Abstract
To model the effect of increasing atmospheric CO2 on semi‐arid grasslands, the gas exchange responses of leaves to seasonal changes in soil water, and how they are modified by CO2, must be understood for C3 and C4 species that grow in the same area. In this study, open‐top chambers were used to investigate the photosynthetic and stomatal responses of Pascopyrum smithii (C3) and Bouteloua gracilis (C4) grown at 360 (ambient CO2) and 720 µmol mol–1 CO2 (elevated CO2) in a semi‐arid shortgrass steppe. Assimilation rate (A) and stomatal conductance (gs) at the treatment CO2 concentrations and at a range of intercellular CO2 concentrations and leaf water potentials (ψleaf) were measured over 4 years with variable soil water content caused by season and CO2 treatment. Carboxylation efficiency of ribulose bisphosphate carboxylase/oxygenase (Vc,max), and ribulose bisphosphate regeneration capacity (Jmax) were reduced in P. smithii grown in elevated CO2, to the degree that A was similar in elevated and ambient CO2 (when soil moisture was adequate). Photosynthetic capacity was not reduced in B. gracilis under elevated CO2, but A was nearly saturated at ambient CO2. There were no stomatal adaptations independent of photosynthetic acclimation. Although photosynthetic capacity was reduced in P. smithii growing in elevated CO2, reduced gs and transpiration improved soil water content and ψleaf in the elevated CO2 chambers, thereby improving A of both species during dry periods. These results suggest that photosynthetic responses of C3 and C4 grasses in this semi‐arid ecosystem will be driven primarily by the effect of elevated CO2 on plant and soil water relations.
Key words: Bouteloua gracilis, Pascopyrum smithii, C3, C4, leaf water potential, photosynthesis, acclimation, stomata, semi‐arid, soil water
INTRODUCTION
Arid and semi‐arid ecosystems occupy 40 % of the Earth’s land surface (Dregne, 1991), so an understanding of the responses of these biomes to increasing atmospheric CO2 is critical. Water‐limited ecosystems are predicted to have relatively large responses to increasing CO2 because elevated CO2 decreases stomatal conductance and leaf transpiration, thereby improving soil water content (Volk et al., 2000). A complicating factor in predicting the responses of grasslands to increasing CO2 is that many grasslands have co‐occuring C3 and C4 photosynthesis species (Wand et al., 1999). Ehleringer et al. (1997) proposed that the global distribution of C3 and C4 plants was related to inherent differences in the quantum yield of photosynthesis. In theory, quantum yield is improved in C3 plants as CO2 increases, potentially expanding the global distribution of C3 species. But the influence of elevated CO2 on photosynthesis may be less important in arid ecosystems where the effects of water may dominate. An understanding of how seasonal and CO2‐induced variation in soil water affects photosynthetic and stomatal responses of competing C3 and C4 species is needed to predict the responses of arid grasslands to increasing CO2.
Although carbon assimilation is stimulated relatively more in C3 than in C4 species when exposed to short‐term increases in CO2, this increase is often not maintained in plants exposed to elevated CO2 for weeks or months (Sage, 1994; Moore et al., 1999; Stitt and Krapp, 1999). This phenomenon has been termed ‘photosynthetic acclimation’. The physiological basis for photosynthetic acclimation is not completely understood, but is often related to reduced leaf nitrogen and photosynthetic enzyme activity (Moore et al., 1999; Stitt and Krapp, 1999). Little research has been done on possible stomatal acclimation to elevated CO2, though reduced stomatal conductance and leaf transpiration may significantly affect ecosystem processes (Drake et al., 1997; Jarvis et al., 1999; Lee et al., 2001).
Field studies are necessary to ascertain the responses of plants to elevated CO2. There are few field studies in which the effects of elevated CO2 on grasslands have been investigated, and even fewer studies in which C3 and C4 species occur in the same ecosystem (Wand et al., 1999). There was no evidence of photosynthetic acclimation in C3 or C4 grasses in a tallgrass prairie (Nie et al., 1992; Knapp et al., 1993), a Texas grassland (Anderson et al., 2001) or in a C3 annual grassland (Jackson et al., 1995). Conversely, photosynthetic acclimation was reported in both C3 and C4 species in a Minnesota grassland, and in C3 grasses, but not a C4 grass, in New Zealand (Lee et al., 2001; von Caemmerer et al., 2001). Therefore, field studies suggest that the photosynthetic responses to elevated CO2 in grasslands are site‐ and species‐dependent. A deficit of resources other than CO2 (soil N, soil water, rooting volume) can limit the production of new sink tissues when assimilation rates are improved (Drake et al., 1997; Rogers et al., 1998; Lee et al., 2001). This source/sink imbalance causes an accumulation of carbohydrates, which is thought to feed back on photosynthetic processes (Sage, 1994; Stitt and Krapp, 1999).
Growth chamber studies have been used to investigate the photosynthetic and growth responses of a C3 [Western wheatgrass; Pascopyrum smithii (Rydb.) A. Love] and a C4 grass [Blue grama; Bouteoloua gracilis (H.B.K.) Lag.] to elevated CO2 (Morgan et al., 1994a, b; Hunt et al., 1996; Read et al., 1997; LeCain and Morgan, 1998; Morgan et al., 1998). These are major species of the semi‐arid shortgrass steppe (SGS) of the North American Great Plains (Milchunas et al., 1989). Photosynthetic acclimation to elevated CO2 was seen in both C3 and C4 species (Morgan et al., 1994a; Read et al., 1997; LeCain and Morgan, 1998). Despite the relative reduction, photosynthesis rates were slightly higher under elevated vs. ambient CO2. Growth improvement in both species was attributed to higher rates of photosynthesis and improved soil and plant water status (Morgan et al., 1994,a, b; Read and Morgan, 1996; Morgan et al., 1998).
To increase understanding of the responses of C3 and C4 grasses to elevated CO2 in a water‐limited ecosystem, a field study was performed on the native SGS in north‐eastern Colorado, USA. We hypothesized that photosynthesis of both P. smithii and B. gracilis would acclimate under elevated CO2, but that there would still be a direct photosynthetic stimulation, especially in P. smithii. Leaf nitrogen, carbohydrates and assimilation at varying intercellular [CO2] were measured to investigate the physiology of photosynthetic adaptations. We also hypothesized that stomatal conductance and transpiration of leaves would be reduced under elevated CO2, and that stomatal acclimation would not occur. Measurements were conducted over four seasons (1997–2000) to document the responses to seasonal and CO2‐induced soil moisture variation.
MATERIALS AND METHODS
The study site is the USDA‐ARS Central Plains Experimental Range, 40°50′N, 104°47′W, in the SGS region of north‐eastern Colorado, USA. Mean annual precipitation (55 years) averaged 320 mm, and air temperatures averaged 15·6 °C in summer and 0·6 °C in winter, with maximum July temperatures of 30·6 °C (Milchunas et al., 1989). The soil at the experimental site is a Remmit fine sandy loam (Ustollic camborthids) to greater than 1 m. This is considered to be a nitrogen‐poor soil (Hunt et al., 1988), with a total nitrogen content of 0·085 % in the upper 30 cm (A. Mosier, pers. comm.).
This experiment was established in spring 1997 on a 6 ha field of native grassland. Although much of the SGS is dominated by B. gracilis (Milchunas et al., 1989), this site was chosen for its nearly equal mix of C3 and C4 grasses (Morgan et al., 2001). The field was divided into three blocks, based on vegetation, and three plots per block were chosen at random. From mid‐March until late October in 1997, 1998, 1999 and 2000, large (60·5 m3) open‐top chambers were placed on two plots in each of the three blocks. One chamber received ambient air (approx. 360 µmol mol–1 CO2), the other received air with elevated CO2 (720 µmol mol–1 CO2). Each block also included an ‘unchambered control’ plot of equal ground area, which was used to monitor the effect of the chamber.
