Abstract
Architectural analyses of temperate tree species using a chronological approach suggest that the expression of epicormic branches is closely related to low growth rates in the axes that make up the branching system. Therefore, sole consideration of epicormic criteria may be sufficient to identify trees with low secondary growth levels or with both low primary and secondary growth levels. In a tropical tree such as Dicorynia guianensis (basralocus), where chronological studies are difficult, this relationship could be very useful as an easily accessible indicator of growth potentials. A simple method of architectural tree description was used to characterize the global structure of more than 1650 basralocus trees and to evaluate their growth level. Measurements of simple growth characters [height, basal diameter, internode length of submittal part (top of the main axis of the tree)] and the observation of four structural binary descriptors on the main stem (presence of sequential branches and young epicormic branches, state of the submittal part, global orientation), indicated that epicormic branch formation is clearly related to a decrease in length of the successive growth units of the main stem. Analysis of height vs. diameter ratios among different tree subgroups, with and without epicormic branching, suggested that trees with epicormic branches generally have a low level of secondary growth compared with primary growth.
Key words: Dicorynia guianensis, architecture, epicormic branch, primary growth, secondary growth, tropical forest, French Guiana, height : diameter ratio
INTRODUCTION
The sprouting of epicormic branches and stool‐shoots from trunks and stumps of juvenile or mature trees is commonly observed in a great number of conifer and angiosperm species. Most research conducted on epicormic branch formation has focused on temperate species and has shown that many factors govern this phenomenon. One of these is the genetic determinism that controls the potential production of epicormic branches by particular species (Brinkman, 1955; Boyce, 1962; Blum, 1963; Ward, 1966; Kozlowsky, 1971; Evans, 1983). Epicormic shoot formation is clearly related to environmental conditions, and occurs on trees growing under stressful conditions. The expression of epicormic branches is linked to abrupt changes in climate or the tree’s environment, such as severe drought, extreme cold, storms or insect attack (Blum, 1963; Kazarjan, 1969; Batzer, 1973; Lanier cited by Roussel, 1978; Roloff, 1989). Therefore, epicormic branches are often associated with stand thinning (Walhenberg, 1950; Huppuch, 1961; Dale and Sonderman, 1984; Sonderman, 1985; Evans, 1987; Wignall and Browning, 1988), which increases exposure to light (Vogt and Cox, 1970; Kramer and Kozlowsky, 1979; Evans, 1987; Hibbs et al., 1989). Epicormic branches may also form following an increase in shade. These branches, known as ‘agony branches’ or ‘shade suckers’ develop on the stem of overtopped individuals of numerous forest species (Büsgen and Münch, 1929; Roussel, 1978; Courraud, 1987). Stem diameter seems to be inversely related to the production of epicormic branches (Bruner, 1964; Hedlund, 1964; Drénou, 1994): individuals producing epicormic branches are generally suppressed trees with low cambial activity (Rohmeder, 1935; Jemison and Schumacher, 1948; Cosens, 1952; Bruner, 1964), irrespective of thinning treatments (Perrin, 1952; Krajicek, 1959; Bruner, 1964; Bachelard, 1969; Kormanik and Brown 1969). Abrupt environmental changes only accentuate this effect (Courraud, 1987).
Architectural analyses using a chronological approach to tree development and a detailed description of both morphological and anatomical characters allowed epicormic branch formation to be considered as an integral part of tree development, e.g. in Fagus sylvatica L. (Nicolini, 1997; Chanson and Nicolini, 2000; Nicolini et al., 2001). These studies also confirmed that this phenomenon is determined by environmental factors, and showed that the expression of epicormic branches was closely related to tree architecture and to low growth levels of the axes that make up the branching system. Therefore, consideration of the presence of epicormic branches may be sufficient to identify trees with low secondary growth levels or with both low primary and secondary growth levels (Nicolini et al., 2001).
In tropical tree species, where chronological studies are often difficult, this relationship between epicormic branch formation and low growth levels could be very useful as an easily accessible indicator of growth potentials, enabling predictions to be made of which individuals will be able to reach the canopy, which will remain suppressed in the understorey, or which will eventually die. To validate this relationship Dicorynia guianensis (basralocus) was used. This is the most heavily logged forest tree species in French Guiana, and its phenology and growth behaviour are well known. The main objective was to point out synthetic descriptors that could be used to assess present growth and physiological stage of basralocus individuals, and to test whether epicormic branch construction constitutes a suitable growth indicator for D. guianensis.
MATERIALS AND METHODS
Study site and sampling
The study was conducted at the Paracou field station (52°08′W, 5°03′N), near Sinnamary, in French Guiana. The site is a lowland tropical rain forest that receives more than 60 % of its annual 3160 ± 161 mm (mean ± s.e.) precipitation between mid‐April and June (Baraloto, 2001). The mean temperature is 26 °C, with minor seasonal variations (Huc et al., 1984). Some topographic variation exists within the reserve, with altitudes varying between 3 and 40 m a.s.l., and slopes infrequently exceeding 50 % (Barthès, 1991). The soils are derived from schist and pegmatite, with some sites characterized by marked podzolization. The woody plant community at Paracou is dominated by Lecythidaceae (17 % of individuals), Chrysobalanaceae (14 %) and Caesalpiniaceae (13 %) (Favrichon, 1995; Molino and Sabatier, 1999).
Paracou’s silvicultural facility comprises four blocks, each with 12 observation plots of 9 ha. Each plot is made up of a central section (250 m × 250 m), leaving a 25‐m‐wide strip as a buffer zone. A Dicorynia guianensis inventory, started in 1999, was conducted by CIRAD‐forêt (research project GIP‐ECOFOR no. 99·09) with the aim of describing the regeneration cycle of this species. The inventory was conducted in the ‘southern block’ (plots 9–12), where trees 1 m tall or more were recorded in the central and buffer zones of each plot.
From this inventory we considered 724 individuals of D. guianensis 1 m tall or more, regardless of environmental conditions. To obtain a better understanding of the successive developmental stages of D. guianensis, we also extended our study to 928 non‐inventoried individuals <1 m tall.
