Abstract
The xylem of Metasequoia glyptostroboides Hu et Cheng is characterized by very low density (average specific gravity = 0·27) and tracheids with relatively large dimensions (length and diameter). The microfibril angle in the S2 layer of tracheid walls is large, even in outer rings, suggesting a cambial response to compressive rather than tensile stresses. In some cases, this compressive stress is converted to irreversible strain (plastic deformation), as evidenced by cell wall corrugations. The heartwood is moderately decay resistant, helping to prevent Brazier buckling. These xylem properties are referenced to the measured bending properties of modulus of rupture and modulus of elasticity, and compared with other low‐to‐moderate density conifers. The design strategy for Metasequoia is to produce a mechanically weak but hydraulically efficient xylem that permits rapid height growth and crown development to capture and dominate a wet site environment. The adaptability of these features to a high‐latitude Eocene palaeoenvironment is discussed.
Key words: Metasequoia, xylem conduction, xylem strength, decay resistance, microfibril angle, plastic deformation, Eocene, palaeoecology
INTRODUCTION
Although many tree species can be readily categorized as either short‐lived, early succession or longer‐lived, late succession cohorts; a few, like redwood [Sequoia sempervirens (D. Don) Endl.], seem to have characteristics of each type and, hence, can colonize a site, grow rapidly, exclude competitors and produce long‐lived pure stands of tall trees (Ornduff, 1998). During the Palaeogene period, Meta sequoia may have exhibited similar characteristics at high latitudes (Francis, 1991; Greenwood and Basinger, 1994). A number of ecophysiological features, such as requirements for seed germination, canopy shading and root competition will determine whether a tree species can capture and maintain site dominance. It is proposed that xylem anatomy and chemistry are among these strategic features.
Utilizing the principles of ‘adequate design’ (Rashevsky, 1973), it is possible to construct a hypothetical woody plant that incorporates the compromises needed for mechanical support, hydraulic conduction, light interception and gas exchange (Niklas, 1992; Romberger et al., 1993; McCulloh et al., 2003). In a previous study, the canopy and leaf design characteristics of Metasequoia glyptostroboides Hu et Cheng have been discussed in relation to light interception and gas exchange (Jagels and Day, 2003). In this paper, the xylem of the main stem is examined in relation to support and hydraulic conduction, and the question is asked whether these provide any evidence to suggest adaptability to a wet palaeoenvironment of continuous but weak intensity light. The current natural range of Metasequoia is restricted to a small, remote area of Hupeh province, near the border of Szechuan, China, at an approximate latitude of 30°10′N (Chu and Cooper, 1950). In this region trees can reach 50 m in height and 13 m in diameter (Florin, 1952). Since its description by botanists in 1945 (Florin, 1952; Li, 1957), Metasequoia has been widely planted throughout the world. In some situations, it has demonstrated a very high growth rate potential, with trees in the eastern United States reaching heights of 38 m in 50 years (Kuser, 1999). Based on the fossil record, Metasequoia forests were once widely distributed in the northern hemisphere (Momohara, 1994). During the Palaeocene and Eocene periods, the range of Metasequoia extended at least to 80°N latitude, and often dominated wet, lowland forests, comprising more than 90 % of stands, and reaching estimated heights of greater than 30 m (Francis, 1991; Greenwood and Basinger, 1994).
Previously, selected properties of Metasquoia wood have been determined from trees growing in China, The Netherlands, Poland and Russia (Li, 1948; Liang et al., 1948; Brazier, 1963; Jaroslavcev and Visnjakova, 1965; Linnard, 1966; Hejnowicz, 1973; Surminski and Bojarczuk, 1973; Wu and Chern, 1995; Polman et al., 1999). The most comprehensive study is that of Polman et al. (1999). These studies have focused on properties of seasoned wood in relation to utilization.
In this study, xylem specific gravity, strength and stiffness for green wood, as found in living trees, were measured, and these are compared with other low‐to‐moderate density conifers to assess probable habitat preferences. Microfibril angle (MFA) of tracheids is often cited as a correlate for mechanical properties (Meylan and Probine, 1969; Cave, 1972; Astley et al., 1998; Booker et al., 1998; Nakada et al., 1998). MFA is measured, not only to relate it to mechanical properties, but also to assess its possible role in counteracting compressive hoop stress in tracheid walls caused by negative hydraulic pressures. In higher density woods, wall collapse may be thwarted by thick walls (Hacke et al., 2001). But in tall, low density conifers compressive hoop stress might be counteracted by adjustment of the MFA (Boyd, 1985). Tracheid lumen diameter and tracheid length are measured and compared with two tall, closely related Cupressaceae—redwood and bald cypress [Taxodium distichum (L.) Rich]. This provides a measure of relative hydraulic efficiencies. Because Metasequoia xylem has very low density and strength, yet grows very rapidly (up to 1·7 m year–1) and produces a tall tree, a novel microscopic method for determining whether irreversible plastic deformation could occur in the living tree was developed. This method provides a historical record during the life of the tree, rather than the short‐term, outer growth ring measure provided with strain gauges (Archer, 1987). The presence of irreversible strain could suggest a tree unsuited to support external static loads, such as ice and snow. Low density trees are more prone to Brazier buckling if heartwood integrity is lost. We tested for heartwood decay resistance and referenced it to redwood and bald cypress. We also examined whether decay resistant heartwood is climate related.
How the wood properties of Metasequoia may have contributed to the success of this species at high latitudes during warmer epochs is discussed here, in combination with previously published data. Since fossil evidence of Sequoia and Taxodium has not been found at the high palaeolatitudes occupied by Metasequoia during the Palaeogene, the working premise is that Metasequoia should have xylem properties similar to these close relatives but with possible modifications for the short growing seasons and low‐intensity continuous light characteristic of the highest latitudes.
MATERIALS AND METHODS
Wood samples were obtained from two trees: PNJ, a tree growing near Princeton, NJ (40°30′N), and JPC, a tree from northern Jiangsu Province, China (approx. 29°N). Both trees were harvested from closed canopy stands, composed mostly of Metasequoia trees. The JPC wood is a breast height sample and was used only for comparative purposes. The PNJ tree was growing in well‐drained silt loam soil on a slope of about 5° with a southerly aspect. After felling, the tree was measured for total height, height to live crown, and discs from various heights were saved for ring analysis and MFA measurement.