The chambers were 3·8 m high and 4·5 m in diameter, with plastic walls and top (Lexan; Regal Plastics, Littleton, CO, USA), with a 0·75‐m opening. An aluminium flange was buried to 0·75 m around the chamber wall. The chambers were aspirated at an exchange rate of 1·5 volumes min–1. The CO2 concentration was monitored using an infrared gas analyser (LI6262; LI‐COR, Lincoln, NE, USA) and was elevated with pure CO2 in three of the chambers (720 ± 20 µmol mol–1). Air temperature (15 cm above the soil) and soil temperature (10 cm depth), measured using thermocouples, light intensity above the plant canopy (LI‐190SA; LI‐COR) and relative humidity at 2 m height (Vaisala HMP45C; Campbell Scientific, Logan, UT, USA) were recorded hourly. Since the chamber top kept out much of the precipitation, rain was captured and returned to the plots using an automatic system. For a more detailed description of the chambers see Morgan et al. (2001). Soil water content (SWC) was measured weekly using a neutron probe (model 4301; Troxler Electronics Lab., Research Triangle Park, NC, USA).
Exchange of CO2 and H2O were measured on recently expanded leaves of B. gracilis and P. smithii using a portable gas analysis system [CIRAS‐1with PLC (N) leaf chamber; PP systems, Hitchin, UK]. Steady‐state measurements of CO2 assimilation rate (A), stomatal conductance to water vapour (gs), transpiration (E) and intercellular CO2 concentration (Ci) at cuvette concentrations (Ca) of 50, 100, 200, 360, 500, 720, 900 (and 1200 in P. smithii) µmol mol–1 CO2 were made on leaves during mid‐May, mid‐July and late August (early, mid‐ and late season) in 1997, 1998 and 1999 in the ambient and elevated CO2 plots. Drought prevented reliable A : Ci measurement in 2000. Cuvette temperature and vapour pressure were set to ambient conditions and kept constant when varying Ca. Measurements were performed in the unchambered plots only at ambient CO2.
Since responses to seasonal and CO2‐induced variation in soil water were of interest, A, gs, E and the leaf water‐use efficiency (WUE; CO2 gain/H2O loss) were measured at the two growth CO2 concentrations every 3 to 4 weeks during 1997, 1998, 1999 and 2000 on all plots. Leaf water potential (ψleaf) was measured on these dates using a pressure chamber (Plant Measurement Systems, Corvalis, OR, USA). The light unit of the gas exchange system provided 1300 µmol m–2 s–1 photosynthetic photon flux (PPF). Photosynthesis was saturated at this light intensity in both species (data not shown). In addition, during early, mid‐ and late season, the photosynthetic response to varying light intensity was measured at the growth CO2 concentration at 0, 50, 100, 160, 290, 590, 850 and 1300 µmol m–2 s–1 PPF.
Leaves similar to those in the cuvette were collected at midday, frozen in liquid nitrogen, lyophylized and ground to 0·5 mm. The content of starch, sucrose, hexose, fructans and total non‐structural carbohydrates (TNC) was determined using a modified method of Hendrix (1993) (Morgan et al., 1998). Nitrogen concentration was measured on a combustion N analyser (PDZ Europa Ltd, Northwich, UK). Leaf carbohydrates and N were calculated on a structural dry mass basis by subtracting TNC from total leaf dry mass.
The mechanistic analysis of A : Ci and A : PPF curves for P. smithii (C3) were made using ‘Photosynthesis Assistant’ software (Dundee Scientific, Dundee, UK). The maximum rate of carboxylation by ribulose‐1,5 bisphosphate carboxylase/oxygenase (Rubisco) (Vc,max), the PPF‐saturated rate of electron transport (Jmax), and the apparent quantum efficiency (Q) were calculated. The light‐ and CO2‐saturated assimilation rate (Asat) was also determined from A : Ci curves. The mechanistic interpretation of A : Ci curves is not well worked out for C4 plants. Therefore, to investigate photosynthetic adaptations in B. gracilis, the carboxylation efficiency (CE), which is an indicator of efficiency of the phosphoenol‐pyruvate carboxylase CO2 pump, was calculated from the initial slope of the A : Ci curves (Long et al., 1993; Sage, 1994). Asat and Q were also calculated for B. gracilis.
Data presented in the figures are means of three replications ± s.e., with statistical significance tested using the SAS ANOVA procedure (SAS Institute Inc., Cary, NC, USA). Data in the tables were analysed using the SAS PROC MIXED procedure, with year as a repeated measures variable. Correlations between parameters were tested using the SAS PROC REG or PROC NLIN procedures, depending on the best fit of the data.
RESULTS
Chamber conditions, precipitation and soil water content
Air and soil temperatures in the chambers were, on average, 2·6 and 1·3 °C higher than in the unchambered plots, but there was no difference in vapour pressure. The plastic covering blocked approx. 5 % of the PPF, while shading from the chamber framework reduced daily PPF by 28 %.
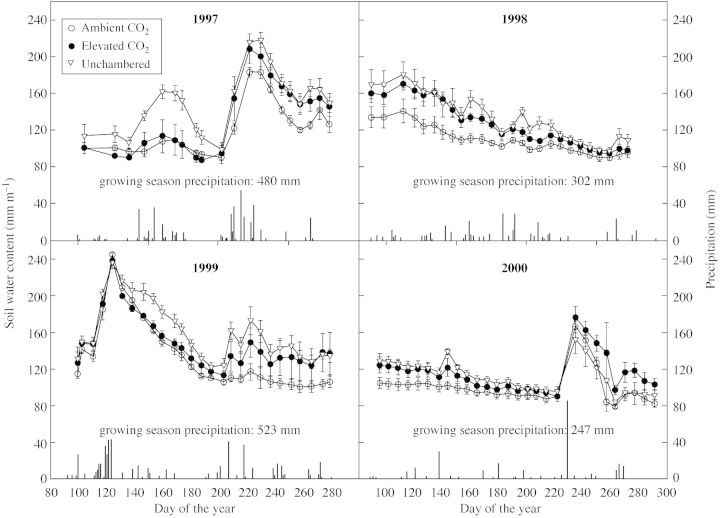
Total growing season precipitation was 480, 302, 523 and 247 mm in 1997, 1998, 1999 and 2000 (long term average = 280 mm). Soil water content was higher in the elevated vs. ambient CO2 chambers on many dates (Fig. 1). When averaged over the 4 years of study, SWC in the upper metre was 129·4 mm in elevated CO2 chambers compared with 113·6 mm in ambient CO2 chambers, an improvement of 14 % (P = 0·02). Ambient CO2 chambers often had lower SWC than the unchambered plots owing to higher air temperatures.
Fig. 1. Soil moisture (to 1 m) and growing season precipitation for 4 years in open‐top chambers with ambient (360 µmol mol–1) and elevated (720 µmol mol–1) CO2 and in plots without chambers on the Colorado shortgrass steppe. Data are means of three replications ± s.e.
Photosynthetic adaptations to elevated CO2
Table 1 shows photosynthetic parameters for P. smithii. Long‐term growth at elevated CO2 reduced Vc,max, Jmax, and Asat by an average of 36, 39, and 36 % respectively (all P < 0·001). This indicates strong and consistent photosynthetic acclimation in this C3 grass. Although there was a significant seasonal effect on these parameters, there was no interaction between CO2 treatment and season or year.
Table 1.