For each of the sampled trees, the height of the main stem and its basal diameter were recorded 1–10 cm above the collar point for trees 0·1–5 m tall, 20–50 cm above the swollen base for trees 5–12 m tall, and at breast height (1·30 m) for trees taller than 12 m.
Architectural description
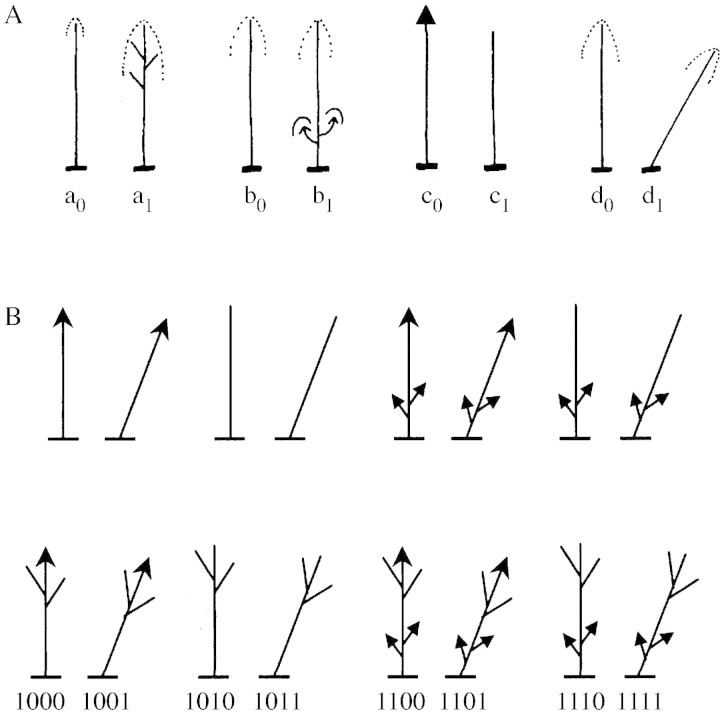
A reduced protocol of architectural description was used, involving a small number of simple criteria which take the global structure and growth of individual trees into account. The branching system of each tree was described using four structural binary descriptors (Fig. 1A). (1) Sequential branches on the main stem (a0 = absence; a1 = presence). Sequential branches are produced throughout tree development, shortly after the bearer growth unit has formed. The girth increment of the two types of axis (main stem and lateral axis) is harmonious, and therefore no bulge occurs at the base of a sequential branch. The colour and texture of the bark of both axes is also similar. (2) Young epicormic branches on the main stem (b0 = absence; b1 = presence). Young epicormic branches appear on old structures and grow from latent buds that remained on the periphery of the stem, embedded just below the bark. Epicormic branches can be distinguished by the bulge formed at their base during growth which is indicative of their superficial insertion on the stem. Their bark is very different to that formed by the bearing stem. (3) State of the submittal part of the main stem (c0 = alive; c1 = dead). (4) Global orientation of the main stem (d0 = vertical; d1 = oblique).
Fig. 1. Architectural descriptors (A) and architectural classes (B) of Dicorynia guianensis. The structural descriptors are: absence (a0) or presence (a1) of sequential branches on the main stem; absence (b0) or presence (b1) of epicormic branches on the main stem; submittal part of the main stem alive (arrow, c0) or dead (x or, c1); vertical (d0) or oblique (d1) orientation of the main stem. The architectural classes result from the combination of the considered modalities for each structural descriptor; for example, the code ‘0010’ corresponds to trees devoid of sequential branches (0∗∗∗, a0), without epicormic branches (∗0∗∗, b0), exhibiting a dead submittal part of the main stem (∗∗1∗, c1) and vertically orientated (∗∗∗0, d0). x, Dead apical meristem; #, broken main axis.
Each branching system was then characterized using a four digit code: for example, ‘0010’ corresponded to trees devoid of sequential branches (0∗∗∗, a0), devoid of epicormic branches (∗0∗∗, b0), with a dead main stem in its submittal part (∗∗1∗, c1), that was vertical (∗∗∗0, d0). All possible combinations defined 16 architectural categories (Fig. 1B). By convention, trees devoid of sequential branches (0***) are called ‘unbranched trees’ whether or not they bear epicormic branches.
Internode length measurements
The architectural description was associated with internode length measurements to evaluate the current growth level of each tree. The current growth level was assessed by averaging internode length from the last six to ten successive internodes at the top of the main stem.
To analyse the relationship between internode length and tree architecture, we also selected 25 individuals that exhibited different heights and architectures, with or without epicormic shoots, for a destructive approach. Internode lengths were then measured from the top to the base of the stem using an a posteriori recognition of internal morphological markers of the activity of the primary meristem, which persist for several years as characters imprinted into the bark or embedded into the wood and pith (Nicolini et al., 2001).
RESULTS
Structure and growth history of some representative individuals
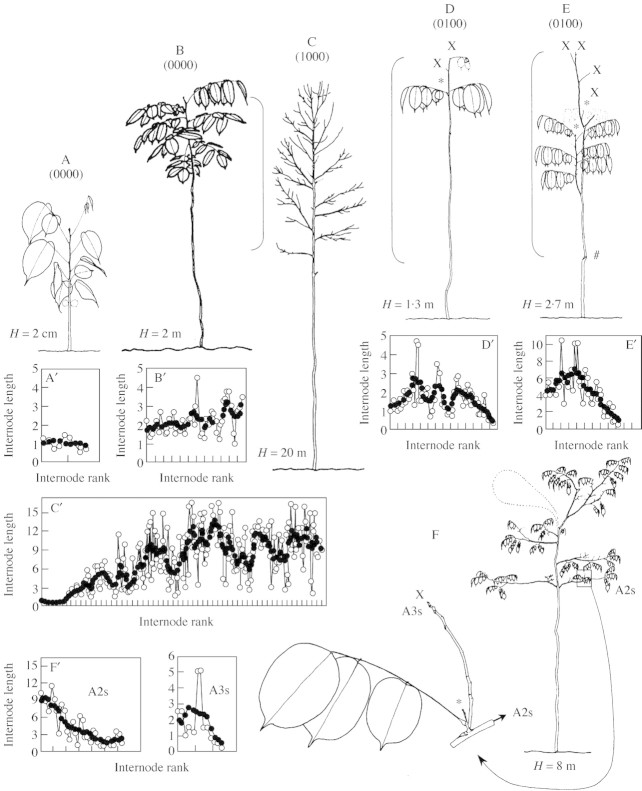
From the combination of a precise architectural analysis and the destructive approach, we reconstructed the complete growth history of 25 selected individuals. Only the most representative cases are presented in Fig. 2.
