Abstract
Aerial images of the high summits of the Spanish Central Range reveal significant changes in vegetation over the period 1957 to 1991. These changes include the replacement of high‐mountain grassland communities dominated by Festuca aragonensis, typical of the Cryoro‐Mediterranean belt, by shrub patches of Juniperus communis ssp. alpina and Cytisus oromediterraneus from lower altitudes (Oro‐Mediterranean belt). Climatic data indicate a shift towards warmer conditions in this mountainous region since the 1940s, with the shift being particularly marked from 1960. Changes include significantly higher minimum and maximum temperatures, fewer days with snow cover and a redistribution of monthly rainfall. Total yearly precipitation showed no significant variation. There were no marked changes in land use during the time frame considered, although there were minor changes in grazing species in the 19th century. It is hypothesized that the advance of woody species into higher altitudes is probably related to climate change, which could have acted in conjunction with discrete variations in landscape management. The pronounced changes observed in the plant communities of the area reflect the susceptibility of high‐mountain Mediterranean species to environmental change.
Key words: Climate change, high mountain vegetation, Spanish Central Range
INTRODUCTION
The current consensus is that global warming will affect the ecophysiological processes of plant systems (Leitonen et al., 1997; Thornley et al., 1997; Wayne et al., 1998; Shaw et al., 2000; Peñuelas and Filella, 2001), leading to complex ecological interactions that need to be studied in detail (Acock, 1992). For instance, shifts in the distribution of plant species have repeatedly been predicted or reported as direct or indirect consequences of such ecophysiological changes, most of these investigations demonstrating the advance of thermophilic species and the retreat of cryophilic or mesophilic taxa in many types of ecosystem (Shaw et al., 2000; Hilbert et al., 2001).
Changes in the distribution of species or communities can be more easily detected at ecotonal boundaries (Harte and Shaw, 1995; Risser, 1995; Pauli et al., 2001). Thus, it has been noted that plants of sub‐alpine areas seem to be especially sensitive to global warming (Shaw et al., 2000; Erschbamer, 2001; Pauli et al., 2001). It is likely that such effects are more intense in mountain systems under limiting conditions (Beniston, 2000), as in many alpine areas of Mediterranean‐type climate, in which most of the orophilous species are relicts from frosts and are considered to be at the limit of their survival.
The advance of thermophilic native and alien plant species from coastal areas towards continental and mid‐mountain Iberian areas has been detected recently, probably as a consequence of the effects of global warming (Sobrino et al., 2001). The present analysis of climatic data suggests that this is not the only effect, and that the apparent absence of ecological alterations in the Iberian Peninsula is more a reflection of a lack of specific studies than an indication that changes in climatic variables have not reached the critical thresholds that could lead to ecological shifts.
The aim of this study was to determine whether the distribution of high‐mountain species of central Spain has changed and, if so, to analyse and discuss the possibility that such alterations could be attributed to global warming.
MATERIALS AND METHODS
Study area
The work was conducted across the Spanish Central Range (some 500 km) in the Peñalara massif (2430 m), the range’s second highest peak (Fig. 1). Geological and palaeohistorical data for the region are given in Muñoz and Sanz‐Herráiz (1995), and a synopsis of the flora and vegetation of this natural reserve in Rivas‐Martínez et al. (1999).
Fig. 1. Location of the Spanish Central Range and of Peñalara massif in the Iberian Peninsula.
Many alpine and sub‐alpine species, and hence their communities, show clear altitudinal distribution gradients in the area, which act as indicators of environmental conditions. Three altitudinal zones have been identified: Supra‐, Oro‐ and Cryoro‐Mediterranean (Rivas‐Martínez and Loidi, 1999). This classification system may be compared with others commonly employed in identifying habitats using the EUNIS Habitat classification (http://mrw.wallonie.be/dgrne/sibw/EUNIS). For the present purposes, it should be mentioned that patches of Juniperus communis ssp. alpina and Cytisus oromediterraneus can be easily identified in aerial photographs. These images are extremely useful for detecting physiognomic changes in vegetation and the type of landscapes they form.
