be regarded as reliable.
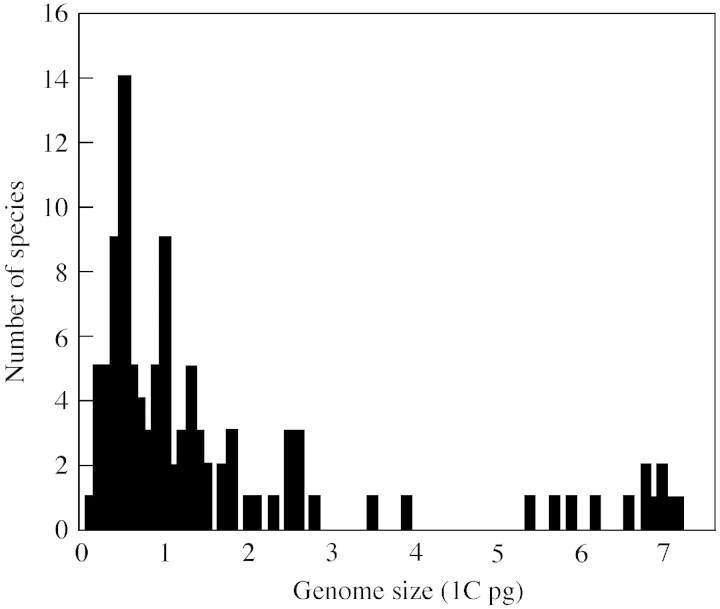
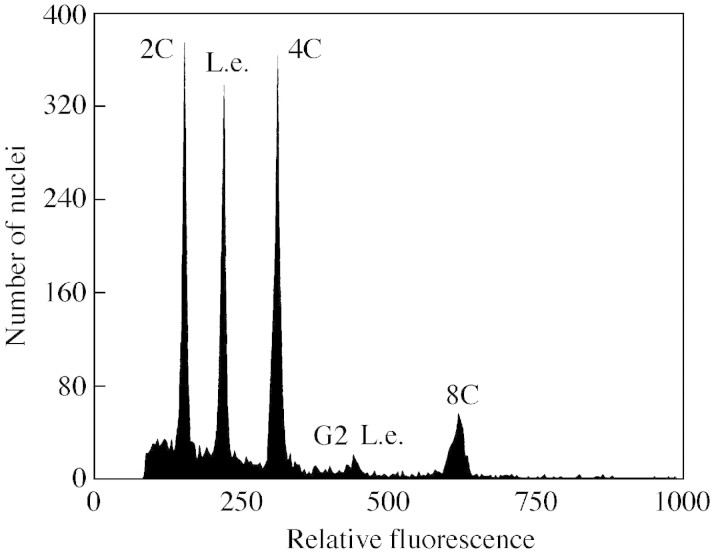
1C nuclear DNA contents obtained in the present study ranged from 0·19 pg in Descurainia bourgeauana to 7·21 pg in Argyranthemum frutescens, representing a difference of approx. 38‐fold (Table 2; Fig. 1). However, the majority of species (71 %) fell into the lower part of the range, with C‐values between 0·2 and 1·6 pg. Genome sizes had a range very similar to that of 1C DNA content, with hexaploid Pericallis appendiculata having the smallest genome (0·18 pg). The highest genome size was found in diploid Argyranthemum frutescens. Comparison of mean values for Macaronesian vs. non‐Macaronesian (taken from Bennett and Leitch, 2001) representatives of individual genera and families affirmed the tendency towards small C‐values and genome sizes in Macaronesian taxa (data not shown). At the rank of family, both C‐values and genome sizes of endemic plants were significantly smaller (P < 0·01, n = 20) than those of non‐Macaronesian taxa, and the comparisons at the generic level brought identical results (P = 0·017, n = 22). Artemisia, Reichardia and Rumex were the most prominent exceptions, with Macaronesian representatives having larger C‐values. Although species from 56 genera were included in the study, only 22 genera were employed in the foregoing comparison as no previous C‐value data were available for the remaining 34 genera. Small nuclear DNA amounts in Macaronesian native flora were also confirmed in data sets taking the phylogenetic position of the species into account. Selected descriptive statistics for major angiosperm lineages clearly indicate that non‐Maca ronesian plants possessed larger C‐values that their Macaronesian counterparts (Table 3). Macaronesian species of particular interest with very low DNA amounts include Nepeta teydea (1C = 0·27 pg; the smallest C‐value among Euasterids II and even the Asterids as a whole), Micromeria spp. (1C = 0·36–0·44 pg; very small C‐values in Euasterids II) and Pericallis appendiculata (1C = 0·55 pg; the second smallest C‐value in Euasterids I). Average 1C nuclear DNA amounts ± s.d. for