Fig. 2. Change in internode length along the main stem and branches of Dicorynia guianensis. Each tree (A–E) or branch (F) is associated with a graph (A′–F′) showing the observed lengths in centimetres (open circles); for each observed value Xi, a mean length value was calculated (‘unweighted moving average of half width 3’; closed circles):, where i is the internode rank on the main stem; the step graduation on the x‐axis is five internodes; a structural code (see Fig. 1) is associated with each tree. x, Dead apical meristem; #, broken main axis; *, epicormic branch; A2s and A3s, sequential branches of second and third order branching, respectively (A1: the tree main axis).
A 20‐cm‐tall seedling with simple leaves (Fig. 2A) showed a main stem made up of successive internodes approx. 1 cm long (A′). A 2‐m‐tall vertical, unbranched tree with compound leaves (Fig. 2B) showed longer internodes, 2–3 cm long, the distal internodes being longer than the basal ones (B′). A 20‐m‐tall young tree with a developed crown (Fig. 2C) illustrates the typical change in length along the main stem of an individual reaching the canopy level: a gradual increase in internode lengths from the base to the mid part of the main stem, followed by regular fluctuations around a constant mean near the top of the tree (C′).
An unbranched tree (Fig. 2D), made up of a vertical main stem with a small epicormic branch shoot, was dying at the time of measurement; the last leaf formed on the main stem was composed of only three leaflets, while the epicormic shoot bore leaves with five to seven leaflets. Internode lengths increased from the base to mid section of the main stem, but then decreased towards the top of the tree (D′). The same trend was observed in a branched tree with two epicormic shoots (Fig. 2E and E′). This tree also showed a dried submittal part of the main stem, but was still bearing a dried epicormic shoot.
Figure 2F shows that epicormic shoot formation was associated with a decrease in branch internode length. The lowest sequential branch (A2s) of the tree had short sequential axes (A3s) that were sometimes dead, and epicormic shoots (*) borne on A3s. The change in internode length along the sequential branches shows that the formation of epicormic branches is associated with a gradual decrease in internode length from the proximal to the distal part of the A2s and A3s (F′).
Epicormic shoot formation and tree height
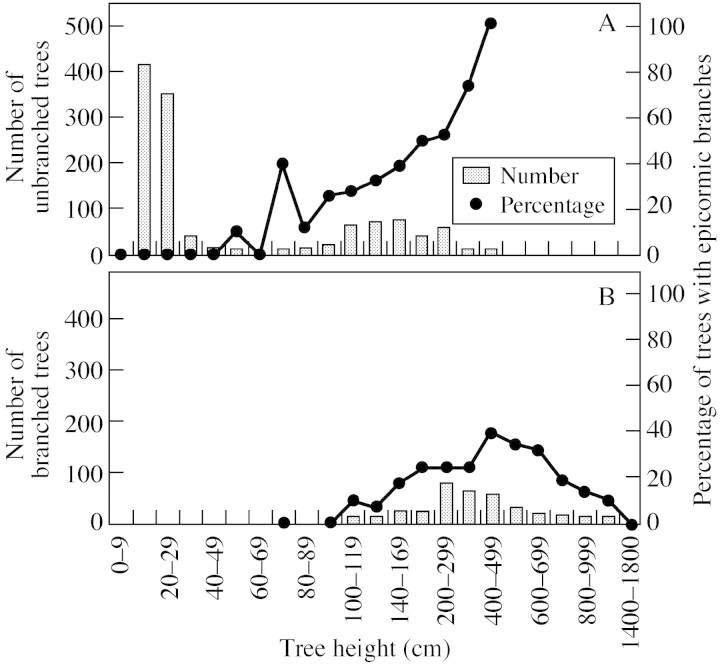
Not all of the 1627 individuals described bore epicormic branches on their trunk (Fig. 3). Of the 867 trees that were between 1 and 70 cm tall, only two formed epicormic branches. In contrast, the percentage of trees with epicormic branches increased for trees >70 cm tall.
Fig. 3. Tree height distribution and percentage of trees with epicormic branches on the main stem for unbranched (A) and branched (B) trees. By convention, unbranched trees were devoid of sequential branches; branched trees had sequential branches.
In the subgroup of unbranched trees, the proportion with epicormic branches gradually increased with height. All trees 4–5 m tall had epicormic branches; no taller unbranched trees were observed.
In the subgroup of branched trees, the proportion with epicormic branches also increased with height. A maximum value (approx. 38 %) was observed for trees 4–5 m tall; beyond 5 m, the proportion of trees bearing epicormic branches decreased with increasing height. Epicormic branches were not observed on trees taller than 14 m, although there were only a few trees in this height category.
Tree architecture and distribution of the structural descriptors
Of the 1627 trees studied, those between 1 and 70 cm tall did not form epicormic branches. Accordingly, only the height classes >70 cm were considered, including trees that formed epicormic branches on their main stem (758 individuals). Table 1 presents the distribution of individuals according to the 16 possible combinations of the four architectural descriptors. About as many trees had sequential branches (1***, 48 %) as those devoid of sequential branches (0***, 52 %). Among the trees with epicormic branches (∗1∗∗), those devoid of sequential branches (01∗∗, 20·7 %) were more numerous than those with sequential branches (11∗∗, 13 %). Similarly, among trees with a main stem that was dead in its submittal part (∗∗1∗), those with epicormic shoots (∗11∗, 16·8 %) were more numerous than those without epicormic shoots (∗01∗, 2·5 %).