Analysis of vegetation shifts during the second half of the 20th century
Aerial photographs taken in May 1957 (1/14 000, conventionally amplified aerial photograph, the first available for the area) and July 1991 (1/5000, ortho‐photomap) were used to investigate possible changes in the alpine communities of the Peñalara massif during the second half of the 20th century. These images facilitated comparison of the distribution patterns of J. communis ssp. alpina and C. oromediterraneus shrublands in these 2 years. The Universal Projection Transverse Mercator system (UTM) was used to represent this cartographic information.
To quantify the patches of the selected shrub species and evaluate their displacement from the Oro‐Mediterranean towards the Cryoro‐Mediterranean zone, two transects (SW–NE and W–E) were drawn from 2300 m towards the summit of the Peñalara massif (2428 m). Consecutive series of 22 and 15 circles (1000 m2 in area) were laid down along each transect. The circles were tangentially arranged with respect to each other. The number of isolated shrubs or continuous shrub patches was recorded at each site for both altitudinal zones (2300–2400 and above 2400 m) in the aerial photographs of 1957 and 1991. These data were compared using Mann–Whitney tests to establish significant differences in the abundance of shrub patches appearing in each altitudinal interval (<2400 and ≥2400 m) between 1957 and 1991 (Zar, 1984). As well as this laboratory work, several field trips to the Peñalara massif were made over the last few years to obtain data on the current distribution of the typical plant communities of the Cryoro‐Mediterranean zone.
Analysis of climatic data
Climatic data were obtained from the neighbouring weather station of Puerto de Navacerrada (at a height of 1890 m a.s.l, within the lower humid and hyper humid Oro‐Mediterranean belt). This station is only 8 km away from the highest peaks of the Peñalara massif. Data from other meteorological stations were not used because of differences in altitude with respect to the study site. Thermometric data (minimum, maximum and mean values) from 1940 to 1999, rainfall and snow‐related information from 1946 to 2001 (days with snowfall, days with hail, days with snow‐covered soil) were grouped into two sets (monthly and annual mean values) and analysed using regression techniques after normalization of the variables when necessary (Zar, 1984). No variable was found to show Studentized residuals exceeding 3·0, indicating that the data fit the expected trend equation reasonably well.
The climatic datasets and temperature anomalies (measured as deviations from 0°C) were searched for possible significant variation from 1940 to 2001. These analyses were performed both on global pooled data and on as many time intervals as possible, the latter spanning at least 20 years, as recommended by the IPCC (2001). The correlation coefficient associated with each regression line was compared with that obtained by curvilinear models. As linear and non‐linear models generated similar results, only the significant results of the linear equations are provided. Also, for the sake of simplicity, only the principal results of the statistical analyses are presented here.
RESULTS
Vegetation changes
The aerial images clearly show a change in the distribution of shrub patches of the Peñalara massif (Fig. 2). In these images, isolated and continuous patches of C. oromediterraneus and J. communis ssp. hemisphaerica appear as dark dots. A considerably higher density of shrubs can be detected in 1991 compared with 1957. This difference is particularly obvious from an altitude of 2200 m upwards: the theoretical limit of the Cryoro‐Mediterranean zone. The small difference in season (May–July) would not be expected to affect the observations made since only evergreen shrubs were considered.
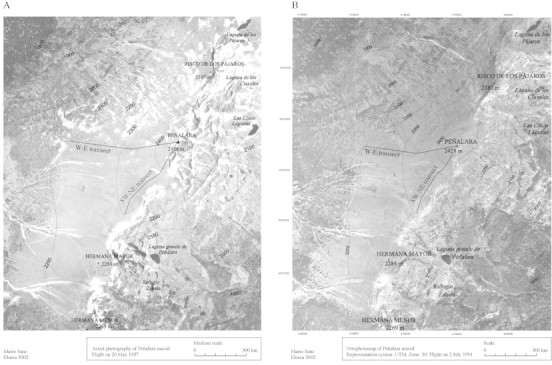
Fig. 2. Aerial image of Peñalara massif in 1957 (A) and 1991 (B) showing the transects analysed.
