Abstract
An automatic gas exchange system was used to continuously measure water and carbon fluxes of attached shoots of Scots pine trees (Pinus sylvestris L.) grown in environment‐controlled chambers for a 3‐year period (1998–2000) and exposed to either normal ambient conditions (CON), elevated CO2 (+350 µmol mol–1; EC), elevated temperature (+2–6 °C; ET) or a combination of EC and ET (ECT). EC treatment enhanced the mean daily total carbon flux per unit projected needle area (Fc.d) by 17–21 %, depending on the year. This corresponds to a 16–24 % increase in light‐use efficiency (LUE) based on incident photosynthetically active radiation. The EC treatment reduced the mean daily total water flux (Fw.d) by 1–12 %, corresponding to a 13–35 % increase in water‐use efficiency (WUE). The ET treatment increased Fc.d by 10–18 %, resulting in an 8–19 % increase in LUE, and Fw.d by 48–74 %, resulting in a reduction of WUE by 19–34 %. There was no interaction between CO2 and temperature elevation in connection with either carbon or water fluxes, as the carbon flux responded similarly in both ECT and EC, while the water flux in the ECT treatment was similar to that in ET. Regressions indicated that the increase in maximum LUE was greater with increasing air temperature, whereas changes in WUE were related only to high vapour pressure deficit. Furthermore, changes in LUE and WUE caused by ECT treatment displayed strong diurnal and seasonal variation.
Key words: Carbon flux, water flux, elevated CO2, elevated temperature, water‐use efficiency, light‐use efficiency, environment‐controlled chamber, Pinus sylvestris, Scots pine
INTRODUCTION
Numerous studies undertaken over the past decade have led to the general conclusion that short‐term increases in CO2 concentration will enhance photosynthesis, reduce transpiration, and increase radiation‐ and water‐use efficiencies of leaves of many species (Curtis, 1996; Drake et al., 1997; Norby et al., 1999), but questions have arisen concerning the longer‐term effects of elevated CO2 on growth and development of plants. Further key questions for conifer species, with their multi‐aged foliage and greater potential for acclimation than non‐coniferous species (Ceulemans and Mousseau, 1994), are how much will photosynthesis increase on an annual basis, and will the trees be more efficient as the duration of exposure to CO2 increases? The answers may be complicated by interactions with other weather conditions, and with soil nutrient supply, and may vary with the source–sink status of the whole tree (Stitt, 1991; Pettersson and McDonald, 1994; Morison and Lawlor, 1999).
In studies of the response of plants to elevated CO2, photosynthesis during the growing season has received more attention than any other physiological process because it was believed that by measuring photosynthesis the responses of plant growth and productivity to atmospheric CO2 enrichment could be predicted. However, photosynthesis in single leaves, fascicles or shoots is not always positively correlated with tree growth (Körner, 1996; Luo et al., 1997; Wang et al., 2002). Although a number of physiological processes are involved (Körner, 1996; Kellomäki and Wang, 2001), the error in determining annual net carbon accumulation of trees appears to be the key factor responsible for the lack of correlation. Under boreal climatic conditions, most conifer needles must survive years of fluctuating temperatures (varying from +35 to –40 °C in Finland; Wang, 1996). There are two important considerations when studying the responses of boreal trees to CO2: acclimation of the photosynthetic apparatus to the prevailing temperature; and the relationship between the response of trees to CO2 and ambient growing temperatures (‘base‐line temperature’). With regards to the former, it has been convincingly documented that low temperature, often accompanied by high irradiance in spring and autumn, can lead to cold acclimation in trees; this may involve changes in membrane composition and Rubisco concentration, so that photosynthesis is depressed (Strand and Öquist, 1985, 1988; Leverenz and Öquist, 1987). Elevated temperatures may, therefore, greatly reduce the period during which natural acclimation results in depression of photosynthetic capacity, allowing trees to utilize radiation intercepted during the spring and autumn with greater efficiency (Long, 1991). In contrast, little is known about the interactions between elevated CO2 and low temperature. Given the temperature dependence of photosynthesis, biochemical models of C3 photosynthesis predict much larger CO2 stimulation of photosynthesis at temperatures above approx. 15 °C, with little benefit below this temperature (Long, 1991). This implies that increased CO2 enrichment will have different impacts on photosynthesis during the growing season and during the ‘non‐growing’ season. Some long‐term field experiments have indeed lent support to these predictions, but there are exceptions and responses vary considerably (Idso and Kimball, 1987; Wang et al., 1996). In Pinus taeda, for example, the relative stimulation of photosynthetic rate over several seasons was found to be correlated with temperature in one study (Tissue et al., 1997), but not in another (Teskey, 1997). Although the information available concerning the response of trees to elevated CO2 under different growing temperatures is still rather limited, it is clear that the separate influences of CO2 and temperature on trees will be superimposed on seasonal variations, so that it is difficult to predict the resultant changes in annual photosynthesis under future climates. This paper reports an analysis of this problem, using Scots pine exposed to elevated CO2 and temperature over a 3‐year period.
Simultaneous and continuous in situ monitoring of environmental factors and gas exchange in shoots of Scots pine trees (Pinus sylvestris L.) was conducted from January 1998 to December 2000 using a set of trap‐type cuvettes. The aims of the study were to investigate seasonal responses of carbon and water fluxes of needles of Scots pine to elevated CO2, temperature and combined treatments, and the effects of weather conditions during the course of 3 years, and to increase understanding of the mechanisms of photosynthetic responses and the changes in light‐ and water‐use efficiencies.
MATERIALS AND METHODS
Tree growth conditions
The experiment was established in a naturally seeded, pure stand of Scots pine (Pinus sylvestris L.) near Mekrijärvi Research Station, University of Joensuu, Finland (62°47′N, 30°58′E, 145 m a.s.l.), in 1996. The trees were 30 years old and had a mean height of 3·5 m. There were approx. 2500 trees per hectare. Mean annual temperature and rainfall at the site are 2·0 ± 0·7 °C and 600 ± 56 mm, respectively. The soil is a sandy loam, with 40 ± 7·2 mm water at field capacity and 20 ± 4·8 mm at the wilting point for the top 30 cm of soil.