Two 1·8‐m‐long logs (basal log from 1 m height, and an upper log from 7 m height) from the PNJ tree were transported to the University of Maine and sawn into 4 cm boards with a Woodmizer bandsaw, and quickly processed into test samples, wrapped in plastic and frozen until testing (within a few weeks). Bending strength was assessed by determining modulus of rupture (MOR), and stiffness by calculating modulus of elasticity (MOE), using an Instron model 4202, following ASTM, D143 (secondary methods) and D2555 standards (Anon., 2001). Measurement of MOE in bending was chosen as a more applicable test for dynamic loading in living trees than Young’s modulus (E), which is a measure of elasticity in pure tension or compression. Samples were tested green since this is more representative of conditions in the living tree, but a subset was tested at 12 % moisture content (MC) for comparison. Comparisons with other conifers, as published in the Wood Handbook, were for the green condition (Anon., 1999). Moisture content and percentage shrinkage from green to oven‐dry (OD) condition were determined on 12 samples, six from each log. Specific gravity (SG) was determined on 81 samples (59 at green, OD basis; 22 at 12 %: OD basis).
For anatomical analysis, radial strips (2 cm × 2 cm) on opposing axes were cut from pith to bark, and every fourth ring was removed and thin‐sectioned (18–22 µm) using an AO model 860 sliding microtome. Imaging and measuring were accomplished using a Zeiss Axioskop and Diagnostic Instruments SPOT RT digital camera and software. MFA of the S2 wall layer, as observed under brightfield microscopy in radial view, was measured from pith to bark at 1 m and 7 m heights, on last‐formed earlywood tracheids, as representative of rings consisting mostly of earlywood (Megraw et al., 1998; Surminski and Bojarczuk, 1973). Eight rings from pith to bark on opposing axes were chosen (ten measurements per ring), and ring width was recorded. Breast height MFAs for JPC tree were determined for comparison.
Rings sampled for MFA were used for tracheid length determination. Segments (approx. 2 mm × 2 cm, including the entire ring) were macerated using Franklin’s method, and stained in Bismarck brown (Berlyn and Miksche, 1976). Fifty tracheids on opposite radial axes, 100 per ring, were measured using the digital camera system and microscope described above. Maximum tangential tracheid diameter was determined on earlywood cells in transverse sections of outermost rings.
A new method for determining the presence of inelastic compressive strain at any location in a tree was devised. The results of examining, with a microscope, thin sections of oak maple and cherry that had been compressed in the green state in the only US version of a patented commercial device used in the furniture industry (Compwood Machines Ltd, Slagelse, Denmark), have been reported in a previous paper (unpublished). Access to the Compwood machine was provided courtesy of Bethel Furniture Stock, Inc., Bethel, ME, USA. This machine constrains radial and tangential movement while a compressive load is applied longitudinally to pre‐steamed wood. The compressed wood, even after partial drying has lost a significant amount of stiffness, and is easily bent in large dimensions. This strain in the plastic range is registered as wall corrugations that are observable in thin radial sections under brightfield microscopy.
For the decay tests, heartwood of three additional conifer species were added: redwood, bald cypress and eastern white pine (Pinus strobus L.). Heartwood of redwood and cypress were chosen because they are close living relatives of Metasequoia, and are moderately to very decay resistant (depending on growth rate). White pine was chosen as a less decay resistant species. The redwood and pine were sampled from ‘old growth’ trees and the bald cypress from ‘second growth’. According to Anon. (1999), the heartwood of redwood should be very decay resistant, cypress moderately resistant, and pine moderately to slightly resistant. The wood samples of pine, redwood and cypress are of unknown origin or position in the tree. The Metasequoia wood was taken from the heartwood of the PNJ tree at 1 m and 7 m. Blocks of all species were prepared to final dimensions of approx. 25 mm × 25 mm × 13 mm with the largest face in the transverse plane.
The blocks were exposed to two brown rot fungi: Postia placenta (Fr.) M. Larsen & Lombard (Mad‐698‐R) and Gloeophylum trabeum (Pers.:Fr.) Murrill (Mad‐617‐R), and two white rot fungi: Trametes versicolor (L.:Fr.) Pil. (Fp‐101664‐Sp) and Irpex lacteus Fries (KTS 003), in a series of modified ‘soil‐block’ wood decay tests, ASTM standard D2017‐81 (1986), with five replicates per test (Anon., 2001). Additional blocks were exposed in uninoculated chambers as control samples. Feeder strips in the decay chambers were inoculated 2 weeks prior to introduction of the test blocks. All blocks were autoclaved prior to testing and placed in a 25–27 °C chamber with transverse surfaces exposed to feeder strips. The initial tests ran for 12 weeks. A follow‐up test using only G. trabeum was conducted for 13 weeks.
RESULTS
Mechanical properties are typically determined for dry (usually 12 % MC) wood, as this condition is representative of lumber used in interior construction. However, in the living tree xylem MC is generally above the fibre saturation point (fsp), and can range from 30 % to more than 200 % (Anon., 1999). Table 1 presents the mechanical properties (MOR and MOE), as well as the physical properties (MC and SG), of Metasequoia wood at green and 12 % MC. Table 2 presents correlation coefficients between mechanical and physical properties for green and dry wood samples. MOR and MOE are not correlated with SG in green wood (above fsp), but are moderately correlated in dry wood. As is generally true MOR is more strongly correlated with SG than MOE (Hirakawa et al., 1998; McAlister et al., 2000). Average shrinkage, from green to OD in radial, tangential and longitudinal directions, was radial = 1·88 %, tangential = 6·13 % and longitudinal = 0·36 %. The large differential between radial and tangential shrinkage is not untypical of low density conifers. Longitudinal shrinkage of 0·36 % is slightly larger than the range of 0·1 to 0·2 % found in ‘normal’ wood, but less than the 2 % reported for some juvenile wood or compression wood (Anon., 1999).
Table 1.
Average property values for green and 12 % moisture content (MC) wood samples
| Wood condition | Sample location | Modulus of rupture (kPa) | Modulus of elasticity (MPa) | Moisture content (%) | Specific gravity |
| Green | All samples (n = 59) | 31 000 (2700) | 4300 (800) | 112·8 (48·8) | 0·262 (0·022) |
| Near pith (n = 8) | 29 000 (3393)a | 3500 (418)a | 121·5 (43·5)a | 0·280 (0·035)a | |
| Near bark (n = 8) | 33 000 (2646)a | 5100 (635)b | 137·0 (42·2)a | 0·266 (0·019)a | |
| 12 % MC | All samples (n = 22) | 48 000 (5600) | 5500 (700) | 12·2 (0·41) | 0·263 (0·036) |
Standard deviation in parentheses.
Significant differences at 0·01 level noted by different superscript letters.
Table 2.
Correlation coefficients for green and 12 % moisture content wood samples
| Moisture content | Specific gravity | Modulus of rupture | Wood condition | |
| Specific gravity | 0·16 | Green | ||
| 0·10 | 12 % MC | |||
| Modulus of rupture | 0·14 | 0·12 | Green | |
| –0·08 | 0·69 | 12 % MC | ||
| Modulus of elasticity | –0·06 | –0·13 | 0·64 | Green |
| –0·14 | 0·59 | 0·67 | 12 % MC |
Numbers in bold are statistically significant.