The maximum rate of carboxylation by Rubisco (Vc,max), the PPF‐saturated rate of electron transport (Jmax), assimilation at saturating light and CO2 (Asat), and the apparent quantum efficiency (Q) in Pascopyrum smithii (C3) at three periods during the growing season
| Vc,max (µmol CO2 m–2 s–1) | Jmax (µmol e– m–2 s–1) | Asat (µmol CO2 m–2 s–1) | Q (CO2/photon) | |
| Early season | ||||
| Ambient CO2 | 63·3 ± 4 | 206 ± 20 | 32·8 ± 2·4 | 0·066 ± 0·01 |
| Elevated CO2 | 40·9 ± 4 | 121 ± 16 | 19·9 ± 2·4 | 0·076 ± 0·007 |
| Mid‐season | ||||
| Ambient CO2 | 43·4 ± 9 | 97 ± 21 | 15·5 ± 3·7 | 0·051 ± 0·009 |
| Elevated CO2 | 28·2 ± 3 | 57 ± 7 | 10·1 ± 1·3 | 0·053 ± 0·008 |
| Late season | ||||
| Ambient CO2 | 62·9 ± 6 | 155 ± 19 | 24·5 ± 3·3 | 0·053 ± 0·013 |
| Elevated CO2 | 39·2 ± 5 | 99 ± 15 | 16·2 ± 3·4 | 0·053 ± 0·004 |
| P | ||||
| CO2trt | 0·0001 | 0·0003 | 0·0006 | 0·4968 |
| Year | 0·8744 | 0·3395 | 0·2531 | 0·6905 |
| Season | 0·0132 | 0·0001 | 0·0001 | 0·1527 |
| CO2trt × year | 0·7552 | 0·5467 | 0·6649 | 0·8450 |
| CO2trt × season | 0·7006 | 0·4207 | 0·4028 | 0·9547 |
Plants were grown in open‐top chambers on native shortgrass steppe of Colorado, USA, with ambient or elevated (360 and 720 µmol mol–1) CO2. Data are averaged across three years and three replications ± s.e.
The CO2 effect on CE and Asat was not significant in B. gracilis (Table 2). Again, there was a significant effect of season, probably due to seasonal differences in soil water (see below). However, the interaction between season and CO2 treatment was again not significant.
Table 2.
The initial slope of the A : Ci curve (carboxylation efficiency, CE), assimilation at saturating light and CO2 (Asat), and the apparent quantum efficiency (Q) in Bouteloua gracilis (C4) at three periods during the growing season
| CE (CO2 m–2 s–1/mol–1 CO2) | Asat (µmol CO2 m–2 s–1) | Q (CO2/photon) | |
| Early season | |||
| Ambient CO2 | 0·1267 ± 0·017 | 25·8 ± 3·7 | 0·047 ± 0·007 |
| Elevated CO2 | 0·1107 ± 0·11 | 22·8 ± 2·2 | 0·064 ± 0·002 |
| Mid‐season | |||
| Ambient CO2 | 0·0697 ± 0·07 | 12·7 ± 3·2 | 0·046 ± 0·007 |
| Elevated CO2 | 0·0726 ± 0·013 | 16·0 ± 2·6 | 0·052 ± 0·009 |
| Late season | |||
| Ambient CO2 | 0·1325 ± 0·024 | 23·9 ± 3·9 | 0·052 ± 0·006 |
| Elevated CO2 | 0·1380 ± 0·021 | 25·3 ± 3·4 | 0·066 ± 0·007 |
| P | |||
| CO2trt | 0·8560 | 0·8948 | 0·2573 |
| Year | 0·2877 | 0·1075 | 0·6451 |
| Season | 0·0048 | 0·0029 | 0·1622 |
| CO2trt × year | 0·9026 | 0·7447 | 0·6857 |
| CO2trt × season | 0·8240 | 0·5861 | 0·5628 |
Plants were grown in open‐top chambers on native shortgrass steppe of Colorado, USA, with ambient or elevated (360 and 720 µmol mol–1) CO2. Data are averaged across three years and three replications ± s.e.
There was no consistent effect of CO2 on the light response curves of photosynthesis (measured at the growth CO2 concentration) at any time or in either species (curves not shown). The apparent quantum efficiency (Q) is summarized in Tables 1 and 2, and shows no significant variable effect in either P. smithii or B. gracilis.
Since there was no CO2 effect, further presentation of A : Ci data from B. gracilis are not shown. The A : Ci curves of P. smithii from 1999 are given (Fig. 2) as this year was representative of the treatment responses. Early in the season, when SWC was high, A was reduced in elevated vs. ambient CO2 leaves at most Ci (Fig. 2A). During mid‐season, when plants were water‐stressed, A was about 50 % of that measured in May and there was a (non‐significant) trend for lower A in leaves grown at elevated CO2 (Fig. 2B). Precipitation in late July improved A, and a significant reduction in the A : Ci curve in leaves at elevated CO2 was again seen late in the season (Fig. 2C). The reduction in photosynthetic capacity is such that A measured at the growth CO2 concentration was similar in grasses grown at ambient and elevated CO2 (Fig. 2, arrows). The assimilation rate in grasses in unchambered plots was similar to that in grasses in ambient CO2 chambers.
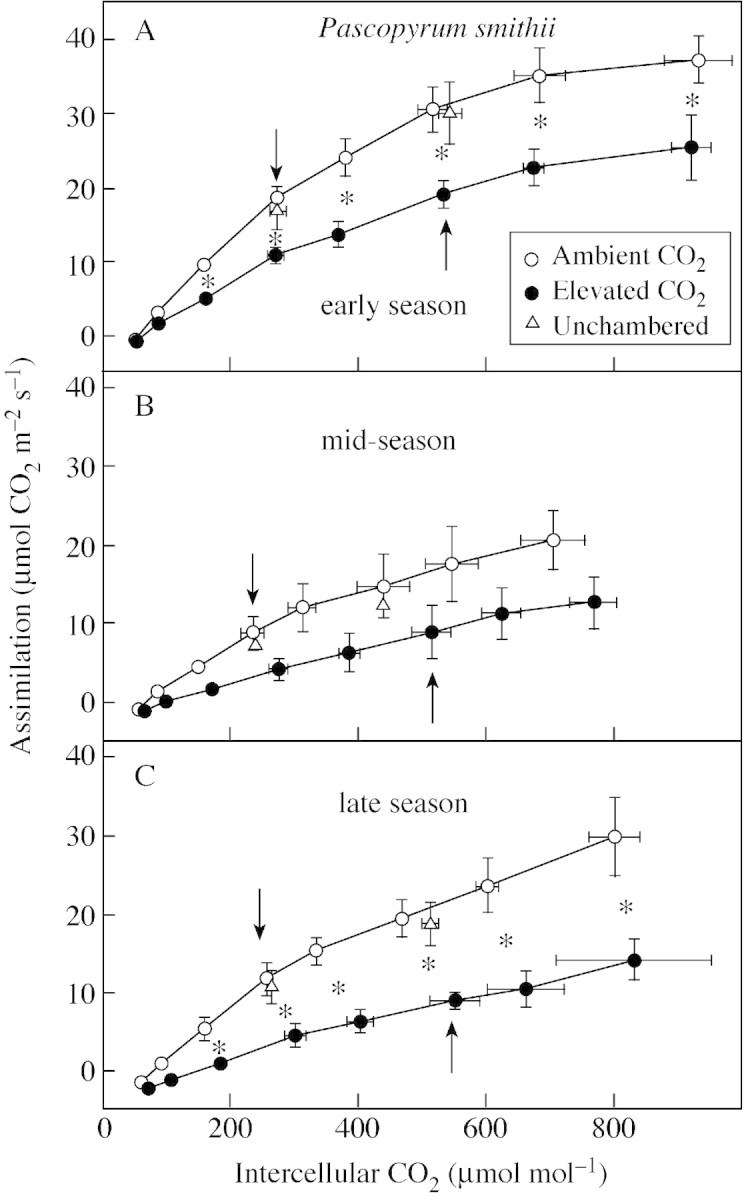
Fig. 2. Assimilation vs. intercellular CO2 concentration of Pascopyrum smithii grown in open‐top chambers with ambient (360 µmol mol–1) and elevated (720 µmol mol–1) CO2, and in plots without chambers on the Colorado shortgrass steppe over the course of the 1999 growing season. Data are means of three replications ± s.e. An asterisk indicates a significant effect of CO2 treatment at P < 0·05. Arrows indicate data at the treatment [CO2].
