Abstract
Effects of girdling on carbohydrate status and carbohydrate‐related gene expression in citrus trees were investigated. Alternate‐bearing ‘Murcott’ (a Citrus reticulata hybrid of unknown origin) trees were girdled during autumn (25 Sep. 2001) and examined 10 weeks later. Girdling brought about carbohydrate (soluble sugar and starch) accumulation in leaves and shoot bark above the girdle, in trees during their fruitless, ‘off’ year. Trees during their heavy fruit load, ‘on’ year did not accumulate carbohydrates above the girdle due to the high demand for carbohydrates by the developing fruit. Girdling caused a strong decline in soluble sugar and starch concentrations in organs below the girdle (roots), in both ‘on’ and ‘off’ trees. Expression of STPH‐L and STPH‐H (two isoforms of starch phosphorylase), Agps (ADP‐glucose pyrophosphorylase, small subunit), AATP (plastidic ADP/ATP transporter), PGM‐C (phosphoglucomutase) and CitSuS1 (sucrose synthase), all of which are associated with starch accumulation, was studied. It was found that gene expression is related to starch accumulation in all ‘off’ tree organs. RNA levels of all the genes examined were high in leaves and bark that accumulated high concentrations of starch, and low in roots with declining starch concentrations. It may be hypothesized that changes in specific sugars signal the up‐ and down‐regulation of genes involved in starch synthesis.
Key words: Alternate bearing, carbohydrates, Citrus, girdling, soluble sugar, starch
INTRODUCTION
Girdling has been, and is still, widely used in citrus, grape, peach and other fruit tree crops, mainly to increase flowering, fruit set and fruit size (Goren et al., 2003). Girdling consists of removal of a strip of bark from the trunk or major limbs of a fruit tree, thereby blocking the downward translocation of photosynthates and metabolites through the phloem.
The best‐known effects of girdling are presumably brought about by accumulation of assimilates above the girdle. Tree parts beneath the girdle, on the other hand, suffer from shortage of assimilates. Following trunk girdling, the roots are gradually depleted of their carbohydrate reserves, sometimes reaching serious root starvation (Weaver and McCune, 1959). Cytokinin and gibberellin content of shoots is also modified by girdling (Cutting and Lyne, 1993).
The common, general explanation for the effects of girdling is that the development of the reproductive organs benefits from the higher concentrations of assimilates available in the canopy following girdling. The precise biochemical changes resulting from girdling have been studied only in some particular cases (Beruter et al., 1997), and a detailed interpretation of the physiological effects of girdling is still lacking.
Progress in plant research during the past decade has led to the understanding that, beyond their nutritional and energetic contribution, sugars play regulatory roles, which involve up‐ and down‐regulation of gene expression (Koch, 1996; Jang and Sheen, 1997; Smeekens, 2000; Koch and Zeng, 2002). The feast/famine hypothesis of Koch (1996), in particular, provides a model of whole plant carbohydrate‐mediated gene regulation.
The main purpose of the present study was to check whether girdling, which modifies carbohydrate levels in canopy and roots of fruit trees, also involves changes in gene expression. Since starch is the main storage carbohydrate in citrus tissues, we focused on the expression of genes related to starch biosynthesis.
MATERIALS AND METHODS
Plant material and experimental design
Field grown Murcott (a Citrus reticulata hybrid of unknown origin) trees grafted onto a sour orange (Citrus aurantium) rootstock, about 5 years old, were selected for these experiments. Trees of this cultivar reveal an extreme alternate‐bearing habit, with ‘off’ (without fruit) and ‘on’ (with a heavy fruit load) trees present in the same orchard each year. Eight uniform ‘off’ and six uniform ‘on’ trees were used, and each group was divided into two sub‐groups (four each for ‘off’ trees and three each for ‘on’ trees); one was girdled (girdling) and the other not girdled (control). Girdling was performed on 25 Sep. 2001 on the trunk 20 cm above the ground. A ring of bark (about 3 mm in width) around the trunk was removed using a sharp knife and the girdle was renewed every 3 weeks to prevent rapid closure of the girdle. Roots (0·5 cm in diameter), bark (peeled from 1‐year‐old shoots) and mature leaves were sampled on the day of girdling (zero time control) and then on 9 Dec. 2001 from girdled and control trees for evaluation of girdling effects.
