Abstract
A variant clone of the tetraploid (2n = 4x = 68) interspecific hybrid Helianthus annuus × H. tuberosus derived by in vitro tissue culture showed a deviation from the usual pattern of organization of the plant body. This variant developed shoot‐like structures and somatic embryos from intact adventitious roots of in vitro‐grown plantlets. The morphogenetic structures were not normally able to differentiate complete plants. They did show cellular proliferation with the inception of additional secondary embryos, leaf‐like structures and unorganized masses of callus. Nevertheless, some ectopic structures isolated from roots and transferred onto fresh basal medium without growth regulators were able to produce plantlets that exhibited the same phenotype as the original clone. Histological analyses demonstrate that they originate from cortical cells in association with the development of lateral root primordia.
Key words: Helianthus annuus × H. tuberosus, interspecific hybrid, regeneration in vitro, root buds, somatic embryogenesis, totipotency
INTRODUCTION
In higher plants, the zygote divides to produce the embryo, a bipolar structure with one, two or several embryonic leaves (cotyledons), the shoot apical meristem (plumule) and the root apical meristem (D’Amato, 1997). Once organized, the two meristems have very different developmental fates (Steeves and Sussex, 1989). However, cell derivatives of each type of meristem can become organized into the other type, i.e. shoots can be rooted and roots can produce shoots. In particular, the natural ability of roots of many species to form buds that develop into new shoots has been long recognized, and lists of species capable of forming ‘root buds’ are extensive (Wittrock, 1884; Holm, 1925; Raju et al., 1966; Donovan, 1976). In some species, shoot buds occur sporadically on roots only after the root has been excised, whereas in other species one of the main functions of the root system appears to be the production of root buds (reviewed in Peterson, 1975). The formation of buds on roots enables the propagation of plants by root cuttings and is an important means of spreading noxious weeds (Hamdoun, 1970; Horvath, 1998, 1999; Donald, 2000). A variety of root tissue may be involved in bud differentiation, and the development pattern therefore varies considerably depending on the region of the root in which bud initiation occurs (Bosela and Ewers, 1997). Root buds of herbaceous species frequently arise endogenously, in a manner similar to initiation of lateral or adventitious roots (Peterson, 1975). Therefore, descriptions of buds arising from both the pericycle and the phellogen or related tissues are frequently reported (Bonnet and Torrey, 1966; Hamdoun, 1970; Peterson, 1975).
The production of root buds is an example of the potentiality (totipotency) of differentiated or partly differentiated plant cells to express a new pattern of differentiation. Likewise, somatic cell totipotency is demonstrated by the ability of plant cells to develop into a complete and fertile plant by in vitro somatic embryogenesis and/or organogenesis. We have previously shown that a high embryogenic potential can be acquired by cells of regenerated plants of the tetraploid (2n = 4x = 68) interspecific hybrid Helianthus annuus × H. tuberosus (Fambrini et al., 1996, 1997, 2001). Despite every clone obtained from these regenerated plants being characterized by a high morphogenetic competence (Fambrini et al., 1997), some phenotypic differences were observed in their in vitro and/or in vivo behaviours, including the ability to form epiphyllous embryos and/or shoots (Fambrini et al., 2000).
In this work, a variant clone (EMB‐9‐RAD) of the hybrid H. annuus × H. tuberosus (Fambrini et al., 1997) is described that develops shoot‐like structures and somatic embryos from intact adventitious roots of in vitro‐grown plantlets. Moreover, by means of histological analysis, it is demonstrated that these ectopic structures originate from cortical cells in association with the development of lateral root primordia.
MATERIALS AND METHODS
Plant material and culture conditions
The variant clone, denominated EMB‐9‐RAD and characterized by in vitro differentiation of ectopic structures on intact roots, was derived from a regenerated plant (R1 generation) of the tetraploid (2n = 4x = 68) interspecific hybrid Helianthus annuus L. (inbred line HA89 cms) × Helianthus tuberosus L. (accession S. Pietro 1457 provided by the Dipartimento di Biologia delle Piante Agrarie, University of Pisa, Italy). EMB‐9‐RAD plants were multiplied by single‐node cuttings on solidified (8 g l–1 Bactoagar; Oxoid Ltd, Basingstoke, UK) MS basal medium (Murashige and Skoog, 1962) supplemented with 30 g l–1 sucrose and without growth regulators in 150 ml Erlen meyer flasks (Fambrini et al., 1997). The cultures were incubated in a controlled environment chamber at 23 ± 1 °C under a 16 h photoperiod and a photosynthetic photon flux of 30 µmol m–2 s–1 provided by cool‐light fluorescent lamps.