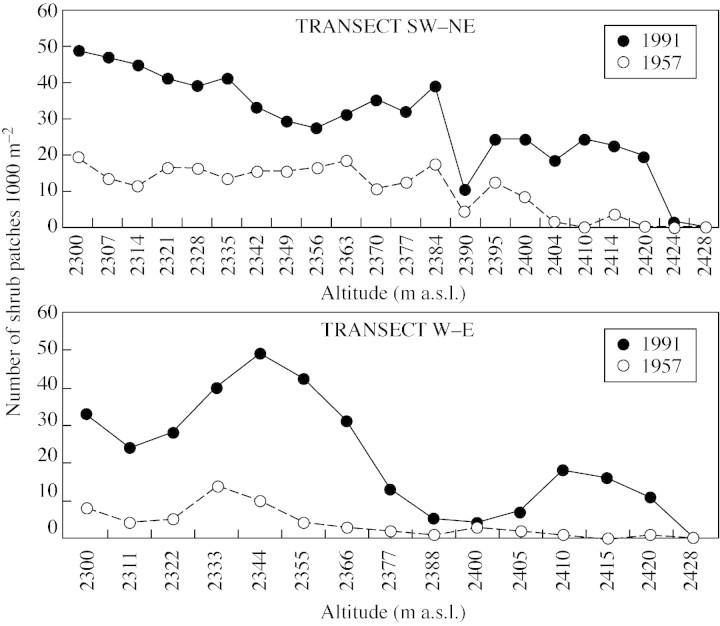
The numerical results from the SW–NE and W–E transects (Fig. 3) show a significant increase in the number of shrubs in 1991 compared with 1957. Data on the area of patches are not shown since the large differences observed make the changes sufficiently apparent. The relatively small number of shrub patches recorded at 2390 m (SW–NE transect) and 2400 m (W–E transect) can be explained by the presence of a rocky outcrop impairing shrub colonization and establishment. In the two transects and in the two altitudinal ranges, the overall number of shrub patches increased significantly from 1957 to 1991. Within the range 2300–2395 m the overall number of shrub patches increased from 207 to 522 in the SW–NE transect and from 48 to 247 in the W–E transect (P < 0·001 in both cases). Within the range 2400–2428 m the overall number of shrub patches increased significantly from 12 to 108 in the SW–NE transect and from 9 to 63 in the W–E transect (P < 0·05 in both cases).
Fig. 3. Number of shrub patches (and single individuals) along the SW–NE and W–E altitudinal transects in 1957 and 1991.
Changes in climatic variables
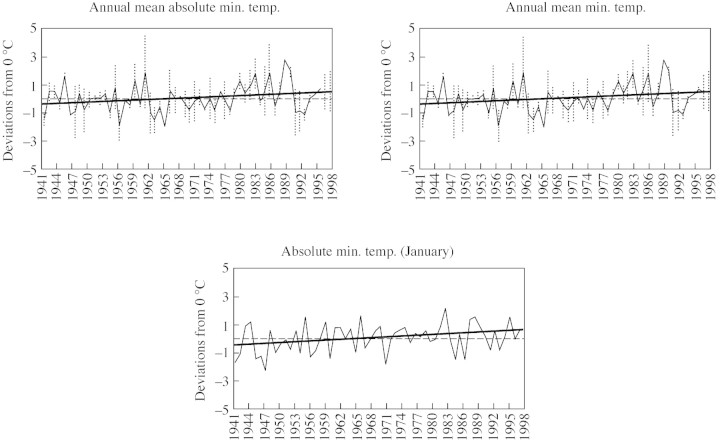
Table 1 and Fig. 4 show a clear climatic trend. The general pattern is one of higher temperatures in winter and early spring, and higher maximum temperatures in most months. Of note is the rise in mean minimum temperatures for January, the coolest month of the year. Also recorded were higher mean maximum and minimum temperatures, and fewer days with snowfall or on which the summits were covered with snow. Total annual rainfall did not change significantly over the time period considered. However, significant decreases (P < 0·05) were observed in monthly rainfall values for February (r = –0·3747, from 1963 to 2000), March (r = –0·4957, from 1964 to 2000), April (r = –0·5120, from 1974 to 2000) and June (r = –0·3706, from 1970 to 2000). Summer rainfall in July (r = 0·3918, 1963–2000) and September (r = 0·3766, 1970–2000) showed a slight yet significant increase (P < 0·05). Thus, since the 1940s, and particularly since the 1960s, the prevailing climate in the summits of the Peñalara massif has moved towards warmer conditions and a seasonal redistribution of rainfall.