Assimilation rates under seasonal and CO2‐induced soil moisture variation
Variability in the patterns of A measured at the growth CO2 concentration was primarily a function of variation in SWC induced by season and CO2. Here we contrast results from a wet (1999) and a dry (2000) year. Data for 1997 and 1998 have been presented previously (Morgan et al., 2001).
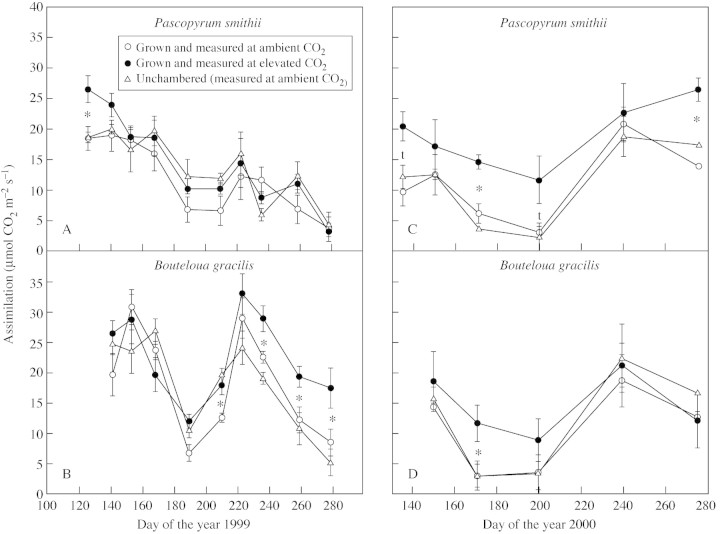
Early in the season of 1999 [day of the year (DOY) 125], P. smithii plants grown at elevated CO2 had higher A than plants grown at ambient CO2 or without chambers (Fig. 3A). By DOY 153, this advantage had disappeared. However, as seasonal water stress developed, there were periods (DOY 188 and 210) when the assimilation rate of P. smithii grown at elevated CO2 tended to be higher, probably due to better SWC in the elevated CO2 plots (Fig. 1). For B. gracili, there was little difference in A early in the season of 1999 (Fig. 3B). However, during drought, and for the later third of the season, A of the elevated CO2 plants was higher than that of ambient CO2 plants (DOY 188, 210 and >225). Again, this was associated with improved SWC under elevated CO2 (Fig. 1).
Fig. 3. Assimilation of Pascopyrum smithii and Boutleoua gracilis grown in open‐top chambers with ambient (360 µmol mol–1) and elevated (720 µmol mol–1) CO2, and in plots without chambers on the Colorado shortgrass steppe during the growing seasons of 1999 and 2000. Measurements were made at the chamber CO2 concentrations. Data are means of three replications ± s.e. An asterisk indicates an effect of CO2 treatment significant at P < 0·05; t, P < 0·10.
During 2000, elevated CO2 plots had higher SWC for much of the season (Fig. 1). Assimilation rates of P. smithii reflect these soil moisture differences, with the elevated CO2 plants having higher A than ambient plants on most dates (Fig. 3C). This effect was less evident in B. gracilis (Fig. 3D). Assimilation in the unchambered plots was similar to that in the ambient plots in both species, even though soil moisture was similar to that in elevated CO2 plots (Fig. 1). This was associated with improved ψleaf in the elevated CO2 plants.
Leaf water potential under seasonal and CO2‐induced soil moisture variation
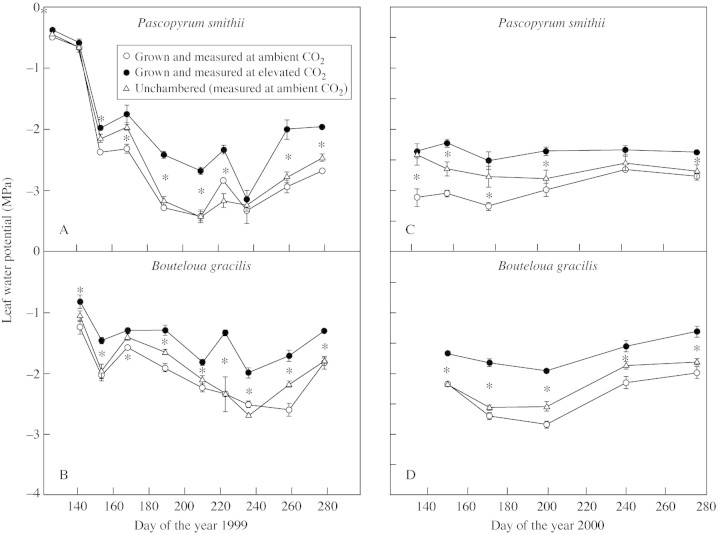
Higher SWC and improved conditions for A under elevated CO2 were also reflected in improved ψleaf. Leaf water potential in both species was higher in plants grown at elevated CO2 on most measurements dates in 1999 and 2000 (Fig. 4).
Fig. 4. Leaf water potential of Pascopyrum smithii and Boutleoua gracilis grown in open‐top chambers with ambient (360 µmol mol–1) and elevated (720 µmol mol–1) CO2, and in plots without chambers on the Colorado shortgrass steppe during the growing seasons of 1999 and 2000. Data are means of three replications ± s.e. An asterisk indicates a significant effect of CO2 treatment at P < 0·05.