Histological analysis
Root and stem segments (1·0–2·0 cm long) of EMB‐9‐RAD plants were collected and fixed for 24 h in FAA (formalin : glacial acetic acid : ethanol : distilled water, 5 : 10 : 50 : 35 v/v). Material was dehydrated in ethanol, cleared in xylene and embedded in paraplast (Sigma Chemical Co., St Louis, MO, USA) (Ruzin, 1999). Serial sections, 10 µm thick, were cut using a rotary microtome (Reichert), and transferred onto glass slides. The paraplast was removed and the material was stained using Delafield’s haematoxylin and mounted in DPX (BDH Chemicals Ltd, Poole, UK). Histological observations were made using a Wild Makroskop M420 inverted microscope (Leica, Heerbrugg, Switzerland) and a Leitz microscope MPV3 (Wetzlar, Germany). Micrographs were taken using Wild MPS 51 equipped with a Wild Photoautomat MPS 45 (Heerbruug, Switzerland).
RESULTS AND DISCUSSION
Morphological characteristics of EMB‐9‐RAD plants propagated in vitro
The interspecific hybrid H. annuus × H. tuberosus is normally propagated by tubers (Pugliesi et al., 1993), but as far as we know there are no reports on the development of root buds and/or somatic embryos on intact roots of in vivo‐ or in vitro‐grown plants. Moreover, the in vitro occurrence of root buds or embryogenic structures on intact roots was never observed in the highly embryogenic clones of this hybrid selected previously (Fambrini et al., 1996, 1997, 2000). By contrast, after development of numerous adventitious roots on MS basal medium, the EMB‐9‐RAD shoots produced a number of root buds (Fig. 1A) and somatic embryos (Fig. 1B) arranged in clusters flanking lateral roots. Individual EMB‐9‐RAD plants differed greatly in the timing and extent of the expression of this phenotypic trait. In some cases, only callus proliferation was observed in association with lateral roots (Fig. 1C). Usually, the morphogenetic structures of EMB‐9‐RAD evolved into additional secondary embryos (Fig. 1D), leaf‐like structures and/or unorganized masses of callus (Fig. 1B and D). It is likely that cells of early ectopic structures retain a morphogenetic potential and can themselves become meristematic growth centres rather than participate in the coordinated growth of shoots or embryos. In addition, many adventitious structures displayed cellular enlargement giving rise to abnormal shoots or somatic embryos (Fig. 1E). The distinct morphology displayed by ectopic structures appeared unrelated to their position along the primary adventitious roots. Some well‐shaped ectopic structures (Fig. 1F), isolated from roots and transferred onto fresh MS basal medium, were able to produce plantlets that exhibited ectopic root buds and somatic embryos, like the original clone (data not shown).