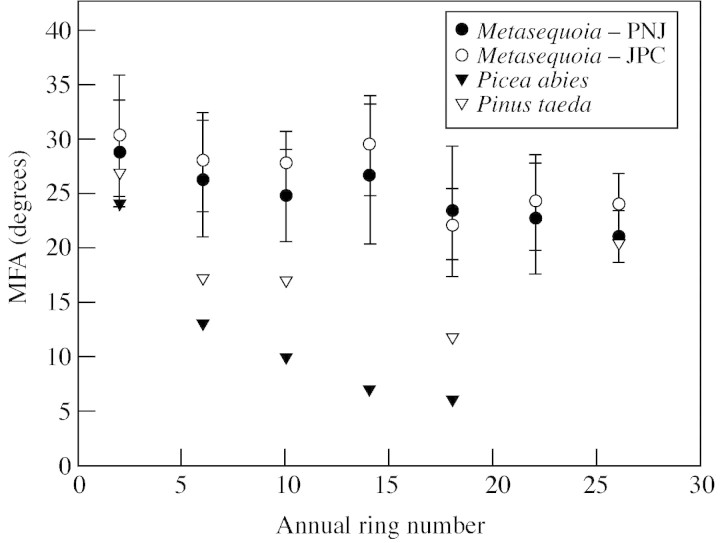
Figure 1 is a plot of MFA from the pith to the bark at breast height for PNJ and JPC trees, and these values are compared with loblolly pine (Pinus taeda L.) (estimated from Hiller and Brown, 1967) and Norway spruce [Picea abies (L.) Karsten] (estimated from Saranpää et al., 1998). For Metasequoia a t‐test showed slight but significant differences between the first and last formed rings: –0·1991 for JPC and –0·153 for PNJ. Most conifers, including the comparison loblolly pine and Norway spruce show much more dramatic changes in MFA within the first 20 years of growth (Wardrop and Preston, 1950).
Fig. 1. Change in tracheid microfibril angle (MFA) from pith to bark in breast height wood from two Metasequoia trees, compared with that of Picea abies and Pinus taeda.
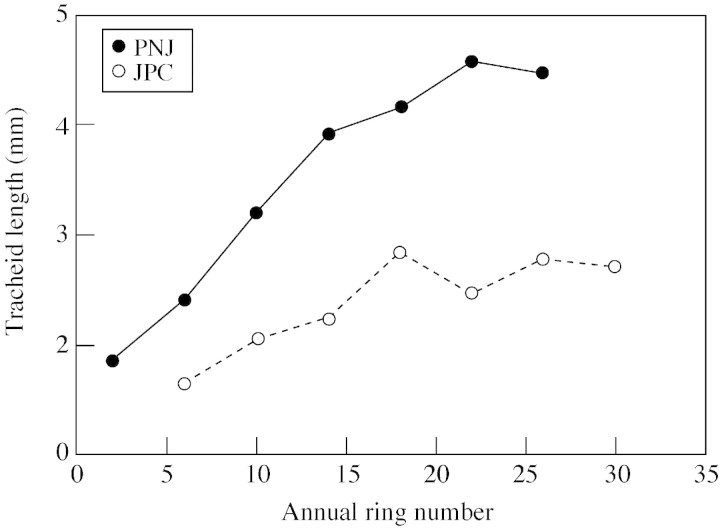
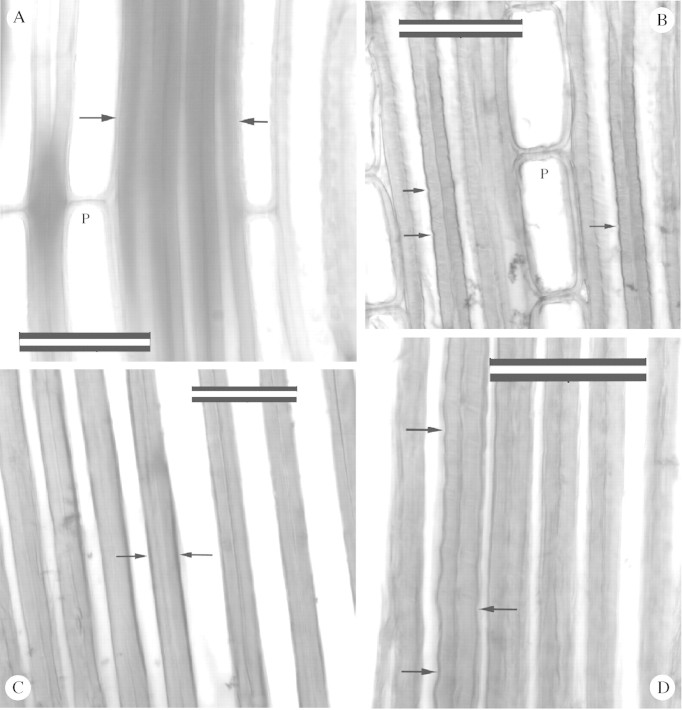
Figure 2 is a plot of mean tracheid length between pith and bark at 1 m height for JPC and PNJ trees. Tracheid length was consistently greater in the PNJ tree, possibly attributable to environmental conditions, although no correlation between ring width and tracheid length was found. Average ring width at 1 m for PNJ is 4·79 mm (s.d. 0·73) and for JPC is 3·50 (s.d. 0·53). To obtain an estimate of tracheid length in the outer rings of Metasequoia wood, tracheid length for rings 22 and 26 of the PNJ tree were averaged, giving a mean of 4·54 (s.d. 0·87). Maximal tracheid diameter was measured in earlywood tracheids of the outer rings of JPC and PNJ trees, and was determined to be 69 µm. Since these trees are young, these values are likely to be conservative estimates of maximum tracheid length and diameter in mature Metasequoia. In Fig. 3, parts A and B are photomicrographs of radial sections of red oak (Quercus sp.). Figure 3A is normal wood, and the latewood fibres are indicated by arrows. Figure 3B is green red oak which has been steamed and compressed to about 85 % of its original length. The walls of latewood fibres are corrugated (arrows) and slip planes can be seen as lighter lines across the fibre walls. Note also that parenchyma cells (P) are shorter than in Fig. 3A. In Fig. 3, parts C and D are photomicrographs of radial sections of latewood of growth rings 26 (C) and 14 (D) from the PNJ Metasequoia tree. Little or no plastic deformation is seen in tracheid walls in ring 26 (Fig. 3C), which is the last ring formed in the tree. Ring 14 (Fig. 3D), by contrast, displays considerable deformation (corrugations and slip planes). Preliminary examination of thin sections of xylem from mature trees of red spruce (Picea rubens Sarg.) and eastern white pine failed to reveal evidence of plastic deformation, but further study with more species is needed.