main phylogenetic lineages of Macaronesian plants were as follows: Eurosids II, 0·55 ± 0·33 pg; Eurosids I, 0·76 ± 0·42 pg; Euasterids I, 0·78 ± 0·52 pg; Basal Eudicots, 2·09 ± 1·85 pg; Monocots, 2·47 ± 1·36 pg; Euasterids II, 2·61 ± 2·44 pg. Apparently, there are some differences in mean C‐values (one group with 1C <1 pg, the other with 1C >2 pg); however, only Eurosids II/Euasterids II and Euasterids I/Euasterids II differed significantly. Somatic tissues of five taxa (Cero pegia dichotoma, C. fusca, Dicheranthus plocamoides, Polycarpaea smithii and Rumex maderensis) underwent endoreduplication, and three peaks corresponding to nuclei with 2C, 4C and 8C DNA content were observed. Flow histograms of particular interest are shown in Figs 2 and 3.
Fig. 1. 1C DNA amount distribution (pg means) of 104 Macaronesian species investigated.
Table 3.
Selected descriptive statistics of 1C‐value sets for major phylogenetic lineages of Macaronesian (M; estimated here) and non‐Macaronesian angiosperms (non‐M; from Bennett and Leitch, 2001)
| Eudicots | Monocots | Basal eudicots | Eurosids I | Eurosids II | Euasterids I | Euasterids II | ||||||||
| Phylogenetic lineage | M (n = 101) | non‐M (n = 1929) | M (n = 3) | non‐M (n = 1487) | M (n = 14) | non‐M (n = 207) | M (n = 9) | non‐M (n = 721) | M (n = 12) | non‐M (n = 152) | M (n = 24) | non‐M (n = 280) | M (n = 42) | non‐M (n = 357) |
| Mean | 1·69 | 3·27 | 2·47 | 10·5 | 2·09 | 6·12 | 0·76 | 2·3 | 0·55 | 1·38 | 0·78 | 2·21 | 2·61 | 3·95 |
| Minimum | 0·19 | 0·05 | 1·28 | 0·15 | 0·44 | 0·25 | 0·32 | 0·1 | 0·19 | 0·05 | 0·27 | 0·33 | 0·55 | 0·4 |
| 25 % quartile | 0·51 | 0·85 | 1·73 | 2·5 | 0·58 | 0·72 | 0·51 | 0·65 | 0·22 | 0·55 | 0·43 | 0·9 | 0·90 | 1·85 |
| Median | 0·99 | 1·65 | 2·18 | 5·8 | 1·95 | 2·1 | 0·61 | 1·15 | 0·55 | 1·12 | 0·51 | 1·4 | 1·4 | 3·05 |
| 75 % quartile | 1·78 | 4·15 | 3·07 | 13·94 | 2·61 | 9·68 | 1·12 | 2·35 | 0·92 | 1·76 | 1·05 | 2·84 | 3·38 | 5·1 |
| Maximum | 7·21 | 79·33 | 3·95 | 127·4 | 6·23 | 79·33 | 1·5 | 27·4 | 0·99 | 8·7 | 2·01 | 15·3 | 7·21 | 24·83 |
| Mode | 0·51 | 0·7 | – | 0·95 | – | 0·48 | 0·51 | 0·55 | 0·22 | 0·58 | 0·37 | 0·85 | 0·57 | 2·9 |
All values in picograms.