The experiment utilized 16 top‐closed chambers built around single trees. Each chamber was 3·5 m tall, of prismatic shape, with eight walls and a conical roof, and covered a ground area of 5·9 m2. The four walls facing south and west were constructed from double‐walled glass (K‐glass+AS Green; Eglas Oy, Imatran, Finland), and the four north‐ and east‐facing walls from double‐walled acrylic sheets.
The CO2 concentration in each chamber was controlled using a CO2 sensor (GMP 111; Vaisala, Helsinki, Finland), a control module (ISM 112; Gantner Electronic, Schruns, Austria) and a proportional valve (MC 110; Gantner Electronic) regulating inflow of pure CO2. The temperature in the chamber was controlled using a heat exchanger in the top of each chamber, linked to a refrigeration unit (CAJ‐4511YHR, 3KW; L’Unite Hermetique, Barentin, France). The computer‐controlled temperature and CO2 supply system enabled the two variables to be adjusted automatically to achieve a specified enrichment in CO2 and/or rise in temperature inside the chamber. This resulted in four treatments: (1) outside ambient temperature and CO2 concentration (CON); (2) elevated temperature at ambient CO2 (ET); (3) elevated concentration of CO2 at ambient temperature (EC); and (4) elevated concentration of CO2 and temperature (ECT). Each treatment had four replicates. CO2 was enriched all day throughout the year. For more details of chamber structure, the environment‐controlled system, as well as long‐term performance in attaining the target environmental treatment, see Kellomäki et al. (2000).
Measurements of CO2 and H2O exchange and environmental variables
Gas exchange was measured in situ using a computer‐controlled system of eight cylindrical plexiglass cuvettes, similar that descripted by Hari et al. (1979), each with an inner volume of 2·37 l. The cuvettes were connected to a gas analyser by copper tubes that were warmed by electric cables to avoid condensation of water vapour. When no measurement was to be made, the two halves of the cuvette remained open. When a measurement was to be made, the two halves were closed simultaneously by two pumps operated by compressed air and magnetic valves. When the measurement had been made the halves of the cuvette opened again. The air flow from each cuvette was adjusted using a rotameter to maintain a relatively stable air flow (approx. 0·8 l min–1). Concentrations of CO2 and H2O vapour in the air in the cuvette were measured using an infrared gas analyser (URAS I; Hartman and Braun, Frankfurt, Germany). To obtain stable conditions, there was a delay of 120 s after the cuvette was closed before four consecutive measurements were taken at 25‐s intervals.
The air temperature inside the cuvette was measured using a 0·2 mm copper–constantan thermocouple, the photosynthetically active radiation (PAR) using a quantum sensor (LI‐190SB; LI‐COR Inc., Lincoln, NB, USA), and global short‐wave radiation using a pyranometer sensor (LI‐200SB; LI‐COR Inc.).
Gas‐exchange measurements were made on eight 1‐year‐old shoot sections (sample shoot) in the four different treatments from January 1998 to December 2000. The sample shoots located in the fourth whorls from the top of the crown faced south and were not shaded by other shoots. Buds were removed and surplus needles were thinned to give equal numbers of needles on all sample shoots before they were inserted into the cuvette. At the end of the each measuring year, the projected needle area of the shoot (the sum of the projection areas of single needles) was measured using a scanner with leaf area analysis software (WinFOLIA™; Regent Instruments Inc., Quebec, Canada).
Net CO2 (Fc, g CO2 m–2 h–1) and H2O (Fw, g H2O m–2 h–1) fluxes were calculated using the mass balance method (Giuliani et al. 1997), assuming that concentrations of CO2 and H2O entering the cuvette remained stable during the 25‐s lag time between two consecutive measurements, i.e.


where ϕ is the air flow rate, Lp the projected needle area of the sample shoot, Vc the volume of the cuvette, Cin and Cout concentrations of CO2 in the air in the cuvette and the outside air, respectively, Win and Wout absolute humidities of the air in the cuvette and outside air, respectively, and t time.
RESULTS
Diurnal course of CO2 and H2O fluxes during the growing season
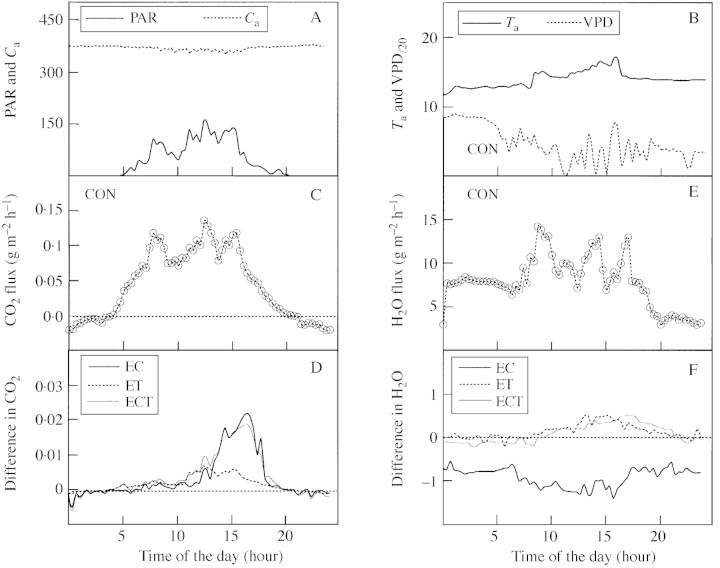
The diurnal courses of CO2 and H2O fluxes on a typical sunny (Day 210 in 2000, Fig. 1) and overcast day (Day 214, Fig. 2) are presented to show the effects of elevated CO2 and temperature treatments. On the sunny day, EC increased the CO2 fluxes and reduced the H2O fluxes during almost all periods of sunshine (from approx. 0600 to 2000 h, Fig. 1D and F), whereas ET increased the CO2 fluxes only after noon at vapour pressure deficits (VPD) >1·2 kPa but increased the H2O fluxes throughout the whole day. ECT treatment led to a similar pattern of CO2 fluxes as EC treatment (Fig. 1D), while H2O fluxes were similar to those produced by ET treatment (Fig. 1F). On the overcast day, EC increased CO2 fluxes only after noon when air temperature (Ta) >14 °C (Fig. 2D), whereas H2O fluxes were reduced throughout the day (Fig. 2F). Following ECT treatment, patterns of diurnal CO2 fluxes were similar to those following EC treatment (Fig. 2D), and diurnal H2O fluxes were similar to those caused by ET treatment (Fig. 2F).