Fig. 2. Change in tracheid length from pith to bark in breast height wood from two Metasequoia trees.
Fig. 3. Fibres of Quercus sp. and latewood tracheids of Metasequoia as seen in radial longitudinal views. A, Non‐compressed (normal) wood of Quercus. Arrows indicate fibres that have smooth walls. P, Parenchyma cell. B, Quercus wood that has been compressed to approx. 85 % of its original length. Arrows point to corrugated walls which also display slip planes (light lines). P, Shortened parenchyma cell. C, Latewood tracheids from the outermost ring (26) of PNJ Metasequoia tree. Arrows point to tracheids where plastic deformation is minimal or absent. D, Latewood tracheids of ring 14 of PNJ Metasequoia. Arrows point to corrugations in tracheid walls, and slip planes are evident. Bars = 50 µm.
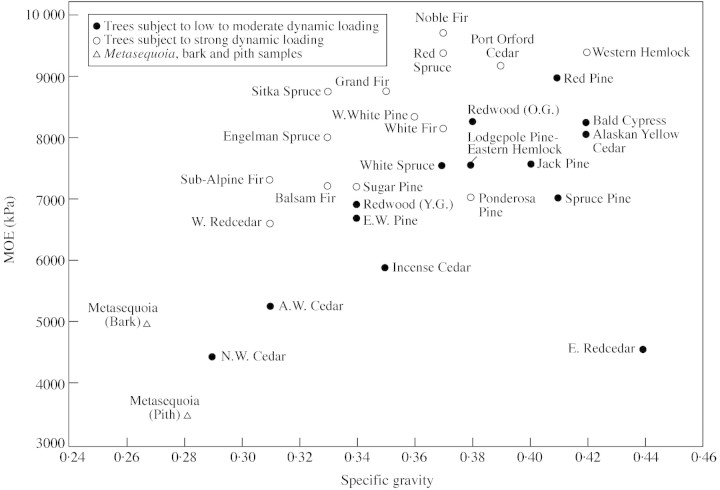
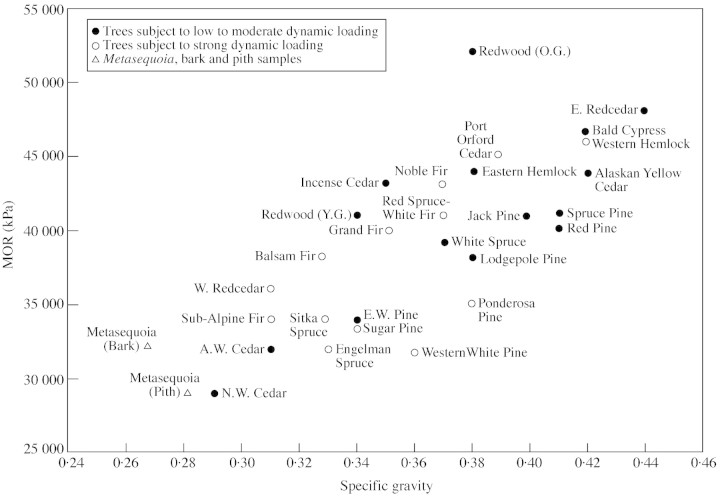
Figures 4 and 5, plots of MOE and MOR against SG for green wood of several low‐to‐moderate density conifers (SG <0·45), are based on data from the Wood Handbook (Anon., 1999) and the data generated for Metasequoia. Using the parameters of habitat exposure, elevation and climate zone, tree species were subjectively placed in one of two categories: exposure to low‐to‐moderate dynamic loading (closed circles on figures), or exposure to strong dynamic loading (open circles). Metasequoia wood (open triangles) from near the centre of the tree or from outer rings was compared with the other species. With the exception of Ponderosa pine, wood from trees exposed to strong dynamic loading had higher density‐specific stiffness (MOE/SG) than trees exposed to weak lateral loading, as seen in Fig. 4, but no relationship was seen between dynamic loading and density‐specific MOR (Fig. 5). Ponderosa pine is very widespread and consists of several races (Elias, 1987); the origin of the wood tested in this study is not known (Anon., 1999).
Fig. 4. Modulus of elasticity (MOE) of low‐to‐moderate density North American conifers plotted against specific gravity (data from Anon., 1999). Species identified according to level of exposure to dynamic (wind) loading.
Fig. 5. Modulus of rupture (MOR) of low‐to‐moderate density North American conifers plotted against specific gravity (data from Anon., 1999). Species identified according to level of exposure to dynamic (wind) loading.
In the decay tests, both of the brown‐rot fungi (P. placenta and G. trabeum) produced significant weight loss in the control pine samples (Table 3). The redwood samples resisted attack by these fungi, but the bald cypress blocks were moderately decayed by P. placenta, with an average weight loss of 12·8 %. P. placenta decayed both the top and bottom blocks of Metasequoia statistically the same as the pine blocks. However, G. trabeum was unable to decay any of the Metasequoia blocks. Because G. trabeum is normally very aggressive in softwoods, the test was repeated with this fungus. Metasequoia continued to show strong decay resistance, with 5 % weight loss compared with 48 % weight loss in the control.
Table 3.
Fungal decay of Metasequoia compared with other conifers using white rot and brown rot fungi in a modified ASTM soil block assay
| Brown rot fungi | White rot fungi | No fungus | |||
| P. placenta | G. trabeum | T. versicolor | I. lacteus | Control | |
| Pinus(control) | 36·0 (3·8) | 59·9 (1·9) | 7·3 (2·1) | 17·5 (2·8) | 7·4 (10·0) |
| Sequoia | 2·6 (0·5) | 2·1 (0·8) | 1·3 (2·9) | 1·0 (0·4) | 1·0 (0·2) |
| Taxodium | 12·8 (9·5) | 0·0 (0·6) | 1·3 (0·5) | 2·8 (0·4) | 0·9 (0·3) |
| Metasequoia (top) | 33·2 (2·6) | –0·5 (0·4) | 8·0 (5·2) | 4·5 (1·6) | 0·4 (1·9) |
| Metasequoia (bottom) | 29·4 (4·3) | –1·1 (0·1) | 3·3 (2·9) | 1·9 (1·3) | 1·6 (0·8) |
Standard deviation in parentheses.
Among the white rot decay fungi, T. versicolor was not very aggressive against any of the softwoods, but produced some weight loss in pine and Metasequoia (less than 10 % in each). Irpex lacteus was moderately aggressive on the pine (18 % weight loss), while the other species, including Metasequoia, were resistant.
