Abstract
The organogenetic cycle of shoots on main branches of 4‐year‐old Juglans regia trees was studied. Mono‐ and bicyclic floriferous and vegetative annual shoots were analysed. Five parent annual shoot types were sampled between October 1992 and August 1993. Organogenesis of summer growth units was monitored between 16 Jun. and 3 Aug. 1993. Variations over time in the number of nodes, cataphylls and embryonic green leaves of terminal buds were studied. The number of nodes of parent shoot buds was compared with the number of nodes of shoots derived from parent shoot buds. The spring growth units of mono‐ and bicyclic shoots consist exclusively of preformed leaves which were differentiated, respectively, during the spring flush of growth (mid‐April until mid‐May) or the summer flush of growth (mid‐June until early August) in the previous growing season. Thus, winter buds may consist of flower and leaf primordia differentiated in two different periods during annual shoot extension. The summer growth units of bicyclic shoots consist of preformed leaves that were differentiated in spring buds during the spring flush of growth in the current growing season. Bud morphology is compared between spring and summer shoots.
Key words: Juglans regia L., Persian walnut tree, preformation, bud formation, leaf primordia, organogenesis
INTRODUCTION
Shoot growth is the result of two complementary components known as organogenesis and extension (Champagnat et al., 1986a). These phases of shoot growth may be synchronous or may take place at different times and be separated by a period of apparent inactivity during which leaves in an embryonic stage are contained in a bud. The leaves of a shoot may thus be preformed in a bud prior to shoot extension or may be formed and simultaneously extended without bud formation (i.e. neoformed organs; Hallé et al., 1978; Caraglio and Barthélémy, 1997). Fully preformed shoots frequently occur in woody species from temperate regions (Moore, 1909; Gill, 1971; Kozlowski, 1971; Allen and Owens, 1972; Abbott, 1977; Owens et al., 1977; Payan, 1982; Macdonald and Mothersill, 1983; Macdonald et al., 1984; Cottignies, 1985; Roloff, 1985; Kremer et al., 1990; Remphrey and Davidson, 1994; Puntieri et al., 2000; Souza et al., 2000; Puntieri et al., 2002a). In some of these species, some shoots, depending on their location within the tree and tree age, may develop neoformed leaves following the extension of preformed leaves (Puntieri et al., 2002b).
The time of the year when leaf differentiation and extension occur is important for understanding the role of climatic factors in leaf and shoot growth (Puntieri et al., 2002a) and in variation in morphology between two successive growth units of an annual shoot. In woody species, this type of knowledge can be used to explain the cause of inter‐annual fluctuations of growth, which may be the result of an endogeneous regulation of plant development or of climatic events (Guédon et al., 1999). Studies considering the periods of the year in which organogenesis takes place are rare, possibly due to the destructive techniques used for organogenesis assessment. Morpho logical categorization of individuals, axes and shoots of a species allows repeated sampling of homogeneous shoots, and thus is a precondition for the evaluation of organogenesis periods (Sabatier et al., 2001a; Puntieri et al., 2002a).
In Juglans regia L., growth is rhythmic and each axis is made up of a succession of annual shoots. Each annual shoot may consist of one or more growth units (i.e. a stem portion extended during an uninterrupted phase of extension; Hallé and Martin, 1968) and, on this basis, can be classified as a mono‐ or polycyclic shoot. Monocyclic annual shoots are built up during one spring flush, whereas bicyclic annual shoots are formed during two successive flushes of growth (spring and summer) separated by a resting phase (Sabatier et al., 1998). Previous studies have revealed that, in the latter case, the spring growth unit is preformed in the winter bud, whereas the summer growth unit is preformed in the spring bud (Sabatier et al., 1995). However, the time of the year when leaf differentiation occurs has not been reported for this species. In mature Juglans regia trees, female flowering is apical on spring shoots. After the development of a terminal female inflorescence, the flowering axis may continue to extend in the same growing season by the development of one to three relay axes which develop from the lateral buds located just below the terminal inflorescence.
Based on recent studies of the architecture and growth dynamics of Juglans regia (Barthélémy et al., 1995; Sabatier et al., 1998), the present study analyses (a) the periods of the year when preformed leaves of mono‐ and bicyclic shoots are differentiated in the bud and (b) variation in bud structure according to the time of bud formation and parent shoot type.
MATERIALS AND METHODS
Study site, plant material and shoot sampling
The study site was an orchard of 4‐year‐old Juglans regia ‘Lara’ at Lalanne nurseries, Gironde, south‐west France (44°34′N, 0°15′W). Trees were obtained from grafts on plants of Juglans regia and were pruned in ‘structured axis’ (Charlot et al., 1989). They thus consisted of a main stem with the lower part bearing a tier of three to four main branches. The buds and shoots selected for study were on these branches.
