Abstract
Seed production and patterns of sex allocation were studied in female and hermaphroditic plants in two gynodioecious populations of Geranium sylvaticum (Geraniaceae). Females produced more flower buds and seeds than hermaphrodites in one of the two study populations. The other female traits measured (pistil biomass, seed number per fruit, individual seed mass) did not differ between the gender morphs. The relative seed fitness of hermaphrodites differed between the study populations, with hermaphrodites gaining less of their fitness through female function in the population with a high frequency of females. However, the amount and size of pollen produced by hermaphrodites did not differ between populations. The number of flower buds was positively correlated with seed production in females, whereas in hermaphrodites a positive correlation between number of buds and seed production was found in only one of the two study populations. These results suggest that fitness gain through female function is labile in hermaphrodites of this species, and is probably affected by environmental factors such as the sex ratio of the population.
Key words: Geranium sylvaticum, Geraniaceae, gynodioecy, sex allocation, pollen production
INTRODUCTION
Most angiosperms are hermaphrodites and achieve their fitness on average equally through male and female function (Lloyd and Bawa, 1984). Only a small proportion of angiosperm species are gynodioecious, i.e. female and hermaphroditic individuals co‐occur in the same populations (Lloyd and Bawa, 1984). Female and hermaphroditic individuals in gynodioecious species may differ in size (Eckhart, 1992; Williams and Fenster, 1998), and there are often differences in flower morphology between the genders. Pistillate flowers are usually smaller than perfect flowers (Vaarama and Jääskeläinen, 1967; Kohn, 1988; Ågren and Willson, 1991; Ashman and Stanton, 1991; Williams et al., 2000; Vaughton and Ramsey, 2002) and produce nectar with lower sugar concentration (Ashman and Stanton, 1991). Dissimilarities between the gender morphs may, in turn, lead to differences in resource allocation between vegetative and reproductive parts.
The genders of a gynodioecious species gain their fitness in different ways, females through ovules and hermaphrodites through both ovules and pollen. This difference in allocation pattern affects the relative fitness and performance of the gender morphs in a population. Fundamentally, females have to compensate for the lack of male function to be maintained in a population, otherwise they transmit their genes only half as frequently as hermaphrodites (Charlesworth and Charlesworth, 1978). Females may gain additional advantage by avoiding inbreeding depression because their flowers are obligatorily outcrossed (Charlesworth and Charlesworth, 1978), thus they may produce seedlings of higher quality compared with those of the hermaphrodites (Shykoff, 1988; Ashman, 1992; Wolfe and Shmida, 1997). Females may also achieve higher fitness by producing more seeds if resources not used for pollen production are allocated to female function (Darwin, 1877; Atlan et al., 1992; Ashman, 1994). Indeed, females are reported to produce more seeds than hermaphrodites in several gynodioecious species (Shykoff, 1988; Kohn, 1989; Delph, 1990; Ågren and Willson, 1991; Delph and Lloyd, 1991; Eckhart, 1992; Klinkhamer et al., 1994; Koelewijn, 1996; Sakai et al., 1997; Williams et al., 2000). However, this has not been observed in all species studied, and the species used in this study, Geranium sylvaticum, has been cited as an example of a case in which females do not produce more seeds than hermaphrodites (Vaarama and Jääskeläinen, 1967).
Females of gynodioecious species are usually constant in their sex expression, whereas hermaphrodites are more labile (Delph and Lloyd, 1991). Hermaphrodites can modify their reproductive allocation by investing varying amounts of resources between seed and pollen production, hence functioning sometimes more as males and sometimes more as females (Delph and Lloyd, 1991; Atlan et al., 1992). Sex allocation theory predicts a trade‐off between male and female function in hermaphrodites (Charnov, 1982), which affects their sex expression and reproductive output. Hermaphrodites are presumed to allocate more resources to the sex function that produces higher fitness gain (Charnov, 1982), i.e. allocation to pollen production should be high if fitness gain through seed production is low.
In this study we focused on the differential sex allocation patterns of female and hermaphroditic individuals of the gynodioecious Geranium sylvaticum. Using two populations that are closely situated but differ in habitat and sex ratio, three specific questions were addressed. First, are there differences in seed production and biomass allocation to female function between the genders? Secondly, is there a difference in pollen production in hermaphrodites between the populations? Thirdly, is there a difference in flower bud production between the genders, and how is flower bud production related to reproductive output in females and hermaphrodites?