Table 4 was created from data in the Wood Handbook (Anon., 1999), and compares heartwood decay resistance of North American tree species, categorized by climate zone, successional status and wood density. In the boreal zone, where the decay hazard is very low, Larix is the only species with decay resistance (only moderate). In the temperate zone species that are long‐lived and have wood with low‐to‐moderate density are likely to have decay resistant heartwood. Some Quercus species with high density develop decay resistance, but many of these are warm temperate to sub‐tropical [i.e. live oak (Quercus virginiana Mill.)]. Species with high density in the temperate zone are less likely to have decay resistant heartwood. Warm temperate species that are members of mostly tropical families tend to have decay resistant heartwood even if they have high density.
Table 4.
Heartwood durability of North American trees
| Short‐lived | Long‐lived | ||
| Early successional | Late successional | ||
| Climate zone | Not durable | Decay resistance† | Not durable |
| Boreal | Populus(L to M) | Larix (M)*, + | Picea(L) |
| Betula (M) | Abies (L) | ||
| Abies (L) | |||
| Temperate | Alnus (M) | Pinus (L)*, + | Tsuga(M) |
| Populus(L) | Pseudotsuga(M)++ | Acer (H) | |
| Prunus (M) | Taxus(M)+++ | Betula (H) | |
| Salix (L to M) | Chamaecyparis (L)++++ | Tilia (L) | |
| Aesculus (L) | Thuja (L)++++ | Fagus(H) | |
| Juglans (L) | Calocedrus(L)++++ | Fraxinus (H)* | |
| Prunus(M to H)*, ++++ | Pinus (M)* | ||
| Quercus(H)++++ | Quercus(H) | ||
| Castanea (M)++++ | Carya(H) | ||
| Juniperus(M)*, ++++ | Ulmus(H)* | ||
| Juglans(M to H)*, ++++ | Celtis (H) | ||
| Morus (L)++++ | Platanus (H) | ||
| Sassafras (L)*, ++++ | Lithocarpus(H) | ||
| Sequoia(L)++++ | Liriodendron(L)* | ||
| Taxodium (L to M)++++ | Liquidambar(M)* | ||
| Magnolia(M) | |||
| Warm temperate (tropical in origin) | Pinus (H)++++ | ||
| Cupressus (H)*, ++++ | |||
| Catalpa(H)*, ++++ | |||
| Gleditsia (H)*, ++++ | |||
| Robinia(H)*, ++++ | |||
| Maclura(H)*, ++++ | |||
| Prosopis (H)*, ++++ | |||
Durability data from Anon. (1999).
Wood density is noted as low (L), moderate (M) or high (H).
* These species are shade intolerant, but can be relatively long‐lived. Genera listed in more than one column and not starred represent different species.
† Decay resistance: moderate (+) through to high (++++).
DISCUSSION
Adequate design in the xylem of trees is predicated on achieving values for strength, stiffness and hydraulic conductivity that provide sufficient functionality within the constraints of the environment (Niklas, 1992; Tyree et al., 1994; Givnish, 1995; Domec and Gartner, 2002). The wood of Metasequoia has an SG of approx. 0·27, one of the lightest and weakest of any conifer, even among the Cupressaceae (Wu and Chern, 1995). The closely related Glyptostrobus pensilis K. Koch has a comparably low SG, but only reaches tree heights of 25 m, while Metasequoia can reach heights of 50 m—comparable with the maximum height of the denser and stronger Taxodium distichum (Henry and McIntyre, 1926; Florin, 1952; Elias, 1987).
Although no relationship exists between wood density and maximum tree height (McMahon, 1973), the exceedingly low density and strength of Metasequoia (Table 1; Fig. 5) suggest that this species may fall below the mechanical strength safety margin usually cited for trees. According to Niklas (1992), trees are mechanically overbuilt for static loads by a design factor of about four. Domec and Gartner (2002) calculated a mechanical safety factor near 2 for Douglas‐fir, and this was more than twice the calculated hydraulic safety factor. Our microscopic confirmation of progressive plastic deformation in a 26‐year‐old Metasequoia suggests that the mechanical strength safety margin may be insufficient for accommodating external loading, such as ice or snow, which increases compression loading by a proportionally greater amount in low density trees. It appears that plastic deformation is a slow accumulative process as evidenced by the differences seen in rings 14 and 26.
Metasequoia, like many other low density trees growing in temperate zones, has a heartwood with significant decay resistance. This helps to offset its low SG, but apparently not enough to prevent some plastic deformation. Perhaps, more importantly, decay resistant heartwood provides a mechanism to counteract Brazier buckling (Niklas, 1992). Based on our decay tests (and those of Polman et al., 1999), the heartwood of Metasequoia is very resistant to the attack of some fungi, but less so of others. Against I. lacteus and G. trabeum, a white rot and brown rot, respectively, decay was limited or completely inhibited in Metasequoia. Gloeophylum trabeum is known to be an aggressive brown rot in many coniferous species, so the almost complete inhibition of decay by this fungus was unexpected. Decay by the other brown rot, P. placenta, was not inhibited and was statistically not different from the pine wood. T. versicolor, which normally attacks hardwoods (dicotyledons), showed limited attack on any of the conifers used in this study.
Since Metasequoia is not native to North America (at least in recent times), as are the other tested species, its variable resistance to the presented decay fungi may indicate that it was exposed to different fungi from those dominant in its current native habitat in remote China. Decay resistance of wood can also be significantly reduced when a tree is grown as an exotic, not only because of different fungi, but also different soil chemistry which may influence the biochemistry of heartwood extractive production (Bultman et al., 1983; Jagels, 1983). Further, it should be noted that the Metasequoia wood had growth rings significantly wider than those of the redwood and cypress. Considering these factors the level of decay resistance demonstrated by the tested Metasequoia wood is remarkably high.
Decay resistance in extant Metasequoia does not ensure the same in Eocene epoch trees. However, an examination of mummified uncompressed logs and unweathered stumps of fossil Metasequoia, unearthed from the Canadian high‐Arctic, revealed little or no evidence of decay fungi activity (Blanchette et al., 1983; Jagels et al., 2001). Microscopic analysis revealed only rarely the presence of fungal hyphae, and most of those had characteristics of Ascomycete rather than Basidiomycete (decay) fungi (unpublished observations).
The lack of any significant increase in bending strength, as measured by MOR, between wood near the centre of the tree and that in outer rings (Table 1) is untypical of conifers, but is consistent with a tree stem only marginally able to support the crown (Haygreen and Boyer, 1989). Consistent with the MOR values is the maintenance of a high MFA in the outer rings—also untypical of stronger conifers (Fig. 2). Boyd (1980, 1985) has suggested that variations in MFA are caused by stresses imposed on tracheids during differentiation in the cambial zone—the greater the compressive stress, the larger the MFA. Moderate to high density conifers develop sufficient mechanical strength to counteract these stresses as the tree enlarges, and consequently MFA decreases (Fig. 2).