Table 1.
Trends of climatic data, temporal interval for which the best fit was obtained and results of the statistical analysis
| Month | RAV ± s.e. | Pearson’s r |
| January | ||
| Minimum absolute temperatures (1941–1999) | 0·054 ± 0·024 | 0·2872* |
| Mean of minimum temperatures (1941–1999) | 0·031 ± 0·014 | 0·2873* |
| February | ||
| Maximum absolute temperatures (1968–1999) | 0·191 ± 0·047 | 0·5984* |
| Mean maximum temperatures (1968–1999) | 0·132 ± 0·037 | 0·5407* |
| Number of days with snowfall (1961–2001) | –0·238 ± 0·087 | –0·4271** |
| March | ||
| Maximum absolute temperatures (1963–1999) | 0·168 ± 0·056 | 0·4596** |
| Mean maximum temperatures (1968–1999) | 0·223 ± 0·067 | 0·5269** |
| Mean minimum temperatures (1968–1999) | 0·126 ± 0·044 | 0·4657** |
| Mean of mean temperatures (1968–1999) | 0·187 ± 0·041 | 0·6484** |
| Number of days with snowfall (1961–2001) | –0·246 ± 0·067 | –0·5129** |
| April | ||
| Maximum absolute temperatures (1971–1999) | 0·178 ± 0·078 | 0·4086* |
| Mean maximum temperatures (1941–1999) | –0·051 ± 0·021 | –0·3094* |
| Mean of mean temperatures (1941–1999) | –0·037 ± 0·0164 | –0·2895* |
| Number of days with snowfall (1973–2001) | –0·293 ± 0·131 | –0·4079* |
| May | ||
| Mean maximum temperatures (1967–1999) | 0·123 ± 0·049 | 0·4164* |
| Mean of mean temperatures (1967–1999) | 0·087 ± 0·039 | 0·3761* |
| Number of days with snowfall (1969–2001) | –0·220 ± 0·085 | –0·4221** |
| June | ||
| Maximum absolute temperatures (1941–1999) | –0·046 ± 0·022 | –0·2719* |
| Maximum absolute temperatures (1973–1999) | 0·160 ± 0·069 | 0·42859* |
| Mean maximum temperatures (1970–1999) | 0·110 ± 0·053 | 0·3669* |
| July | ||
| Maximum absolute temperatures (1969–1999) | 0·098 ± 0·035 | 0·4773** |
| Mean maximum temperatures (1976–1999) | 0·147 ± 0·041 | 0·5743** |
| Mean of mean temperatures (1976–1999) | 0·122 ± 0·051 | 0·4613* |
| August | ||
| Maximum absolute temperatures (1968–1999) | 0·100 ± 0·035 | 0·4704** |
| Mean maximum temperatures (1968–1999) | 0·122 ± 0·031 | 0·5957** |
| Mean minimum temperatures (1941–1999) | 0·029 ± 0·010 | 0·3506** |
| Mean minimum temperatures (1963–1999) | 0·052 ± 0·019 | 0·4184* |
| Mean of mean temperatures (1968–1999) | 0·090 ± 0·027 | 0·5246** |
| September | ||
| Maximum absolute temperatures (1972–1999) | 0·176 ± 0·065 | 0·4743* |
| Mean minimum temperatures (1977–1999) | –0·213 ± 0·065 | –0·5921** |
| Mean of mean temperatures (1977–1999) | 0·176 ± 0·069 | –0·4925* |
| November | ||
| Maximum absolute temperatures (1961–1999) | 0·095 ± 0·043 | 0·3438* |
| Minimum absolute temperatures (1962–1999) | 0·083 ± 0·036 | 0·3644* |
| Mean maximum temperatures (1961–1999) | 0·092 ± 0·029 | 0·4656** |
| Mean minimum temperatures (1941–1999) | 0·031 ± 0·014 | 0·2803* |
| Mean minimum temperatures (1958–1999) | 0·074 ± 0·021 | 0·4858** |
| Mean of mean temperatures (1961–1999) | 0·084 ± 0·026 | 0·4697** |
| December | ||
| Minimum absolute temperatures (1962–1999) | 0·161 ± 0·046 | 0·5070** |
| Mean maximum temperatures (1962–1999) | 0·088 ± 0·026 | 0·4935** |
| Mean minimum temperatures (1962–1999) | 0·072 ± 0·023 | 0·4670** |
| Mean of mean temperatures (1962–1999) | 0·079 ± 0·024 | 0·4893** |
| Number of days with snowfall (1962–2001) | –0·197 ± 0·082 | –0·3900** |
| Number of days with soil covered by snow (1962–2001) | –0·272 ± 0·115 | –0·3576** |