Carbohydrate determinations
Samples were dried in the oven (80 °C) for 48 h and then ground into fine powder. Dry tissue samples (100 mg each) were extracted four times with 80 % (v/v) ethanol at 55 °C. All the extract solutions were collected and brought to a final volume of 50 ml. Soluble sugar was determined using anthrone, according to the method of Yemm and Willis (1964). Starch was measured after glucoamylase hydrolysis of the insoluble faction and the released glucose determined according to Hassid and Neufeld (1964). Three tissue samples were extracted for each treatment. The amount of total non‐structural carbohydrates (TNC) was calculated by summing the amount of starch and soluble sugar. Where appropriate, Student’s t‐test was used to determine the statistical significance.
Detection of starch with iodine
Roots (0·5 cm in diameter) and 1‐year‐old shoots (0·5 cm in diameter) were collected from girdled and non‐girdled ‘on’ and ‘off’ trees on 11 Dec. 2001. Samples were hand‐sectioned into 4‐mm‐thick cylinders and immediately immersed in Lugol solution (Sigma, St Louis, MO, USA) containing iodine and potassium iodide. Optimal staining was obtained within 3 min, after which the cylinders were photographed under an Olympus binocular system (Olympus SZX12).
RNA isolation and Northern blot analysis
Total RNA was extracted using a standard SDS–phenol method described in Current Protocol Unit 4·3 (Ausubel et al., 1989). For RNA blot analysis, total RNA samples (20 µg per lane) were separated by electrophoresis in 1 % agarose gels containing formaldehyde and blotted onto Hybond‐NX nylon membrane (Amersham Pharmacia Biotech, Little Chalfont, UK) by capillary transfer. The RNA blots were hybridized with specific cDNA probes (Agps, AF184597; CitSuS1, AB022092; STPH‐L, AY098892; STPH‐H, AY098895; AATP, AY098893; and PGM‐C, AY112996) labelled using a random priming method (Boehringer Mannheim, Germany). Hybridization was carried out at 42 °C in a solution containing 10 % dextran sulfate (Na salt, MW 500 000), 1 % SDS, 2× SSC, 50 % formamide and 5× Denhardt’s solution for 16 h followed by high stringent wash. The hybridized membranes were exposed and analysed by Phosphorimager (FUJI BAS 1000). Each membrane was re‐probed several times after stripping and finally re‐hybridized with citrus 18S rRNA. All hybridization experiments have been carried out at least twice, with identical results.
RESULTS
Carbohydrate concentrations in leaves and bark of girdled trees
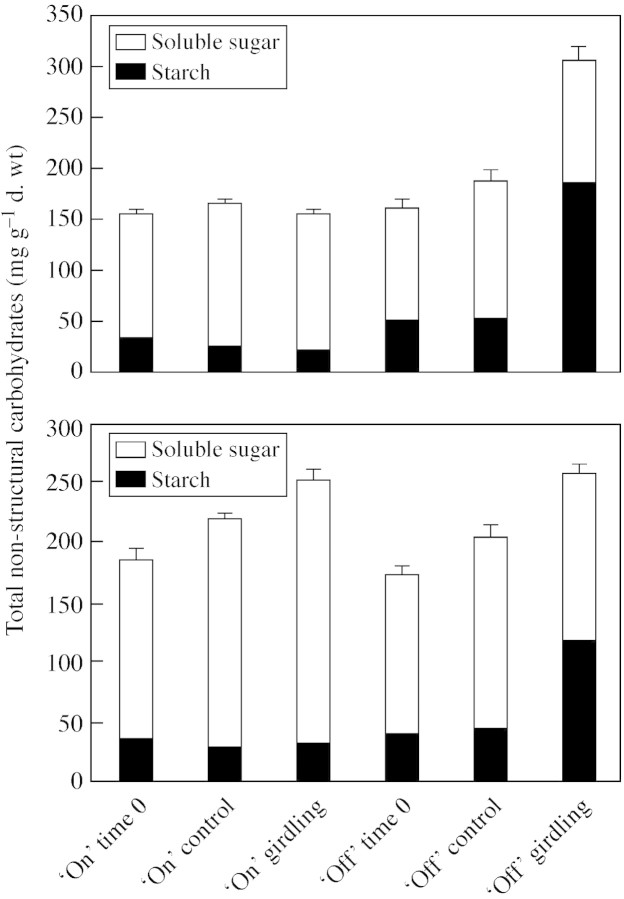
Removal of a strip of phloem from the main trunk by girdling actually blocks the transport of sugars to the roots; large amounts of carbohydrates produced by photosynthesis will accumulate in vegetative organs above the girdle or be utilized for fruit development. In ‘on’ trees, no increase in TNC concentration was found in girdled tree leaves and only a small increase in TNC was found in girdled tree bark (Fig. 1), which was mainly the result of accumulation of soluble sugar (Fig. 1). In ‘off’ trees, TNC concentration increased in leaves and bark following girdling (Fig. 1), with leaves showing a remarkable increase (P < 0·01) in comparison with non‐girdled control trees. The increase in carbohydrates was mainly in the form of starch. The concentration of starch in girdled tree leaves was three times that of non‐girdled control tree leaves, and in girdled tree bark twice that of control tree bark (Fig. 1). Soluble sugar concentrations in girdled tree leaves and bark did not change significantly (P > 0·05). Iodine staining of shoot cross‐sections showed that girdled ‘off’ trees accumulated a large amount of starch in the bark and in the pith (Fig. 2A and C). ‘On’ shoots, on the other hand (Fig. 2B and D), appeared to be devoid of starch, except for the outer bark in girdled trees as well as in controls.