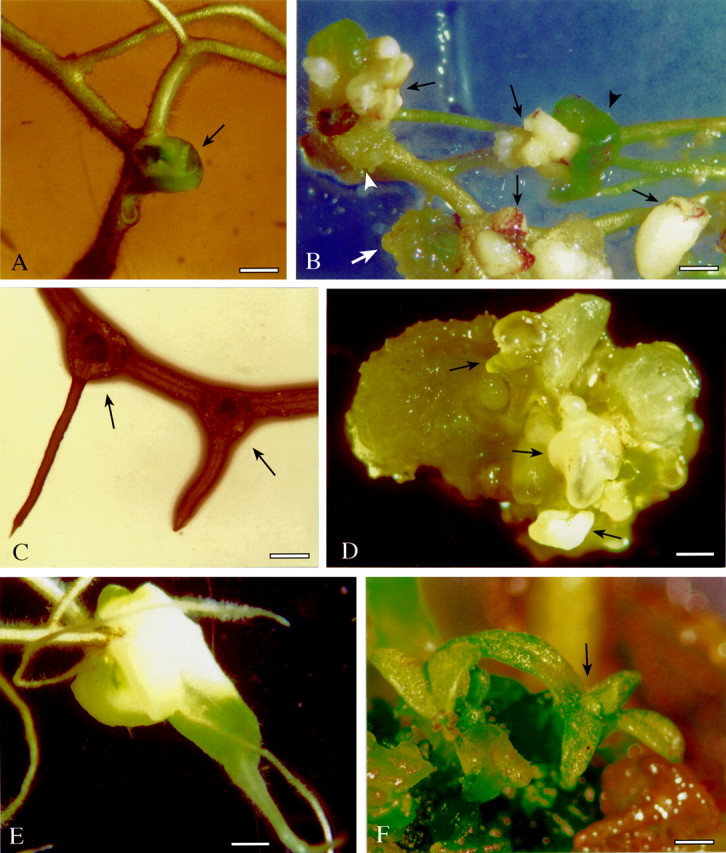
Fig. 1. Development of ectopic structures from roots of EMB‐9‐RAD plants of the interspecific hybrid H. annuus × H. tuberosus. A, Root bud primordia (arrow) flanking a lateral root. B, Embryogenic structures (arrows) and unorganized aggregates of callus (arrowheads) initiate on intact roots. C, Callus proliferation (arrows) at the site of lateral root initiation. D, Mass of embryogenic callus with polyembryonic structures (arrows). E, Teratological structure developed on a lateral root. F, Shoot with normal appearance (arrow). Bars = 3 mm (A and B), 2·5 mm (C and D), 2 mm (E) and 1 mm (F).
Histological analysis
Root buds often occurred in association with the basal region of lateral root primordia from various tissues of the main root, including the phellogen (Emery, 1955), the cortex (Bakshi and Coupland, 1960; Peterson and Thomas, 1971) or the sub‐epidermal layer (Ossenbeck, 1927). In other cases, the origin of root buds could be traced back to outer tissues of the lateral root itself when it was still within the parent root (Charlton, 1965). In EMB‐9‐RAD buds, primordia started with active cell division in a cell (or cells) at the junction of the parent and some of the lateral roots (Fig. 2A). Two or more bud meristems could be initiated in the superficial layer of the lateral root primordium within the cortex of the parent root (Fig. 2A and B), although parent and lateral root tissues could often not be distinguished in this region. The primordia, initially detected as clusters of densely staining cells with large nuclei (Fig. 2C), produced