Table 1.
Distribution of individuals of Dicorynia guianensis according to the 16 possible combinations of four architectural descriptors
| Architectural decriptor | 0000 | 0001 | 0010 | 0011 | 0100 | 0101 | 0110 | 0111 | 1000 | 1001 | 1010 | 1011 | 1100 | 1101 | 1110 | 1111 |
| Number of individuals | 216 | 11 | 9 | 1 | 59 | 11 | 71 | 15 | 240 | 17 | 8 | 1 | 44 | 14 | 35 | 6 |
Each branching system was characterized using a four digit code (see text): absence (0***) or presence (1***) of sequential branches on the main stem; absence (*0**) or presence (*1**) of epicormic branches on the main stem; submittal part of the main stem alive (**0*) or dead (**1*); vertical (***0) or oblique (***1) orientation of the main stem. For example, the code ‘0010’ corresponds to trees devoid of sequential branches, devoid of epicormic branches, with a dead submittal part of the main stem and orientated vertically.
Independence between pairs of criteria was tested using χ2 statistics applied to 2 × 2 contingency tables (Sokal and Rohlf, 1995). This showed that: (1) the absence of sequential branches was highly significantly related to the presence of epicormic shoots (P < 0·001), as well as to the presence of a dead submittal part of the main stem (P < 0·001); (2) the presence of a dead submittal part was significantly related to an oblique orientation of the main stem (P < 0·05), and highly significantly related to the presence of epicormic shoots (P < 0·001); and (3) the presence of epicormic shoots was highly significantly related to an oblique orientation of the main stem (P < 0·001).
Tree architecture and height vs. diameter relationship
A Tukey–Kramer HSD procedure (Sokal and Rohlf, 1995) was used to perform multiple comparisons among mean H : D ratios within the different architectural categories. In this analysis, the architectural categories were defined from a three digit code only, criterion ‘d’ (global orientation of the main stem) being disregarded. Moreover, categories 001 and 101 were excluded because they contained too few individuals. Results are summarized in Table 2. This allowed us to distinguish three different groups: (1) unbranched trees (0**), which exhibited non‐significantly different H : D ratios, whether or not they bore epicormic branches; (2) branched trees bearing epicormic branches (11*), with non‐significantly different H : D ratios regardless of the state of their submittal part (dead or alive); and (3) branched trees devoid of epicormic branches (10*), with non‐significantly different H : D ratios from trees in categories 010, 011 and 111.
Table 2.
Multiple comparisons among mean height : diameter ratios of Dicorynia guianensis in different architectural categories
| Architectural descriptor | 000 | 010 | 011 | 100 | 110 | 111 |
| Number of individuals | 227 | 70 | 86 | 257 | 58 | 41 |
| Mean height : diameter ratio | 122·7a | 131·9a, b | 124·6a, b | 134·2b, c | 156·2d | 145·7b, c, d |
Each branching system was characterized using a three digit code (see text): absence (0**) or presence (1**) of sequential branches on the main stem; absence (*0*) or presence (*1*) of epicormic branches on the main stem; submittal part of the main stem alive (**0) or dead (**1). For example, the code ‘010’ corresponds to trees devoid of sequential branches, bearing epicormic branches, with a dead main stem on its submittal. Significant differences are indicated by different superscript letters (Tukey–Kramer HSD test, P < 0·05).
Moreover, the mean H : D ratios of these three groups was highly statistically significant (Tukey–Kramer HSD, P < 0·001). Thus, for a given diameter, branched trees (H : D = 139·6) are taller than unbranched trees (H : D = 121·5), and branched trees bearing epicormic branches (H : D = 152·1) are taller than branched trees devoid of epicormic branches (H : D = 134·7).
Tree architecture and internode length
Trees whose submittal part of the main stem was dead (**1*) were excluded from this analysis because, logically, they showed no increase in internode length because the terminal meristem was dead.
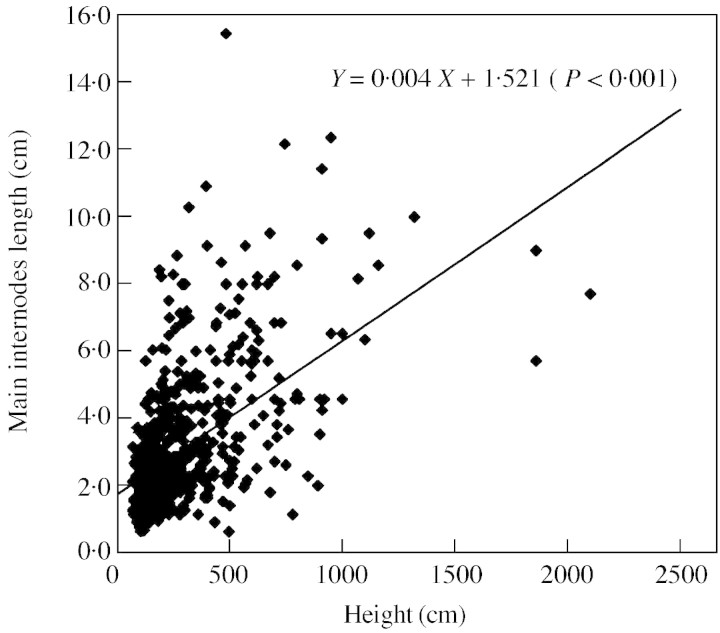
For trees still producing growth units on their main stem (∗∗0∗), the mean internode length changed significantly with tree height: the taller the tree, the more likely was its apical meristem to produce longer internodes (Fig. 4).
Fig. 4. Mean internode lengths as a function of tree height.