Fig. 1. Daily pattern of CO2 (C and D) and H2O (E and F) fluxes in attached shoots of Scots pine trees grown in environment‐controlled chambers and exposed to either normal ambient conditions (CON), elevated CO2 (+350 µmol mol–1; EC), elevated temperature (+2–6 °C; ET) or a combination of EC and ET (ECT), measured on a sunny day (Day 210 in 2000). The measured air temperature (Ta, °C), vapour pressure deficit (Dv, kPa), incident PAR (µmol m–2 s–1) and CO2 concentration in the chamber (Ca, µmol mol–1) are also presented (A and B). Values are means of two shoot measurements for each treatment.
Fig. 2. Daily pattern of CO2 (C and D) and H2O (E and F) fluxes in attached shoots of Scots pine trees grown in environment‐controlled chambers and exposed to either normal ambient conditions (CON), elevated CO2 (+350 µmol mol–1; EC), elevated temperature (+2–6 °C; ET) or a combination of EC and ET (ECT), measured on an overcast day (Day 214 in 2000). The measured air temperature (Ta, °C), vapour pressure deficit (Dv, kPa), incident PAR (µmol m–2 s–1) and CO2 concentration in the chamber (Ca, µmol mol–1) are also presented (A and B). Values are means of two shoot measurements for each treatment.
Compared with CON, the diurnal net flux of CO2 increased by 22, 5 and 21 % for EC, ET and ECT on the sunny day, respectively, and by 8, 3 and 7 % on the overcast day (Table 1). In contrast, the diurnal net H2O flux increased by 11 % for ET and by 9 % for ECT, but decreased by 9 % in the EC treatment. The hourly mean light‐use efficiency (LUE) varied from 0·01 to 0·06 mol CO2 mol–1 PAR, and the water use efficiency (WUE) from 0·002 to 0·047 g CO2 g–1 H2O over all treatments. Treatment‐induced percentage changes in LUE and WUE were similar to those in CO2 and H2O fluxes regardless of the weather conditions (Table 1).
Table 1.
Daily net fluxes of CO2 and H2O and hourly mean light‐ (LUE) and water‐use efficiencies (WUE) for attached needles of Scots pine trees grown in environment‐controlled chambers and exposed to either normal ambient conditions (CON), elevated CO2 (+350 µmol mol–1; EC), elevated temperature (+2–6 °C; ET) or a combination of EC and ET (ECT)
| Sunny day (Day 210) | Overcast (Day 214) | |||||||
| Treatments | CO2 flux(g m–2 d–1) | H2O flux(g m–2 d–1) | LUE(mol CO2 mol–1 incident PAR) | WUE(g CO2 g–1 H2O) | CO2 flux(g m–2 d–1) | H2O flux(g m–2 d–1) | LUE(mol CO2 mol–1 incident PAR) | WUE(g CO2 g–1 H2O) |
| CON | 3·088 | 420·1 | 0·039 | 0·012 | 1·195 | 188·6 | 0·031 | 0·008 |
| EC–CON | 0·692 | –39·6 | 0·009 | –0·002 | 0·091 | –19·7 | 0·002 | –0·001 |
| ET–CON | 0·159 | 45·9 | 0·002 | 0·001 | 0·036 | 7·2 | 0·001 | 0·000 |
| ECT–CON | 0·642 | 41·4 | 0·008 | 0·001 | 0·083 | 5·3 | 0·002 | 0·000 |
Measurements made on two typical days in summer 2000.
Recovery of CO2 and H2O fluxes from winter dormancy
Measurements showed that the net CO2 flux (= photosynthetic CO2 uptake – respiration) became positive on various dates in the 3 years. For example, in the case of CON, continuous positive CO2 fluxes began in late April, although positive CO2 fluxes were also recorded during warmer days in March (Fig. 3, Day 74). A rapid increase in CO2 flux occurred in May, and the maximum occurred in late June. The EC treatment did not alter the date of onset of the positive net CO2 flux relative to CON, but it did lead to a mean increase of 11 % in total daily CO2 flux (Fc.t) between April and May 2000. In contrast, the warmer temperature treatments (EC and ECT) advanced the onset of positive net CO2 fluxes by 15–20 d, i.e. CO2 flux started in early April, reaching a maximum in the middle of June. The measurements made in April and May 2000 showed that ET and ECT increased the mean Fc.t by 16 and 17 %, respectively.
Fig. 3. Daily courses of CO2 and H2O fluxes for attached shoots of Scots pine trees grown in environment‐controlled chambers and exposed to either normal ambient conditions (CON), elevated CO2 (+350 µmol mol–1; EC), elevated temperature (+2–6 °C; ET) or a combination of EC and ET (ECT). Measurements made on 5 d in spring 2000. Incident PAR and air temperature (Ta) of the CON treatment are presented in the top‐most panel. Values are means of two measurements for each treatment.