meristematic domes (Fig. 2D). Subsequently, these young adventitious primordia originated organized shoot‐like structures (Fig. 2E and F) that showed a clear double‐layered tunica, leaf primordia and differentiating procambial traces. However, the meristematic centres often produced deformed structures arranged in clusters (Fig. 2G) or precociously de‐differentiated into unorganized callus aggregates.
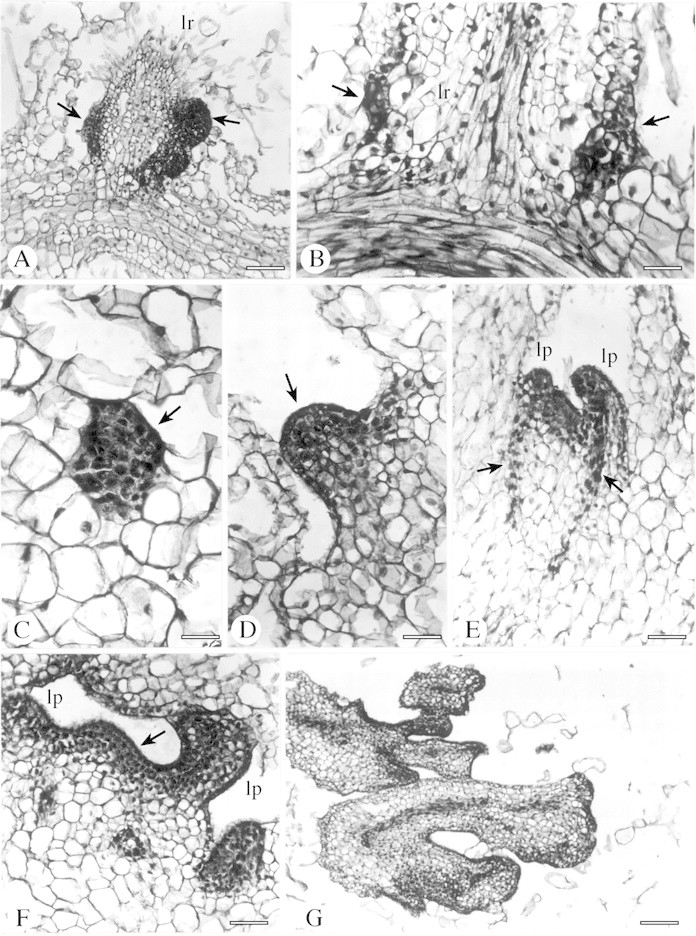
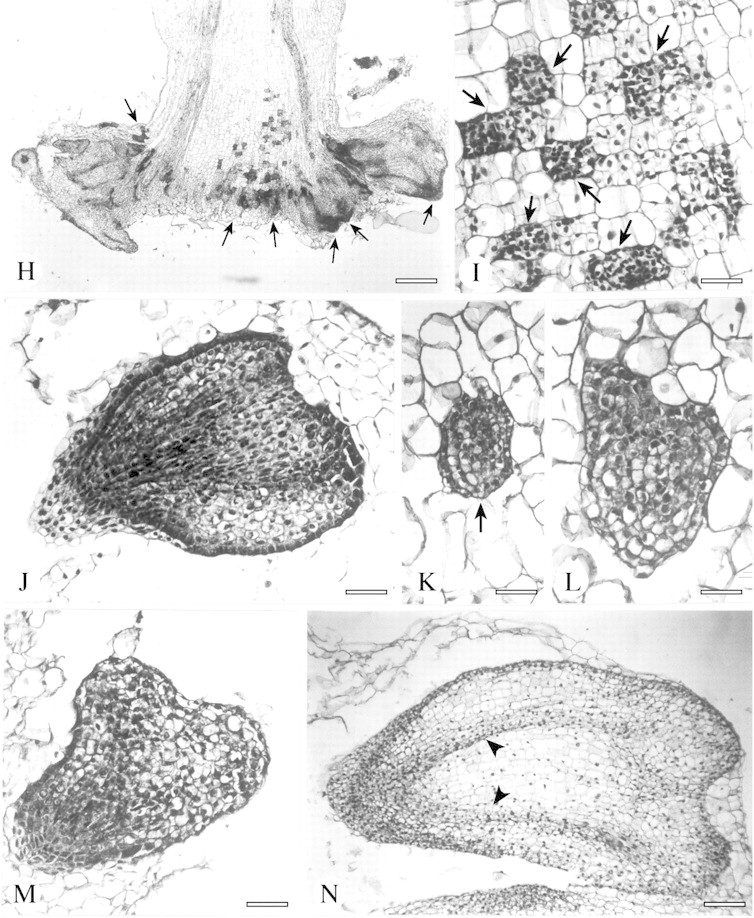
Fig. 2. Origin and development of root buds and somatic embryos in EMB‐9‐RAD plants of the interspecific hybrid H. annuus × H. tuberosus, as indicated by root and stem sections stained with haematoxylin. A and B, Bud primordia (arrows) at different stages of development flanking lateral roots (lr). C, Initial stage of a bud meristem (arrow) in the cortex adjacent to a lateral root. D, Meristematic dome (arrow). E, Root bud with leaf primordia (lp) and differentiating procambial strands (arrows). F, Root bud showing a double stratified tunica (arrow) and leaf primordia (lp). G, Cluster of abnormal morphogenetic structures. H, Longitudinal section of stem showing meristematic primordia (arrows) initiated at the cut edge. I, Magnification of H. Parenchyma cells after repeated divisions (arrows). J, Torpedo‐stage of a somatic embryo. K, Late globular‐stage of a somatic embryo (arrow). L and M, Abnormal embryo‐like structures. N, Late torpedo‐stage of a somatic embryo with procambial strands evident (arrowheads). Bars = 120 µm (A), 70 µm (B, C, E, F, J, M), 40 µm (D, K, L), 300 µm (G), 500 µm (H), 50 µm (I) and 200 µm (N).