Fig. 1. Total non‐structural carbohydrates (TNC) (soluble sugar and starch) in leaves (top) and bark (bottom) of girdled and non‐girdled ‘on’ and ‘off’ citrus trees (means ± s.d. of three independent samples, n = 3).
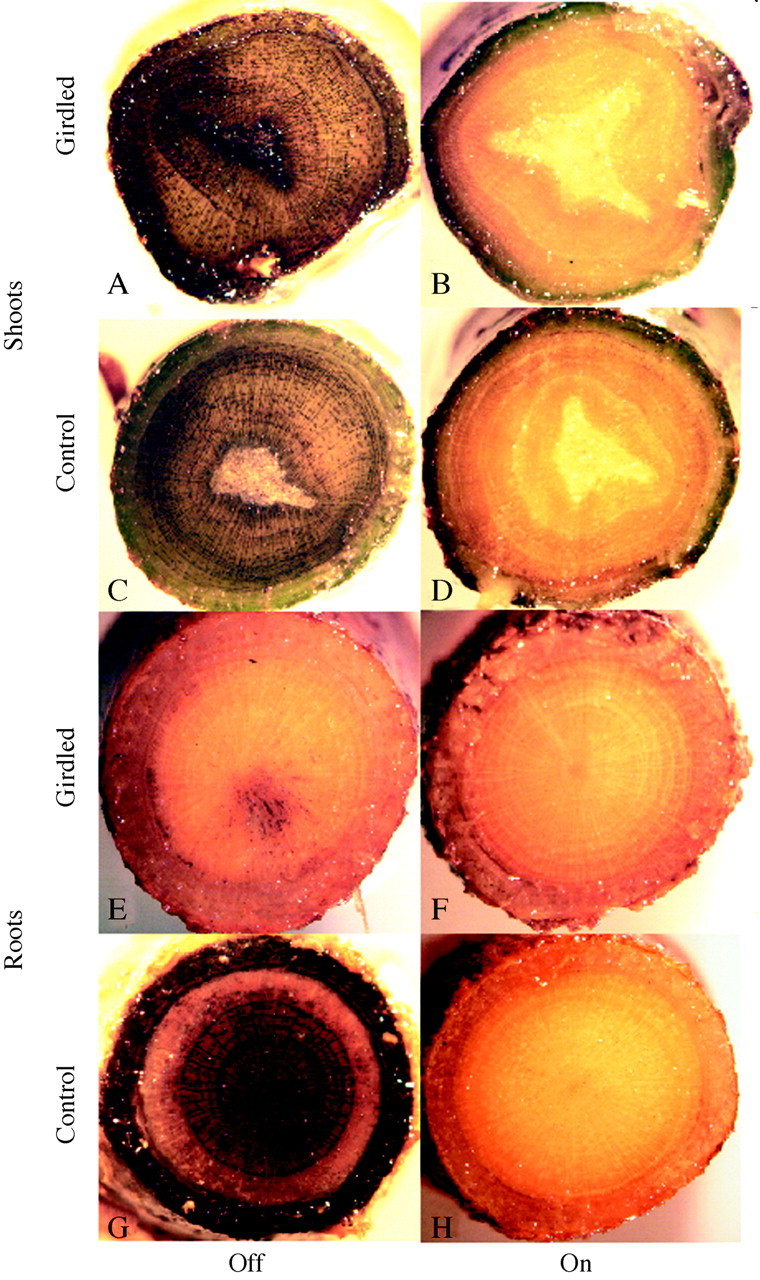
Fig. 2. Iodine staining of shoot (A–D) and root (E–H) cross‐sections. A and E, Girdled ‘off’ trees; B and F, girdled ‘on’ trees; C and G, non‐girdled ‘off’ trees; D and H, non‐girdled ‘on’ trees. The cross‐sections of shoot and roots are 0·5 cm in diameter.