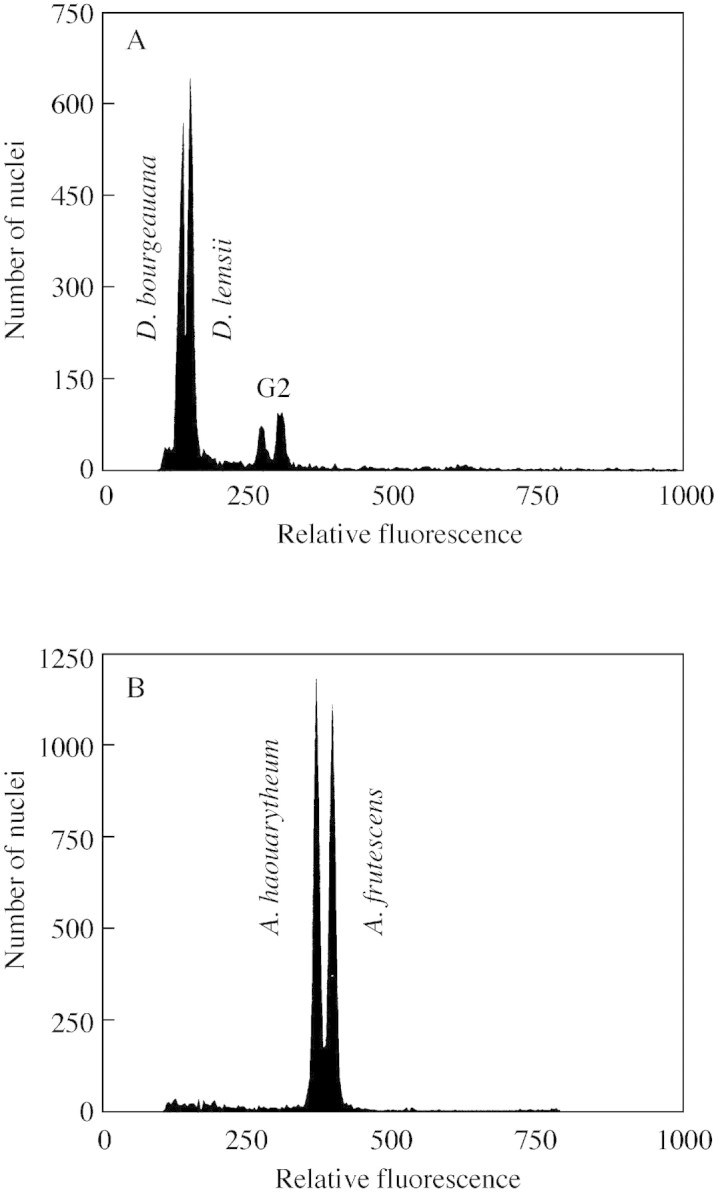
Fig. 2. Flow‐cytometric histograms showing the difference in 2C nuclear DNA content for species with the smallest DNA amounts, Descurainia bourgeauana and D. lemsii (A), and species with the highest DNA amounts, Argyranthemum haouarytheum and A. frutescens (B). Nuclei of both species in the genus were isolated and stained with propidium iodide simultaneously. Small G2 peaks represent the nuclei of Descurainia with doubled (4C) nuclear DNA content.
Fig. 3. Flow‐cytometric histogram obtained after simultaneous analysis of propidium iodide‐stained nuclei of Lycopersicum esculentum (L.e.; internal standard) and Rumex maderensis. Occurrence of nuclei with 2C, 4C and 8C DNA amounts indicates endoreduplication.