MATERIALS AND METHODS
Study species
Geranium sylvaticum is a gynodioecious, self‐compatible (Vaarama and Jääskeläinen, 1967), common perennial herb that occurs in deciduous forests, moist meadows and roadsides in most parts of Europe. Populations of G. sylvaticum may contain hermaphroditic plants with ten functional stamens per flower, female plants with rudimentary stamens or intermediate plants with one to nine functional stamens in their flowers (Vaarama and Jääskeläinen, 1967). Plants with several types of flowers also occur, i.e. there may be intermediate and female flowers on the same individual (S. Ramula, pers. obs.). Female frequency varies from 0·4 to 27·2 % among populations in Finland (Asikainen and Mutikainen, 2003). A flowering plant consists of a rosette of hairy basal leaves with long petioles, and from one to several flowering shoots, up to 50 cm tall, each bearing a dichasium of several to many flowers. Flower buds are formed in the year before flowering (Ågren and Willson, 1994). In the study area, G. sylvaticum plants flower in June and July for approx. 3 weeks; the colour of flowers varies from purple to white. Fruits are 5‐carpellate and seeds are dispersed through explosive dehiscence 3–4 weeks after pollination. Fruits may contain up to five seeds (Ågren and Willson, 1994), although there are ten ovules per flower (P. Mutikainen, pers. obs.). Perfect flowers are protandrous, hence the stigmas open after anthesis, which reduces the probability of autogamous self‐pollination. Flowers are visited by generalist bees, bumblebees and syrphid flies (S. Ramula, pers. obs.).
Study populations
The study was performed in two open‐pollinated G. sylvaticum populations on Seili island, SW Finland (60°N, 22°E) during summer 2000. Population 1 occurs in a mixed forest patch and consists of 1120 individuals, whereas population 2 is situated in a meadow next to a forest edge and consists of 703 individuals; the proportions of females are 23·0 and 9·2 %, respectively. The populations are situated approx. 300 m apart and are separated by a dense conifer forest. Herbaceous vegetation in both habitats is quite similar consisting of, for example, Anthriscus, Phleum and Urtica species. There was a slight phenological difference between the populations, with population 1 starting to flower in the first week of June, whereas population 2 began flowering a week later. Seeds also ripened 1 week later in July in population 2 compared with population 1. As a result of the forest barrier and the difference in flowering phenology, gene flow between the populations is likely to be limited. However, the populations may be considered as subpopulations of one G. sylvaticum metapopulation on Seili island because of their close location.
Female allocation
To measure biomass allocation to female function, a total of 20 female and 40 hermaphroditic plants per population was randomly selected and marked when the first flower was opening (in the first and second weeks in June for populations 1 and 2, respectively). Thus, all of the marked individuals were at the same phenological stage at the beginning of the experiment. At the same time, the number of flower buds was recorded to be used later as a covariate in statistical analyses to exclude the effect of plant size. The selected plants consisted of one to six flowering shoots.
For each of 20 female and 20 hermaphroditic marked plants, the first three flowers to open were collected. The flowers were bagged before collection to prevent visitation by pollinators. On females, flowers were collected when their styles had elongated but before the five stigma lobes had opened. On hermaphroditic plants, flowers were collected when they were at anthesis. Bags were removed after flower collection, hence they did not disturb pollination of other flowers on the plant. The pistils were separated from the flowers in the laboratory and were dried at 65 °C for 48 h and weighed to the nearest 0·01 mg. The mean of the three pistils of each individual was used in the statistical analyses.
All marked female plants and 20 of the marked hermaphroditic plants were bagged after flowering and pollination to measure total seed production. The hermaphroditic plants used differed to those used to measure pistil biomass. Before bagging, the first three opened flowers on each hermaphroditic plant were removed so that they had been treated the same as female plants. Three randomly selected fruits were subsequently collected from each bagged plant, and the average seed number per fruit was calculated for both genders. The fruits were collected in July, approx. 4 weeks after flowering, when the seeds had ripened. Seeds were dried at room temperature (20 °C), counted, and weighed to the nearest 0·01 mg. The average mass of an individual seed was determined by dividing the total seed mass of undamaged seeds by the number of undamaged seeds produced per plant. Seeds damaged by seed predators were excluded when analysing individual seed mass.
Differences in female allocation between the genders were determined by comparing seed production of females with that of hermaphrodites. Seed number per fruit, individual seed mass and pistil biomass were also compared between the gender morphs. Furthermore, to study the female investment of hermaphrodites in relation to that of females, the relative seed fitness of hermaphrodites was calculated in both populations by dividing the average seed production of hermaphrodites by the average seed production of females. When the seed fitness of females and hermaphrodites is equal, the relative seed fitness of hermaphrodites equals one. The present estimate of relative seed fitness is based on seed production only because the performance of the resulting seedlings was not measured. However, this estimate gives a realistic picture of relative seed fitness because no difference in germination or juvenile survival has been observed between seedlings produced by female and hermaphroditic individuals of G. sylvaticum (Asikainen and Mutikainen, 2003).