Frequently, meristematic primordia developed at the cut edge of the stem of EMB‐9‐RAD shoots (Fig. 2H), when roots were not yet differentiated. Repeated divisions of parenchyma cells of the pith produced 40‐ to 50‐celled meristemoids and/or somatic embryos (Fig. 2I). Morpho genetic potential is probably not restricted to the cortical root cells.
In addition to root buds, embryo‐like structures associated with lateral roots were regularly recognizable (Fig. 2J–N), which indicates that some cells of EMB‐9‐RAD roots could acquire different patterns of competence. It has previously been shown that adventitious organogenesis and embryogenesis could occur in parallel from in vitro‐cultured tissues of Helianthus (Bronner et al., 1993; Laparra et al., 1997; Charrière et al., 1999; Fambrini et al., 2000). Induction of both shoots and somatic embryos from the same cell type was often dependent on the endogenous hormonal auxin : cytokinin ratio and/or on the sugar concentration of the induction medium (Bronner et al., 1993; Charrière et al., 1999; Thomas et al., 2002). In contrast, in EMB‐9‐RAD, exogenous hormonal treatments were unnecessary to allow the expression of both morphogenetic responses.
Although some of these ectopic structures developed through typical embryogenic stages (Fig. 2J and N), abnormal patterns of development were frequently observed (e.g. callus‐like proliferation, polyembryony). Moreover, the internal cellular differentiation was lacking or quickly lost in most of these embryo‐like structures (Fig. 2L and M), and their ability to evolve into complete plants (data not shown) was reduced in comparison with somatic embryogenesis induced from other type of organs, e.g. cotyledons, leaves or immature zygotic embryos (Fambrini et al., 1996, 1997; Fiore et al., 1997; Laparra et al., 1997; Sujatha and Prabakaran, 2001). Generally, a prolonged dark treatment and/or a high sugar content in the medium was required to induce somatic embryogenesis in Helianthus (Bronner et al., 1993; Fiore et al., 1997; Laparra et al., 1997; Charrière et al., 1999; Sujatha and Prabakaran, 2001; Vasic et al., 2001). In contrast, EMB‐9‐RAD, like other highly morphogenic clones of H. annuus × H. tuberosus (Fambrini et al., 1997), produced numerous ectopic structures on roots under a 16 h photoperiod in a medium with a moderate sucrose content (30 g l–1).
Somatic embryos are obtained from in vitro‐cultured root explants in many species (Litz and Gray, 1995), but formation of embryo‐like structures from intact roots is an astounding result. Roots of the pickle (pkl) mutant of arabidopsis retained embryogenic characteristics, such as the synthesis of particular seed‐type fatty acids, the presence of densely packed oil bodies and the expression of other embryo‐specific markers (Ogas et al., 1997). However, globular‐ and torpedo‐stage embryo‐like structures were produced in the pkl mutant on growth regulator‐free medium only after dissection of roots (Ogas et al., 1997). The EMB‐9‐RAD clone had some features in common with plants overexpressing a homeodomain protein WUSCHEL (WUS; Mayer et al., 1998). In arabidopsis, WUS transient overexpression caused high embryogenic callus formation in the presence of auxin, whereas it directly induced somatic embryo formation from root tips of intact root systems in the absence of any exogenous auxin. Therefore, besides of the direct role of WUS in maintaining the central pool of stem cells in both shoot and floral meristems, this homeotic gene plays a predominant role by promoting the vegetative‐to‐embryogenic transition and/or maintaining the identity of embryonic stem cells (Zuo et al., 2002). In fact, ectopic expression of genes essential for meristem formation and/or maintenance is a key event to ensure adventitious morphogenesis too (Fambrini et al., 2001). Up‐regulation of SHOOTMERISTEMLESS (STM), WUS and CLAVATA1 (CLV1) was demonstrated at the time of adventitious shoot commitment during in vitro culture of arabidopsis root explants on a medium with a hormonal composition suitable for regeneration (Cary et al., 2002). In addition, constitutive expression of class I of KNOTTED homeobox genes (KNOX) induced the adventitious differentiation of ectopic meristems in leaves of transgenic plants (Reiser et al., 2000). This class of genes is implicated in maintaining indeterminancy in meristems and/or repressing differentiation. There is evidence to support the hypothesis of a link between KNOX overexpression and endogenous hormonal balance, which is a key factor in determining in vitro morphogenetic competence (Fambrini et al., 2001). In particular, Frugis et al. (2001) showed that the dramatic alteration of leaf shape in lettuce, induced by the ectopic expression of a KNOX gene, was associated with accumulation of cytokinins. Similarly, mutant tissue lines of arabidopsis forming shoot‐like structures and capable of hormone autotrophic growth were characterized by an increased level of KNOX genes (Frank et al., 2000). More recently, Scanlon et al. (2002) suggested that the strong reduction of polar auxin transport is a downstream effect of ectopic expression of this class of genes.