Correlation of C‐value with environmental conditions
Correlations between C‐value and altitude, average annual temperature, humidity and rainfall were calculated in genera where at least three species from Tenerife were available. The nuclear DNA amount in Argyranthemum was negatively correlated with altitude (r = –0·806, P < 0·0001, n = 8) and annual rainfall (r = –0·783, P < 0·0001, n = 8), and positively correlated with mean annual temperature (r = 0·704, P < 0·0001, n = 8). The number of species in other genera was too small to permit meaningful comparisons so the results were treated only as tendencies. The DNA amount in Silene and Micromeria followed the same trends as in Argyranthemum (with the exception of a non‐significant correlation with altitude). A completely different pattern of variation was observed in Crambe and Sonchus where nuclear DNA content increased with higher mean altitude and rainfall, and decreased with average annual temperature. The DNA amount in Pericallis was negatively correlated with relative humidity (r = –0·616, P = 0·0005, n = 4). However, this relationship may be biased to a certain extent as the genus comprised species of two growth‐forms (chamaephytes P. appendiculata and P. lanata possessed significantly smaller genomes than hemicryptophytes P. echinata and P. cruenta).
DISCUSSION
Nuclear DNA amounts for 104 Macaronesian angiosperms from 56 genera and 20 families were estimated in this study. Species from this phytogeographic region have been under‐represented in previous investigations of nuclear DNA amount, and only 12 estimates were available (Cerbah et al., 1999; Bennett and Leitch, 2001; Hanson et al., 2001; Ellul et al., 2002). The present paper therefore increases knowledge of nuclear DNA content in Macaronesian angiosperms by almost nine times. Thirty‐four of the 56 genera (60·7 %) were previously without C‐values and the present estimates thus represent the first data on nuclear DNA content. A huge majority (93 %) of taxa analysed was restricted to the Canary Islands. A comparison with recent figures on Canarian flora (Francisco‐Ortega et al., 2000) indicates that information on C‐value and genome size for more than one‐sixth (approx. 17·9 %) of Canarian endemics was obtained. It should, however, be emphasized that there are significant differences concerning both the total number and the proportion of endemic plants given by various authors. Although Macaronesia lacks any endemic family, more than 30 endemic genera can be found there (Bramwell, 1976; Kunkel, 1993). This study encompassed species from 11 endemic genera, thus representing about one‐third of generic coverage.
The overall 1C‐values obtained here varied from 0·19 pg in Descurainia bourgeauana to 7·21 pg in Argyranthemum frutescens (about 38‐fold difference). Nuclear DNA amounts for 12 Macaronesian angiosperms published previously differed approx. 16‐fold, from 0·54 pg in the Tenerife‐endemic Aeonium haworthii to 8·63 pg in the Macaronesian Ranunculus cortusifolius. By merging both lists, we can conclude that C‐values of Macaronesian vascular plants currently differ at least 45‐fold. This is a relatively narrow range compared with the approx. 1000‐fold variation in angiosperms as a whole (Bennett et al., 2000). However, it should be noted that very few Macaronesian monocots have been studied and there is a strong possibility that the inclusion of additional taxa, e.g. from Liliaceae s.l., would extend the C‐value range. Two species (Hypochoeris oligocephala, Plocama pendula) from our collection have been analysed by previous authors (Bennett and Leitch, 1995; Cerbah et al., 1999). Both values were similar and differed by only 3·5 % in the former and by 4·3 % in the latter taxon, with our estimates being negligibly smaller.
The majority of taxa analysed here possessed small genomes, with 1C nuclear DNA amounts less than 1·6 pg. They fall into the lowest third of the C‐value database comprising about 3500 records of flowering plants (Bennett and Leitch, 2001). The estimates for Descurainia (0·19–0·23 pg) even approached the minimum known for the angiosperms. 1C DNA amount for Nepeta teydea represented the smallest value in the Asterids, and Pericallis appendiculata had the second smallest C‐value in Euasterids II (only Leontodon longirostris possessed a smaller 1C nuclear DNA amount). Comparisons of average C‐values and genome sizes for Macaronesian vs. non‐Macaronesian representatives of individual genera and families confirmed the significantly smaller estimates for the former group. Obvious differences in C‐values were also found when matching data for major phylogenetic lineages of Macaronesian and non‐Macaronesian taxa (e.g. mean and median for the Macaronesian Eudicots were 1·93 and 1·67 times smaller, respectively).