Compounding the problem for Metasequoia is its deciduous habit. When the cambial zone is reactivating at the beginning of the growing season new short shoots and leaves are adding weight to the crown, increasing the compressive load on unlignified cells (Boyd, 1974; Saka and Thomas, 1982). By contrast, Taxodium and Larix, two other deciduous conifers, have wood SG values approaching twice that of Metasequoia.
Typically, wood with a high MFA, characteristic of juvenile wood, displays significant longitudinal shrinkage when dried (Haygreen and Boyer, 1989). Longitudinal shrinkage, from green to oven dry of 0·36 %, was found to be greater than that typically found in mature wood, but considerably less than the 2 % often reported for juvenile wood. The permanent strain (plastic deformation) we observed might explain the less than expected shrinkage value (Meylan, 1972).
Like MOR, MOE was not correlated with SG in green wood (Table 2). Unlike MOR, MOE increased between wood near the centre and that formed later. Previous research has demonstrated that MOE and SG are often poorly correlated, but several researchers have found a correlation between MOE and MFA (Cowdrey and Preston, 1966; Astley et al., 1998; Booker et al., 1998). However, only a small change in MFA with increasing ring number was found (Fig. 1). Tracheid length has been linked to strength properties in wood, but since tracheid length normally increases while MFA decreases, the true relationship may be with MFA rather than tracheid length (Wellwood, 1962; Dinwoodie, 1965; Kaya and Smith, 1993). At this time, there is no definitive explanation for the increase in MOE with increasing distance from the pith, but it is possible that the observed plastic deformation in rings closer to the tree centre significantly reduced MOE in this area compared with wood closer to the bark which was not strained in the plastic range (Tabarsa and Chui, 2000).
Some of the characteristics that reduce mechanical strength of a stem might improve hydraulic conductance potential. Recently, McCulloh et al. (2003) provided evidence that water transport in plants fits the ‘aorta model’ (Murray’s law for cardiovascular hydraulics) better than the traditionally used ‘pipe model’ (Shinozaki et al., 1964). In this new model the optimum network requires wide conduits at the base of a tree feeding smaller diameter conduits distally. Measurement of conduit diameter in outer rings near the base of a tree should, therefore, provide surrogate values for hydraulic conductance potential. Furthermore, a comparison of maximum conduit diameter among conifers should provide evidence to indicate growth potential and maximum tree height (see table 4‐3 in Panshin and deZeeuw, 1980). Since tracheids have finite lengths that are orders of magnitude shorter than vessels, tracheid length as well as diameter must be considered. Tyree and Zimmermann (2002) estimate that tracheid length contributes about half to hydraulic resistance. We measured maximum tracheid diameter of 69 µm and average tracheid length of 4·54 mm in outer rings of the 26‐year‐old Metasequoia. This compares with values of 70 µm and 4·72 mm in bald cypress (a tree of similar maximum height), and 80 µm and 6·59 mm in redwood, the tallest conifer (Panshin and deZeeuw, 1980).
Hacke et al. (2001) reported a correlation between wood density and cavitation and implosion resistance. They concluded that hoop stresses in cell walls are small and, therefore, implosion resistance is counteracted primarily by thick cell walls. However, they only examined branches, which in conifers contain a high proportion of compression wood, which has abnormally high density and very low conductance, and they restricted their survey to trees with moderate to high densities. It is suggested that, in low density conifers with high MFAs, hoop stresses may provide significant restraint to xylem implosion by transferring (Poisson’s ratio) radial/tangential stresses to axial ones (Boyd, 1980).
How do the measured xylem characteristics fit with the palaeoenvironment of high latitudes where Metasequoia thrived and dominated on many wet sites during the Eocene? The constraining and unique environmental factors would have been a short growing season under low‐intensity but constant illumination. In a previous paper (Jagels and Day, 2003), it was demonstrated how photosynthesis in Metasequoia was adapted to these conditions. Also noted was the high water use efficiency (WUE) at canopy level, a feature of adaptive value for a tree that, in theory, is transpiring continuously during the growing season. Even with a high WUE and a wet site environment, water potentials must have been continuously strongly negative, with no dark period to restore or repair the hydraulic system (Pallardy et al., 1995). Yet based on logs unearthed from the Eocene palaeosite at Axel Heiberg (Jagels et al 2001) tree height has been estimated to have exceeded 30 m (Francis, 1991; Greenwood and Basinger, 1994). Large diameter and long tracheids would, theoretically have provided hydraulic advantages, and a large MFA in the S2 wall could have helped to counteract compressive hoop stress in the presence of thin walls (Boyd, 1985; Tyree et al., 1994; Tyree and Zimmermann, 2002).
Since wood density and strength have been sacrificed for increased hydraulic efficiency in Metasequoia, the consequent reduction in ‘design margin’ strength suggests that the palaeosite was not normally impacted by significant externally applied stem loads (snow, ice, wind). Koch (1963) characterized the Palaeocene climate in north‐western Greenland as warm temperate, humid and weakly winter‐dry. This fits with the current, mostly snow free, relict habitat for Metasequoia in China (Chu and Cooper, 1950).
Wind loading at the palaeosite is unknown. During the dormant season Metasequoia presents a minimal loading surface and mass by shedding both leaves and short shoots. Where Metasequoia trees are currently exposed to greater wind loads (open grown or edge of stand) they develop highly fluted stems, which, by increasing stem cross‐sectional area, have a large positive influence on mechanical strength (Domec and Gartner, 2002). Fluting is reduced to a minimum in closed stands. Thus, it appears that Metasequoia has developed strategies other than increased stem density to adapt to mechanical environmental stresses. The presence of durable heartwood is consistent with adaptation to a temperate or warmer climate, as shown in Table 4, and provides stem resistance to Brazier buckling in an excessively low‐density conifer.
The hypothesis that minimizing stem density is important in gaining site dominance at the weak‐light, highest latitudes, is supported by noting that two close relatives of Metasequoia, bald cypress and redwood also can develop fluted stems, have stem hydraulic efficiencies that are theoretically as high or higher than Metasequoia, and have been found as Eocene epoch fossils—but not at the high latitudes dominated by Metasequoia. Both of these species have higher wood densities than Metasequoia, and redwood is not deciduous, a liability during a warm, dark winter (Ornduff, 1988; Anon., 1999). A Larix sp., a higher density conifer, was a cohort of Metasequoia at the high latitude Eocene palaeosites, but only as a minor component (Jagels et al., 2001).