Regardless of the treatment, H2O fluxes were closely coupled with CO2 fluxes during spring (Fig. 3), although there was wider variation between the treatments in H2O fluxes than in CO2 fluxes, and less variation in H2O fluxes between measuring dates. For example, the total daily H2O flux (Fw.t) increased by 104 % for ET and by 110 % for ECT, but decreased by 6 % for EC on Day 136, whereas Fw.t increased by only 38 and 41 % for ET and ECT, respectively, on Day 180, demonstrating that treatments led to disproportional changes in H2O and CO2 fluxes in the spring.
Daily course of CO2 and H2O fluxes during late autumn
Under favourable weather conditions, particularly at warm temperatures, needles grown under different treatments maintained their high rate of photosynthesis at the beginning of September, as seen on Day 242 (Fig. 4). After mid‐September, however, the CO2 flux decreased as air cooled in the case of CON, so that the continual positive net CO2 flux had disappeared by the end of October. However, positive CO2 fluxes were still recorded when short warm spells occurred (mean daytime temperature above 0 °C for several days). Thus, the decrease in positive net CO2 flux from the maximum to zero in autumn seems to be slower than the recovery of maximum CO2 flux following winter dormancy (Fig. 3).
Fig. 4. Daily courses of CO2 and H2O fluxes for attached shoots of Scots pine trees grown in environment‐controlled chambers and exposed to either normal ambient conditions (CON), elevated CO2 (+350 µmol mol–1; EC), elevated temperature (+2–6 °C; ET) or a combination of EC and ET (ECT). Measurements made on 6 d in autumn 2000. Incident PAR and air temperature (Ta) of the CON treatment are presented in the top‐most panel. Values are means of two shoot measurements for each treatment.
The EC‐induced increase in CO2 fluxes declined with acclimation to low temperature, whereas the EC‐induced reduction in H2O fluxes showed a smaller change over the whole acclimation period (Day 240–290) relative to CO2 fluxes. For example, EC increased Fc.t by 17 % on Day 242, and reduced Fw.t by 64 %, but the changes on Day 255 were only 9 % and 53 %, respectively (Fig. 4). The warmer temperature treatments prolonged the duration of the positive net CO2 flux, extending it until the end of November, but they also led to a greater increase in H2O flux during the autumn. Furthermore, measurements in September and October showed that all three treatments greatly increased the sensitivity of CO2 fluxes to frost during the previous night. An example is given in Fig. 4, where three daily courses of CO2 flux, on Days 265, 266 and 270, with similar incident PAR, daytime air temperature and vapour pressure deficit (not shown), are presented. The freezing night‐time temperatures (mean –9 °C) on Day 265 resulted in a 24 % decrease in Fc.t on Day 266 for CON, a 35 % decrease for EC, a 41 % decrease for ET and a 39 % decrease for ECT, while on Day 270, Fc.t showed a recovery of 16 % for CON, 10 % for EC, 12 % for ET and 13 % for ECT. The H2O flux was also reduced significantly by frost (Fig. 4).
Seasonal and annual variation in CO2 and H2O fluxes
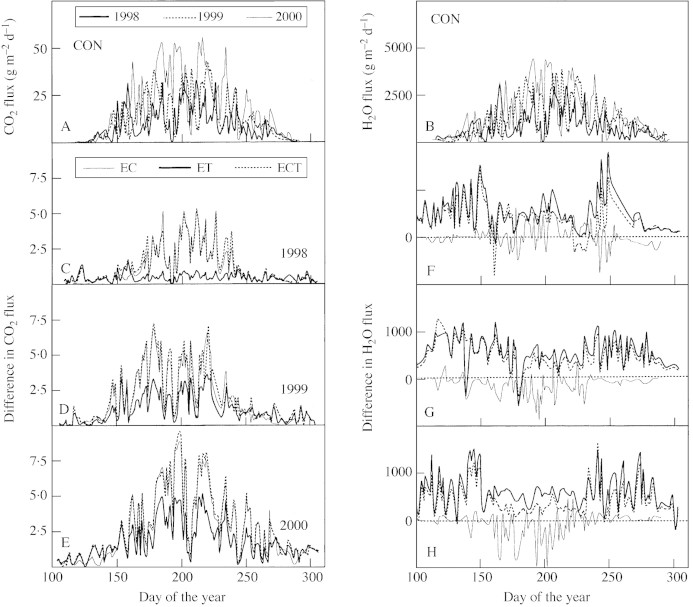
A dependence of Fc.t and Fw.t on season is evident during the 3 years of the study (1998–2000). In the control treatment, maximum Fc.t and Fw.t varied from 27 to 53 g m–2 d–1 and from 2600 to 4800 g m–2 d–1, respectively (Fig. 5A and B). Maximum mean values occurred in July, and ranged from 23 to 46 g m–2 d–1 and from 1200 to 3700 g m–2 d–1, respectively. Owing to difference in fluxes between growing‐ and non‐growing seasons (Fig. 5), the data were analysed separately for the growing season (Day 150–240) and the non‐growing season (Table 2). Results indicated that: (1) EC significantly increased mean Fc.t during the 3 years and reduced Fw.t only in 1999 and 2000; (2) EC had a larger influence on both Fc.t and Fw.t in the growing seasons than in the non‐growing seasons; (3) the higher temperature increased mean Fc.t as exposure proceeded, but decreased the mean Fw.t independent of growing season; and (4) ECT led to similar percentage change in Fc.t as the EC treatment, and a similar percentage change in Fw.t as the ET treatment.
Fig. 5. Seasonal variation in daily total CO2 and H2O fluxes in attached shoots of Scots pine trees grown in environment‐controlled chambers and exposed to normal ambient conditions (CON, A and B) over 3 years (1998, 1999 and 2000), and differences (treatment–control) in daily net CO2 (C–E) and H2O (F–H) fluxes resulting from the treatments (EC, ET and ECT). Data points are means of measurements on two trees in the same treatment. EC, Elevated CO2 (+350 µmol mol–1); ET, elevated temperature (+2–6 °C); ECT, combination of EC and ET.