Pollen production in hermaphrodites
To measure pollen production, the first three opened, bagged flowers were collected from each of 20 hermaphroditic plants per population. There were ten functional stamens in each flower collected. The hermaphroditic plants and flowers used for this purpose were the same as those used in the measurement of pistil biomass (see above). For each plant, ten anthers in total were collected from the three flowers; these anthers were immediately stored in 0·5 ml ethanol in an Eppendorf tube to release the pollen grains (Kearns and Inouye, 1993). The total amount of pollen produced by each plant was determined using a haemocytometer. The haemocytometer slide was filled with a subsample of pollen suspension (from all ten anthers) and the number of pollen grains per grid was counted under a microscope. The procedure was repeated three times for each pollen sample. Since the haemocytometer slide holds a standard quantity of liquid, it is possible to determine the total number of pollen grains in a known volume of pollen suspension (Kearns and Inouye, 1993). Therefore, the total number of pollen grains was estimated in the 0·5 ml pollen suspension, based on the three subsamples, to give the number of pollen grains per flower (i.e. ten anthers). That amount of pollen was then multiplied by the number of flower buds produced by a hermaphroditic plant to give the total number of pollen grains produced per plant. It was assumed that the number of flower buds in each plant equals the number of flowers each containing ten functional stamens. The size of pollen grains was measured under a compound microscope (magnification ×40) by measuring the diameter of three pollen grains per plant to the nearest micrometre. The means of the three replicates for amount and size of pollen per plant were used in the statistical analyses.
Flower bud production and reproductive output
The difference in flower bud production between genders was studied. Furthermore, the relationship between the number of flower buds and reproductive output was studied separately for each gender in both populations. Correlations were calculated between the number of flower buds and amount of seed produced for both genders, and between flower buds and pollen production for hermaphrodites.
Data analyses
The effects of gender, population and their interaction on pistil biomass, total seed production, seed number per fruit and individual seed mass were analysed with mixed‐model two‐way analyses of covariance (ANCOVAs) using the number of flower buds as a covariate to exclude the effect of plant size. Gender was treated as a fixed factor and population as a random factor. The population × gender interaction was used as the error term for gender (Zar, 1984). Total seed production was square‐root transformed to normalize the distributions of residuals. Owing to the somewhat heterogeneous variances after the transformation, non‐parametric Wilcoxon tests were performed by using population and gender separately as a class factor; however, these results were qualitatively identical to the results of parametric ANCOVA. We preferred the parametric ANCOVA because it was possible to include the population × gender interaction in the model; therefore, only the results of the ANCOVA are presented here. Since the population × gender interaction was significant for total seed production, pairwise comparisons between the genders were performed separately for the two populations using two‐tailed t‐tests.
One‐way ANCOVAs were run to examine differences in pollen production of hermaphrodites between the populations. Size and number of pollen grains were used as dependent variables, population as an independent variable, and the number of flower buds as a covariate. Pollen grain size was square‐root transformed and the number of pollen grains was natural‐log transformed to normalize the distributions.
A mixed‐model two‐way ANOVA was conducted to test for differences in the number of flower buds between the genders and populations. Gender was treated as a fixed and population as a random factor. Population × gender interaction was used as the error term for gender (Zar, 1984). The number of flower buds was natural‐log transformed.
Type III sums of squares were used in all ANCOVAs and ANOVA. Normality of the data was checked with Shapiro–Wilks’ test, and homogeneity of the variances with Levene’s test. The number of flower buds was used as a covariate in all ANCOVAs to exclude the effect of plant size. Possible interactions between the covariate and main factors were studied for each model; because none of the covariate × main factor interactions was significant, they were removed and the number of flower buds was included as a covariate.
To study the relationship between the number of flower buds and reproductive output, correlation coefficients were calculated. Due to the heterogeneous data (F‐test), Spearman’s rank correlations were calculated between the number of flower buds and seed production separately for females and hermaphrodites in both populations. In addition, Spearman’s correlations were calculated between the number of flower buds and pollen production for hermaphrodites in both populations. The significance of the correlations was adjusted by sequential Bonferroni corrections (Rice, 1989) treating the correlations of the two populations as two separate data sets.