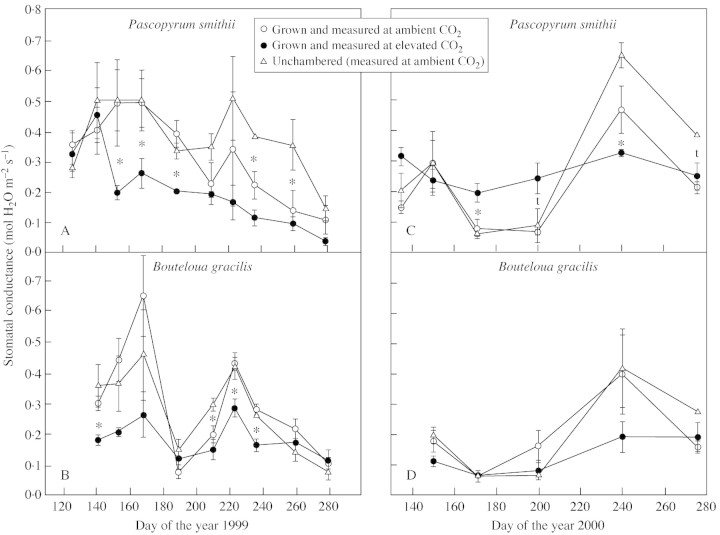
Stomatal responses to elevated CO2
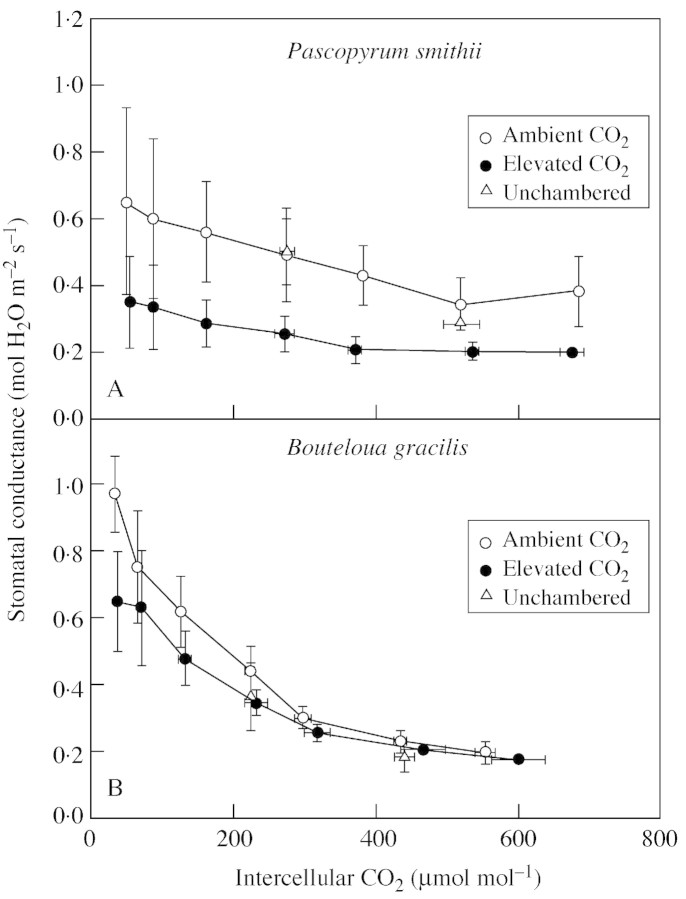
Responses of gs to varying Ci early in the season of 1999 were representative of responses when interactions with drought were not occurring (see below) and thus are discussed here (Fig. 5). Stomatal conductance of P. smithii decreased from 0·5 to 0·28 mol m–2 s–1 over a range of Ci from 50 to 670 µmol mol–1; this variation was less than that seen in B. gracilis which varied from 0·8 to 0·18 over similar values of Ci. In P. smithii there was a trend for lower gs in leaves grown in elevated CO2 (Fig. 5A). This trend was observed on most measurement dates. There was no indication of stomatal adaptation to elevated CO2 in B. gracilis (Fig. 5B).
Fig. 5. Stomatal conductance vs. intercellular CO2 of Pascopyrum smithii and Boutleoua gracilis grown in open‐top chambers with ambient (360 µmol mol–1) and elevated (720 µmol mol–1) CO2 and in plots without chambers on the Colorado shortgrass steppe. Measurements made during May 1999. Data are means of three replications ± s.e. The CO2 treatment effect was not significant.
During much of the growing season of 1999, stomatal conductance of P. smithii (measured at the growth CO2 concentration) was lower in plants grown at elevated CO2 (Fig. 6A). Stomatal conductance of B. gracilis in elevated CO2 chambers also tended to be lower, although the difference was significant on fewer dates (Fig. 6B). During the dry spring of 2000, gs was sometimes higher in P. smithii plants from elevated CO2, and there were no differences in gs in B. gracilis (Fig. 6C and D). Leaf water‐use efficiency of both species was always higher when measured at elevated CO2 (data not shown).
Fig. 6. Stomatal conductance of Pascopyrum smithii and Boutleoua gracilis grown in open‐top chambers with ambient (360 µmol mol–1) and elevated (720 µmol mol–1) CO2, and in plots without chambers on the Colorado shortgrass steppe during the growing seasons of 1999 and 2000. Measurements were made at the chamber CO2 concentrations. Data are means of three replications ± s.e. An asterisk indicates an effect of CO2 treatment significant at P < 0·05; t, P < 0·10.
Leaf nitrogen and carbohydrates responses to elevated CO2
There was a significant CO2 × season interaction for leaf N and TNC. Therefore, statistics are shown for each measurement period (Table 3). Early in the season, there was no CO2 effect on the leaf N of P. smithii, but N was reduced under elevated CO2 in mid‐ and late season. Leaf N was reduced in B. gracilis in elevated CO2 in all three periods.
Table 3.
Percentage nitrogen and total non‐structural carbohydrates (TNC) on a structural dry mass basis of leaves of Pascopyrum smithii (C3) and Bouteloua gracilis (C4) grown in open‐top chambers on native shortgrass steppe of Colorado, USA, with ambient or elevated (360 and 720 µmol mol–1) CO2, or on plots with no chamber
| Nitrogen (%) | TNC (mg g–1) | |||
| P. smithii | B. gracilis | P. smithii | B. gracilis | |
| Early season | ||||
| No chamber | 1·59 ± 0·05 | 1·89 ± 0·03 | 111·6 ± 18·9 | 121·0 ± 10·9 |
| Ambient CO2 | 1·42 ± 0·03 | 1·65 ± 0·02 | 119·6 ± 6·6 | 110·9 ± 2·7 |
| Elevated CO2 | 1·33 ± 0·04 | 1·40 ± 0·05 | 193·1 ± 14·2 | 140·4 ± 11·3 |
| P | ||||
| CO2trt | 0·1391 | 0·0001 | 0·0003 | 0·0103 |
| Year | 0·1106 | 0·0372 | 0·0001 | 0·0007 |
| CO2trt × year | 0·1909 | 0·0723 | 0·1845 | 0·5604 |
| Mid‐season | ||||
| No chamber | 1·33 ± 0·08 | 1·24 ± 0·06 | 111·0 ± 16·3 | 100·5 ± 1·2 |
| Ambient CO2 | 1·31 ± 0·05 | 1·15 ± 0·04 | 97·4 ± 7·9 | 106·7 ± 5·6 |
| Elevated CO2 | 1·10 ± 0·02 | 1·02 ± 0·02 | 176·5 ± 25·3 | 117·0 ± 5·8 |
| P | ||||
| CO2trt | 0·0029 | 0·0013 | 0·0007 | 0·0868 |
| Year | 0·6238 | 0·0014 | 0·0310 | 0·0001 |
| CO2trt × year | 0·9010 | 0·2490 | 0·1593 | 0·3753 |
| Late season | ||||
| No chamber | 1·05 ± 0·03 | 1·26 ± 0·04 | 72·9 ± 10·1 | 76·5 ± 3·0 |
| Ambient CO2 | 0·96 ± 0·06 | 1·20 ± 0·03 | 56·3 ± 7·1 | 74·7 ± 2·2 |
| Elevated CO2 | 0·77 ± 0·06 | 1·06 ± 0·02 | 83·5 ± 9·3 | 73·1 ± 6·2 |
| P | ||||
| CO2trt | 0·0096 | 0·0250 | 0·0670 | 0·8580 |
| Year | 0·0001 | 0·0001 | 0·0001 | 0·0001 |
| CO2trt × year | 0·3399 | 0·0029 | 0·0691 | 0·8497 |
Data are averaged over 4 years and three replications ± s.e. The season × CO2 interaction was significant.
CO2trt is the No chamber, ambient CO2 and elevated CO2 variables.
The content of non‐structural carbohydrates was higher in P. smithii leaves grown in elevated vs. ambient CO2 in all three periods (Table 3). An increase in all of the individual sugar fractions was found, but fructans and sucrose were the dominant carbohydrates in P. smithii (data not shown). In contrast, B. gracilis grown in elevated CO2 had significantly more carbohydrates only early in the season (primarily starch). In both species, unchambered plants had TNC and N contents similar to those of plants in the ambient CO2 chambers.