Relative mRNA levels in leaves and bark of girdled trees
To investigate whether girdling effects involve regulation at the RNA level, expression of several genes related to carbohydrate metabolism was analysed: STPH‐H, STPH‐L, Agps, PGM‐C, AATP and CitSuS1. STPH‐H and STPH‐L code for two isoforms of starch phosphorylase, type‐H and type‐L, respectively, Agps codes for a small subunit of ADP glucose pyrophosphorylase, PGM‐C encodes a cytosolic phosphoglucomutase, AATP is a plastidic ATP/ADP transporter gene and CitSuS1 encodes a sucrose synthase. Girdling induced the expression of STPH‐H, STPH‐L, Agps, PGM‐C and CitSuS1 in both leaves and bark of ‘off’ trees (Fig. 3), corresponding to starch accumulation in these organs (Fig. 1). In ‘on’ trees, however, the changes in mRNA transcription levels did not correspond to the changes in starch concentration.
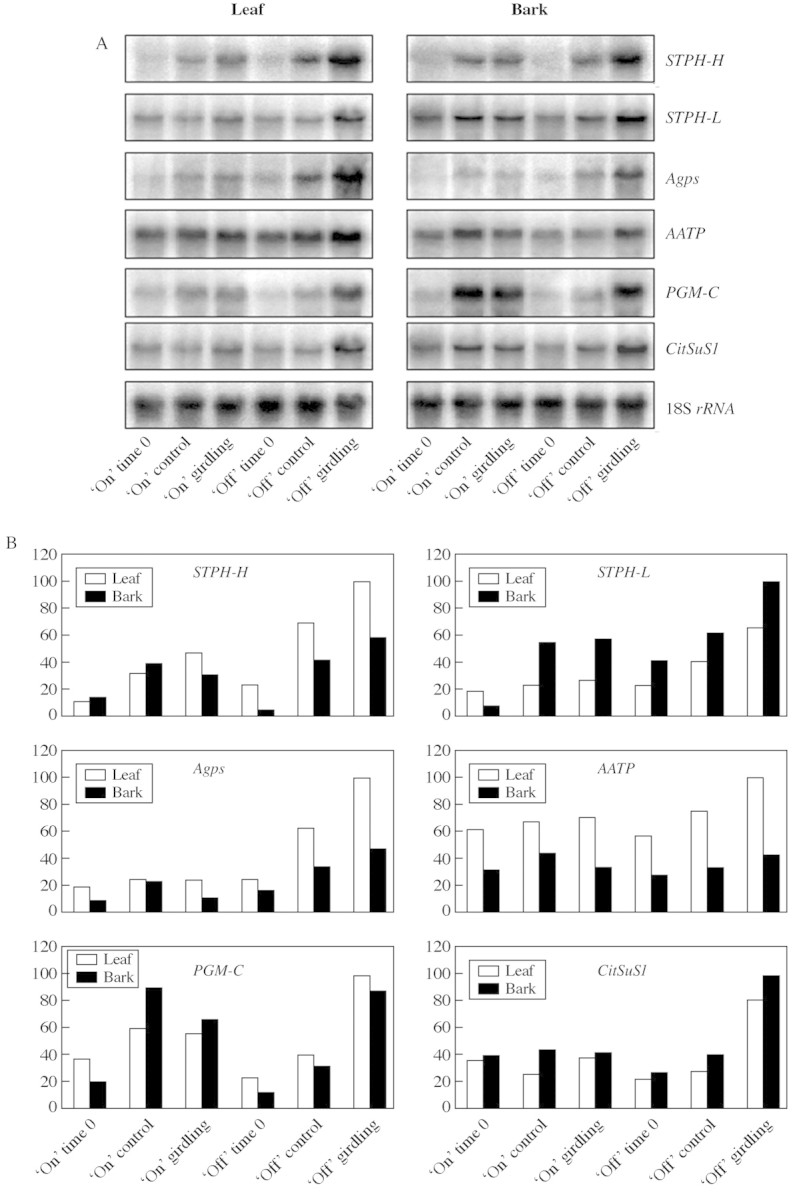
Fig. 3. Relative transcript levels of STPH‐H, STPH‐L, Agps, AATP, PGM‐C and CitSuS1 in leaves and bark of girdled and non‐girdled ‘on’ and ‘off’ citrus trees (A). Total RNAs (20 µg) isolated from leaves and bark were separated and analysed by RNA blot. 18S rRNA from citrus was used as an internal control for loading differences. Relative levels of transcripts (B) were estimated by quantitation of signals on the blotted membranes with a Phosphorimager BAS1000 (Fuji Photo Film, Tokyo, Japan). The results are expressed as a percentage of the respective maximum level for each gene. Results are representative data of two independent experiments, the same as for Fig. 5.