All data were analysed using the SAS Statistical Package (SAS 8.1). In the figures and tables, all means and standard errors are presented in the untransformed scale. In both populations, one female plant died before the seeds were ripe, thus these females were included in the analysis of pistil biomass only.
RESULTS
Female allocation
Plants in population 1 produced heavier pistils than plants in population 2 (F1,75 = 6·10, P = 0·0158). Pistil biomass did not differ between the genders (F1,75 = 17·02, P = 0·1514) (Table 1).
Table 1.
Reproductive allocation for females and hermaphrodites in two Geranium sylvaticum populations
| Population 1 | Population 2 | |||
| Females | Hermaphrodites | Females | Hermaphrodites | |
| Pistil biomass (mg) | 1·6 (0·09, 20) | 1·7 (0·09, 20) | 1·3 (0·09, 20) | 1·4 (0·10, 20) |
| Seeds per fruit | 3·4 (0·23, 17) | 3·2 (0·23, 20) | 3·8 (0·21, 19) | 3·4 (0·25, 18) |
| Seed mass (mg) | 4·5 (0·17, 19) | 4·4 (0·21, 20) | 4·7 (0·21, 19) | 4·6 (0·18, 18) |
| Pollen grain size (µm) | – | 32·7 (0·37, 20) | – | 33·1 (0·30, 20) |
| Pollen number per plant | – | 241 369 (78 632, 20) | – | 215 066 (25 256, 20) |
Values are plant size‐adjusted least‐square means; standard error and number of replicates are given in parentheses.
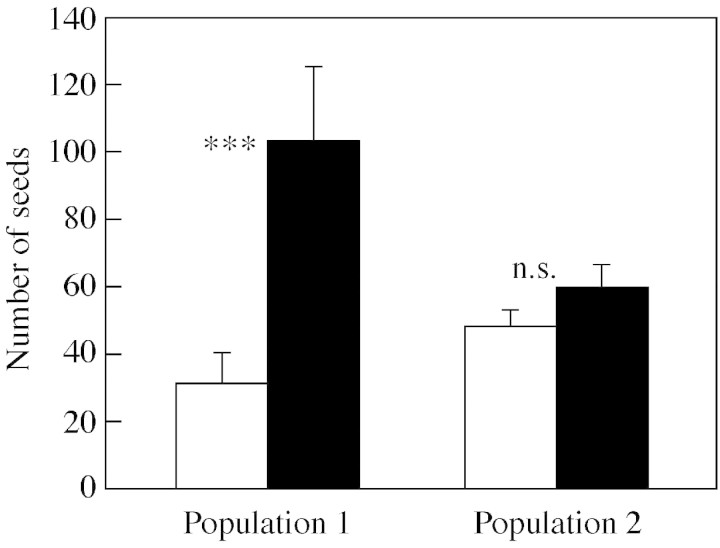
There was no difference in total seed production between the populations (F1,73 = 0·70, P = 0·407) or between the genders (F1,73 = 2·73, P = 0·347), but the population × gender interaction was significant (F1,73 = 7·94, P = 0·006) (Fig. 1). When plant size was controlled for, females produced 3·3 times more seeds than hermaphrodites in population 1 (Fig. 1). In population 2, the seed production of females was only 1·2 times higher than that of hermaphrodites (Fig. 1). The relative seed fitness of hermaphrodites was more than 2·5 times lower in population 1 than in population 2 (0·31 and 0·81, respectively). Two hermaphroditic plants in population 2 failed to produce any seeds although they produced flower buds normally; these plants were also included in the analyses. Seed number per fruit and individual seed mass did not differ between the populations (F1,69 = 1·10, P = 0·299 and F1,71 = 0·81, P = 0·372, respectively) or between the genders (F1,69 = 8·25, P = 0·213 and F1,71 = 4·84, P = 0·272, respectively) (Table 1).
Fig. 1. Total seed production of hermaphroditic (unshaded bars) and female (shaded bars) Geranium sylvaticum individuals in two populations (plant size‐adjusted least‐square mean plus s.e.). *** Significant difference between females and hermaphrodites at P < 0·0001; n.s., P > 0·05 using a two‐tailed t‐test.
Male allocation
There was no difference in pollen grain size and pollen production of hermaphroditic plants between the populations (F1,37 = 1·32, P = 0·258 and F1,37 = 0·91, P = 0·347, respectively) (Table 1).