Leitch et al. (1998) analysed angiosperm C‐values in a phylogenetic context and concluded that ancestral taxa probably possessed small genomes with 1C ≤3·5 pg. A considerable proportion (86·5 %) of Macaronesian plants meet this criterion, and more than two‐thirds of them had very small genomes (defined as 1C ≤1·4 pg). These findings would support the relictual nature of Macaronesian flora (Bramwell, 1976). However, recent phylogenetic investigations of several endemic groups (e.g. Aeonium, Argyranthemum, Bencomia, Echium) revealed the derived position of Macaronesian representatives indicating their recent origin (Emerson, 2002). In most cases, there has been only a single colonization of the archipelago (Francisco‐Ortega et al., 1997; Barber et al., 2000; Helfgott et al., 2000) followed by a rapid speciation. Two theories may explain the smaller C‐values of Macaronesian taxa in comparison with their non‐Macaronesian counterparts: (1) there has been a loss of DNA since the archipelago was colonized; (2) ancestral species possessed small genomes and only negligible changes have occurred during subsequent speciation processes. Chromosomal surveys on both oceanic and more continental islands revealed chromosomal stasis even though speciation has often been accompanied by striking morphological divergence (Stuessy and Crawford, 1998). Data obtained in the present study support a working hypothesis that small C‐values are an evolutionary advantage under insular selection pressures. However, further targeted work is essential to test this theory.
Although several molecular analyses of Macaronesian angiosperms have been published, meaningful comparison between the phylogenetic position and nuclear DNA content is possible only in a few cases. In the genus Sideritis, two major clades were identified (Barber et al., 2000): one comprised S. macrostachys (1C = 2·01 pg) and S. oroteneriffae (1C = 1·82 pg); the other S. canariensis from La Palma (1C = 1·78 pg). C‐values rather reflected the sectional classification: S. macrostachys (sect. Creticae) had a substantially larger genome than the two remaining species belonging to sect. Marrubiastrum. Nuclear DNA content variation that parallels the taxonomic classification is also known in the genus Pinus, for example (Hall et al., 2000). Six Pericallis species from the present study occupied four clades on the phylogenetic tree based on ITS sequences (Panero et al., 1999). P. cruenta (Tenerife, 1C = 0·68 pg), P. echinata (Tenerife, 1C = 0·73 pg) and P. papyraceae (La Palma, 1C = 0·6 pg) were grouped together, and P. webbii (Gran Canaria, 1C = 0·57 pg), P. lanata (Tenerife, 1C = 0·57 pg) and P. appendiculata (Tenerife, 1C = 0·55 pg) were each situated on a different branch (the position of the latter two species with woody stems was closer). Along with higher C‐values for hemicryptophytes, the present data also indicate that there could be an inter‐island differentiation in 2C‐values between species of the same life form. Similar traces of inter‐island diversification can also be found in other genera (e.g. Argyranthemum), and this question merits further study.
An increasing number of papers have investigated correlations between DNA content and environmental conditions. Altitude, average annual temperature, humidity and rainfall on Tenerife were included as environmental variables in the present study. Positive correlation between nuclear DNA amount and mean annual temperature was found in Argyranthemum; C‐values in this genus were negatively correlated with altitude and annual rainfall. The same trends were also observed in Silene and Micromeria. Presented results are in accordance with the pattern of DNA amount variation in Berberis, where diploids with lower DNA content grew in high‐elevation habitats with greater rainfall, and species with higher DNA content preferred sites with higher temperatures (Bottini et al., 2000). However, the nuclear DNA content divergence in Crambe and Sonchus followed a completely opposite trend and their C‐values increased with altitude and rainfall and decreased with temperature. As concluded by Grime and Mowforth (1982), large genomes in British flora have probably evolved under low temperature conditions. In the last group of genera (Descurainia, Polycarpaea), no correlation with environmental traits was found, as previously noted e.g. in the genus Lonchocarpus (Palomino and Sousa, 2000). Taken together, these data suggest that the nuclear DNA amount of Macaronesian endemics on Tenerife was negatively correlated with altitude in genera distributed over a large altitudinal range (from lowland to supracanarian zone); on the contrary, genera with a limited range in altitude (from lowland to laurel forests) showed a positive correlation between DNA content and altitude. However, more data are required for reliable conclusions.