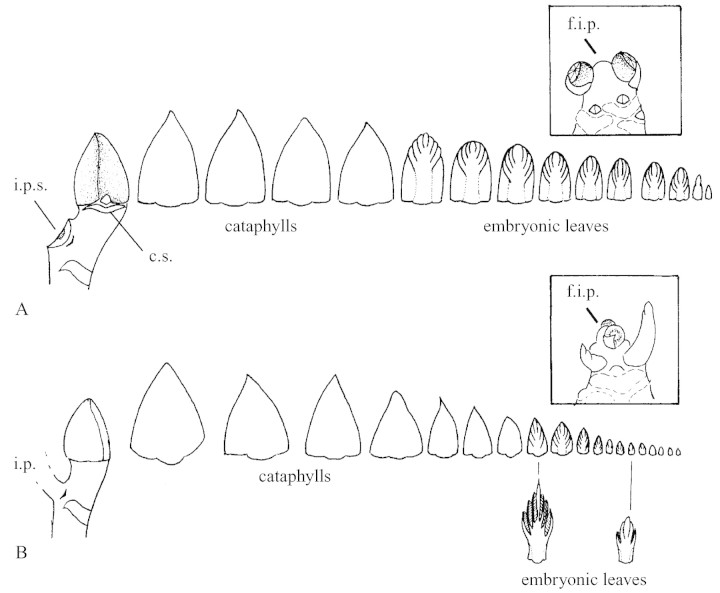
This study was centred on four types of previously identified annual shoots (Sabatier et al., 1998). These were: (1) monocyclic floriferous shoots: axillary buds placed on the first and second nodes from the terminal inflorescence were observed (Fig. 1A); (2) bicyclic floriferous shoots with one relay axis formed on the first node counted from the terminal inflorescence: the apical bud of the summer growth unit was analysed (Fig. 1B); (3) bicyclic floriferous shoots with two relay axes localized on the first and second nodes from the terminal inflorescence: the apical bud of the summer growth unit of each relay axis was analysed (Fig. 1C); and (4) bicyclic vegetative shoots: the apical bud was observed (Fig. 1D).
Fig. 1. Diagrammatic representation of studied types of parent annual shoots at different sampling times. A, Monocyclic floriferous shoot: axillary buds located on the first and second nodes from the terminal inflorescence. B, Bicyclic floriferous shoot with one relay axis. C, Bicyclic foriferous shoot with two relay axes. D, Bicyclic vegetative shoot. Only the measured buds are indicated (open triangles). 1, October 1992 or March 1993; 2, 16 Jun. 1993; 3, 3 Aug. 1993; 4, 26 Jul. 1993. Open circles, Terminal female inflorescence; parallel lines, inter‐annual growth‐unit limit; arrows, intra‐annual growth‐unit limit.
The number of buds collected per shoot type at each sampling time is indicated in Tables 1 and 2.
Table 1.
Mean (± s.e.; sample size in parenthesis) numbers of cataphyll scars (cat. scars), cataphylls (cat.), embryonic green leaves (leaves) and total nodes (cataphylls + embryonic green leaves) per bud of monocyclic floriferous shoots (m.f.s.) at different sampling dates
| October 1992 | 30 Mar. 1993 | 18 May 1993 | 16 Jun. 1993 | 26 Jul. 1993 | ||
| End of growth season | Winter‐bud break | End of spring flush | Spring‐bud break | End of summer flush | ||
| m.f.s. | Cat. scars | 4·2 ± 2·0 | n.m. | 0 | 0 | 2·5 ± 2·2 |
| p1 | Cat. | 3·9 ± 0·9 | 3·5 ± 0·8 | n.m. | 7·7 ± 1·1 | 5·8 ± 1·0 |
| Leaves | 12·7 ± 1·8 | 13·5 ± 1·6 | n.m. | 8·4 ± 1·0 | 12·8 ± 1·7 | |
| Total nodes | 16·7 ± 1·7 (26) | 16·7 ± 1·6 (53) | 13·1 ±1·4 (50) | 16·1 ± 1·0 (25) | 18·6 ± 1·8 (24) | |
| m.f.s. | Cat. scars | 1·1 ± 0·7 | n.m. | 0 | 0 | 0·5 ± 0·8 |
| p2 | Cat. | 4·7 ± 0·7 | 3·9 ± 0·5 | n.m. | 7·3 ± 0·9 | 6·4 ± 1·0 |
| Leaves | 10·3 ± 1·3 | 12·7 ± 1·3 | n.m. | 8·3 ± 1·0 | 11·9 ± 1·7 | |
| Total nodes | 14·9 ± 1·4 (26) | 16·6 ± 1·3 (53) | 12·9 ±1·4 (50) | 15·5 ± 1·4 (25) | 18·3 ± 1·6 (24) |
p1 and p2, Axillary buds localized on the first or second node below the terminal inflorescence; n.m., not measured.
Table 2.