Carbohydrate concentrations in roots of girdled trees
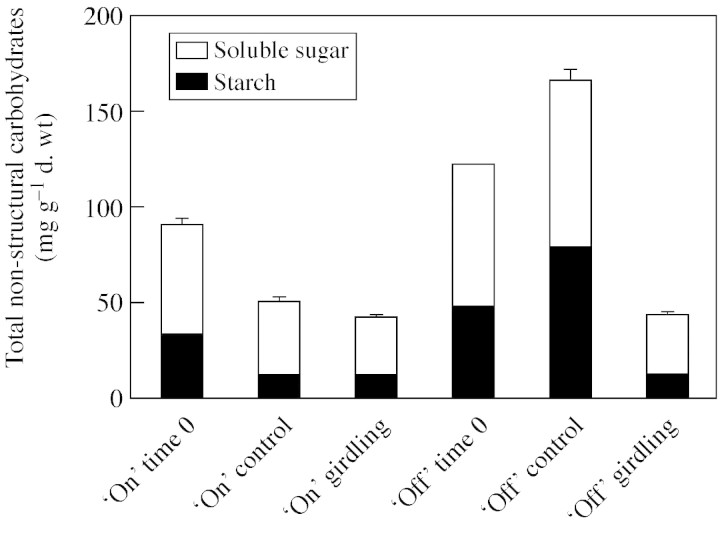
Opposite seasonal trends of root carbohydrate concentrations were found in ‘on’ and ‘off’ trees (Fig. 4). In ‘on’ trees the concentration of TNC and each of its components, starch and soluble sugar, declined between ‘time 0’ (25 Sep. 2001) and the end of the experiment (9 Dec. 2001). Girdling caused a further, slight decline. In ‘off’ tree roots, TNC concentrations, and those of starch in particular, were already high at ‘time 0’ and continued to rise in non‐girdled trees during the experiment. However, girdling markedly reduced both starch (P < 0·01) and soluble sugar (P < 0·01) concentrations, down to levels comparable with those of ‘on’ roots. These findings were also confirmed by the iodine staining of root cylinders (Fig. 2E–H). Non‐girdled ‘off’ tree roots (Fig. 2G) were loaded with starch in the bark as well as in the vascular cylinder of root cross‐sections. Girdled ‘off’ tree roots (Fig. 2E), and girdled as well as non‐girdled ‘on’ tree roots (Fig. 2F and H) stained poorly, indicating a low concentration of starch.
Fig. 4. Total non‐structural carbohydrates (TNC) (soluble sugar and starch) in roots of girdled and non‐girdled ‘on’ and ‘off’ citrus trees (means ± s.d. of three independent samples, n = 3).
Relative mRNA levels in roots of girdled citrus roots
Expression of STPH‐H, STPH‐L, Agps, AATP, PGM‐C and CitSuS1 in girdled tree roots was analysed by Northern blot analysis. Results showed that all the genes examined were down‐regulated in girdled ‘off’ tree roots (Fig. 5). Among them, STPH‐H, STPH‐L and Agps showed an 80–95 % reduction, whereas AATP, PGM‐C and CitSuS1 had only a <50 % reduction in transcript levels compared with non‐girdled control roots. The transcript levels of all the genes examined increased in girdled ‘on’ tree roots in comparison with non‐girdled control roots (Fig. 5), with Agps and STPH‐L showing a marked increase.