Flower bud production and reproductive output
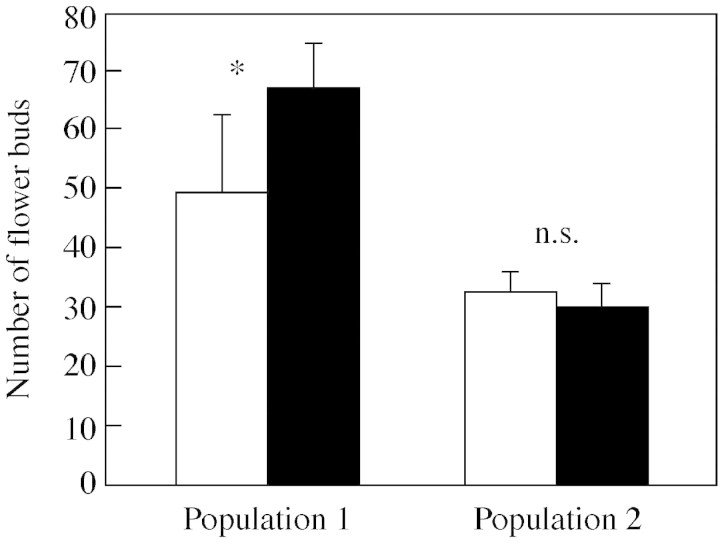
The number of flower buds did not differ significantly between the genders (F1,76 = 0·19, P = 0·738), but plants in population 1 produced more flower buds than plants in population 2 (F1,76 = 14·30, P = 0·0003). There was also a significant population × gender interaction (F1,76 = 5·73, P = 0·019): females produced more flower buds than hermaphrodites in population 1, whereas in population 2 the difference was not significant (Fig. 2).
Fig. 2. Number of flower buds of hermaphroditic (unshaded bars) and female (shaded bars) Geranium sylvaticum individuals in two populations (least‐square mean plus s.e.). * Significant difference between females and hermaphrodites at P < 0·05; n.s., P > 0·05 using a two‐tailed t‐test.
In females, the number of flower buds and seed production correlated positively in both populations, whereas in hermaphrodites the number of flower buds correlated positively with seed production only in population 2 (Table 2). There was no correlation between the number of flower buds and pollen production of hermaphrodites in population 2. In population 1, there was a weak positive correlation between the number of flower buds and pollen production; however, this trend disappeared after sequential Bonferroni correction (Table 2).
Table 2.
Correlations between flower bud production and reproductive output for females and hermaphrodites in two G. sylvaticum populations
| Population 1 | Population 2 | |||||
| r s | P | n | r s | P | n | |
| Females | ||||||
| Seed production | 0·73 | 0·0004* | 19 | 0·78 | <0·0001* | 19 |
| Hermaphrodites | ||||||
| Seed production | 0·24 | 0·3015 | 20 | 0·55 | 0·0113* | 20 |
| Pollen production | 0·42 | 0·0593 | 20 | 0·03 | 0·8945 | 20 |
Spearman’s correlation coefficients that remain significant (<0·05) after sequential Bonferroni corrections are indicated with an asterisk.
DISCUSSION
The aim of this study was to examine sex allocation patterns in female and hermaphroditic individuals of the gyno dioecious Geranium sylvaticum. According to our results, the genders allocated different amounts of resources to female function depending on the population, i.e. there was a significant population × gender interaction for seed production. The number of flower buds correlated positively with seed production in females, but in hermaphrodites the relationship between flower buds and reproductive output was labile. The amount and size of pollen produced by hermaphrodites did not differ between the populations.
Sex allocation
On average, total seed production was similar in the two study populations but was distributed differently between the genders. Females produced significantly more seed in population 1, where the seed production of females was 3·3 times higher than that of hermaphrodites. In population 2, the genders produced almost equal numbers of seeds. Theoretical models presume a seed production advantage for females, and depending on the mode of sex determination, the advantage needed for the maintenance of females in a population varies from slight to two‐fold (e.g. Gouyon and Couvet, 1987). The present results suggest that the increased seed production by females may contribute significantly to their maintenance in some G. sylvaticum populations. However, in accordance with previous studies (Asikainen and Mutikainen, 2003), the present results also indicate that among‐population variation in relative seed production is significant and thus suggest that the relative roles of seed fitness, inbreeding depression and ecological factors in the maintenance of females are likely to vary significantly among populations of G. sylvaticum.
We propose three potential explanations for the significant interaction observed between population and gender for seed production in the present study. First, pollen limitation may have decreased the seed production of females in population 2. Generally, seed production decreases more in females than hermaphrodites under pollen limitation (e.g. Maurice and Fleming, 1995). Nevertheless, due to the high frequency of hermaphrodites in population 2, pollen limitation seems to be an unlikely explanation for the population × gender interaction observed in seed production.