RAV, Estimated rate of annual variation of temperature (°C) per year.
Only significant results at P < 0·05 (*) and P < 0·01 (**) are shown.
Fig. 4. Temperature anomalies, standard deviations and regression lines describing the general pattern of variation. Only variables showing the most representative changes are shown. R2 values were significant at the 5 % probability level.
DISCUSSION
In studies of climate warming it has been difficult to establish a single link between vegetation change and climate change (Archer et al., 1995). Thus, to identify the underlying cause of the advance in shrub species towards higher altitudes over the last decades established here, it is necessary to consider all possible causes of vegetation change including the effects of land use (fire regime, grazing mode or grazing pressure) and air or soil pollution (e.g. acid rain). According to the records of the Spanish Ministry of the Environment, there have been no fires in mountain‐top regions over the last two centuries. Furthermore, the most recent study of this area (Peña‐Martínez, 2002) reports no detectable pollution of any sort in the Peñalara summit area. Indeed, the environmental quality of this enclave is reflected by the fact that it is currently a candidate for the status of ‘natural reserve’ under Spanish legislation.
There has been no apparent change in grazing pressure or in grazing practices (Montserrat and Fillat, 1990). The high Peñalara areas have not been intensively exploited as pastures, given the low palatability of their plant communities. Consequently, the area has traditionally been under low grazing pressure (0·7 animal ha–1) since the beginning of the 20th century (Vías, 2001). Today, each herd includes 20 animals on average (field observations), which, based on the records available, probably represents the level for many decades. The only historical evidence of change is the replacement of the few existing sheep at the end of the 19th century by an equivalent number of cattle. Indeed, it seems that variables related to land use have remained quite constant over the past 100 years, which is twice the time frame examined here. In contrast, a clear effect of global warming on Mediterranean ecosystems has been described (Peñuelas et al., 2002), and differences in maximum and minimum temperatures shown here are in close agreement with the findings of others (e.g. Brázdil et al., 1996; Easterling et al., 1997).
Although at a first glance the rapid changes in vegetation of the Peñalara summits appear surprising, there are several reports in the literature of rapid encroachment by shrub or tree communities (Ansley et al., 1995; Bellingham, 1998 for Cytisus; Archer and Stokes, 2000 for Juniperus). Such rapid changes seem to be caused by intense modifications in land use (fencing, fires, abandoned crops, etc.), but this does not appear to be the case in the Peñalara massif. It seems reasonable to assume that the changes in grazing that took place over 15 years before the start of the period examined here played a minor role compared with changes in climatic variables. Moreover, the time intervals over which these climate changes occurred in Peñalara are similar to those noted by Peñuelas et al. (2002) for the Mediterranean Basin.