DISCUSSION
Reports of photosynthetic acclimation under elevated CO2 have been considered the exception in field studies (Sage, 1994; Drake et al., 1997). Contrary to this, our hypothesis that P. smithii would develop photosynthetic acclimation was confirmed in this study. Although there were periods, in early spring, that reflected a direct photosynthetic stimulation in elevated CO2, 3 years of A : Ci measurements showed consistent photosynthetic acclimation in P. smithii. These findings agree with previous results of growth chamber studies (Morgan et al., 1994a, 1998; Read et al., 1997), but the photosynthetic acclimation was greater in the field.
Previous field studies of CO2 enrichment in grassland ecosystems show contrasting evidence for photosynthetic acclimation. In the tall grass prairie and a Texas grassland there was no photosynthetic acclimation in either C3 or C4 species (Nie et al., 1992; Hamerlynck et al., 1997; Anderson et al., 2001). However, photosynthetic acclimation was reported in C3 species in a New Zealand pasture (von Caemmerer et al., 2001) and in several diverse grassland species in the Minnesota prairie (Lee et al., 2001).
From these reports it is difficult to generalize about the basis of photosynthetic acclimation under elevated CO2, as species, soils, climate and experimental conditions vary. However, it seems clear that the reduction in photosynthetic capacity helps to restore the balance between carbon assimilation, other resources (water, nutrients, rooting volume) and carbon demand (Sage, 1994; Midgley et al., 1999; Moore, 1999). In C3 plants, this typically includes a reduction in Rubisco amount or activity, and reduced leaf N (Sage, 1994; Midgley et al., 1999; Moore et al., 1999; Stitt and Krapp, 1999).
Consistent with these studies, Vc,max and leaf N concentration of P. smithii were significantly reduced under elevated CO2. We suspect that improved growth of SGS species under elevated CO2 (Morgan et al., 2001) has further limited the available soil N in this N‐limited ecosystem (Hunt et al., 1988). The resultant inability of P. smithii plants to produce new sinks causes an imbalance in ‘supply and demand’, which feeds back on photosynthetic processes. Our leaf TNC data support this, as strong accumulation (average 61 %) of carbohydrates occurred. These results agree with those of Rogers et al. (1998) who concluded that photosynthetic acclimation results primarily from sink limitation in low N soils.
The A : Ci curves obtained also show a reduction in the regeneration capacity for ribulose‐1,5 bisphosphate (RuBP) in P. smithii, as indicated by reduced Jmax (Table 1) (Long et al., 1993). The light response data also support this (Table 1). The apparent quantum efficiency (Q) of photosynthesis is related to the RuBP regeneration capacity of C3 plants (Long et al., 1993). An increase in Ca should increase Q, owing to less oxygenation of RuBP. The lack of improvement in Q when measured at elevated CO2 provides more evidence for a reduction in RuBP regeneration capacity (Table 1). These results agree with those of Midgley et al. (1999), who reported an integrated reduction in both carboxylation and RuBP regeneration capacity under elevated CO2. However, these findings contradict the theory that Q will be improved in C3 plants under increasing atmospheric CO2 (Ehleringer et al., 1997).
Contrary to our hypothesis, B. gracilis did not display photosynthetic acclimation under elevated CO2, although we have previously reported acclimation of well‐watered and fertilized B. gracilis (Read et al., 1997; LeCain and Morgan, 1998). The A : Ci curves in this field study show the short‐term, ‘potential’ photosynthetic improvement at elevated CO2 is only approx. 10 % in B. gracilis (data not shown), compared with approx. 65 % in P. smithii. Therefore, B. gracilis is less likely to experience source/sink imbalances. Compared with growth chamber studies, the low soil N availability in the field may have limited the potential photosynthetic response of B. gracilis, which precluded photosynthetic acclimation to elevated CO2. Our results agree with those of von Caemmerer et al. (2001) who reported photosynthetic acclimation in C3 species, but not C4 species, under elevated CO2. Due to acclimation, there was little improvement in A of P. smithii grown at elevated CO2 during periods of adequate soil moisture. However, during dry periods, the improved soil moisture at elevated CO2 resulted in improved A in both species. Averaged over the study, A was 30 and 21 % higher in P. smithii and B. gracilis, respectively, grown at elevated compared with ambient CO2 (Table 4).
Table 4.
Means of gas exchange data of Pascopyrum smithii and Bouteloua gracilis measured at the growth CO2 concentration, and averaged over all of the data (4 years, 36 dates)
| Assimilation (µmol CO2 m–2 s–1) | Conductance (mol H2O m–2 s–1) | Transpiration (mmol H2O m–2 s–1) | Water‐use efficiency (mmol CO2/mol H2O) | |
| P. smithii | ||||
| Ambient CO2 | 12·5 | 0·29 | 5·66 | 2·07 |
| Elevated CO2 | 16·2 | 0·21 | 4·32 | 3·76 |
| P | ||||
| CO2trt | 0·0002 | 0·0038 | 0·0058 | 0·0001 |
| Year | 0·0330 | 0·0068 | 0·8393 | 0·0051 |
| CO2trt × year | 0·4216 | 0·0681 | 0·0998 | 0·2757 |
| B. gracilis | ||||
| Ambient CO2 | 15·9 | 0·25 | 6·33 | 2·79 |
| Elevated CO2 | 19·3 | 0·16 | 5·16 | 4·89 |
| P | ||||
| CO2trt | 0·0097 | 0·0001 | 0·0001 | 0·0001 |
| Year | 0·0008 | 0·0096 | 0·0035 | 0·2667 |
| CO2trt × year | 0·9997 | 0·5184 | 0·9716 | 0·7657 |
Plants were grown in open‐top chambers on native shortgrass steppe of Colorado, USA, with ambient or elevated (360 and 720 µmol mol–1) CO2.
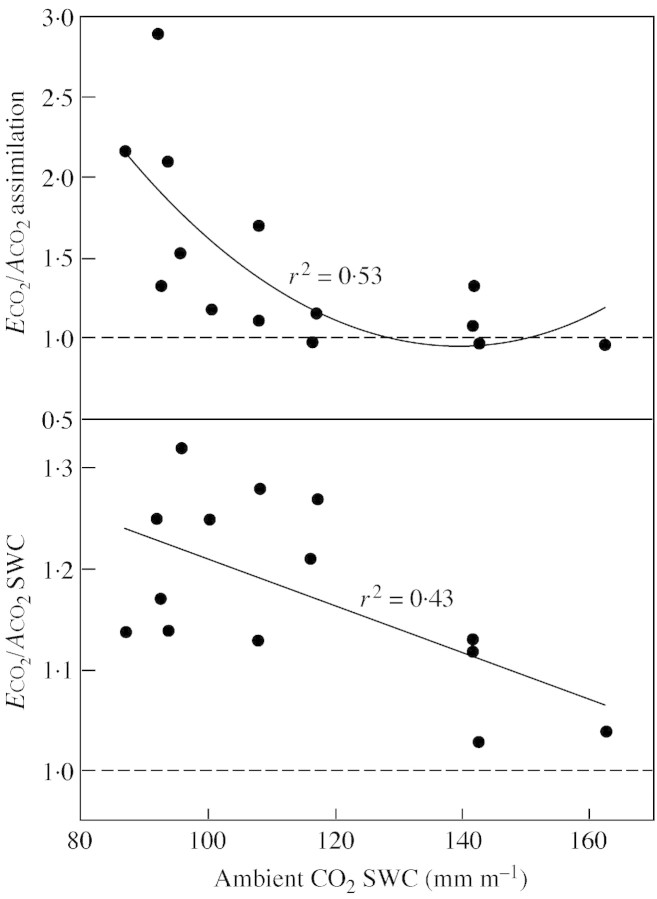
Figure 7 shows data for SWC and A in plants grown at elevated relative to ambient CO2, plotted against the ambient CO2 SWC, for 1999 and 2000. These plots demonstrate that the improvement in SWC under elevated CO2 occurred primarily as soils dried out. Also, the improvement in A under elevated CO2 was expressed primarily at low SWC. These results conflict with the views of Wand et al. (1999) that water stress reduces the relative photosynthetic enhancement of grasses grown at elevated CO2. But our findings agree with those of Owensby et al. (1993) and Hamerlynck et al. (1997) for a tallgrass prairie, where responses were greater in drought seasons. Huxman et al. (1998), conducting a field study in the desert, also reported photosynthetic acclimation in Larrea tridentata Cav. when soil water was available, but assimilation was higher under elevated CO2 during drought periods.