Secondly, differences in habitat quality between the populations might explain the differences in seed production between the genders. Habitat quality has been shown to have a significant impact on reproductive investment in plants (e.g. Zimmerman and Lechowicz, 1982; Eckhart and Chapin, 1997; Meyer, 2000; Stanton et al., 2000). In gynodioecious species, females (Delph, 1990; Wolfe and Shmida, 1997; Ashman, 1999) or hermaphrodites (Sakai and Weller, 1991) have been observed to be more successful in harsher environments; other studies report no differences between the genders (Kohn, 1989). To our knowledge, gender‐specific habitat preferences have not been studied in G. sylvaticum. Since the study populations were closely situated and the herbaceous vegetation was quite similar in both populations, the differences in gender‐specific seed production patterns between the populations were probably not related to habitat quality.
The third possible explanation for the significant interaction between population and gender in seed production is based on the difference in population sex ratio. According to Lloyd and Bawa (1984), the characteristics of other plants in a population may affect fitness gains of hermaphrodites through seed and pollen production, which may lead to sex modification in hermaphrodites because of frequency‐dependent selection. When female frequency is high in a population, sex allocation in hermaphrodites is proposed to evolve towards the male function because hermaphrodites receive higher fitness through acting as pollen donors than through producing seeds (Lloyd, 1976). In the present study, the relative seed fitness of hermaphrodites was remarkably lower in population 1 than in population 2, suggesting that hermaphrodites achieved less of their fitness through female function in the population with high female frequency compared with the population with low female frequency. This may indicate that the reproductive allocation and fitness of hermaphrodites is affected by the population sex ratio. This suggestion is further supported by the fact that the seed fitness of hermaphrodites correlates negatively with the frequency of females in G. sylvaticum populations (Asikainen and Mutikainen, 2003), as is also the case in four other gynodioecious species (Delph, 1990; Wolfe and Shmida, 1997; Ashman, 1999; Delph and Carroll, 2001). In accordance with the sex allocation theory (Charnov, 1982), a higher allocation to male function in hermaph roditic plants could have been expected in population 1, where the relative seed fitness of hermaphrodites was low. However, we did not find any difference in pollen production between the populations. Thus, differences in population sex ratio did not affect the amount of male allocation in hermaphrodites at the population level. Nevertheless, the present estimate of pollen production is based on the three first flowers of each plant only, and therefore pollen production may be estimated incorrectly if it varies in time within plants.
Females and hermaphrodites of G. sylvaticum produced an equal number of seeds per fruit. In contrast to the results of an earlier study by Vaarama and Jääskeläinen (1967) in which hermaphrodites produced more and bigger seeds than females, individual seed mass in the present study was very similar in both genders. This conflicting result may be explained by the rather small sample size at the population level or by the fact that Vaarama and Jääskeläinen (1967) measured seed length and breadth, not seed biomass. However, Asikainen and Mutikainen (2003) found that seed mass varied significantly among populations and years but not between the genders in G. sylvaticum.
Flower bud production and reproductive output
Plants in population 1 produced more flower buds compared with plants in population 2. There was no significant difference between the genders in the number of flower buds produced, but female plants produced more flower buds in population 1. Several other studies have reported no difference between female and hermaphroditic plants in the number of flower buds or flowers produced (Ågren and Willson, 1991; Klinkhamer et al., 1994; Sakai et al., 1997; Wolfe and Shmida, 1997; Barrett et al., 1999), although this is not always the case (Maki, 1996; Vaughton and Ramsey, 2002).
In both populations a strong positive correlation was observed between seed production and the number of flower buds produced by female plants. In hermaphrodites, a significant positive correlation was noted between the number of flower buds and seed production only in population 2. There was no statistically significant correlation between the number of flower buds and pollen production in hermaphrodites, although the positive correlation coefficient was relatively high in population 1. It might still be beneficial for hermaphrodites to produce many flowers, even if not all of the flowers produce seeds, because hermaphrodites may increase their male fitness through pollen donation (Sutherland, 1987) since the number of pollinator visits increases with the number of flowers (e.g. Willson and Price, 1977; Strauss et al., 1996). Unfortunately, it was not possible to test for a trade‐off between male and female allocation in hermaphroditic plants at the individual level because seed and pollen production were not measured using the same hermaphroditic individuals.
To conclude, significant differences were observed in sex allocation within and between the gender morphs and between the two closely situated populations of G. sylvaticum. The present results suggest that fitness gain through female function in hermaphrodites in this gynodioecious species is labile, and they show that it may vary over a limited spatial scale.