Table 2.
Seasonal mean (± s.e.) of daily total CO2 and H2O fluxes of attached shoots of Scots pine as a function of year (1998, 1999 and 2000) and treatments (CON, EC, ET and ECT)
| Treatments | CO2 flux (g m–2 d–1) | H2O (g m–2 d–1) | ||||||||||
| 1998 | 1999 | 1999 | 1998 | 1999 | 1999 | |||||||
| Season: | Growing | Non‐growing | Growing | Non‐growing | Growing | Non‐growing | Growing | Non‐growing | Growing | Non‐growing | Growing | Non‐growing |
| CON | 15·10 ± 1·62a | 2·51 ± 0·13a | 20·19 ± 1·20a | 4·89 ± 0·26a | 24·80 ± 1·52a | 5·62 ± 0·52a | 1503 ± 58a | 704 ± 26a | 1750 ± 113a | 783 ± 27a | 1981 ± 113a | 1010 ± 46a |
| EC | 19·31 ± 1·71b | 2·86 ± 0·20b | 25·21 ± 1·66b | 5·40 ± 0·24b | 31·24 ± 1·44b | 6·76 ± 0·46b | 1615 ± 62a | 755 ± 28a | 1529 ± 102b | 751 ± 45a | 1728 ± 101b | 909 ± 53b |
| ET | 15·85 ± 1·78a | 2·92 ± 0·19cb | 22·61 ± 1·18c | 5·78 ± 0·51b | 28·30 ± 1·91b | 7·16 ± 0·56b | 1996 ± 135b | 1524 ± 133b | 2204 ± 147c | 1523 ± 221b | 2433 ± 126c | 1764 ± 224c |
| ECT | 19·15 ± 1·50b | 2·98 ± 0·24c | 26·37 ± 1·74b | 6·04 ± 0·42b | 31·88 ± 1·88b | 7·37 ± 0·62b | 1953 ± 214b | 1456 ± 152b | 2152 ± 132c | 1404 ± 189b | 2405 ± 195c | 1713 ± 209c |
CON, Normal ambient conditions; EC, elevated CO2 (+350 µmol mol–1); ET, elevated temperature (+2–6 °C); ECT, combination of EC and ET.
Different superscripts within a column indicate statistically different values at P < 0·05 (multifactorial ANOVA).
Seasonal variation in light‐ and water‐use efficiencies
The hourly means of CO2 flux, H2O flux and incident PAR during the 3 years of study were used to calculate the long‐term LUE and WUE (Figs 6 and 7). In the case of CON, LUE varied from 0·005 to 0·054 mol CO2 mol–1 PAR, with a mean of 0·045 mol mol–1 in the growing season (Fig. 6A) and 0·018 mol mol–1 in the non‐growing season (Fig. 6E). Similarly, WUE ranged from 0·002 to 0·047 g CO2 g–1 H2O, with a mean of 0·012 g g–1 in the growing season (Fig. 7A) and 0·0045 g g–1 in the non‐growing season (Fig. 7E). Hourly means of LUE and WUE are plotted against those of air temperature (Fig. 6) and VPD (Fig. 7). Since initial plots gave a wide scatter, only points for hourly mean PAR >600 µmol m–2 s–1 and VPD <1·0 kPa were used to calculate the response of maximum LUE to temperature (Fig. 6) and, correspondingly, points for hourly mean PAR >600 µmol m–2 s–1 and hourly mean temperature >5 °C were used to estimate the response of maximum WUE to VPD (Fig. 7). Results indicate that maxima for LUE and WUE were significantly correlated with temperature and VPD, respectively, with R2 varying from 0·63 to 0·84 and P < 0·05 for the four treatments. The differences between the treatments (EC, ET and ECT) and CON (based on the fitted lines) indicated that: (1) the differences in LUE increased with air temperature regardless of treatment (Fig. 6B–D and F–H); (2) ECT increased LUE most and ET least at high temperatures during the growing season, but ET (1998 and 1999) and ECT (2000) increased LUE most during the non‐growing season, when EC had least effect; (3) EC increased WUE more in the growing season than in the non‐growing season, but ET and ECT reduced WUE more in the non‐growing season than in the growing season (Fig. 7B–D and F–H); (4) both the dependence of WUE on VPD and the difference in WUE were sensitive to changes at low VPDs, but approached constancy when VPD exceeded approx. 1·2 kPa; and (5) the effect of EC on WUE increased with exposure time, regardless of season, but the effect of ET and ECT on WUE decreased with exposure time.
Fig. 6. Hourly values for light‐use efficiency (LUE, mol CO2 mol–1 incident PAR) of attached shoots of Scots pine trees grown in environment‐controlled chambers and exposed to normal ambient conditions (CON), plotted against hourly mean temperature for the growing season (A) and the non‐growing season (E) of 2000. Each point represents the mean of two measurements for each treatment. Points obtained under hourly mean PAR >600 µmol m–2 s–1 and VPD <1·0 kPa (closed circles) are fitted by a second‐order function. Percentage differences in LUE between the treatments and CON (based on the fitted lines) are presented as functions of seasons (A–D, growing season; E–H, non‐growing season), treatments (EC, ET and ECT) and year of measurement (1998, 1999 and 2000). EC, Elevated CO2 (+350 µmol mol–1); ET, elevated temperature (+2–6 °C); ECT, combination of EC and ET.