For trees more than 70 cm tall, mean internode lengths of branched (1***) and unbranched (0***) trees were significantly different (2·79 vs. 1·68 cm; Mann–Whitney U‐test P < 0·001). Within the unbranched trees as well as within the branched trees, the mean internode length of those trees with epicormic shoots was significantly different to that of trees without epicormic branches (0·87 vs. 2·22 cm for unbranched trees, and 1·78 vs. 3·17 cm for branched trees; Mann–Whitney U‐test, P < 0·001).
DISCUSSION
The present results help to define architectural characteristics of trees that show epicormic branches. Typically, tree height is greater than 70 cm. In a few cases, the main stem is oblique. The trees do not generally bear sequential branches and frequently show a dry and dead submittal main stem and, consequently, are unable to continue height growth. Trees devoid of sequential branches tend to be taller than trees devoid of epicormic branches. No such distinction was established for the group of trees with sequential branches, which generally had shorter internodes than trees devoid of epicormic branches; this trend was less obvious for trees bearing sequential branches. The subgroup of branched trees appeared to have a greater height : diameter ratio than that of branched trees devoid of epicormic branches. However, no distinction was found between trees with and without epicormic branches in the group of trees devoid of sequential branches.
Epicormic branch formation and primary growth
Unbranched basralocus trees and epicormic branch formation.
In this subgroup, the proportion of trees bearing epicormic branches increased with increasing tree height (Table 1): the taller the unbranched tree, the greater was its ability to form epicormic branches. It should also be noted that the smallest branched trees were approximately 1 m high, highlighting the fact that basralocus trees are able to form sequential branches early. Many architectural studies have shown that unfavourable environmental conditions delay branching (Barthélémy et al., 1995, 1997; Grosfeld et al., 1999). Similarly, an architectural study of Dicorynia guianensis showed that trees growing in open conditions formed their first sequential branches earlier and lower than trees growing in forest conditions (pers. comm.). We can consider the tallest unbranched trees as being individuals whose branching is very much delayed, because they were unable to form sequential branches under their growth conditions. Epicormic branching in basralocus may be a mechanism for continuing architectural development without sequential branching or when sequential branches cannot be maintained. This could explain the positive relationship between trees devoid of sequential branches (a1) and trees with epicormic branches (b1).
The delay in branch expression is generally associated with slow growth (Nicolini and Caraglio, 1994; Nicolini et al., 2000): under unfavourable conditions, forest trees growing slowly in height do not form sequential branches or form only reduced sequential branches. If the growth dynamics (internode length trend) of different unbranched basralocus individuals are considered (Fig. 2), we can associate the presence of epicormic shoots with a long phase during which internode length decreases and also with the death of the submittal part of the main stem. These chronological facts observed in a small number of trees could explain: (1) the significant differences observed between mean internode length values recorded for the two subgroups of trees with and without epicormic branches; and (2) the positive relationship between trees with a dead submittal part of the main stem (c1) and trees with epicormic branches (b1).
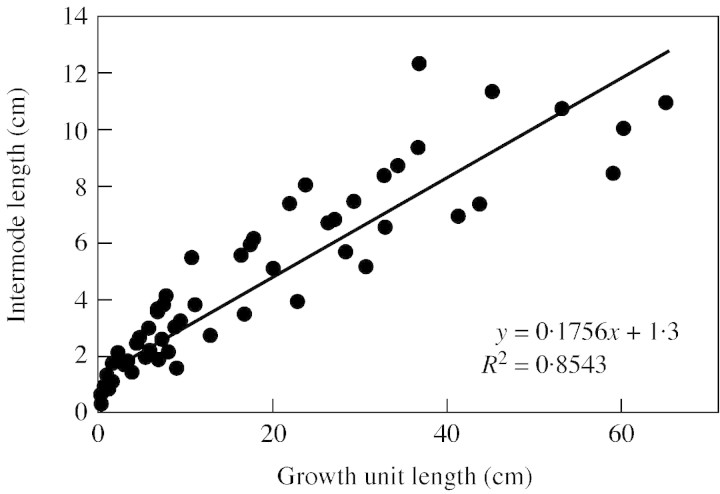
The primary growth of D. guianensis is rhythmic (Drénou, 1994): the primary meristem periodically produces new successive growth units (GU; from Hallé and Martin, 1968). The length of growth units changes as a function of tree height. During juvenile development, the taller the tree, the more its apical meristem is able to produce longer GUs (Drénou, 1994). Internode length is indicative of GU length (Fig. 5): the longer the GU, the longer its internodes. The fact that internode length increases with tree height (Figs 2C′ and ) agrees with Drénou’s results and clearly illustrates the establishment phase of D. guianensis, also observed in many other plant species (Barthélémy et al., 1997). In D. guianensis, epicormic branch formation is associated with a decrease in length in the successive GUs. Therefore, during the establishment phase, the young unbranched basralocus individual may interrupt GU length progression (environmental changes) and form shorter new GUs, as epicormic branches appear on the main stem. It may also be assumed that death of the submittal part of the main stem results from a gradual decrease in primary growth.
Fig. 5. Relationships between growth unit length (cm) and internode length (cm) in Dicorynia guianensis.
Epicormic branch formation and secondary growth
Unbranched trees and epicormic shoot formation.
The decrease in primary growth is probably associated with a decrease in secondary growth. Unfortunately, we did not measure girth increments. The height : diameter ratio analysis is an alternative method to assess secondary growth. No difference was found between trees with and without epicormic branches. These trees are monocaulous and height growth is due solely to the activity of a single terminal meristem. Since the girth increment is proportional to the height increment, the H : D ratio does not change with tree growth dynamics, i.e. rapid or slow. This may explain the inability of this indicator to distinguish between trees with and without epicormic branches in the subgroup of unbranched trees.
Branched trees and epicormic shoot formation.