Mean (± s.e.; sample size in parenthesis) numbers of cataphylls (cat.), embryonic green leaves (leaves) and total nodes (cataphylls + embryonic green leaves) per bud of bicyclic shoots at different sampling dates
| October 1992 | 30 Mar. 1993 | 18 May 1993 | 16 Jun. 1993 | 3 Aug. 1993 | ||
| End of growth season | Winter‐bud break | End of spring flush | Spring‐bud break | End of summer flush | ||
| b.f.s.1 | Cat. | 3·8 ± 0·7 | 2·9 ± 0·6 | n.m. | 9·7 ± 1·7 | 4·8 ± 1·2 |
| Leaves | 11·5 ± 1·5 | 12·0 ± 1·0 | n.m. | 9·4 ± 2·0 | 10·4 ± 1·0 | |
| Total nodes | 15·3 ± 1·5 (50) | 14·9 ± 1·1 (40) | 13·5 ± 1·4 (49) | 19·1 ± 2·0 (28) | 15·3 ± 1·2 (49) | |
| b.f.s.2 | Cat. | 4·2 ± 0·6 | n.m. | n.m. | 10·3 ± 1·5 | 4·9 ± 1·2 |
| p1 | Leaves | 11·6 ± 1·2 | n.m. | n.m. | 10·1 ± 1·4 | 10·1 ± 0·7 |
| Total nodes | 15·8 ± 1·4 (50) | n.m. | n.m. | 20·3 ± 1·7 (25) | 14·8 ± 1·5 (21) | |
| b.f.s.2 | Cat. | 3·9 ± 0·6 | n.m. | n.m. | 9·1 ± 1·3 | 4·9 ± 1·0 |
| p2 | Leaves | 11·6 ± 1·5 | n.m. | n.m. | 9·8 ± 1·0 | 10·1 ± 1·0 |
| Total nodes | 15·6 ± 1·5 (50) | n.m. | n.m. | 18·9 ± 1·2 (26) | 15·2 ± 0·9 (23) | |
| b.v.s. | Cat. | 3·7 ± 0·7 | 3·1 ± 0·4 | n.m. | 6·9 ± 0·9 | 4 ± 1·1 |
| Leaves | 12·6 ± 1·5 | 12·7 ± 1·3 | n.m. | 10·4 ± 1·4 | 10·8 ± 1 | |
| Total nodes | 16·3 ± 1·6 (50) | 15·9 ± 1·3 (40) | n.m. | 17·3 ± 1·4 (25) | 14·9 ± 1 (25) |
b.f.s.1, Bicyclic floriferous shoot with one relay summer shoot; b.f.s.2, bicyclic floriferous shoot with two relay summer shoots; p1 and p2, buds localized on the first and second node below the terminal inflorescence; b.v.s., bicyclic vegetative shoot; n.m., not measured.
Data collection and analysis
Buds were sampled destructively from each parent shoot type and used for the analysis of bud content. Five samples were collected at the different phenological stages of parent annual shoots (Sabatier et al., 1998): at the end of the growing season (October 1992); at the winter‐bud swelling (30 Mar. 1993); at the end of the spring growth flush (18 May); at the spring‐bud swelling (16 Jun.); and at the end of the summer growth flush (26 Jul. and 3 Aug.).
To study the variation in the number of embryonic green leaves of buds during the second flush of growth, weekly bud samples of bicyclic shoots were collected from 16 Jun. until 3 Aug.
The numbers of nodes (cataphylls and leaves included) and the length of each parent shoot were recorded at each sampling date. The numbers of cataphylls (scaly leaves) and embryonic green leaves of each bud were recorded after manual dissection under a stereomicroscope (×40). Embryonic green leaves were distinguished from cataphylls by the presence of well‐differentiated leaflet and lamina primordia. In spring buds, undifferentiated organs surrounding the apical meristematic dome were considered as leaf primordia. The total number of nodes of a bud was obtained by adding the numbers of cataphylls, embryonic green leaves and leaf primordia. The number of cataphyll scars was noted.
Student’s t‐test for paired samples using a significance level of 0·01 (Saporta, 1990) was carried out to compare total numbers of nodes, cataphylls and embryonic leaves per bud between (a) bud positions on the shoot, (b) sampling dates, and (c) parent annual shoot types.
RESULTS
Number of nodes of first and second axillary buds of monocyclic floriferous shoots
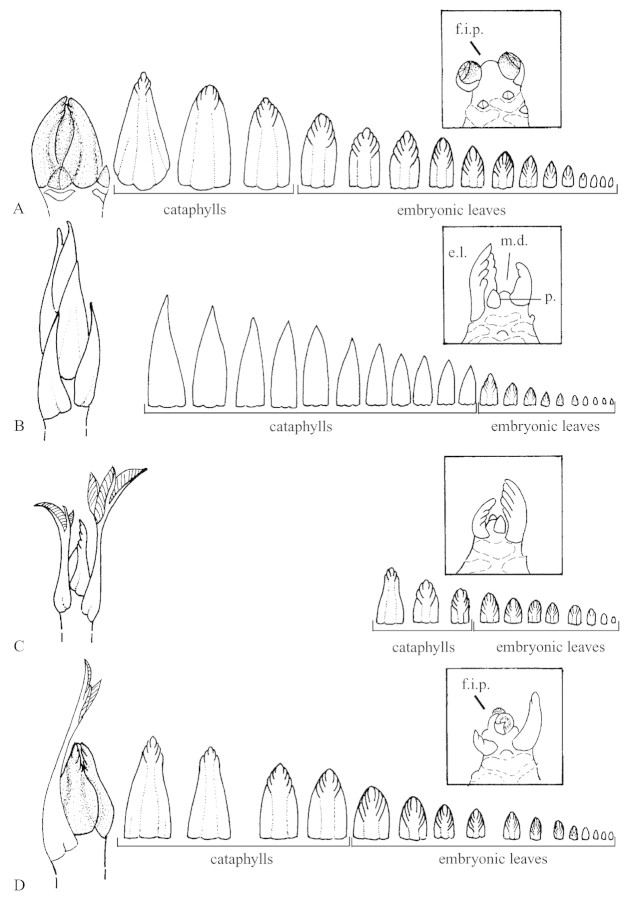
External cataphylls of lateral winter buds were round and coriaceous. In March, winter buds contained a small axis bearing a series of cataphylls surrounding well‐developed embryonic leaves (Fig. 2A) and terminated in a female inflorescence primordium. The axillary buds were visible in the bud. On 1 July, the new winter buds resembled those observed in March (Fig. 2B).