Fig. 7. Hourly values for water use efficiency (WUE, g CO2 g–1 H2O) for attached shoots of Scots pine trees grown in environment‐controlled chambers and exposed to normal ambient conditions (CON), plotted against hourly mean VPD for the growing season (A) and the non‐growing season (E) of 2000. Each point represents the mean of two measurements for each treatment. Points obtained under hourly mean PAR >600 µmol m–2 s–1 and hourly mean temperature >5 °C (closed circles) are fitted by means of an exponential function. Percentage differences in WUE between the treatments and CON (based on the fitted lines) are presented as functions of seasons (A–D, growing season; E–H, non‐growing season), treatments (EC, ET and ECT) and year of measurement (1998, 1999 and 2000). EC, Elevated CO2 (+350 µmol mol–1); ET, elevated temperature (+2–6 °C); ECT, combination of EC and ET.
DISCUSSION
Measurements made continually over 3 years of gas exchange in attached shoots of Scots pine show the long‐term acclimation of CO2 and H2O fluxes to elevated CO2 and temperature under (almost) natural conditions. Mean CO2 fluxes in the EC treatment increased by 28, 24 and 25 % during the 1998, 1999 and 2000 growing seasons, respectively (Table 2). It has been concluded that CO2‐induced stimulation in photosynthesis can vary with exposure time, leaf age and other environment variables (Ceulemans and Mousseau, 1994; Wang et al., 1995; Saxe et al., 1998; Tissue et al., 2001). The decline in CO2 fluxes with exposure time that were observed here cannot be attributed to the effect of needle age because the gas‐exchange measuring system was moved to another 1‐year‐old shoot in the November of each year. Also, it was noticed that both total radiation and mean daily temperature were higher in 1999 and 2000 than in 1998, when the absolute rates of CO2 uptake were also larger (Fig. 5A), implying that the decline could not be attributed to the differences in growing temperatures between the years, and may therefore be related to CO2‐induced ‘down‐regulation’ in photosynthetic capacity (Pettersson et al., 1993; Van Oosten and Besford, 1994; Griffin et al., 2000). As has been discussed intensively, this down‐regulation can often be related to CO2‐induced adjustments in leaf biochemical capacity, changes in leaf morphology and nutritional limitations (Stitt, 1991; Van Oosten and Besford, 1994; Wang and Kellomäki, 1997b). The present results support the view that long‐term regulation in photosynthetic capacity by elevated CO2 reflects an indirect effect through source–sink relationships within the whole tree (Tissue et al., 2001) because exposure times were the same for sample shoots (needles) used in the different measuring years, but differed for the sample trees growing in the chambers.
Little is known about the response of CO2 fluxes to CO2 enrichment at cool growing temperatures. Murray et al. (1994) showed that CO2 delayed bud burst in Sitka spruce in spring, and advanced bud set in autumn, indicating some shift in bud phenology. However, the present results showed no significant change in the date at which positive net CO2 fluxes (photosynthetic rate > respiration rate) occurred in trees growing in the control or elevated‐CO2 chambers over the 3 years (Fig. 3), although a mean enrichment of 11 % in the total diurnal CO2 flux was observed in early spring and 13 % in late autumn. By examining the diurnal course of CO2 flux in relation to the corresponding weather conditions, it was found that the increase in CO2 flux during early spring resulted mainly from the higher photosynthetic rate on warmer days. One significant finding is that rates of CO2 flux in chambers with elevated CO2 may have been more affected by frost the previous night than those in the control chambers in both spring and autumn. As a result, full recovery of photosynthetic capacity may be delayed in spring, and the positive net CO2 flux may disappear early in autumn, as observed in 2000 (Fig. 4).
Considering only the treatment‐induced differences in CO2 flux, the theoretically expected positive interaction between elevated CO2 and temperature can be seen in the growing seasons of 1999 and 2000 as a greater percentage increase in the mean CO2 flux in the case of ECT relative to CON (Fig. 5D and E). However, from the diurnal performance of CO2 fluxes on two typical days in summer 2000 (Figs 1 and 2), the increased CO2 flux in ECT relative to EC could be attributed mainly to the increase in CO2 flux during the afternoon. Elevated temperature alone progressively stimulated CO2 flux by 5 % in the growing season of 1998 to 14 % in 2000 (Table 1), suggesting that elevated CO2 could have a ‘fertilization’ effect only, whereas elevated temperature could modify some processes of photosynthesis in the case of ECT.
In the context of climate change, there have fewer studies of tree transpiration compared with those of photosynthesis. Transpiration rates are expected to decline as a result of decreased stomatal conductance (Morison, 1996), but this is not always the case in experiments (Morison, 1996; Wang and Kellomäki, 1997a), and low transpiration rates may also lead to higher leaf temperatures, particularly under conditions of high irradiance and low windspeed (Idso et al., 1987). The present results showed that elevated CO2 had less effect on H2O flux than on CO2 flux, although a more pronounced reduction in H2O flux was found with increasing exposure time. This seems to reflect acclimation of stomatal conductance to CO2 (Morison, 1996) or modifications in the resistance of the whole‐tree water system (Atkinson and Taylor, 1996). Assuming that these are the only causes, then a constant and lower H2O flux should be recorded throughout the measurement period in the case of EC. However, in the 3 years of the study, total daily H2O fluxes (Fig. 5F–H) declined mainly during the growing season, and differences in total diurnal H2O fluxes showed wide variation. Thus, one interpretation is that the decrease in H2O flux, in the case of elevated CO2, is largely attributable to greater sensitivity of stomata to heat stress in the summer. Similarly, the increase in total diurnal H2O flux throughout the measurement period in the case of elevated temperatures, despite its reduction with increasing exposure time, suggests possible acclimation of stomata or of the resistance of the whole tree to water flux with warming, in addition to a direct effect of rising temperature on transpiration.