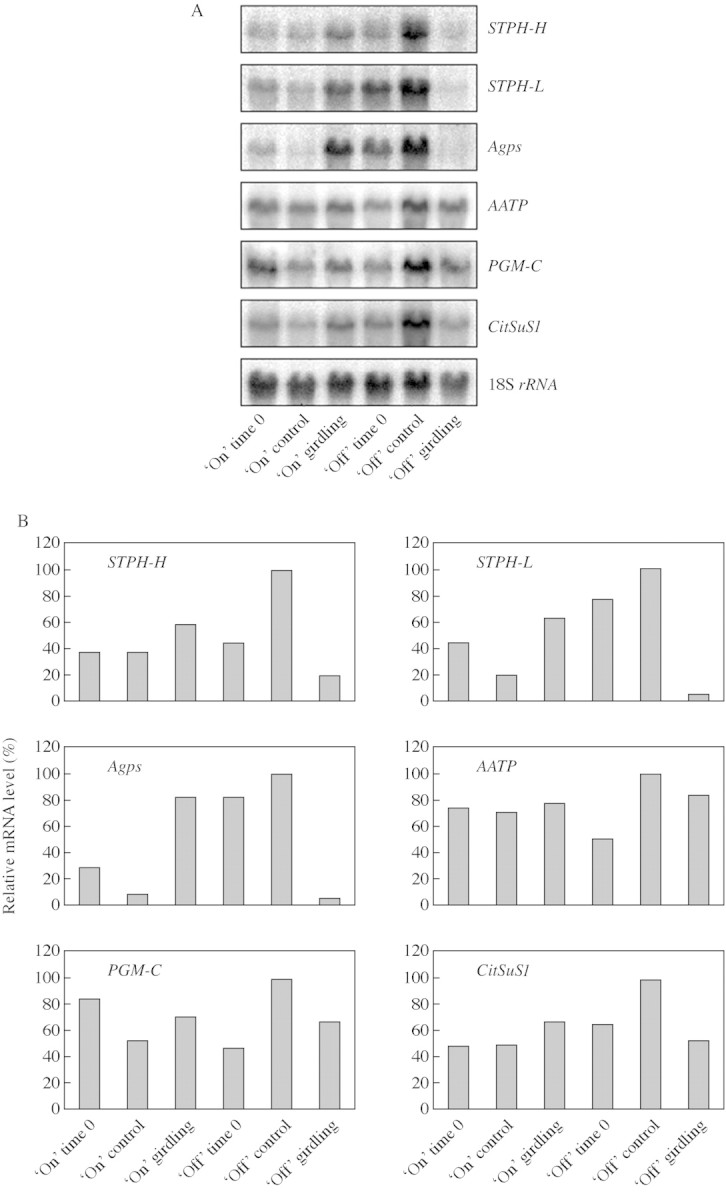
Fig. 5. Relative transcript levels of STPH‐H, STPH‐L, Agps, AATP, PGM‐C and CitSuSy1 in roots of girdled and non‐girdled ‘on’ and ‘off’ citrus trees (A). Total RNAs (20 µg) isolated from roots were separated and analysed by RNA blot. 18S rRNA from citrus was used as an internal control for loading differences. Relative levels of transcript (B) were estimated as described in Fig. 3.
DISCUSSION
The results of the present investigation confirm earlier observations but, at the same time, provide some new insight into the study of girdling. Girdling results in accumulation of carbohydrates above the girdle, but such an accumulation takes place only when there is an excess of carbohydrates, as occurs during ‘off’ years. As shown by Garcia‐Luis et al. (1995), the presence of fruit prevents the girdling‐induced accumulation of carbohydrates. During ‘on’ years the high, barely saturable demand by the developing fruit (Fishler et al., 1983; Goldschmidt, 1999) consumes all the available carbohydrates and prevents any accumulation of carbohydrates in leaves and bark (Fig. 1).
Starch has been shown to be the major storage carbohydrate in citrus (Goldschmidt and Golomb, 1982). Assuming that starch accumulation in shoots is confined to the bark, bark samples were used for shoot carbohydrate determinations. However, the iodine staining of shoot sections revealed that starch accumulation in the shoot is not confined to the bark; considerable starch accumulation takes place in the xylem rays (Fig. 2C). Indeed, carbohydrates can be stored in ray parenchyma and other parenchymatous cells associated with the xylem. When there is a large excess of carbohydrates, as in ‘off’ trees, starch accumulates even in the innermost part of the shoot, the pith (Fig. 2A).
The effects of girdling on root metabolism have only rarely been studied in detail (Wallerstein et al., 1978). During ‘on’ years root carbohydrate concentrations are relatively low and the effects of girdling are minimal (Fig. 4). During ‘off’ years, there is a dramatic increase in root starch and soluble sugar concentrations in non‐girdled control trees (Fig. 4). Girdling curtails this accumulation, and carbohydrates drop to very low levels, presumably due to the respiratory and metabolic needs of the root system. Starch quantitative determinations are corroborated by iodine staining: in non‐girdled control roots large amounts of starch are deposited in the bark and in the vascular cylinder of the root (Fig. 2G).