The chromosome number in somatic cells was counted in about one‐third of the species; eight counts represented new species records. Almost all the present counts agreed with one or more previously published values for the same taxon. The only exception was Descurainia gonzalesii where a diploid number with 14 chromosomes was ascertained. Borgen (1969) and Bramwell (1977) observed 21 (= 3x) and 28 (= 4x) chromosomes in somatic cells, respectively. All records might be correct since the species could comprise diploid, triploid and tetraploid individuals. Seeds from two different localities were sown in the present study but only one batch of seeds germinated successfully (non‐viable seeds from the other site might have come from triploid plants). Borgen (1969) also reported the tetraploid number (2n = 44) for Ceropegia fusca. Flow‐cytometric histograms revealed endoreduplication in somatic tissues of this species, and it is likely that cells with doubled chromosome number were counted in Borgen’s study. Endopoly ploidization also occurred in four other Macaronesian taxa; the (sub)succulent leaves were a common feature in all of them. Their low nuclear DNA amounts support the hypothesis that somatic polyploidization is a general property of succulents that have small genomes (De Rocher et al., 1990).
ACKNOWLEDGEMENTS
We thank J. Brabec, V. Hadincová, T. Herben, S. Pecháčková, K. Vincenecová and J. Wild for their help with collecting seed; T. Herben and Z. Münzbergová for critical comments on the manuscript; J. Dolez̆el (Olomouc) for fruitful discussions on flow‐cytometry; E. Ibermayerová for technical help; and R. Malcová, J. Lazarová and P. Nová for care of the seedlings. The work was supported by the Grant Agency of the Czech Republic (project no. 206/00/1445), and partly by the Ministry of Education, Youth and Sports (project no. 313 004) and the programme ‘Biosféra’ (research project VaV/610/3/00).
Supplementary Material
Received: 25 October 2002; Returned for revision: 30 January 2003; Accepted: 4 March 2003
References
- BarberJC, Francisco‐Ortega J, Santos‐Guerra A, Marrero A, Jansen RK.2000. Evolution of endemic Sideritis (Lamiaceae) in Macaronesia: insights from a chloroplast DNA restriction site analysis. Systematic Botany 25: 633–647. [Google Scholar]
- BennettMD.1972. Nuclear DNA content and minimum generation time in herbaceous plants. Proceedings of the Royal Society of London 181: 109–135. [DOI] [PubMed] [Google Scholar]
- BennettMD, Leitch IJ.1995. Nuclear DNA Amounts in Angiosperms. Annals of Botany 76: 113–176. [Google Scholar]
- BennettMD, Leitch IJ.2001.Angiosperm DNA C‐values database (release 3·1, Sept. 2001). http://www.rbgkew.org.uk/cval/home page.html [Google Scholar]
- BennettMD, Bhandol P, Leitch IJ.2000. Nuclear DNA amounts in angiosperms and their modern uses – 807 new estimates. Annals of Botany 86: 859–909. [Google Scholar]
- BorgenL.1969. Chromosome numbers of vascular plants from the Canary Islands, with special reference to the occurrence of polyploidy. Nytt Magasin for Botanikk 16: 81–121. [Google Scholar]
- BorgenL.1974. Chromosome numbers of Macaronesian flowering plants. II. Norwegian Journal of Botany 21: 195–210. [Google Scholar]
- BottiniMCJ, Greizerstein EJ, Aulicino MB, Poggio L.2000. Relationships among genome size, environmental conditions and geographical distribution in natural populations of NW Patagonian species of Berberis L. (Berberidaceae). Annals of Botany 86: 565–573. [Google Scholar]