It is likely that possible consequences of climate change in the area include diminished frost damage and an extension of the period of vegetative activity (Peñuelas et al., 2002). The spread of woody vegetation towards alpine meadows favours further advances as a consequence of higher temperatures in scrubland canopies and their surroundings (Betts, 2000). This type of feed‐forward process may have favoured the spread of species typical of lower altitudes, and a change from alpine to sub‐alpine communities has been observed in many areas worldwide (Holten and Carey, 1992; Grabherr et al., 1994; Henry and Molau, 1997; Molau and Alatalo, 1998; Gottfried et al., 2000). Reduced snow cover and a redistribution of monthly rainfall could also have altered water relations at the soil surface, perhaps affecting seed dispersal and the survival of some species. For instance, J. comunis ssp. communis is sensitive to summer drought, which is considered to be the main abiotic factor regulating recruitment in high‐mountain regions of southern Spain (García, 2001). This author also points out that the number of days on which the soil and plants are covered by a layer of snow limits the availability of fruit for animal dispersal.
The small areas of Cryoro‐Mediterranean communities in the Spanish Central Range make them vulnerable to environmental change, including changes caused by global warming. Some authors predict that a mean temperature increase of 1–2 °C could be within the tolerance limits of most alpine species, although an increase of 3–4 °C would not (Körner, 1995; Theurillat, 1995). This is of particular significance in the case of endemic plants confined to high summits. At the species level, the changes observed must have not only affected Juniperus and Cytisus, but also modified the distribution of associated taxa that are less easily detectable in aerial images. Thus, if the trend observed in the present study is common to other high summits of the Mediterranean Iberian Peninsula, this could be a sign of the partial or complete disappearance, in critical cases, of many relict ecosystems and plant communities, if species are not able to adapt their life‐cycles or migrate (Gottfried et al., 2000). The immediate effect of the disappearance of the typical Cryoro‐Mediterranean zone is a clear simplification of the high‐mountain landscapes of central Spain, since many of these species are also found at lower altitudes.
In conclusion, the available evidence suggests that global warming may have triggered vegetation changes in the high Peñalara region. However, a possible synergistic effect including discrete changes in grazing practices that took place in the 19th century (Archer et al., 1995) cannot be ruled out. Whatever the cause, the pronounced change in alpine vegetation reflects the susceptibility of high‐ mountain Mediterranean species to environmental alterations. If climate change is the main factor responsible for the trend observed, a reduction in landscape diversity can be expected since most high‐peak taxa also thrive at lower altitudes.
ACKNOWLEDGEMENTS
We acknowledge the assistance provided by Professor Francisco González Bernardo (University of León, Spain) in obtaining the aerial maps of the area. We also thank Professor Elisabeth Holland (National Center for Atmospheric Research, USA) for her valuable insight into the encroachment of woody vegetation.
Supplementary Material
Received: 28 February 2003; Returned for revision: 20 March 2003; Accepted: 30 April 2003 Published electronically: 18 June 2003
References
- AcockB.1992. Effects of carbon dioxide on photosynthesis, plant growth and other processes. In: Kimball BA, Rosemberg N, Hartwell L, eds. Impact of carbon dioxide, trace gases and climate change on global agriculture Madison, Wisconsin, USA: ASA, CSSA, SSA, 156–153. [Google Scholar]
- AnsleyJA, Pinchak WE, Ueckert DN.1995. Changes in redberry juniper distribution in northwest Texas (1948–1982). Rangelands 17: 49–53. [Google Scholar]
- ArcherS, Stokes CJ.2000. Stress, disturbance and change in rangeland ecosystems. In: Arnalds O, Archer S, eds. Rangeland desertification Advances in vegetation science Vol. 19. Dordrecht: Kluwer Pub lishing Company, 17–38. [Google Scholar]