Fig. 2. Morphology and content of lateral buds located on the first node from the terminal inflorescence of monocyclic shoots before winter‐bud swelling on 30 Mar. (A) and after spring flush of growth on 1 Jul. (B). Magnification ×2. Insets show details of the corresponding embryonic shoot apex at magnification ×4. c.s., Cataphyll scar; p., peduncle; i.p.s., inflorescence peduncle scar; i.p., inflorescence peduncle; f.i.p., female inflorescence primordium.
Differences in the mean numbers of total nodes, cataphylls and embryonic leaves per bud generally were not significant (P > 0·02) between the two most distal buds of the monocyclic floriferous shoots except in October (Table 1).
Buds sampled at the end of the spring flush consisted, on average, of 13 nodes. Between this and the last sample (26 Jul.), as shoot extension finished, the mean number of nodes in these buds increased by about three (Table 1). The mean number of embryonic leaves of winter buds was not significantly different (P > 0·02) between 30 Mar. and 26 Jul. samples (Table 1). Differences in the number of cataphylls between buds at winter‐bud break and in the following summer are linked to the early fall of the first cataphylls formed during the growth season (Table 1; see the number of cataphyll scars).
No significant differences (P ≥ 0·30) were found between buds sampled in October 1992 and July 1993 with regard to the number of embryonic leaves of buds on the first distal node of monocyclic floriferous shoots (Table 1). In winter buds located on the second node from the terminal inflorescence, significant differences (P = 0·002) in the number of embryonic leaves were found between buds sampled in October and July. The difference in the number of embryonic leaves was not significant (P = 0·03) between buds sampled in March and July (Table 1).
Number of nodes of terminal buds of bicyclic shoots
Terminal winter buds of bicyclic shoots sampled in March consisted of a small axis with a series of distally incised and thick cataphylls followed by well‐developed embryonic green leaves, and terminated in a female inflorescence primordium (Fig. 3A). At the end of the spring flush of growth, spring buds, which are located on the first or second node from the terminal inflorescence, showed a lengthened form. At spring‐bud swelling (16 Jun.), buds contained a series of long, thin and green cataphylls (i.e. resembling embryonic green leaves without a lamina primordium) covering a series of embryonic green leaves, and terminated in one or two leaf primordia below the meristematic dome (Fig. 3B). Terminal buds of bicyclic shoots sampled during the summer growth flush consisted of embryonic leaves surrounded by some immature leaves (Fig. 3C). At the end of the summer growth flush (3 Aug.), buds contained the inflorescence primordium surrounded by a series of embryonic leaves and, more externally, by a series of cataphylls with leaflet primordia, classified as distally incised cataphylls (Fig. 3D).
Fig. 3. Morphology and content of the apical bud of bicyclic annual shoots on 30 Mar. before winter‐bud swelling (A), on 16 Jun. before the summer flush of growth (B), on 8 Jul., during the summer flush of growth (C) and on 3 August at the end of the summer flush of growth (D). Magnification ×2. Insets show details of the corresponding embryonic shoot apex at magnification ×4. e.l., Embryonic leaf; p., leaf primordium; m.d., meristematic dome; f.i.p., female inflorescence primordium.
For each sampling time, the differences in numbers of nodes, cataphylls and embryonic green leaves were not significant (P > 0·02) between the terminal bud of bicyclic shoots with one relay axis and those of bicyclic shoots with two relay axes (Table 2). Terminal spring buds of vegetative bicyclic shoots contained fewer cataphylls than spring buds of floriferous bicyclic shoots (Table 2). This difference is explained by the presence of two prophylls at the proximal end of the summer growth flush of floriferous bicyclic shoots.
Terminal buds sampled at the end of the spring flush of growth had, on average, 13 nodes. Between the end of spring and summer flushes of growth, as shoot extension finished, the mean number of nodes increased by about six (Table 2). The additional leaves correspond to the end of the spring‐bud development. Differences in the number of nodes between winter buds sampled in October, March and August were not significant (P > 0·02).
Between 16 and 24 Jun., as the number of unfolded leaves of summer shoots increased, the number of leaves of their terminal buds decreased rapidly. The number of leaves of buds increased between 24 Jun. and 3 Aug., whereas the number of unfolded leaves remained relatively constant in that period (Fig. 4). Similar behaviour was found for the four types of summer growth units (Fig. 4).