- BramwellD.1976. The endemic flora of the Canary Islands; distribution, relationships and phytogeography. In: Kunkel G, ed. Biogeography and ecology in the Canary Islands The Hague: Dr W. Junk b.v. Publishers, 207–240. [Google Scholar]
- BramwellD.1977. A revision of Descurainia Webb & Berth. section Sisymbriodendron (Christ) O.E. Schulz in the Canary Islands. Botanica Macaronesica 4: 31–53. [Google Scholar]
- BramwellD.1986. Contribución a la biogeografia de las Islas Canarias. Botanica Macaronesica 14: 3–34. [Google Scholar]
- BramwellD, Perez de Paz J, Ortega J.1976. Studies in the flora of Macaronesia: some chromosome numbers of flowering plants. Botanica Macaronesica 1: 9–16. [Google Scholar]
- CarracedoJC.1994. The Canary Islands: an example of structural control on the growth of large oceanic‐island volcanoes. Journal of Volcanology and Geothermal Research 60: 225–241. [Google Scholar]
- CerbahM, Coulaud J, Brown SC, Siljak‐Yakovlev S.1999. Evolutionary DNA variation in the genus Hypochaeris Heredity 82: 261–266. [DOI] [PubMed] [Google Scholar]
- CrawfordDJ, Whitkus R, Stuessy TF.1987. Plant evolution and speciation on oceanic islands. In: Urbanska KM, ed. Differentiation patterns in higher plants London: Academic Press, 183–199. [Google Scholar]
- DalgaardV.1991. Chromosome studies in flowering plants from Macaronesia II. Willdenowia 20: 139–152. [Google Scholar]
- De RocherEJ, Harkins KR, Galbraith DW, Bohnert HJ.1990. Developmentally regulated systemic endopolyploidy in succulents with small genomes. Science 250: 99–101. [DOI] [PubMed] [Google Scholar]
- Dolez̆elJ, Sgorbati S, Lucretti S.1992. Comparison of 3 DNA fluorochromes for flow cytometric estimation of nuclear‐DNA content in plants. Physiologia Plantarum 85: 625–631. [Google Scholar]
- EllulP, Boscaiu M, Vicente O, Moreno V, Rosselló JA.2002. Intra‐ and interspecific variation in DNA content in Cistus (Cistaceae). Annals of Botany 90: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EmersonBC.2002. Evolution on oceanic islands: molecular phylogenetic approaches to understanding pattern and process. Molecular Ecology 11: 951–966. [DOI] [PubMed] [Google Scholar]
- FernandopulléD.1976. Climatic characteristics of the Canary Islands. In: Kunkel G, ed. Biogeography and ecology in the Canary Islands The Hague: Dr W. Junk b.v. Publishers, 185–206. [Google Scholar]
- Francisco‐OrtegaJ, Santos‐Guerra A, Hines A, Jansen RK.1997. Molecular evidence for a Mediterranean origin of the Macaronesian endemic genus Argyranthemum (Asteraceae). American Journal of Botany 84: 1595–1613. [PubMed] [Google Scholar]
- Francisco‐OrtegaJ, Santos‐Guerra A, Kim SC, Crawford DJ.2000. Plant genetic diversity in the Canary Islands: a conservation perspective. American Journal of Botany 87: 909–919. [PubMed] [Google Scholar]
- GrantV.1971.Plant speciation. New York: Columbia University Press. [Google Scholar]
- GreilhuberJ, Speta F.1985. Geographic variation of genome size at low taxonomic levels in the Scilla bifolia alliance (Hyacinthaceae). Flora 176: 431–438. [Google Scholar]
- GrimeJP, Mowforth MA.1982. Variation in genome size – an ecological interpretation. Nature 229: 151–153. [Google Scholar]
- HallSE, Dvorak WS, Johnston JS, Price HJ, Williams CG.2000. Flow cytometric analysis of DNA content for tropical and temperate New World pines. Annals of Botany 86: 1081–1086. [Google Scholar]