The expression of several genes (STPH‐H, STPH‐L, Agps, AATP, PGM‐C and CitSuS1) was investigated in the present study; the proteins encoded by all these genes seem to play a role in starch synthesis. Both STPH‐L and STPH‐H encode for starch phosphorylase, an enzyme that catalyses the reversible reaction for degrading or synthesizing starch polymers (Stitt and Steup, 1985). Starch phosphorylase activity has been shown to correlate with rapid starch synthesis in buttercup squash (Irving et al., 1999). High expression of plastidic starch phosphorylase genes (STPH‐L) was found to correlate with starch biosynthesis in potato (Duwenig et al., 1997a), spinach (Duwenig et al., 1997b), pea (van Berkel et al., 1991) and maize (Yu et al., 2001). ADP‐glucose pyrophosphorylases (AGPs), encoded by Agps, catalyse the rate‐limiting step for starch biosynthesis in both photosynthetic and non‐photosynthetic tissues (Weber et al., 2000). The plastidic ATP/ADP translocator, encoded by AATP, is involved in starch synthesis by providing ATP, which is required for AGPs activity, to the plastid (Neuhaus et al., 1997). The cytosolic phosphoglucomutase, encoded by PGM‐C, was found to play a critical role in starch accumulation in the potato tuber (Fernie et al., 2002). Sucrose synthase, encoded by SuSy, is also associated with starch synthesis (Dejardin et al., 1997).
All six genes were highly expressed in leaves and bark of girdled ‘off’ trees (Fig. 3), as well as in non‐girdled ‘off’ roots (Fig. 5), all of which accumulated high concentrations of starch. The up‐regulation of these genes in starch‐accumulating organs is supported by the scheme of Kossmann and Lloyd (2000) that illustrates the pathway for starch accumulation. When there is an excess supply of carbohydrates, as in the case of ‘off’ trees, sucrose can be converted into starch via a series of reactions involving sucrose synthase, cytoplasmic phosphoglucomutase and AGPs. Recent evidence indicates that the CitSuS1 gene is up‐regulated by exogenous sucrose and glucose in citrus leaf discs (C.‐Y. Li, D. Weiss and E. E. Goldschmidt, unpubl. res.). Agps expression is also increased by sugars (Sokolov et al., 1998). In girdled ‘off’ tree roots, the low concentrations of starch and soluble sugar (Fig. 4) are accompanied by down‐regulation of STPH‐H, STPH‐L and Agps, which are apparently very responsive to sugar concentrations. AATP, on the other hand, is only slightly affected by the carbohydrate status in leaves, bark (Fig. 3) and root (Fig. 5) and seems to be constitutively expressed. PGM‐C and, to a lesser extent, both forms of STPH, are strongly expressed in ‘on’ tree leaves and bark in spite of their low starch content, corresponding perhaps to the high concentration of soluble sugar in these tissues (Fig. 3).
In conclusion, it has been demonstrated that even seemingly simple agrotechnical manipulations like girdling involve extensive changes in gene expression. Following recent literature (Smeekens, 2000; Koch and Zeng, 2002; Rolland, 2002) it might be hypothesized that changes in specific sugar components, which have yet to be identified in citrus, signal the regulation of genes involved in starch biosynthesis (and other carbohydrate‐related genes) in citrus tree organs.
ACKNOWLEDGEMENTS
Thanks are due to Professor J. Riov (The Hebrew University of Jerusalem, Israel) for critical reading of the manuscript. This work was supported by funds from Israeli Citrus Marketing Board to E.E.G.
Supplementary Material
Received: 10 February 2003; Returned for revision: 10 March 2003; Accepted: 21 March 2003 Published electronically: 21 May 2003
References
- AusubelFM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K.1989.Current protocols in molecular biology, Vol. 1. Wiley, New York. [Google Scholar]
- BeruterJ, Feusi MES.1997. The effect of girdling on carbohydrate partitioning in the growing apple fruit. Journal of Plant Physiology 151: 277–285. [Google Scholar]
- CuttingJGM, Lyne MC.1993. Girdling and the reduction in shoot xylem sap concentrations of cytokinins and gibberellins in peach. Journal of Horticultural Science 68: 619–626. [Google Scholar]
- DejardinA, Rochat C, Wuilleme S, Boutin JP.1997. Contribution of sucrose synthase, ADP‐glucose pyrophosphorylase and starch synthase to starch synthesis in developing pea seeds. Plant and Cell Environment 20: 1421–1430. [Google Scholar]
- DuwenigE, Steup M, Kossmann J.1997a. Induction of genes encoding plastidic phosphorylase from spinach (Spinacia oleracea L.) and potato (Solanum tuberosum L.) by exogenously supplied carbohydrates in excised leaf discs. Planta 203: 111–120. [DOI] [PubMed] [Google Scholar]