Fig. 7. 1999 and 2000 assimilation (A) and soil water content (SWC) data in elevated (E: 720 µmol mol–1) relative to ambient (A: 360 µmol mol–1) CO2 chambers, plotted against the ambient CO2 SWC. Lines are a linear regression for SWC and a second order polynomial for assimilation. Both correlations are significant (P < 0·05).
A reduction in gs is an almost universal response to elevated CO2 (Drake et al., 1997; Jarvis et al., 1999; Wand et al., 1999). Consistent with our hypothesis, stomatal conductance was lower under elevated CO2 compared with ambient CO2 when soil moisture was adequate. Averaged over the study, stomatal conductance was reduced by 27 and 36 %, and transpiration was reduced 24 and 20 % in leaves of P. smithii and B. gracilis (Table 4). Reduced transpiration is probably the reason for improved SWC under elevated CO2 (Field et al., 1995).
An unexpected finding was that during periods in which the soil was very dry, gs was sometimes higher in P. smithii grown under elevated CO2 than that grown at ambient CO2. The best example of this occurred in 2000, where higher SWC in elevated CO2 plots was carried over from the previous year (Fig. 1). The lack of spring precipitation led to such extended drying of the soil that a point was reached where very low SWC under ambient CO2 conditions actually reduced gs below that of plants grown at elevated CO2. Knapp et al. (1996) also reported an interaction between SWC and gs during a dry year.
A consistent trend for lower gs at an equivalent Ci was seen in P. smithii grown at elevated CO2 (Fig. 5A). There is evidence that stomatal functioning is coupled with photosynthetic activity to maintain a relatively stable Ci : Ca ratio (Jarvis et al., 1999). If stomata adapt to elevated CO2 independently of photosynthetic adaptations, the Ci : Ca ratio will differ (Ball and Berry, 1982). We found no effect of CO2 treatment on Ci : Ca in either species (data not shown). Our results agree with those of Drake et al. (1997) and Jarvis et al. (1999) that apparent stomatal acclimation is a result of stomatal dependence on photosynthetic processes.
Leaf water potential was higher in elevated CO2 plots on most dates. This is probably a result of improved SWC and gs during much of the growing season (Tyree and Alexander, 1993; Hamerlynck et al., 1997). However, the regression analysis showed poor correlations between the improvement in ψleaf and improved A and gs (data not shown). This is because ψleaf was improved on nearly all dates, whereas A was improved primarily during periods of low SWC, and gs interacted with SWC. Factors such as osmotic adjustment and rooting dynamics will complicate the relationship between ψleaf and A and gs (Tyree and Alexander, 1993). Still, we believe that the strikingly consistent improvement in ψleaf is evidence that plant water status was more favourable for assimilation in the elevated CO2 plots. The present results agree with those of Hamerlynck et al. (1997) who reported that elevated CO2 improved ψleaf and A in both C3 and C4 species during drought years.
Implications for grassland ecosystems
In this semi‐arid, N‐limited ecosystem, the C3 grass was unable to produce adequate new sinks to maintain higher A under elevated CO2. Despite the lack of direct photosynthetic stimulation in both the C3 and C4 species, the present results provide support for a positive growth response to elevated CO2 in this ecosystem. Averaging the gas exchange data over the study, A was improved by 30 and 21 %, gs was reduced by 27 and 36 %, E was reduced by 24 and 20 %, and WUE was increased by 80 and 75 % in P. smithii and B. gracilis under elevated compared with ambient CO2 (Table 4). A 26–47 % increase in above‐ground biomass was found in the elevated CO2 plots in 1997 and 1998 (Morgan et al., 2001). In 1999, a year with nearly twice the average seasonal precipitation, biomass was improved by only 17 % in elevated compared with ambient CO2 (J. A. Morgan, pers. comm.). But during the drought year of 2000, biomass was increased by 95 % in elevated CO2 plots. This large improvement in biomass was possible because of higher SWC and improved A and ψleaf in elevated CO2 plots.
The present study suggests that improvements in WUE, soil water conservation and plant water relations will primarily affect responses to elevated CO2 in this semi‐arid ecosystem, rather than a direct effect on assimilation. Although the improvement in SWC (relative average 14 %) may seem small, the effect is large in this water‐limited ecosystem. These results agree with those of Volk et al. (2000) that the effects of elevated CO2 on soil moisture account for most of the variability in production. Improved soil moisture should extend the favourable growing season in arid and semi‐arid ecosystems (Knapp et al., 1996).
This study provides no evidence for a competitive advantage of the C3 over the C4 grass under elevated CO2. Earlier notions that C3 species would have a large advantage as global levels of CO2 rise are not being supported by field studies (Owensby et al., 1993; Wand et al., 1999; Anderson et al., 2001). The comparative response of C3 vs. C4 species will depend on phenology, morphology, root distribution, reproduction strategies and nutrient‐use efficiency, as well as photosynthetic and stomatal responses.
ACKNOWLEDGEMENTS
The authors thank Scott Andre, Barry Weaver, Dean Ackerman and Dennis Mueller for designing and building the chambers, and for technical assistance, and Clenton Owensby, Jay Ham, Lisa Auen and Fred Caldwell of Kansas State University for guidance on chamber design. We also thank Alan Knapp and Lewis Ziska for critically reviewing the manuscript. This research was supported, in part, by funding from the NSF‐TECO awards IBN‐9524068 and USDA/NRICGP‐98‐134, NSF award DEB‐9708596 and from the Shortgrass Steppe LTER project DEB 9350273. Mention of a trademark or manufacturer by the USDA does not imply its approval to the exclusion of other products or manufacturers that may also be suitable.