ACKNOWLEDGEMENTS
We thank Eija Asikainen for her cooperation. This study was funded by the Academy of Finland.
Supplementary Material
Received: 9 December 2002; ; Returned for revision: 7 March 2003. Accepted: 25 April 2003 Published electronically: 18 June 2003
References
- ÅgrenJ, Willson MF.1991. Gender variation and sexual differences in reproductive characters and seed production in gynodioecious Geranium maculatum American Journal of Botany 78: 470–480. [Google Scholar]
- ÅgrenJ, Willson MF.1994. Cost of seed production in the perennial herbs Geranium maculatum and G. sylvaticum: an experimental field study. Oikos 70: 35–42. [Google Scholar]
- AshmanT‐L.1992. The relative importance of inbreeding and maternal sex in determining fitness in Sidalcea oregana ssp. spicata, a gynodioecious plant. Evolution 46: 1862–1874. [DOI] [PubMed] [Google Scholar]
- AshmanT‐L.1994. Reproductive allocation in hermaphrodite and female plants of Sidalcea oregana ssp. spicata (Malvaceae) using four currencies. American Journal of Botany 81: 433–438. [Google Scholar]
- AshmanT‐L.1999. Determinants of sex allocation in gynodioecious wild strawberry: implications for the evolution of dioecy and sexual dimorphism. Journal of Evolutionary Biology 12: 648–661. [Google Scholar]
- AshmanT‐L, Stanton M.1991. Seasonal variation in pollination dynamics of sexually dimorphic Sidalcea oregana ssp. spicata (Malvaceae). Ecology 72: 993–1003. [Google Scholar]
- AsikainenE, Mutikainen P.2003. Female frequency and relative fitness of females and hermaphrodites in gynodioecious Geranium sylvaticum (Geraniaceae). American Journal of Botany 90: 224–232. [DOI] [PubMed] [Google Scholar]
- AtlanA, Gouyon P‐H, Fournial T, Pomente D, Couvet D.1992. Sex allocation in hermaphroditic plant: the case of gynodioecy in Thymus vulgaris L. Journal of Evolutionary Biology 5: 189–203. [Google Scholar]
- BarrettSCH, Case AL, Peters GB.1999. Gender modification and resource allocation in subdioecious Wurmbea dioica (Colchicaceae). Journal of Ecology 87: 123–137. [Google Scholar]
- CharlesworthB, Charlesworth D.1978. A model for the evolution of dioecy and gynodioecy. American Naturalist 112: 975–997. [Google Scholar]
- CharnovEL.1982.The theory of sex allocation. Princeton, NJ, USA: Princeton University Press. [Google Scholar]
- DarwinCR.1877.The different forms of flowers on plants of the same species. London: J. Murray. [Google Scholar]
- DelphLF.1990. Sex‐ratio variation in the gynodioecious shrub Hebe strictissima (Scrophulariaceae). Evolution 44: 134–142. [DOI] [PubMed] [Google Scholar]
- DelphLF,Carroll SB.2001. Factors affecting relative seed fitness and female frequency in a gynodioecious species (Silene acaulis). Evolutionary Ecology Research 3: 487–505. [Google Scholar]
- DelphLF,Lloyd DG.1991. Environmental and genetic control of gender in the dimorphic shrub Hebe subalpina Evolution 45: 1957–1964. [DOI] [PubMed] [Google Scholar]
- EckhartVM.1992. Resource compensation and the evolution of gynodioecy in Phacelia linearis (Hydrophyllaceae). Evolution 46: 1313–1328. [DOI] [PubMed] [Google Scholar]
- EckhartVM,Chapin FS.1997. Nutrient sensitivity of the cost of male function in gynodioecious Phacelia linearis (Hydrophyllaceae). American Journal of Botany 84: 1092–1098. [PubMed] [Google Scholar]
- GouyonP‐H,Couvet D.1987. A conflict between two sexes, females and hermaphrodites. In: Stearns SC, ed. The evolution of sex and its consequences Boston: Birhäuser Verlag Basel, 245–261. [DOI] [PubMed] [Google Scholar]
- KearnsCA, Inouye DW.1993.Techniques for pollination biologists. Boulder, CO: The University Press of Colorado, USA. [Google Scholar]
- KlinkhamerPGL, de Jong TJ, Nell HW.1994. Limiting factors for seed production and phenotypic gender in the gynodioecious species Echium vulgare (Boraginaceae). Oikos 71: 469–478. [Google Scholar]
- KoelewijnHP.1996. Sexual differences in reproductive characters in gynodioecious Plantago coronopus Oikos 75: 443–452. [Google Scholar]