The increase in H : D is generally a result of intense self‐pruning. This regulates either the leaf area or the number of active meristems in the crown of individuals that do not stop growing vertically but which produce narrower, smaller rings (Bormann, 1965; Houllier and Leban, 1991; Blaise et al., 1998). These growth dynamics give the dominant trees a typical shape: a long, slim stem with a small, narrow crown. The formation of epicormic branches is often associated with this typical shape (Bruner, 1964; Stern, 1971; Evans, 1982; Holmsgard, 1985; Nicolini et al., 2001). This relationship between secondary growth dynamics and epicormic branches also exists in the development of D. guianensis. The H : D ratio therefore allows us to distinguish branched trees without epicormic branches (H : D ≈ 134) from those with epicormic branches (H : D ≈ 152). The same values were recorded in branched beech trees 5–10 m tall growing in dense understorey, i.e. H : D ≈ 154 for trees bearing epicormic branches (Nicolini et al., 2001) vs. H : D ≈ 131 for trees devoid of epicormic branches (Nicolini and Caraglio, 1994).
In D. guianensis, the total height vs. diameter equilibrium changes significantly with height and architectural stage in trees that do not produce epicormic branches (pers. obs.). Therefore, the mean value for seedlings 20 cm tall (Fig. 2A) is 80 ± 14, whereas for a metamorphosed basralocus tree, 20 m tall and reaching the canopy (Fig. 2C), the mean value is 159 ± 27. This gradient characterizes ‘normal’ development of a basralocus tree in a forest situation. It also means that: (1) trees reaching such a high value do not automatically form epicormic branches; and (2) the total H : D ratio must not be interpreted without information about tree height. This progression may explain the significantly higher H : D values in unbranched trees (H : D ≈ 121, H = 161 ± 68 cm) compared with branched trees (H : D ≈ 139, H = 394 ± 225 cm). The branched trees were therefore taller than unbranched trees. No significant height difference was noted between branched trees devoid of epicormic branches (H = 390 ± 237 cm) and those with epicormic branches (H = 401 ± 188 cm). Therefore, we may assume that the significant difference in H : D ratio between these two subgroups is due not to tree height but instead to different growth dynamics. Branched trees with epicormic shoots are trees that have a low rate of secondary growth compared with primary growth.
Epicormic branches: suitable indicators of global or local growth conditions
This study of Dicorynia guianensis has shown: (1) that epicormic branch formation is an organized phenomenon closely related to a gradual reduction in cambial activity and often associated with a reduction in primary growth; and (2) a relationship between growth dynamics and architectural stages. However, the evidence that trees that form epicormic branches also show low levels of growth does not hide the fact that variability was observed when results were expressed using punctual indicators, such as H : D ratio and internode length, underlining their relative unsuitability; branched trees that form epicormic branches do not always have a high H : D ratio. Here, we should recall the example of a ‘suppressed moribund’ [according to Kraft’s (1884) definition; see Lanier, 1986] beech tree (Nicolini et al., 2001, tree ‘Chavi3’). This tree had a H : D ratio of 100 when it formed the first epicormic branches on its main stem. We cannot explain why this well‐balanced tree suddenly did this, but epicormic branch formation may be associated with a global decrease in both primary and secondary growth without changing the H : D ratio. This clearly highlights the limited use of the H : D descriptor, which does not detect the change in growth dynamics of a tree. In other words, sole consideration of the H : D ratio is insufficient to identify all trees expressing low growth levels. Likewise, sole consideration of internode length is insufficient to identify all trees expressing low secondary growth. On the other hand, the presence or absence of epicormic branches on the main stem seems to be a very good indicator of low growth levels for trees or parts of trees, e.g. lateral branches. It would therefore be most useful in the description of forest structure and particularly in diagnosing tree growth potentials in the early stages of development.
To conclude, it seems necessary to describe epicormic branch formation relative to architectural development. Based on the present results and observations, we may order the different structural classes observed and propose possible patterns of development for D. guianensis. Because several classes were seldom observed (rarity of classes with an oblique orientation of the main stem, ***1: 10 %; improbable combinations between presence of a dead submittal part and absence of epicormic shoots: 2 %), we ignore them and focus on the most frequently observed structural classes for D. guianensis (0000, 0100, 0110, 1000, 1100, 1110; 88 % of the trees described, Table 1). Three possible patterns of development for D. guianensis are presented in Fig. 6: trees reaching the canopy (A), and trees surviving and dying in the understorey (B and C).
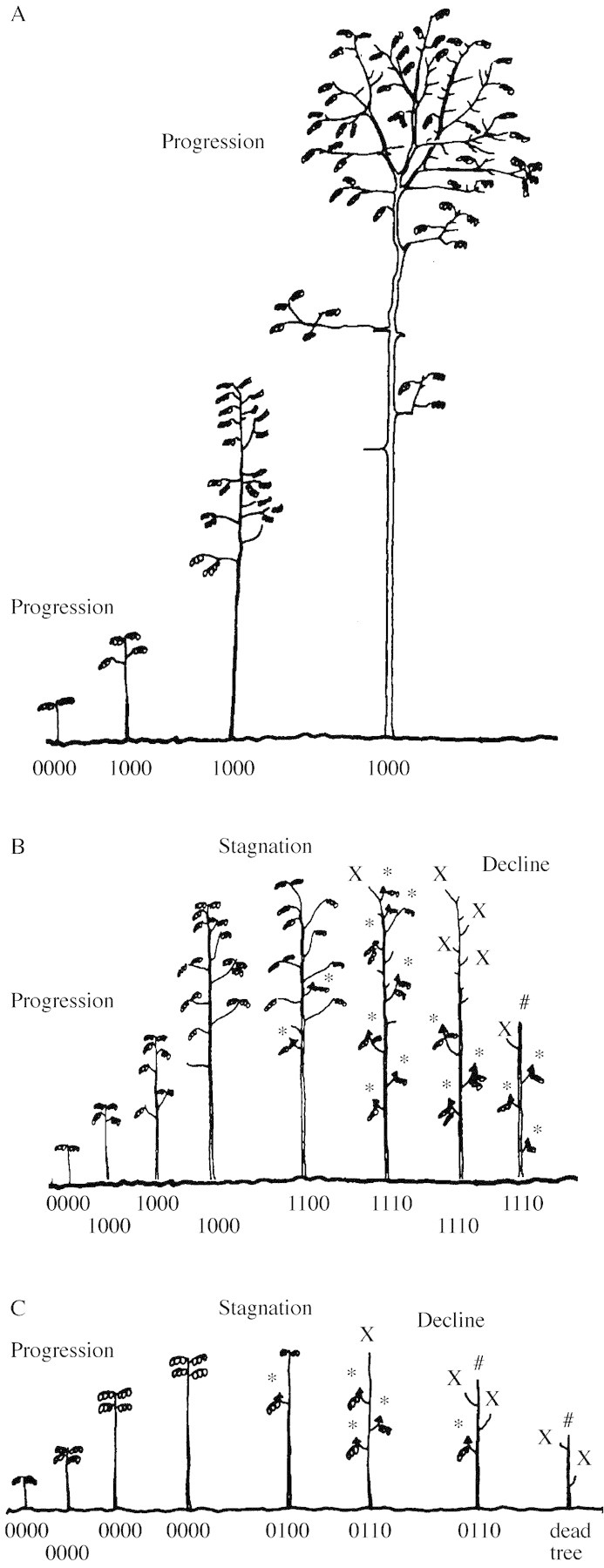
Fig. 6. Development patterns for Dicorynia guianensis. A, Young branched tree reaching the canopy level; B and C, young branched (B) and unbranched (C) trees dying in the understorey. A structural code (see Fig. 1) is associated with each development stage. x, Dead apical meristem; #, broken dried main axis; *, epicormic branch.