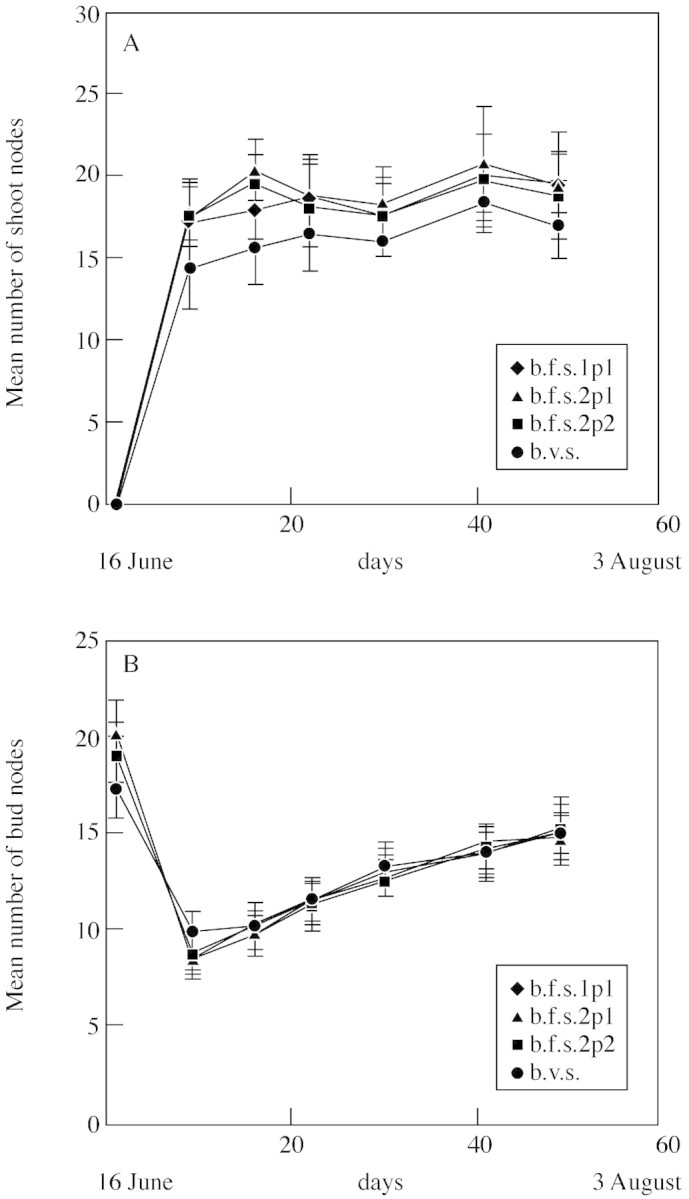
Fig. 4. Mean (± s.e.) number of nodes of parent shoots (A) and winter buds (B) according to annual shoot type during the summer shoot extension. m.f.s., Monocyclic floriferous shoot; b.f.s.1, bicyclic floriferous shoot with one relay summer shoot; b.f.s.2, bicyclic floriferous shoot with two relay summer shoots; b.v.s., bicyclic vegetative shoot. Buds or shoots are located on the first (p1) or second (p2) node from the terminal inflorescence. Calendar dates and number of days from date of first observation (i.e. 16 Jun. 1993) are indicated.
The total number of nodes of winter buds of bicyclic floriferous shoots was similar between October 1992 and August 1993 (P > 0·02) (Table 2). Winter buds of October 1992 had, on average, one cataphyll less than winter buds of August 1993 (P < 0·001). The opposite was true for the number of embryonic green leaves (Table 2).
Comparisons of the number of nodes between winter and spring buds and between terminal and lateral buds
The numbers of nodes, cataphylls and embryonic green leaves were significantly different between spring buds sampled in June and winter buds sampled in March or August (Table 2). Spring buds, which give rise to summer shoots, had more than twice as many cataphylls as the winter buds, which give rise to next spring shoots (Table 2).
The numbers of nodes and cataphylls in lateral winter buds of monocyclic shoots (Table 1) were significantly (P < 0·02) higher than those in terminal winter buds of bicyclic shoots (Table 2).