Hormones are essential for the induction of adventitious buds and somatic embryos from roots (Peterson, 1975; Ogas et al., 1997). In particular, auxins are inhibitory to bud initiation, whereas cytokinins are stimulatory in several species (Peterson, 1975). The role played by other growth regulators, such as gibberellic acid, ethylene and abscisic acid, are even less clear. For example, gibberellic acid induced growth of root buds in Euphorbia esula (Horvath, 1999); by contrast, the penetrance of the pkl phenotype was increased by gibberellic acid inhibitors (Ogas et al., 1997). The EMB‐9‐RAD clone, which is derived from a single somatic embryo induced in a medium with a low cytokinin concentration (Fambrini et al., 1997), was maintained by single‐node cutting for more than 3 years in MS basal medium without growth regulators. It appears that the cell fate of some root cells of EMB‐9‐RAD can be reprogrammed, bypassing the requirement for exogenous hormonal treatments, as demonstrated by the profuse regeneration of intact roots in MS basal medium. Nevertheless, since hormones are very often key inducers of adventitious morphogenesis, the EMB‐9‐RAD phenotype could be the result of an altered endogenous hormonal level or, conversely, an increased sensitivity to growth regulators due to genetic or epigenetic variations induced throughout the in vitro regeneration process. Moreover, the hybrid nature of EMB‐9‐RAD means that it is also possible that genomic imbalance is involved in the ectopic proliferation on the root system of this variant. It has often been observed that interspecific hybridization, such as that between Nicotiana glauca × N. langsdorffii, is accompanied by tumour formation on leaves or stems (Bayer, 1982). Tumour formation has been related to an altered gene expression due to the combination of genes from different species interacting to induce greater than normal phytohormone production. Recently, it has also been demonstrated that members of class I of KNOX genes were specifically expressed in genetic tumours with ‘shooty’ phenotype of Nicotiana hybrids (Matveeva et al., 2001). The altered activity of regulatory gene(s) involved in the switch from indeterminate to determinate cell fate (e.g. KNOX, WUS and PKL) may be responsible for the phenotype expressed by EMB‐9‐RAD plants.
Further studies will be performed to identify the biochemical basis of this phenomenon through a detailed physiological characterization, i.e. endogenous hormonal levels or hormonal sensitivity. Moreover, crosses between the interspecific hybrid and H. annuus could help elucidate the nature (genetic or epigenetic) of the phenotype expressed by EMB‐9‐RAD plants. In our opinion, the EMB‐9‐RAD variant may be a valuable tool in the study of the physiological and molecular basis of cell totipotency.