- HansonL, McMahon KA, Johnson MAT, Bennett MD.2001. First nuclear DNA C‐values for 25 angiosperm families. Annals of Botany 87: 251–258. [DOI] [PubMed] [Google Scholar]
- HelfgottDM, Francisco‐Ortega J, Santos‐Guerra A, Jansen RK, Simpson BB.2000. Biogeography and breeding system evolution of the woody Bencomia alliance (Rosaceae) in Macaronesia based on ITS sequence data. Systematic Botany 25: 82–97. [Google Scholar]
- HobohmC.2000. Plant species diversity and endemism on islands and archipelagos, with special reference to the Macaronesian Islands. Flora 195: 9–24. [Google Scholar]
- I.U.C.N.1983. List of rare, threatened and endemic plants in Europe. 2nd edn. Nature and Environmental Series, 27. Strasbourg: Council of Europe. [Google Scholar]
- KunkelG.1993.Die Kanarischen Inseln und ihre Pflanzenwelt. Stuttgart: Gustav Fischer Verlag. [Google Scholar]
- LarsenK.1963. Contribution to the cytology of the Endemic Canarian Element. Botaniska Notiser 116: 410–424. [Google Scholar]
- LeitchIJ, Bennett MD.1997. Polyploidy in angiosperms. Trends in Plant Science 2: 470–476. [Google Scholar]
- LeitchIJ, Chase MW, Bennett MD.1998. Phylogenetic analysis of DNA C‐values provides evidence for a small ancestral genome size in flowering plants. Annals of Botany 82 (Suppl. A): 85–94. [Google Scholar]
- LysákMA, Dolez̆el J.1998. Estimation of nuclear DNA content in Sesleria (Poaceae). Caryologia 51: 123–132. [Google Scholar]
- MacGillivrayCW, Grime JP.1995. Genome size predicts frost‐resistance in British herbaceous plants – implications for rates of vegetation response to global warning. Functional Ecology 9: 320–325. [Google Scholar]
- MishibaKI, Ando T, Mii M, Watanabe H, Kokubun H, Hashimoto G, Marchesi E.2000. Nuclear DNA content as an index character discriminating taxa in the genus Petunia sensu Jussieu (Solanaceae). Annals of Botany 85: 665–673. [Google Scholar]
- OttoF.1990. DAPI staining of fixed cells for high‐resolution flow cytometry of nuclear DNA. In: Crissman HA, Darzynkiewicz Z, eds. Methods in cell biology, vol. 33 New York: Academic Press, 105–110. [DOI] [PubMed] [Google Scholar]
- PalominoG, Sousa SM.2000. Variation of nuclear DNA content in the biflorus species of Lonchocarpus (Leguminosae). Annals of Botany 85: 69–76. [Google Scholar]
- PaneroJL, Francisco‐Ortega J, Jansen RK, Santos‐Guerra A.1999. Molecular evidence for multiple origins of woodiness and a New World biogeographic connection of the Macaronesian Island endemic Pericallis (Asteraceae: Senecioneae). Proceedings of the National Academy of Sciences of the USA 96: 13886–13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShmidaA, Werger MJA.1992. Growth form diversity on the Canary Islands. Vegetation 102: 183–199. [Google Scholar]
- StevensPF.2002.Angiosperm phylogeny website Version 3, May 2002. http://www.mobot.org/MOBOT/research/APweb/
- StuessyTF, Crawford DJ.1998. Chromosomal stasis during speciation in angiosperms of oceanic islands. In: Stuessy TF, Ono M, eds. Evolution and speciation of island plants Cambridge: Cambridge University Press, 307–324. [Google Scholar]
- World Conservation Monitoring Centre.1992.Global biodiversity: status of the Earth’s living resources. London: Chapman and Hall. [Google Scholar]
- ZonneveldBJM.2001. Nuclear DNA contents of all species of Helleborus (Ranunculaceae) discriminate between species and sectional divisions. Plant Systematics and Evolution 229: 125–130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.