- KohnJR.1988. Why be female? Nature 335: 431–433. [Google Scholar]
- KohnJR.1989. Sex ratio, seed production, biomass allocation, and the cost of male function in Cucurbita foetidissima HBK (Cucurbitaceae). Evolution 43: 1424–1434. [DOI] [PubMed] [Google Scholar]
- LloydDG.1976. The transmission of genes via pollen and ovules in gynodioecious angiosperms. Theoretical Population Biology 9: 299–316. [DOI] [PubMed] [Google Scholar]
- LloydDG, Bawa KS.1984. Modification of gender of seed plants in varying conditions. Evolutionary Biology 17: 255–338. [Google Scholar]
- MakiM.1996. Differences in plant size and flower production between hermaphrodites and females of two gynodioecious Chionographis (Liliaceae). Canadian Journal of Botany 74: 150–153. [Google Scholar]
- MauriceS, Fleming TH.1995. The effect of pollen limitation on plant reproductive systems and the maintenance of sexual polymorphisms. Oikos 74: 55–60. [Google Scholar]
- MeyerGA.2000. Interactive effects of soil fertility and herbivory on Brassica nigra Oikos 88: 433–441. [Google Scholar]
- RiceWR.1989. Analyzing tables of statistical tests. Evolution 43: 223–225. [DOI] [PubMed] [Google Scholar]
- SakaiAK, Weller SG.1991. Ecological aspects of sex expression in subdioecious Shiedea globosa (Caryophyllaceae). American Journal of Botany 78: 1280–1288. [Google Scholar]
- SakaiAK, Weller SG, Chen M‐L, Chou S‐Y, Tasanont C.1997. Evolution of gynodioecy and maintenance of females: the role of inbreeding depression, outcrossing rates, and resource allocation in Shiedea adamantis (Caryophyllaceae). Evolution 51: 724–736. [DOI] [PubMed] [Google Scholar]
- SAS Institute.1999.SAS/STAT user’s guide, version 8.1. Cary, North Carolina: SAS Institute. [Google Scholar]
- ShykoffJA.1988. Maintenance of gynodioecy in Silene acaulis (Caryophyllaceae): stage‐specific fecundity and viability selection. American Journal of Botany 75: 844–850. [Google Scholar]
- StantonML, Roy BA, Thiede DA.2000. Evolution in stressful environments. I Phenotypic variability, phenotypic selection, and response to selection in five distinct environmental stresses. Evolution 54: 93–111. [DOI] [PubMed] [Google Scholar]
- StraussSY, Conner JK, Rush SL.1996. Foliar herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. American Naturalist 147: 1098–1107. [Google Scholar]
- SutherlandS.1987. Why hermaphroditic plants produce many more flowers than fruits: experimental tests with Agave mckelveyana Evolution 41: 750–759. [DOI] [PubMed] [Google Scholar]
- VaaramaA, Jääskeläinen O.1967. Studies on gynodioecism in the Finnish populations of Geranium silvaticum L. Annales Academiae Scientiarum Fennicae. Series A. IV. Biologica 108: 1–39. [Google Scholar]
- VaughtonG, Ramsey M.2002. Evidence of gynodioecy and sex ratio variation in Wurmbea biglandulosa (Colchicaceae). Plant System atics and Evolution 232: 167–179. [Google Scholar]
- WilliamsHL, Fenster CB.1998. Ecological and genetic factors contributing to the low frequency of male sterility in Chamaecrista fasciculata (Fabaceae). American Journal of Botany 85: 1243–1250. [PubMed] [Google Scholar]
- WilliamsCF, Kuchenreuther MA, Drew A.2000. Floral dimorphism, pollination, and self‐fertilization in gynodioecious Geranium richardsonii (Geraniaceae). American Journal of Botany 87: 661–669. [PubMed] [Google Scholar]
- WillsonMF, Price PW.1977. The evolution of inflorescence size in Asclepias (Asclepiadaceae). Evolution 31: 495–511. [DOI] [PubMed] [Google Scholar]
- WolfeLM, Shmida A.1997. The ecology of sex expression in a gynodioecious Israeli desert shrub (Ochradenus baccatus). Ecology 78: 101–110. [Google Scholar]
- ZarJH.1984.Biostatistical analysis, 2nd edn. Englewood Cliffs, New Jersey, USA: Prentice‐Hall. [Google Scholar]
- ZimmermanJK, Lechowicz MJ.1982. Responses on moisture stress in male and female plants of Rumex acetosella L. (Polygonaceae). Oecologia 53: 305–309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.