A question that remains is why have Metasequoia populations now been reduced to relict status in protected wet valley bottoms in a small region of China (Chu and Cooper, 1950). In addition to ecophysiological factors previously discussed (Jagels and Day, 2003), it could be speculated that a mechanically weak stem may have become a liability in harsher (drier and more windy) higher light environments, where shade intolerant diffuse‐porous dicotyledons would have a competitive edge.
ACKNOWLEDGEMENTS
We thank John E. Kuser for allowing us to cut a Metasequoia tree on his property, and Bethel Furniture Stock, Inc., Bethel, Maine for permission to use their Compwood machine for compressing red oak. This paper is Maine Experimental Station Report No. 2619.
Supplementary Material
Received: 4 October 2002; Returned for revision: 10 February 2003; Accepted: 11 April 2003 Published electronically: 21 May 2003
References
- Anon.2001. Test method D2017‐81 (1994). Standard test method for accelerated laboratory test of natural decay resistance of woods. West Conhohocken, PA: American Society for Testing and Materials. [Google Scholar]
- Anon.1999.Wood handbook—wood as an engineering material. Madison, WI: Forest Products Society. [Google Scholar]
- ArcherRA.1987.Growth stresses and strains in trees. Berlin: Springer‐Verlag. [Google Scholar]
- AstleyRJ, Harrington JJ, Tang S, Neuman J.1998. Modeling the influence of microfibril angle on stiffness and shrinkage on radiata pine. In: Butterfield BG, ed. Microfibril angle in wood. Christchurch: University of Canterbury. [Google Scholar]
- BeckerP, Tyree MT, Tsuda M.1999. Hydraulic conductances of angiosperms versus conifers: similar transport sufficiency at the whole‐plant level. Tree Physiology 19: 445–452. [DOI] [PubMed] [Google Scholar]
- BlanchetteRA, Cease KR, Abad AR, Burnes TA, Obst JR.1983. Ultrastructural characterization of wood from Tertiary fossil forests in the Canadian Arctic. Canadian Journal of Botany 69: 560–568. [Google Scholar]
- BerlynGP, Miksche JP.1976.Botanical microtechnique and cyto chemistry. Ames, IA: Iowa State University Press. [Google Scholar]
- BookerRE, Harrington J, Shiokura T.1998. Variation of Young’s modulus with microfibril angle, density and spiral grain. In: Butterfield BG, ed. Microfibril angle in wood. Christchurch: University of Canterbury. [Google Scholar]
- BoydJD.1972. Tree growth stresses. V. Evidence of an origin in differentiation and lignification. Wood Science and Technology 6: 251–262. [Google Scholar]
- BoydJD.1974. Relating lignification to microfibril angle differences between tangential and radial wall faces of all wall layers in wood cells. Drevarsky Vyskum 19: 41–54. [Google Scholar]
- BoydJD.1980. Relationships between fibre morphology, growth strains and wood properties. Australian Forest Research 10: 337–360. [Google Scholar]
- BoydJD.1985.Biophysical control of microfibril orientation in plant cell walls. Dordrecht: Martinus Nijhoff/Dr W. Junk. [Google Scholar]
- BrazierJD.1963. The timber of young plantation‐grown Metsequoia. Quarterly Journal of Forestry 57(2): 151–153. [Google Scholar]
- BultmanJD, Beal RH, Huffman JB, Parrish KK.1983. An investigation of the natural decay resistance of Melaleuca quinquenervia to tropical and terrestrial wood‐destroying organisms. Forest Products Journal 33(3): 39–43. [Google Scholar]
- CaveID.1972. A theory of the shrinkage of wood. Wood Science and Technology 6: 284–292. [Google Scholar]
- ChuK, Cooper WS.1950. An ecological reconnaissance in the native home of Metasequoia glyptostroboides Ecology 31: 260–278. [Google Scholar]
- CowdreyDR, Preston RD.1966. Elasticity and microfibril angle in the wood of sitka spruce. Proceedings of the Royal Society London, Series B. 166: 245–272. [Google Scholar]
- DinwoodieJM 1965. The relationship between fiber morphology and paper properties, a review of literature. Technical Asssociation of the Pulp and Paper Industry 48(8): 440–447. [Google Scholar]
- DomecJC, Gartner BL.2002. Age‐ and position‐related changes in hydraulic versus mechanical dysfunction of xylem: inferring the design criteria for Douglas‐fir wood structure. Tree Physiology 22: 91–104. [DOI] [PubMed] [Google Scholar]
- EliasTS.1987.Trees of North America. New York, NY: Gramercy Publishing Co. [Google Scholar]
- FlorinR.1952. On Metasequoia, living fossil. Botaniska Notiser 105: 1–29. [Google Scholar]
- FrancisJE.1991. The dynamics of polar fossil forests of Axel Heiberg island, Canadian Arctic Archipelago. Geological Survey of Canada Bulletin 403: 29–38. [Google Scholar]
- GivnishTJ.1995. Plant stems: biomechanical adaptation for energy capture and influence on species distributions. In: Gartner BL, ed. Plant stems: physiology and functional morphology. New York, NY: Academic Press. [Google Scholar]
- GreenwoodDR, Bassinger JF.1994. The paleoecology of high‐latitude Eocene swamp forests of Axel Heiberg island, Canadian Arctic Archipelago. Review of Paleobotany and Palynology 81: 83–97. [Google Scholar]
- HackeUG, Sperry JS, Pockman WT, Davis SD, McCulloh KA 2001. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461. [DOI] [PubMed] [Google Scholar]
- HaygreenJG, Bowyer JL.1989.Forest products and wood science, 2nd edn. Ames, IA: Iowa State University Press. [Google Scholar]
- HejnowiczA 1973. Anatomical studies on the development of Metasequoia glyptostroboides Hu et Cheng wood. Acta Societatis Botanicorum Poloniae 62(3): 437–490. [Google Scholar]
- .HenryA, McIntyre M.1926. The swamp cypresses, Glyptostrobus of China and Taxodium of America, with notes on allied genera. Proceedings of the Royal Irish Academy 37(13): 90–124. [Google Scholar]
- HillerCH, Brown RS.1967. Comparison of dimensions and fibril angles of loblolly pine tracheids formed in wet or dry growing seasons. American Journal of Botany 54: 453–460. [Google Scholar]
- HirakawaY, Yamashita K, Fujisawa Y, Nakada R, Kijidani Y.1998. The effects of S2 microfibril angles and density on MOE in Sugi trees. In: Butterfield BG, ed. Microfibril angle in wood. Christchurch: University of Canterbury. [Google Scholar]