DISCUSSION
Leaf differentiation periods
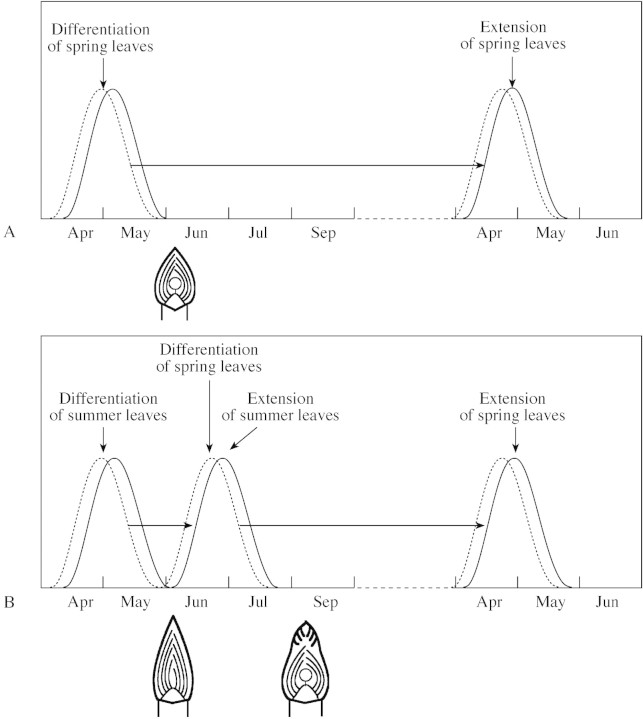
The period of differentiation of spring shoots in winter buds of Juglans regia may be either mid‐spring or early summer, depending on whether the parent shoot had, respectively, a monocyclic or a bicyclic growth pattern. Monocyclic shoots consist of leaves that were differentiated in the previous spring and that remained enclosed in a bud from summer up to early spring (Fig. 5A). Bicyclic shoots consist of leaves that were differentiated in the previous summer and extended in spring, and leaves that were differentiated in spring and extended in the summer of the same growing season (Fig. 5B). The bicyclic shoots of Juglans regia derive from two organogenesis periods separated by a resting period. In bicyclic shoots of oak, a single growth unit is also preformed in each terminal bud (Champagnat et al., 1986b; Fontaine et al., 1999). On the other hand, in some species of Pinaceae, two successive growth units may be preformed during an uninterrupted period (Lanner, 1970; Kozlowski, 1971). This may explain the fact that the expression of polycyclism appears to be more strongly linked to climatic conditions of the current year of growth in some species, e.g. Juglans regia, than in others, e.g. Pinus spp. In J. regia, the possibility of exhibiting several periods of leaf and female inflorescence preformation during one growing season may be regarded as a feature related to the tree’s capacity to respond to environmental fluctuations.
Fig. 5. Diagrammatic representation of bud structure and ontogenetic cycle for Juglans regia for a monocyclic floriferous shoot (A) and a bicyclic floriferous shoot (B). The rates of leaf differentiation and extension are indicated by curves with broken and unbroken lines, respectively. Open circles, Female terminal inflorescence.
The present results show that a large number of leaves of further shoots are differentiated at the inception of the current shoot extension. The organogenetic timing found here for J. regia resembles that found for apple trees (Abbott, 1977), Theobroma cacao L. (Greathouse et al., 1971) and some species of the Guyana forest (Comte, 1993), which show a high organogenetic activity at early stages of shoot extension. A different behaviour was found for several species of Pinaceae (Parker, 1959; Cannell et al., 1976; Owens et al., 1977; Pillai and Chacko, 1978; Hejnowicz and Obarska, 1995), some species of Taxaceae (Tomlinson and Zacharias, 2001), Callistemon viminalis (Gaertner) G. Don f. (Purohit and Nanda, 1968), Cephalotaxus drupacea Sieb. et Zucc. (Bompar, 1974), short shoots of Populus trichocarpa Torrey et A. Gray (Critchfield, 1960), Fraxinus americana L. (Gill, 1971), Fagus sylvatica L. (Fromard, 1982) and Nothofagus dombeyi (Mirb.) Oersted (Puntieri et al., 2002a), which present a high organogenetic activity at the end of shoot extension.
Winter and spring buds of Juglans regia
In J. regia, the lateral buds of monocyclic floriferous shoots possess round and undivided cataphylls without leaflet primordia (Fig. 5A), while the terminal bud of bicyclic shoots has cataphylls with distal incisions (Fig. 5B). This difference in form of bud protective leaves is in accordance with previous results on form variation between terminal and lateral buds of vegetative monocyclic shoots of J. regia (Sabatier and Barthélémy, 2001a).
In this species, winter buds, which give rise to spring growth units (Fig. 5A), and spring buds, from which summer growth units derive (Fig. 5B), differ in their composition. The cataphylls are longer, thinner and more numerous for spring buds than for winter buds. Spring buds are present during a short resting period of about 4 weeks between two flushes of growth in summer (Sabatier et al., 1998), whereas winter buds remain dormant during several months in autumn and winter. Thus, winter and spring buds differ in the length of their resting periods and in the time of extension of the derived shoots. In Eurya japonica Thunb., an evergreen tree species of warm‐temperate rain forests, two types of terminal buds were found for different seasons of the year, in association with variations in temperature conditions, day length and the length of resting periods (Nitta and Ohsawa, 1998). Differences in bud form between two successive flushes have also been observed in Quercus rubra L. (Collin et al., 1996). These results suggest that the morphology of the protective leaves of buds may be a marker of the length of the period between two successive shoot‐extension phases.
In Juglans regia, the capacity of a shoot to develop a summer growth unit is closely linked with tree ontogeny, the architectural position of the parent shoot within the tree (Sabatier and Barthélémy, 2001b), climatic conditions during the growing season and environmental conditions of growth (Barthélémy et al., 1995). The specific morphology of spring and winter buds allows an early prediction (i.e. during the spring flush) of the time of bud development and of the type of annual shoot produced.
ACKNOWLEDGEMENTS
We are grateful to J. G. Puntieri for helpful comments on previous versions of this paper.