ACKNOWLEDGEMENTS
We thank Caroline Loup, Catherine Coutant, Javier Grosfeld and Thierry Claude for their help with the tree measurements; Sylvie Gourlet‐Fleury for coordinating the GIP‐ECOFOR project (GIP‐ECOFOR project no. 99·09); and Cirad‐forêt for managing the Paracou silvicultural facility and the basralocus tree inventory.
Supplementary Material
Received: 23 December 2002; Returned for revision: 21 February 2003; Accepted: 28 March 2003
References
- BachelardEP.1969. Studies on the formation of epicormic shoots on eucalypt stem segments. Australian Journal of Biological Sciences 22: 1291–1296. [Google Scholar]
- BaralotoC.2001.Tradeoffs between neotropical tree seedling traits and performance in contrasting environments. PhD Thesis, University of Michigan, USA. [Google Scholar]
- BarthélémyD, Caraglio Y, Costes E.1997. Architecture, gradients morphogénétiques et âge physiologique chez les végétaux. In: Bouchon J, Reffye de P and Barthelemy D, eds. Modélisation de l’architecture des végétaux Paris: INRA édition, 89–136. [Google Scholar]
- BarthélémyD, Sabatier S, Pascal O.1995. Le développement architectural du noyer commun Juglans regia L. (Juglandaceae). Forêt‐Entreprise 103: 61–68. [Google Scholar]
- BarthèsB.1991. Influence des caractères pédologiques sur la répartition spatiale de deux espèces du genre Eperua (Caesalpiniaceae) en forêt Guyanaise. Bois et Forêts des Tropiques 46: 303–320. [Google Scholar]
- BatzerHO.1973. Defoliation by the spruce budworm stimulates epicormic shoots on balsam fir. Environmental Entomology 2: 727–728. [Google Scholar]
- BlaiseF, Barczi JF, Jaeger M, Dinouard P, Reffye de Ph.1998. Simulation of the growth of plants. Modeling of metamorphosis and spatial interactions in the architecture and development of plants. In: Kunii TL, Luciani A, eds. Cyberworlds Tokyo: Springer Verlag, 81–109. [Google Scholar]
- BlumBM.1963. Excessive exposure stimulates epicormic branching in young northern hardwoods. USDA Forest Service, Research Note NE‐9. [Google Scholar]
- BoyceSG.1962. Selecting white oaks as parent stock for growing high quality logs. Proceedings of the 3rd Central States Forest Tree Improvement Conference, Purdue University, Lafayette, Indiana, USA. [Google Scholar]
- BormannFH.1965. Changes in growth pattern of white pine trees undergoing suppression. Ecology 46: 269–277. [Google Scholar]
- BrinkmanKA.1955. Epicormic branching on oaks in sprout stands. Central States Forest Experimental Station Technical Bulletin 146. [Google Scholar]
- BrunerMH.1964. Epicormic sprouting on released Yellow‐Poplar. Forestry 62: 754–755. [Google Scholar]
- BüsgenM, Münch E.1929. The structure and life of forest trees. London: Chapman and Hall, Ltd (translated from German). [Google Scholar]
- ChansonB, Nicolini E.2000. Les relations entre la croissance primaire et la croissance secondaire: antagonisme ou complémentarité des méristèmes dans le plan d’organisation des arbres. In: Labrecque M, ed. L’arbre 2000 – The tree Montréal: Isabelle Quentin Editeur, 71–79. [Google Scholar]
- CosensRD.1952. Epicormic branching on pruned white fir. Forestry 50: 939–940. [Google Scholar]
- CourraudR.1987. Les gourmands sur les chênes ‘rouvres’ et ‘pédonculés’. Forêt Entreprise 45: 20–33. [Google Scholar]
- DaleME, Sonderman DL.1984. Effect of thinning on growth and potential quality of young white oak crop trees. USDA Forest Service, Research Note NE‐539. [Google Scholar]
- DrénouC.1994.Approche architecturale de la sénescence des arbres. Le cas de quelques angiospermes tempérées et tropicales. PhD Thesis, University of Montpellier II, France. [Google Scholar]
- EvansJ.1982. Tree growth and control of epicormics. Proceedings: Broadleaves in Britain – future management and research Loughborough: Institute of Chartered Foresters, 183–190. [Google Scholar]
- EvansJ.1983. Le contrôle des gourmands. Etat actuel des recherches en Grande‐Bretagne. Revue Forestière Française 35: 369–375. [Google Scholar]
- EvansJ.1987. The control of epicormic branches. In: Patch D, ed. Advances in practical arboriculture Forestry Commission bulletin 65 London: HMSO, 115–120. [Google Scholar]
- FavrichonV.1995.Modèle matriciel déterministe en temps discret. Application à l’étude de la dynamique d’un peuplement forestier tropical humide (Guyane Française). PhD Thesis, University Claude Bernard (Lyon I), France. [Google Scholar]
- GrosfeldJ, Barthélémy D, Brion C.1999. Architectural variations of Araucaria araucana (Molina) K. Koch (Araucaruaceae) in its natural habitat. In: Kurmann MH, Hemsley AR, eds. The evolution of plant architecture. London: Royal Botanic Gardens, Kew. [Google Scholar]