- JagelsR.1983. How reliable are wood durability ratings? WoodenBoat 54: 120–122. [Google Scholar]
- JagelsR, Day ME.2003. The adaptive physiology of Metasequoia to Eocene high‐latitude environments. In: Hemsley AR, Poole, I, eds. Evolution of plant physiology Vol. 2. Evolutionary physiology from whole plant to ecosystem. London: Academic Press (in press). [Google Scholar]
- JagelsR, LePage BA, Jiang M.2001. Definitive identification of Larix (Pinaceae) wood based on anatomy for the middle Eocene, Axel Heiberg Island, Canadian High Arctic. IAWA Journal 22: 73–83. [Google Scholar]
- JaroslavcevGD, Visnjakov TN.1965. Wood of Metasequoia (in Russian). Bjulleten Glavnogo Botanicheskogo Sada 59: 97–99. [Google Scholar]
- KayaF, Smith I.1993. Variation in crushing strength and some related properties of red pine. Wood Science and Technology 27: 229–239. [Google Scholar]
- KochEB.1963. Fossil plants from the early Paleocene of the Agatdalen (Angmârtussut) area, central Nagssuag penninsula, northwest Greenland. Unders Grönlands Geology 172: 1–20. [Google Scholar]
- KuserJE.1999.Metasequoia glyptostroboides: fifty years of growth in North America. Arnoldia 59: 76–79. [Google Scholar]
- LiH.1957. The discovery and cultivation of Metasequoia Morris Arboretum Bulletin 8(4): 49–53. [Google Scholar]
- LiJY.1948. Anatomical study of the wood of ‘Shuisha’ (Metasequoia glyptostroboides Hu et Cheng). Tropical Woods 94: 28–29. [Google Scholar]
- LiangH, Chow KY, Au CN.1948. Properties of a living fossil wood. National Central University Forestry Institute (Nanking) Research Notes 1: 1–6. [Google Scholar]
- LinnardW.1966. A note on the wood of Metasequoia Wood 31: 46. [Google Scholar]
- McAlisterRH, Powers HR, Pepper WD.2000. Mechanical properties of stemwood and limbwood of seed orchard loblolly pine. Forest Products Journal 50(9): 91–94. [Google Scholar]
- McCullohKA, Sperry JS, Adler FR.2003. Water transport in plants obeys Murray’s law. Nature 421: 939–942. [DOI] [PubMed] [Google Scholar]
- McMahonTA.1973. On size and shape in biology. Science 179: 1201–1204. [DOI] [PubMed] [Google Scholar]
- MegrawRA, Leaf G, Bremer D.1998. Longitudinal shrinkage and microfibril angle in Loblolly pine. In: Butterfield BG, ed. Microfibril angle in wood. Christchurch: University of Canterbury. [Google Scholar]
- MeylanBA.1972. The influence of microfibril angle on the longitudinal shrinkage‐moisture content relationship. Wood Science and Tech nology 6: 293–301. [Google Scholar]
- MeylanBA, Probine MC.1969. Microfibril angle as a parameter in timber quality assessment. Forest Products Journal 19(4): 30–34. [Google Scholar]
- MomoharaA.1994. Paleoecology and paleobiogeography of Meta sequoia Fossils 57: 24–30. [Google Scholar]
- NakadaR, Fujisawa Y, Nishimura K, Hirakawa Y.1998. Variation in S2 microfibril angle of latewood among plus‐tree clones and test stands in Cryptomeria japonica D. Don. In: Butterfield BG, ed. Microfibril angle in wood. Christchurch: University of Canterbury. [Google Scholar]
- NiklasKJ.1992.Plant biomechanics, an engineering approach to plant form and function. Chicago, IL: University of Chicago Press. [Google Scholar]
- OrnduffR.1998. The Sequoia sempervirens (Coast Redwood) forest of the pacific coast, USA. In: Laderman AD, ed. Coastally restricted forests. New York, NY: Oxford University Press. [Google Scholar]
- PallardySG, Cermak J, Ewers FW, Kaufman MR, Parker WC, Sperry JS.1995. Water transport dynamics in trees and stands. In: Smith WK, Hinkley TM, eds. Resource physiology of conifers; aquisition, allocation and utilization. New York, NY: Academic Press. [Google Scholar]
- PanshinAJ, deZeeuw C.1980.Textbook of wood technology, 4th edn. New York, NY: McGraw‐Hill. [Google Scholar]
- PolmanJE, Michon SGL, Militz H, Helmink ATF.1999. The wood of Metasequoia glyptostroboides (Hu et Cheng) of Dutch origin. Holz als Roh‐und Werkstoff 57: 215–221. [Google Scholar]
- RashevskyN 1973. The principle of adequate design. In Rosen R, ed. Foundations of mathematical biology. New York, NY: Academic Press. [Google Scholar]
- RombergerJA, Hejnowicz Z, Hill JF.1993.Plant structure: function and development. Berlin: Springer‐Verlag. [Google Scholar]
- SakaS, Thomas RJ.1982. A study of lignification of loblolly pine tracheids by SEM‐EDXA technique. Wood Science and Technology 16: 167–179. [Google Scholar]
- SaranpääP, Serimaa R, Anderson S, Pesonen E, Suni T, Paakkari T.1998. Variation in microfibril angle in Norway spruce (Picea abies (L.) Karst.) and Scots pine (Pinus sylvestris L.) – comparing X‐ray diffraction and optical methods. In: Butterfield BG, ed. Microfibril angle in wood. Christchurch: University of Canterbury. [Google Scholar]
- ShinozakiK, Yoda K, Hozumi K, Kira TA.1964. A quantitative analysis of plant form – the pipe model theory. I. Basic analysis. Japanese Journal of Ecology 14: 97–105. [Google Scholar]
- SurminskiJ, Bojarczuk T.1973. Drewno metasekwol chinskiej (Metasequoia glyptostroboides Hu et Cheng) polskiego pochod zenia. Rocznik Dendrologiczny 27: 159–168. [Google Scholar]
- TabarsaT, Chui YH.2000. Stress‐strain response of wood under radial compression. Part I. Test method and influences of cellular properties. Wood and Fiber Science 32(2): 144–152. [Google Scholar]
- TyreeMT, Zimmermann MH.2002.Xylem structure and the ascent of sap, 2nd edn. Berlin: Springer. [Google Scholar]
- TyreeMT, Davis SD, Cochard H.1994. Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA Journal 14(4): 335–360. [Google Scholar]
- WardropAB, Preston RD.1950. The fine structure of the wall of the conifer tracheid. V. The organization of the secondary wall in relation to the growth rate of the cambium. Biochimica et Biophysica Acta 6: 36–47. [Google Scholar]
- WellwoodRW.1962. Tensile strength of small wood samples. Pulp and Paper Magazine of Canada 63(2): T61–T67. [Google Scholar]
- WuS, Chern J‐H.1995. Group analysis as applied to wood anatomy of Taxodeaceae members. University of Taiwan Agriculture College Research Reports 35(3): 360–374. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.