Supplementary Material
Received: 2 December 2002; Returned for revision: 28 January 2003; Accepted: 12 March 2003 Published electronically: 9 May 2003
References
- AndersonLJ, Maherali H, Johnson HB, Polley HW, Jackson RB.2001. Gas exchange and photosynthetic acclimation over subambient to elevated CO2 in a C3‐C4 grassland. Global Change Biology 7: 693–707. [Google Scholar]
- BallHT, Berry JA.1982. The Ci/Cs ratio: a basis for predicting stomatal control of photosynthesis. Carnegie Institute of Washington Yearbook 81: 88–92. [Google Scholar]
- CampbellBD, Laing WA, Greer DH, Crush JR, Clarke H, Williamson DY, Given MDJ.1995. Variations in grassland populations and species and the implications for community responses to elevated CO2 Journal of Biogeography 22: 315–322. [Google Scholar]
- DrakeBG, Gonzalez‐Meler MA, Long SP.1997. More efficient plants: a consequence of rising atmospheric CO2 Annual Review of Plant Physiology and Plant molecular Biology 48: 607–637. [DOI] [PubMed] [Google Scholar]
- DregneHE.1991. Global status of desertification. Annals of Arid Zone 30: 179–185. [Google Scholar]
- EhleringerJR, Cerling TE, Helliker BR.1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112: 285–299. [DOI] [PubMed] [Google Scholar]
- FieldCB, Jackson RB, Mooney HA.1995. Stomatal responses to increased CO2: implications from the plant to the global scale. Plant, Cell and Environment 18: 1214–1225. [Google Scholar]
- HamerlynckEP, McAllister CA, Knapp AK, Ham JM, Owensby CE.1997. Photosynthetic gas exchange and water relation responses of three tallgrass prairie species to elevated carbon dioxide and moderate drought. International Journal of Plant Sciences 158: 608–616. [Google Scholar]
- HendrixDL.1993. Rapid extraction and analysis of nonstructural carbohydrates in plant tissues. Crop Science 33: 1306–1311. [Google Scholar]
- HuntHW, Elliott ET, Detling JK, Morgan JA, Chen DX.1996. Responses of a C3 and a C4 perennial grass to elevated CO2 and temperature under different water regimes. Global Change Biology 2: 35–47. [Google Scholar]
- HuntHW, Ingham ER, Coleman DC, Elliott ET, Reid CP.1988. Nitrogen limitation of production and decomposition in prairie, mountain meadow, and pine forest. Ecology 69: 1009–1016. [Google Scholar]
- HuxmanTE, Hamerlynck EP, Moore BD, Smith SD, Jordan DN, Zitzer SF, Nowak RS, Coleman JS, Seeman JR.1998. Photosynthetic down‐regulation in Larrea tridentata exposed to elevated atmospheric CO2: interaction with drought under glasshouse and field (FACE) exposure. Plant, Cell and Environment 21: 1153–1161. [Google Scholar]
- JacksonRB, Luo Y, Cardon ZG, Sala OE, Field CB, Mooney HA.1995. Photosynthesis, growth and density for the dominant species in a CO2‐enriched grassland. Journal of Biogeography 22: 221–225. [Google Scholar]
- JarvisAJ, Mansfield TA, Davies WJ.1999. Stomatal behavior, photosynthesis and transpiration under rising CO2 Plant, Cell and Environment 22: 639–648. [Google Scholar]
- KnappAK, Hamerlynck EP, Owensby CE.1993. Photosynthetic and water relations responses to elevated CO2 in the C4 grass Andropogon gerardii International Journal of Plant Sciences 154: 459–466. [Google Scholar]
- KnappAK, Hamerlynck EP, Ham JM, Owensby CE.1996. Responses in stomatal conductance to elevated CO2 in 12 grassland species that differ in growth form. Vegetation 125: 31–41. [Google Scholar]
- LeCainDR, Morgan JA.1998. Growth, gas exchange, leaf nitrogen and carbohydrate concentrations in NAD‐ME and NADP‐ME C4 grasses grown in elevated CO2 Physiologia Plantarum 102: 297–306. [Google Scholar]
- LeeTD, Tjoelker MG, Ellsworth DS, Reich PB.2001. Leaf gas exchange responses of 13 prairie grassland species to elevated CO2 and increased nitrogen supply. New Phytologist 150: 405–418. [Google Scholar]
- LongSP, Baker NR, Raines CA.1993. Analyzing the responses of photosynthetic CO2 assimilation to long‐term elevation of atmospheric CO2 concentration. Vegetation 104/105: 33–45. [Google Scholar]
- MidgleyGF, Wand SJE, Pammenter NW.1999. Nutrient and genotypic effects on CO2‐responsiveness: photosynthetic regulation in Leucadenron species of a nutrient‐poor environment. Journal of Experimental Botany 50: 533–542. [Google Scholar]
- MilchunasDG, Laurenroth WK.1992. Carbon dynamics and estimates of primary production by harvest,14C dilution and 14C turnover. Ecology 73: 593–607. [Google Scholar]
- MilchunasDG, Lauenroth WK, Chapman PL, Kazempour MK.1989. Effect of grazing, topography, and precipitation on the structure of a semiarid grassland. Vegetation 80: 11–23. [Google Scholar]
- MooreBD, Cheng SH, Sims D, Seemann JR.1999. The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2 Plant, Cell and Environment 22: 567–582. [Google Scholar]
- MorganJA, Hunt HW, Monz CA, LeCain DR.1994a. Consequences of growth at two carbon dioxide concentrations and two temperatures for leaf gas exchange in Pascopyrum smithii (C3) and Bouteloua gracilis (C4). Plant Cell and Environment 17: 1023–1033. [Google Scholar]
- MorganJA, Knight WG, Dudley LM, Hunt HW.1994b. Enhanced root system C‐sink activity, water relations and aspects of nutrient acquisition in mycotrophic Bouteloua gracilis subjected to CO2 enrichment. Plant and Soil 165: 139–146. [Google Scholar]
- MorganJA, LeCain DR, Mosier AR, Milchunas DG.2001. Elevated CO2 enhances water relations and productivity and affects gas exchange in C3 and C4 grasses of the Colorado shortgrass steppe. Global Change Biology 7: 451–466;. [Google Scholar]
- MorganJA, LeCain DR, Read JJ, Hunt HW, Knight WG.1998. Photosynthetic pathway and ontogeny affect water relations and the impact of CO2 on Bouteloua gracilis (C4) and Pascopyrum smithii (C3). Oecologia 114: 483–493. [DOI] [PubMed] [Google Scholar]
- NieD, He H, Kirkham MB, Kanemasu ET.1992. Photosynthesis of a C3 and an C4 grass under elevated CO2 Photosynthetica 26: 189–198. [Google Scholar]
- OwensbyCE, Coyne PI, Ham JM, Auen LA, Knapp AK.1993. Biomass production in a tallgrass prairie ecosystem exposed to ambient and elevated CO2 Ecological Applications 3: 644–653. [DOI] [PubMed] [Google Scholar]
- ReadJJ, Morgan JA.1996. Growth and partitioning in Pascopyrum smithii (C3) and Bouteloua gracilis (C4) as influenced by carbon dioxide and temperature. Annals of Botany 77: 487–496. [Google Scholar]
- ReadJJ, Morgan JA, Chatterton NJ, Harrison PA.1997. Gas exchange and carbohydrate and nitrogen concentrations in leaves of Pascopyrum smithii (C3) and Bouteloua gracilis (C4) at different carbon dioxide concentrations and temperatures. Annals of Botany 79: 197–206. [Google Scholar]
- RogersA, Fischer BU, Bryant J, Frehner M, Blum H, Raines CA, Long SP.1998. Acclimation of photosynthesis to elevated CO2 under low‐nitrogen nutrition is affected by the capacity for assimilate utilization. Perennial Ryegrass under free‐air CO2 enrichment. Plant Physiology 188: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SageRF.1994. Acclimation of photosynthesis to increasing atmospheric CO2: The gas exchange perspective. Photosynthesis Research 39: 351–368. [DOI] [PubMed] [Google Scholar]
- StittM, Krapp A.1999. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant, Cell and Environment 22: 583–621. [Google Scholar]
- TyreeMT, Alexander JD.1993. Plant water relations and the effects of elevated CO2: a review and suggestions for future research. Vegetation 104/105: 47–62. [Google Scholar]
- VolkM, Niklaus PA, Korner C.2000. Soil moisture effects determine CO2 responses of grassland species. Oecologia 125: 380–388. [DOI] [PubMed] [Google Scholar]
- Von CaemmererS, Ghannoum O, Conroy J, Clark H, Newton PCD.2001. Photosynthetic responses of temperate species to free air CO2 enrichment (FACE) in a grazed New Zealand pasture. Australian Journal of Plant Physiology 28: 439–450. [Google Scholar]
- WandSJE, Midgley GF, Jones MH, Curtis PS.1999. Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta‐analytic test of current theories and perceptions. Global Change Biology 5: 723–741. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.