Supplementary Material
Received: 15 November 2002; Returned for revision: 24 February 2003; Accepted: 8 April 2003
References
- BakshiTS, Coupland RT.1960. Vegetative propagation in Linaria vulgaris Canadian Journal of Botany 38: 243–249. [Google Scholar]
- BayerMH.1982. Genetic tumors: physiological aspects of tumor formation in interspecific hybrids. In: Kahl G, Schell JS, eds. Molecular biology of plant tumors New York: Academic Press, 33–67. [Google Scholar]
- BonnetHT, Torrey JG.1966. Comparative anatomy of endogenous bud and lateral root formation in Convulvulus arvensis roots cultured in vitro American Journal of Botany 53: 496–507. [Google Scholar]
- BoselaMJ, Ewers FW.1997. The mode of origin of root buds and root sprouts in the clonal tree Sassafras albidum (Lauraceae). American Journal of Botany 84: 1466–1481. [PubMed] [Google Scholar]
- BronnerR, Jeannin G, Hahne G.1993. Early events during organogenesis and somatic embryogenesis induced on immature embryos of sunflower (Helianthus annuus). Canadian Journal of Botany 72: 239–248. [Google Scholar]
- CaryAJ, Che P, Howell SH.2002. Developmental events and shoot apical meristem gene expression patterns during shoot development in Arabidopsis thaliana Plant Journal 32: 867–877. [DOI] [PubMed] [Google Scholar]
- CharltonWA.1965. The root system of Linaria vulgaris Mill. I. Morphology and anatomy. Canadian Journal of Botany 44: 1111–1116. [Google Scholar]
- CharrièreF, Sotta B, Miginiac E, Hahne G.1999. Induction of adventitious shoots or somatic embryos on in vitro cultured zygotic embryos of Helianthus annuus: variation of endogenous hormone levels. Plant Physiology and Biochemistry 37: 751–757. [Google Scholar]
- D’AmatoF.1997. Role of somatic mutations in the evolution of higher plants. Caryologia 50: 1–15. [Google Scholar]
- DonaldWW.2000. A degree‐day model of Cirsium arvense shoot emergence from adventitious root buds in spring. Weed Science 48: 333–341. [Google Scholar]
- DonovanMD.1976. A list of plants regenerating from root cuttings. Plant Propagator 22: 7–8. [Google Scholar]
- EmeryAEH.1955. The formation of buds on the roots of Chamaenerion angustifolium (L.) Scop. Phytomorphology 5: 139–145. [Google Scholar]
- FambriniM, Cionini G, Pugliesi C.1996. Development of somatic embryos from morphogenetic cells of the interspecific hybrid Helianthus annuus × Helianthus tuberosus Plant Science 114: 205–214. [Google Scholar]
- FambriniM, Cionini G, Pugliesi C.1997. Acquisition of high embryogenic potential in regenerated plants of Helia nthus anuuus × H. tuberosus Plant Cell, Tissue and Organ Culture 51: 103–110. [Google Scholar]
- FambriniM, Fisichella M, Pugliesi C.2001. Enhanced morphogenetic potential from in vitro regenerated plants of genus Helianthus: an overview. Recent Research in Development Plant Biology 1: 35–54. [Google Scholar]
- FambriniM, Cionini G, Bianchi R, Pugliesi C.2000. Epiphylly in a variant of Helianthus annuus × H. tuberosus induced by in vitro tissue culture. International Journal of Plant Science 161: 13–22. [DOI] [PubMed] [Google Scholar]
- FioreMC, Trabace T, Sunseri F.1997. High frequency of plant regeneration in sunflower from cotyledons via somatic embryo genesis. Plant Cell Reports 16: 295–298. [DOI] [PubMed] [Google Scholar]
- FrankM, Rupp H‐M, Prinsen E, Motyka V, van Onckelen H, Schmülling T.2000. Hormone autotrophic growth and differentation identifies mutant lines of Arabidopsis with altered cytokinin and auxin content or signaling. Plant Physiology 122: 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FrugisG, Giannino D, Mele G, Nicolodi C, Chiappetta A, Bitonti MB, Innocenti AM, Dewitte W, van Onckelen H, Mariotti D.2001. Overexpression of KNAT1 in lettuce shifts leaf determinate growth to a shoot‐like indeterminate growth associated with an accumulation of isopentenyl‐type cytokinins. Plant Physiology 126: 1370–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HamdounAM.1970. The anatomy of subterranean structures of Cirsium arvense (L.) Scop. Weed Research 12: 128–136. [Google Scholar]