Theoretically, leaf LUE depends on the maximum leaf photosynthetic rate and is influenced by many factors, such as PAR, temperature, VPD and plant development stage (Marscal et al., 2000). In the present study, LUE was defined as the ratio of shoot CO2 flux to incident PAR and it is therefore not surprising that annual variation of LUE under conditions of CO2 enrichment almost paralleled the annual course of CO2 flux. Even so, some regulation of LUE, induced by elevated CO2, can be seen when LUE is plotted against temperature (Fig. 6), i.e. there were considerable differences in maximum LUE between years and between seasons in the same year, suggesting that there is some dependence of LUE on other environmental variables, and that the link between LUE and CO2‐induced modifications in leaf structure and development may be more significant for understanding the response of LUE to CO2 at the leaf level.
It is widely assumed that elevated atmospheric CO2 concentrations will reduce stomatal conductance and, therefore, transpiration rates, and that WUE will be enhanced as a result (Morison, 1996; Drake et al., 1997). However, there is increasing evidence that the stomatal conductance of many woody species is relatively unresponsive to elevated CO2 in the field, so that the increase in WUE may be due entirely to increased assimilation rates and will bring about no improvement in water economy. Compared with LUE, the CO2‐induced increase in WUE was more evident here and showed more enhancement with exposure time (Fig. 7). It is clear that apart from the ‘fertilizer effect’ of CO2, the CO2‐induced increase in stomatal sensitivity to high VPD and the consequent reduction of water loss (Fig. 5) could be largely attributable to the increase in WUE.
As expected, elevated temperature increased LUE and reduced WUE for much of the time over all 3 years. Two points should be mentioned here. First, the percentage decrease in WUE became smaller with exposure time (Fig. 7B–D), and maximum LUE increased (Fig. 6B–D). Secondly, LUE and WUE were more sensitive to temperature and VPD, respectively, in the non‐growing season than in the growing season. The former indicates that regulation may take place in the structure of the water system in trees, while the latter may imply that temperature or VPD stress during early spring and autumn may be more harmful to physiological processes in the tree that involve carbon and water.
CONCLUSIONS
(1) Long‐term, continual monitoring of CO2 and H2O fluxes in situ has shown significant acclimation of the daily and seasonal responses of trees to elevated CO2 and temperature; (2) increases in temperature and CO2 concentration had quite different consequences in the growing season and the non‐growing season, with a larger relative effect of temperature on carbon and water fluxes in the non‐growing season than in the growing season, but a larger relative effect of CO2 on carbon and water fluxes in the growing season; (3) there was no additional stimulation of carbon assimilation by temperature at elevated CO2 compared with CO2 elevation alone, and H2O fluxes in trees exposed to elevated temperatures and CO2 were similar to those in trees exposed to elevated temperatures alone; (4) elevated CO2 and increased temperature markedly altered light‐ and water‐use efficiencies, with the extent depending greatly on air temperature and VPD, respectively.
ACKNOWLEDGEMENTS
This work was part of the China–Finland cooperation project ‘Responses of the Ecosystem Processes of High‐Frigid Coniferous Forest to Climate Change’ no. 302(1130504), the Finnish Centre of Excellence Programme (no. 64308), Key Project of Ecology and Environment in Western China (no. 90202010), and ‘100 Distinguished Experts’ Programme of the Chinese Academy of Sciences. Funding provided by the Academy of Finland, Chinese Academy of Sciences, National Natural Science Foundation of China (NSFC), National Technology Agency (Tekes) of Finland, and University of Joensuu is gratefully acknowledged. Thanks also to the Mekrijärvi Research Station for providing access and logistical support at the research site, and Mr Matti Lemettinen and Mr Alpo Hassinen for help in the field.
Supplementary Material
Received: 15 January 2003; Returned for revision: 27 February 2003; Accepted: 18 March 2003 Published electronically: 9 May 2003
References
- AtkinsonCJ, Taylor JM.1996. Effects of elevated CO2 on stem growth, vessel area and hydraulic conductivity of oak and cheery seedlings. New Phytologist 133: 617–626. [Google Scholar]
- CeulemansR, Mousseau M.1994. Effects of elevated atmospheric CO2 on woody plants. New Phytologist 127: 425–446. [Google Scholar]
- CurtisPS.1996. A meta‐analysis of leaf gas exchange and nitrogen in trees under elevated carbon dioxide. Plant, Cell and Environment 19: 127–137. [Google Scholar]
- DrakeBG, Gonzales‐Meler M, Long SP.1997. More efficient plants: a consequence of rising atmospheric CO2? Annual Review of Plant Physiology and Plant Molecular Biology 48: 609–639. [DOI] [PubMed] [Google Scholar]
- GiulianiR, Nerozzi F, Magnanni E, Corelli‐Grappadelli L.1997. Influence of environmental and plant factors on canopy photo synthesis and transpiration of apple trees. Tree Physiology 17: 367–645. [DOI] [PubMed] [Google Scholar]
- GriffinKL, Tissue DT, Turnbull MH, Whitehead D.2000. The onset of photosynthetic acclimation to elevated CO2 partial pressure in field‐grown Pinus radiata D. Don. after 4 years Plant, Cell and Environment 23: 1089–1098. [Google Scholar]
- HariP, Kanninen M, Kellomäki S, Luukkanen O, Pelkonen P, Salminen R, Smolander H.1979. An automatic system for measurements of gas exchange and environmental factors in a forest stand, with special reference to measuring principles. Silva Fennica 13: 94–100. [Google Scholar]