Supplementary Material
Received: 3 January 2003;; Returned for revision: 3 April 2003. Accepted: 28 April 2003 Published electronically: 12 June 2003
References
- AbbottDL.1977. Fruit‐bud formation in Cox’s orange pippin. Long Ashton Research Station, Annual Report for 1976, 167–176. [Google Scholar]
- AllenGS, Owens JN.1972.The life history of Douglas fir Ottawa: Department of Forestry. [Google Scholar]
- BarthélémyD, Sabatier S, Pascal O.1995. Le développement archi tectural du Noyer commun, Juglans regia L. (Juglandaceae). Bulletin de Vulgarisation Forestière, Forêt Entreprise 103: 61–68. [Google Scholar]
- BomparJL.1974. Morphologie et ontogenèse du rameau plagiotrope du Cephalotaxus drupacea Sieb. et Zucc. mâle. Revue Générale de Botanique 81: 5–39. [Google Scholar]
- CannellMGR, Thompson S, Lines R.1976. An analysis of inherent differences in shoot growth within some north temperate conifers. In: Cannell MGR and Last FT eds. Tree physiology and yield improvement London, New York: Academic Press, 173–205. [Google Scholar]
- CaraglioY, Barthélémy D.1997. Revue critique des termes relatifs à la croissance et à la ramification des tiges des végétaux vasculaires. In: Bouchon J, de Reffye P, Barthélémy D, eds. Modélisation et simulation de l’architecture des végétaux Paris: INRA, 11–87. [Google Scholar]
- ChampagnatP, Barnola P, Lavarenne S.1986a. Quelques modalités de la croissance rythmique endogéne des tiges chez les végétaux ligneux. In: Compte rendu du Colloque International de l’Arbre, Naturalia Monspeliensia, no. h.s: 279–302. [Google Scholar]
- ChampagnatP, Payan E, Champagnat M, Barnola P, Lavarenne S, Bertholon C.1986b. La croissance rythmique de jeunes chênes pédonculés en conditions contrôlées et uniformes. In: Compte rendu du Colloque International de l’Arbre, Naturalia Monspeliensia, no. h.s: 303–337. [Google Scholar]
- CharlotG, Germain E, Prunet P.1989.Le Noyer. Nouvelles techniques. Paris: CTIFL, ed. [Google Scholar]
- CollinP, Badot PM, Millet B.1996. Croissance rythmique et développement du Chêne rouge d’Amérique, Quercus rubra L. cultivé en conditions contrôlées. Annales des Sciences Forestières 53: 1059–1069. [Google Scholar]
- ComteL.1993.Rythmes de croissance et structures spatiales périodiques d’arbres tropicaux. Exemple de cinq espèces de forêt équatoriale. Thèse Doctorat Physiol. Ecosystèmes forestiers tropicaux, Université des Sciences et Techniques du Languedoc, Montpellier II. [Google Scholar]
- CottigniesA.1985.Dormance et croissance active chez le Frêne. Thèse de Doctorat, Université de Paris 6. [Google Scholar]
- CritchfieldWB.1960. Leaf dimorphism in Populus trichocarpa American Journal of Botany 47: 699–711. [Google Scholar]
- FontaineF, Chaar H, Colin F, Clément C, Burrus M, Druelle JL.1999. Preformation and neoformation of growth units on 3‐year‐old seedlings of Quercus petraea Canadian Journal of Botany 77: 1623–1631. [Google Scholar]
- FromardL.1982.Croissance rythmique et variabilité chez le Hêtre (Fagus sylvatica L.). Diplôme d’Etudes Approfondies, Université Clermont‐Ferrand II. [Google Scholar]
- GillAM.1971. The formation, growth and fate of buds of Fraxinus americana L. in central Massachusetts. Harvard Forest Paper 20: 1–16. [Google Scholar]
- GreathouseDC, Laetsch WM, Phinney BO.1971. The shoot growth rhythm of a tropical tree, Theobroma cacao Biotropica 3(2): 109–124. [Google Scholar]
- GuédonY, Barthélémy D, Caraglio Y.1999. Analyzing spatial structures in forest tree architectures. In: Amaro A, Torné M, eds. Empirical and process‐based models for forest tree and stand growth simulation Lisbon: Ediçioes Salamandra, 23–42. [Google Scholar]
- HalléF, Martin R.1968. Etude de la croissance rythmique chez Hévéa (Hevea brasiliensis Müll. Arg. Euphorbiaceae‐Crotonoidees). Adansonia, ser. 2, 8(4): 475–502. [Google Scholar]
- HalléF, Oldeman RAA, Tomlinson PB.1978.Tropical trees and forests. Berlin: Springer Verlag. [Google Scholar]
- HejnowiczA, Obarska E.1995. Structure and development of vegetative buds, from the lower crown of Picea abies Annales des Sciences Forestières 52: 433–447. [Google Scholar]
- KozlowskiTT.1971.Growth and development of trees. Vol. 1. Seed germination, ontogeny and shoot growth. New York: Academic Press. [Google Scholar]
- KremerA, Nguyen A, Lascoux M, Roussel G.1990. Morphogénèse de la tige principale et croissance primaire du Pin maritime (Pinus pinaster Ait.). In: Actes du 3ème Colloque Sciences et Industries du Bois, Bordeaux: ARBORA, 333–349. [Google Scholar]