- HalléF, Martin R.1968. Étude de la croissance rythmique chez l’Hévéa (Hevea brasiliensis Mull. Arg.). Adansonia 8: 475–503. [Google Scholar]
- HedlundA.1964. Epicormic branching in North Louisiana delta. USDA Forest Service, Research Note SO‐8. [Google Scholar]
- HibbsDE, Emmingham WH, Bondi MC.1989. Thinning red alder: effects of method and spacing. Forest Science 35: 16–29. [Google Scholar]
- HolmsgardE.1985. Self pruning and formation of epicormic shoots during twenty‐five years in a non‐thinned and two thinned plots of a thinning experiment in beech. Det forstlige Forsogsvaesen 40: 3–51. [Google Scholar]
- HoullierF, Leban JM.1991.Modèle théorique de croissance des arbres en peuplement équien et monospécifique. Nancy: INRA‐ Station de Recherches sur la Qualité des Bois‐ENGREF (Internal Document). [Google Scholar]
- HucR, Ferhi A, Guehl JM.1984. Pioneer and late stage tropical rainforest tree species (French Guiana) growing under common conditions differ in leaf gas exchange regulation, carbon isotope discrimination and leaf water potential. Oecologia 99: 297–305. [DOI] [PubMed] [Google Scholar]
- HuppuchCD.1961. Epicormic branching on sycamore. USDA Forest Service, Research Note SO‐166. [Google Scholar]
- JemisonGM, Schumacher FX.1948. Epicormic branching in old‐growth Appalachian hardwoods. Forestry 46: 252–255. [Google Scholar]
- KarzarjanVO.1969. Le vieillissement des plantes supérieures. In: Navka, eds, Moscou. Traduction française Riedacker, A. Nancy: Centre National de Recherches Forestières. [Google Scholar]
- KormanikP, Brown CL.1969. Origin and development of epicormic branches in sweetgum. USDA Forest Service, Research Note SE‐54. [Google Scholar]
- KozlowskyTT.1971.Growth and development in trees. Vols 1 and 2. London: Academic Press. [Google Scholar]
- KrajicekJE.1959. Epicormic branching in even‐aged, undisturbed white oak stands. Forestry 57: 372–373. [Google Scholar]
- KramerPJ, Kozlowsky TT.1979.Physiology of woody plants. London: Academic Press. [Google Scholar]
- LanierL.1986.Précis de sylviculture. Nancy: Ecole Nationale des Eaux et Forêts. [Google Scholar]
- MolinoJF, Sabatier D.1999.Liste des taxa et morphotaxa arborescents identifiés sur le site expérimental de Paracou. Montpellier: Institut de Recherche et Développement. [Google Scholar]
- NicoliniE.1997.Approche morphologique du développement du hêtre(Fagus sylvatica L.). PhD Thesis, University of Montpellier II, France. [Google Scholar]
- NicoliniE, Caraglio Y.1994. L’influence de divers caractères architecturaux sur l’apparition de la fourche chez Fagus sylvatica L. en fonction de l’absence ou de la présence d’un couvert. Canadian Journal of Botany 72: 1723–1734. [Google Scholar]
- NicoliniE, Barthélémy D, Heuret P.2000. Influence de la densité du couvert forestier sur le développement architectural de jeunes chênes sessiles, Quercus petraea (Matt.) Liebl. (Fagaceae), en régénération forestière. Canadian Journal of Botany 78: 1531–1544. [Google Scholar]
- NicoliniE, Chanson B, Bonne F.2001. Stem growth and epicormic branch formation in understorey beech trees (Fagus sylvatica L.). Annals of Botany 87: 737–750. [Google Scholar]
- PerrinH.1952.Sylviculture. Vol. 1. Nancy: Ecole Nationale des Eaux et Forêts. [Google Scholar]
- RohmederE.1935. Relation between classification of tree classes and the development of watersprouts in young oak stands. USDA Forest Service translation 276. [Google Scholar]
- RoloffA.1989.Kronenentwicklung und Vitalitätsbeurteilung ausgewählter Baumarten der gemäßigten Breiten. Frankfurt am Main: J.D. Sauerländers Verlag. [Google Scholar]
- RousselJ.1978. Lumière, gourmands et rejets de souche. Revue Forestière Française 30: 186–200. [Google Scholar]
- SokalRR, Rohlf CJF.1995.Biometry. New York: W.H. Freeman Co. [Google Scholar]
- SondermanDL.1985. Stand density – A factor affecting stem quality of young hardwoods. USDA Forest Service, Northeastern Forest Experiment Station, Research Note NE‐561, 1–8. [Google Scholar]
- SternRC.1971. Pruning free‐grown hardwoods. Quarterly Journal of Forestry 65: 1–5. [Google Scholar]
- VogtAR, Cox GS.1970. Evidence for the hormonal control of stump sprouting by oak. Forest Science 16: 165–171. [Google Scholar]
- WalhenbergWG.1950. Epicormic branching of young yellow poplar. Journal of Forestry 48: 417–419. [Google Scholar]
- WardWW.1966. Epicormic branching of black and white oak. Forest Science 12: 290–296. [Google Scholar]
- WignallTA, Browning G.1988. The effects of stand thinning and artificial shading on epicormic bud emergence in pedunculate oak (Quercus robur L.). Forestry 61: 45–59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.