- IdsoSB, Kimball BA, Mauney JR.1987. Atmospheric carbon dioxide enrichment effects on cotton midday foliage temperature: implications for plant water use and crop yield. Agronomy Journal 79: 667–672. [Google Scholar]
- KellomäkiS, Wang K‐Y.2001. Growth and resource use of birch seedlings under elevated carbon dioxide and temperature. Annals of Botany 87: 669–682. [Google Scholar]
- KellomäkiS, Wang K‐Y, Lamittene M.2000. Controlled environment chambers for investigating tree response to elevated CO2 and temperature under boreal conditions. Photosynthetica 38: 69–81. [Google Scholar]
- KörnerC.1996. The response of complex multispecies systems to elevated CO2 In: Walker BH, Steffen WL, eds. Global changes and terrestrial ecosystems Cambridge: Cambridge University Press, 20–42. [Google Scholar]
- LeverenzJ, Öquist G.1987. Quantum yields of photosynthesis at temperatures between –2 °C and 35 °C in a cold tolerant C3 plant (Pinus sylvestris) during the course of one year. Plant, Cell and Environment 10: 287–295. [Google Scholar]
- LongSP.1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 con centrations: has its importance been underestimated? Plant, Cell and Environment 14: 729–739. [Google Scholar]
- LuoY, Chen J, Reynolds JF, Field CB, Mooney HA.1997. Disproportional increases in photosynthesis and plant biomass in a Californian grassland exposed to elevated CO2: a simulation analysis. Functional Ecology 11: 696–704. [Google Scholar]
- MarscalML, Orgaz F, Villalobos FJ.2000. Radiation‐use efficiency and dry matter partitioning of a young olive (Olea europeae) orchard. Tree Physiology 20: 65–72. [DOI] [PubMed] [Google Scholar]
- MorisonJIL.1996. Global environment change impacts on crop growth and production in Europe. Implications of global environmental change for crops in Europe. Aspects of Applied Biology 45: 62–74. [Google Scholar]
- MorisonJIL, Lawlor DW.1999. Interactions between increasing CO2 concentration and temperature on plant growth. Plant, Cell and Environment 22: 659–682. [Google Scholar]
- MurrayMB, Smith RL, Leith ID, Fowler D, Lee HSL, Friend AD, Jarvis PJ.1994. Effects of elevated CO2, nutrition and climatic warming on bud phenology in Sitka Spruce (Picea sitchensis) and their impact on the risk of frost damage. Tree Physiology 14: 691–706. [DOI] [PubMed] [Google Scholar]
- NorbyRJ, Wullschleger SD, Gunderson CA, Johnson DW, Ceulemans R.1999. Tree responses to rising CO2 in field experiments: implications for the future forest. Plant, Cell and Environment 22: 683–714. [Google Scholar]
- PetterssonR, McDonald AJS.1994. Effects of nitrogen supply on the acclimation of photosynthesis to elevated CO2 Photosynthesis Research 39: 389–400. [DOI] [PubMed] [Google Scholar]
- PetterssonR, McDonald AJS, Stadenberg I.1993. Response of small birch plants (Betula pendula Roth.) to elevated CO2 and nitrogen supply. Plant, Cell and Environment 16: 1115–1121. [Google Scholar]
- SaxeH, Ellsworth DS, Heath J.1998. Tree and forest functioning in an enriched CO2 atmosphere. New Phytologist 139: 395–435. [Google Scholar]
- StittM.1991. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant, Cell and Environment 14: 741–762. [Google Scholar]
- StrandM, Öquist G.1985. Inhibition of photosynthesis by freezing temperatures and high light levels in cold‐acclimated seedlings of Scots pine (Pinus sylvestris). II. Effects on chlorophyll fluorescence at room temperature and 77 K. Physiologia Plantarum 65: 117–123. [Google Scholar]
- StrandM, Öquist G.1988. Effects of forest hardening, dehardening and freezing stress on in vivo chlorophyll fluorescence of Scots pine seedings (Pinus sylvestris). Plant, Cell and Environment 11: 231–238. [Google Scholar]
- TeskeyRO.1997. A field‐study of the effects of elevated CO2 on carbon assimilation, stomatal conductance and leaf and branch growth of Pinus taeda trees. Plant, Cell and Environment 18: 565–573. [Google Scholar]
- TissueDT, Thomas RB, Strain BR.1997. Atmospheric CO2 enrichment increases growth and photosynthesis of Pinus taeda: a four year experiment in the field. Plant, Cell and Environment 20: 1123–1134. [Google Scholar]
- TissueDT, Griffin K, Turnbull MH, Whitehead D.2001. Canopy position and needle age affect photosynthetic response in field‐grown Pinus radiata after five years of exposure to elevated carbon dioxide partial pressure Tree Physiology 21: 915–923. [DOI] [PubMed] [Google Scholar]
- Van OostenJJ, Besford RT.1994. Sugars feeding mimics effect of acclimation to high‐CO2‐rapid down regulation of Rubisco small subunit transcripts but not of the large subunits transcripts. Journal of Plant Physiology 143: 306–312. [Google Scholar]
- WangK‐Y.1996. Canopy CO2 exchange of Scots pine and its seasonal variation after four‐year exposure to elevated CO2 and temperature. Agricultural and Forest Meteorology 82: 1–27. [Google Scholar]
- WangK‐Y, Kellomäki S.1997a. Stomatal conductance and transpiration in shoots of Scots pine after 4‐year exposure to elevated CO2 and temperature. Canadian Journal of Botany 75: 552–561. [Google Scholar]
- WangK‐Y, Kellomäki S.1997b. Effects of elevated CO2 and soil‐nitrogen supply on chlorophyll fluorescence and gas exchange in Scots pine, based on a branch‐in‐bag experiment. New Phytologist 136: 277–286. [Google Scholar]
- WangK‐Y, Kellomäki S, Laitinen K.1995. Effects of needle age, long‐term temperature and CO2 treatments on the photosynthesis of Scots pine. Tree Physiology 15: 211–218. [DOI] [PubMed] [Google Scholar]
- WangK‐Y, Kellomäki S, Laitinen K.1996. Acclimation of photosynthetic parameters in Scots pine after three years exposure to elevated temperature and CO2 Agricultural and Forest Meteorology 82: 195–217. [Google Scholar]
- WangK‐Y, Zha T, Kellomäki S.2002. Measuring and simulating crown respiration of Scots pine with increased temperature and carbon dioxide enrichment. Annals of Botany 90: 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.