- DuwenigE, Steup M, Willmitzer L, Kossmann J.1997b. Antisense inhibition of cytosolic phosphorylase in potato plants (Solanum tuberosum L.) affects tuber sprouting and flower formation with only little impact on carbohydrate metabolism. The Plant Journal 12: 323–333. [DOI] [PubMed] [Google Scholar]
- FernieAR, Tauberger E, Lytovchenko A, Roessner U, Willmitzer L, Trethewey RN.2002. Antisense repression of cytosolic phos phoglucomutase in potato (Solanum tuberosum) results in severe growth retardation, reduction in tuber number and altered carbon metabolism. Planta 214: 510–520. [DOI] [PubMed] [Google Scholar]
- FishlerM, Goldschmidt EE, Monselise SP.1983. Leaf‐area and fruit size on girdled grapefruit branches. Journal of the American Society for Horticultural Science 108: 218–221. [Google Scholar]
- Garcia‐LuisA, Fornes F and Guardiola JL 1995. Leaf carbohydrates and flower formation in Citrus Journal of the American Society for Horticultural Science 120: 222–227. [Google Scholar]
- GoldschmidtEE.1999. Carbohydrate supply as a critical factor for citrus fruit development and productivity. Hortscience 34: 1020–1024. [Google Scholar]
- GoldschmidtEE, Golomb A.1982. The carbohydrate balance of alternate‐bearing citrus trees and the significance of reserves for flowering and fruiting. Journal of the American Society for Horticultural Science 107: 206–208. [Google Scholar]
- GorenR, Huberman M, Goldschmidt EE.2003. Girdling: physiological and horticultural aspects. Horticultural Reviews (in press). [Google Scholar]
- HassidWZ, Neufeld EF.1964. Quantitative determination of starch in plant tissue. Methods in Carbohydrate Chemistry 4: 33–36. [Google Scholar]
- IrvingDE, Shingleton GJ, Hurst PL.1999. Starch degradation in buttercup squash (Cucurbita maxima). Journal of the American Society for Horticultural Science 124: 587–590. [Google Scholar]
- JangJC, Sheen J.1997. Sugar sensing in higher plants. Trends in Plant Science 2: 208–214. [Google Scholar]
- KochKE.1996. Carbohydrate modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 509–540. [DOI] [PubMed] [Google Scholar]
- KochKE, Zeng Y.2002. Molecular approaches to altered C partitioning: genes for sucrose metabolism. Journal of the American Society for Horticultural Science 127: 474–483. [Google Scholar]
- KossmannJ., Lloyd J.2000. Understanding and influencing starch biochemistry. Critical Reviews in Plant Science 19: 171–226. [PubMed] [Google Scholar]
- NeuhausHE, Thom E, Möhlmann T, Steup M, Kampfenkel K.1997. Characterization of a novel eukaryotic ATP/ADP translocator located in the plastid envelope of Arabidopsis thaliana L. The Plant Journal 11: 73–82. [DOI] [PubMed] [Google Scholar]
- RollandF, Moore B and Sheen J.2002. Sugar sensing and signaling in plants. The Plant Cell 14: S185–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SmeekensS.2000. Sugar‐induced signal transduction in plants. Annual Review of Plant Molecular Biology and Plant Physiology 51: 49–81. [DOI] [PubMed] [Google Scholar]
- SokolovLN, Déjardin A, Kleczkowski LA.1998. Sugars and light/dark exposure trigger differential regulation of ADP‐glucose pyro phosphorylase genes in Arabidopsis thaliana (thale cress). Bio chemical Journal 336: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StittM, Steup M.1985. Starch and sucrose degradation. In: Douce R and Day DA, eds. Encylopedia of plant physiology (new series). Vol. 18. Higher plant cell respiration Springer‐Verlag, Berlin, 347–390. [Google Scholar]
- vanBerkelJ, Conradsstrauch J, Steup M.1991. Glucan‐phosphorylase forms in cotyledons of Pisum sativum L. – localization, develop mental‐change, in vitro translation, and processing. Planta 185: 432–439. [DOI] [PubMed] [Google Scholar]
- WallersteinI, Goren R, Bental Y.1978. Effect of ringing on root starvation in sour orange seedlings. Journal of Horticultural Science 53: 109–113. [Google Scholar]
- WeaverRJ, McCune SB.1959. Girdling: its relation to carbohydrate nutrition and development of Thompson Seedless, Red Malaga and Ribier grapes. Hilgardia 28: 421–456. [Google Scholar]
- WeberH, Rolletschek H, Heim U, Golombek S, Gubatz S, Wobus U.2000. Antisense‐inhibition of ADP‐glucose pyrophosphorylase in developing seeds of Vicia narbonensis moderately decreases starch but increases protein content and affects seed maturation. The Plant Journal 24: 33–43. [DOI] [PubMed] [Google Scholar]
- YemmEW, Willis AJ.1964. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal 57: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YuY, Mu HH, Wasserman BP, Carman GM.2001. Identification of the maize amyloplast stromal 112‐kD protein as a plastidic starch phosphorylase. Plant Physiology 125: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.