- LannerRM.1970. Origin of the summer shoot of pinyon pines. Canadian Journal of Botany 48: 1759–1765. [Google Scholar]
- MacdonaldAD, Mothersill DH.1983. Shoot development in Betula papyrifera I. Short‐shoot organogenesis. Canadian Journal of Botany 61: 3049–3065. [Google Scholar]
- MacdonaldAD, Mothersill DH, Caesar JC.1984. Shoot development in Betula papyrifera III. Long‐shoot organogenesis. Canadian Journal of Botany 62: 437–445. [Google Scholar]
- MooreE.1909. The study of winter buds with reference to their growth and leaf content. Bulletin of the Torrey Botanical Club 37(3): 117–145. [Google Scholar]
- NittaI. and Ohsawa M.1998. Bud structure and shoot architecture of canopy and understorey evergreen broad‐leaved trees at their northern limit in East Asia. Annals of Botany 81: 115–129. [Google Scholar]
- OwensJN, Molder M, Langer H.1977. Bud development in Picea glauca I. Annual growth cycle of vegetative buds and shoot extension as they relate to date and temperature sums. Canadian Journal of Botany 55: 2728–2745. [Google Scholar]
- ParkerJ.1959. Growth periodicity and the shoot tip of Abies concolor American Journal of Botany 46: 110–118. [Google Scholar]
- PayanE.1982.Contribution à l’étude de la croissance rythmique chez de jeunes Chênes pédonculés (Quercus pedunculata Ehrh.). Thèse Doctorat, Université de Clermont‐Ferrand II. [Google Scholar]
- PillaiSK, Chacko B.1978. Anatomical and histochemical studies of the shoot apex of Cedrus deodara Phytomorphology 28(3): 275–283. [Google Scholar]
- PuntieriJ, Souza MS, Barthélémy D, Brion C, Nunez M, Mazzini C.2000. Preformation, neoformation and shoot structure in Nothofagus dombeyi (Fagaceae). Canadian Journal of Botany 78: 1044–1054. [Google Scholar]
- PuntieriJG, Barthélémy D, Mazzini C, Brion C.2002a. Periods of organogenenis in shoots of Nothofagus dombeyi (Mirb.) Oersted (Nothofagaceae). Annals of Botany 89: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PuntieriJG, Stecconi M, Barthélémy D.2002b. Preformation and neoformation in shoots of Nothofagus antarctica (G. Forster) Oerst. (Nothofagaceae) shrubs from Northern Patagonia. Annals of Botany 89: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PurohitAM, Nanda KK.1968. Morphological studies of shoot apex. I. Recurrent growth flushes and their relationship with structural changes in growing apex of Callistemon viminalis Canadian Journal of Botany 46: 1287–1295. [Google Scholar]
- RemphreyWR, Davidson CG.1994. Shoot preformation in clones of Fraxinus pennsylvanica in relation to site and year of bud formation. Trees 8: 126–131. [Google Scholar]
- RoloffA.1985.Morphologie der Kronentwicklung von Fagus sylvatica L. (Rotbuche) unter besonderer Berücksichtigung möglicherweise neuartiger Veränderungen. Thèse Doctorat, Georg‐August‐universität, Göttingen. [Google Scholar]
- SabatierS, Barthélémy D, Ducousso I, Germain E.1995. Nature de la pousse annuelle chez le Noyer commun, Juglans regia var. Lara (Juglandaceae): préformation hivernale et printanière. In: Bouchon J, ed. Comptes Rendus du Colloque ‘‘Architecture des arbres fruitiers et forestiers’’, INRA, Les Colloques no. 74, 109–123. [Google Scholar]
- SabatierS, Barthélémy D, Ducousso I, Germain E.1998. Modalités d’allongement et morphologie des pousses annuelles chez le Noyer commun, Juglans regia L. cv. ‘‘Lara’’ (Juglandaceae). Canadian Journal of Botany 76: 1253–1264. [Google Scholar]
- SabatierS, Barthélémy D.2001a. Bud structure in relation to shoot morphology and position on the vegetative annual shoots of Juglans regia L. (Juglandaceae). Annals of Botany 73: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SabatierS, Barthélémy D.2001b. Annual shoot morphology and architecture in Persian walnut, Juglans regia L. (Juglandaceae). Acta Horticulturae 544: 255–264. [Google Scholar]
- SaportaD.1990.Probabilités des données et statistique. Paris: Technip. [Google Scholar]
- SouzaMS, Puntieri JP, Barthélémy D, Brion C.2000. Bud content and its relation to shoot size and structure in Nothofagus pumilio (Poepp. et Endl.) Krasser (Fagaceae). Annals of Botany 85: 547–555. [Google Scholar]
- TomlinsonPB, Zacharias EH.2001. Phyllotaxis, phenology and architecture in Cephalotaxus, Torreya and Amentotaxus (Coniferale). Botanical Journal of Linnean Society 135: 215–228. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.