- HolmT.1925. On the development of buds upon roots and leaves. Annals of Botany 39: 867–881. [Google Scholar]
- HorvathDP.1998. The role of specific organs and polar auxin transport in correlative inhibition of leafy spurge (Euphorbia esula) root buds. Canadian Journal of Botany 76: 1227–1231. [Google Scholar]
- HorvathDP.1999. Role of mature leaves inhibition of root bud growth in Euphorbia esula L. Weed Science 47: 544–550. [Google Scholar]
- LaparraH, Bronner R, Hahne G.1997. Histological analysis of somatic embryogesis induced in leaf explants of Helianthus smithii Heiser. Protoplasma 196: 1–11. [Google Scholar]
- LitzRE, Gray DJ.1995. Somatic embryogenesis for agricultural improvement. World Journal of Microbiology and Biotechnology 11: 416–425. [DOI] [PubMed] [Google Scholar]
- MatveevaTV, Lutova LA, Nester, Yu.2001. Tumor formation in plants. Russian Journal of Genetics 37: 993–1001. [PubMed] [Google Scholar]
- MayerKFX, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T.1998. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815. [DOI] [PubMed] [Google Scholar]
- MurashigeT, Skoog F.1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum 15: 473–497. [Google Scholar]
- OgasJ, Cheng J‐C, Sung ZR, Somerville C.1997. Cellular differentiation regulates by gibberellin in the Arabidopsis thaliana pickle mutant. Science 277: 91–93. [DOI] [PubMed] [Google Scholar]
- OssenbeckC.1927. Kritische und experimentelle Utersuchungen an Bryophyllum Flora 122: 342–387. [Google Scholar]
- PetersonRL.1975. The initiation and development of root buds. In: Torrey JG, Clarkson DT, eds. The development and function of roots New York: Academic Press, 125–161. [Google Scholar]
- PetersonRL, Thomas AG.1971. Buds on the roots of Hieracium florentinum (hawkweed). Canadian Journal of Botany 49: 53–54. [Google Scholar]
- PugliesiC, Megale P, Cecconi F, Baroncelli S.1993. Organogenesis and embryogenesis in Helianthus tuberosus and in the interspecific hybrid Helianthus annuus × Helianthus tuberosus Plant Cell, Tissue and Organ Culture 33: 187–193. [Google Scholar]
- RajuMVS, Steeves TA, Coupland RT.1966. On the occurrence of root buds on perennial plants in Saskatchewan. Canadian Journal of Botany 44: 33–37. [Google Scholar]
- ReiserL, Sánchez‐Baracaldo P, Hake S.2000. Knots in the family tree: evolutionary relationships and functions of knox homeobox genes. Plant Molecular Biology 42: 151–166. [PubMed] [Google Scholar]
- RuzinSE.1999. Infiltrating and embedding tissues. In: Ruzin SE, ed. Plant microtechnique and microscopy New York, Oxford: Oxford University Press, 61–72. [Google Scholar]
- ScanlonMJ, Henderson DC, Bernstein B.2002. SEMAPHORE1 functions during the regulation of ancestrally duplicated knox genes and polar auxin transport in maize. Development 129: 2663–2673. [DOI] [PubMed] [Google Scholar]
- SteevesTA, Sussex IM.1989.Patterns in plant development, 2nd edn. Cambridge: Cambridge University Press. [Google Scholar]
- SujathaM, Prabakaran AJ.2001. High frequency embryogenesis in immature zygotic embryos of sunflower. Plant Cell, Tissue and Organ Culture 65: 23–29. [Google Scholar]
- ThomasC, Bronner R, Molinier J, Prinsen E, van Onckelen H, Hahne G.2002. Immuno‐cytochemical localization of indole‐3‐acetic acid during induction of somatic embryogenesis in cultured sunflower embryos. Planta 215: 577–583. [DOI] [PubMed] [Google Scholar]
- VasicD, Alibert G, Skoric D.2001. Protocols for efficient repetitive and secondary somatic embryogenesis in Helianthus maximiliani (Schrader). Plant Cell Reports 20: 121–125. [DOI] [PubMed] [Google Scholar]
- WittrockVB.1884. Om Rootskott hos Ortartade Vaxter, med Sarskild Hansyn till deras olika Biologiska Betydelse. Botaniska Notiser 34: 21–37. [Google Scholar]
- ZuoJ, Niu Q‐W, Frugis G, Chua N‐H.2002. The WUSCHEL gene promotes vegetative‐to embryonic transition in Arabidopsis Plant Journal 30: 349–359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