- ArcherS, Schimel DS, Holland E.1995. Mechanisms of shrubland expansion: land use, climate or CO2? Climate Change 29: 91–99. [Google Scholar]
- BellinghamPJ.1998. Shrub succession and invasibility in a New Zealand montane grassland. Australian Journal of Ecology 23: 562–573. [Google Scholar]
- BenistonM.2000.Environmental change in mountains and uplands. London: Arnold/Hodder and Stoughton: Chapman and Hall Publishers. [Google Scholar]
- BettsRA.2000. Offset of the potential carbon sink from boreal forestation by decreases in surface albedo. Nature 408: 187–190. [DOI] [PubMed] [Google Scholar]
- BrázdilR, Pudiková M, Aver I, Bohm R, Cegnar T, Tasko P, Lapin M, Gajik‐Capka M, Zaninovic K, Koleva Eet al.1996. Trends of maximum and minimum daily temperatures in central and southeastern Europe. International Journal of Climatology 16: 765–782. [Google Scholar]
- EasterlingDR, Horton B, Jones PD, Peterson TC, Karl TR, Parker DE, Salinger MJ, Razuvayev V, Plummer N, Jameson Pet al.1997. Maximum and minimum temperature trends for the globe. Science 277: 364–367. [Google Scholar]
- ErschbamerB.2001. Responses of some Austrian glacier foreland plants to experimentally changed microclimatic conditions. In: Walther GR, Burga CA, Edwards PJ, eds. Fingerprints of climate change. Adapted behaviour and shifting species range New York: Kluwer Academic/Plenum Publishers, 263–279. [Google Scholar]
- GarcíaD.2001. Effects of seed dispersal on Juniperus communis recruitment on a Mediterranean mountain. Journal of Vegetation Science 12: 839–848. [Google Scholar]
- GottfriedM, Pauli H, Reiter K, Grabherr G.2000. A fine‐scaled predictive model for climate warming‐induced changes of high mountains plant species distribution patterns. Diversity and Distribution 8: 10–21 [Google Scholar]
- GrabherrG, Gottfried M, Pauli H.1994. Climate effects on mountain plants. Nature 369: 448. [DOI] [PubMed] [Google Scholar]
- HarteJ, Shaw MR.1995. Shifting dominance within a montane vegetation community: Results from a climate‐warming experiment. Science 267: 876–880. [DOI] [PubMed] [Google Scholar]
- HenryGHR, Molau U.1997. Tundra plants and climate change: The International Tundra Experiment (ITEX). Global Change Biology 3: 1–9. [Google Scholar]
- HilbertDW, Ostendorf B, Hopkins MS.2001. Sensitivity of tropical forests to climate change in the humid tropics of north Queensland. Austral Ecology 26: 590–603. [Google Scholar]
- HoltenJI, Carey PD.1992. Responses of climate change on natural terrestrial ecosystems in Norway. NINA Institute Research Report 29: 1–59. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). 2001. Contribution of Working Groups I, II and III to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- KörnerC.1995. Impact of atmospheric changes on alpine vegetation: the ecophysiological perspective. In: Guisan A, Holten JI, Spichiger R, Tessier L, eds. Potential ecological impacts of climate change in the Alps and Fennoscandian Mountains Geneva: Conservatoire et Jardin Botaniques de la ville de Genève, 113–120. [Google Scholar]
- LeitonenI, Repo T, Hänninen H.1997. Changing environmental effects on frost hardiness of Scots pine during dehardening. Annals of Botany 79: 133–138. [Google Scholar]
- MolauU, Alatalo JM.1998. Responses of subarctic‐alpine plant communities to simulated environmental change: biodiversity of bryophytes, lichens and vascular plants. Ambio 27: 322–329. [Google Scholar]
- MontserratP, Fillat F.1990. The systems of grassland management in Spain. In: Breymeyer AI, ed. Managed grassland: regional studies Amsterdam: Elsevier, 37–70. [Google Scholar]
- MuñozJ, Sanz‐Herráiz C.1995.Guía física de España 5:Las montañas. Madrid: Alianza Editorial. [Google Scholar]
- PauliH, Gottfried M, Grabherr G.2001. High summits of the Alps in a changing climate. The oldest observation series on high mountain plant diversity in Europe. In: Walther GR, Burga CA, Edwards PJ, eds. Fingerprints of climate change. Adapted behaviour and shifting species range New York: Kluwer Academic/Plenum Publishers, 139–149. [Google Scholar]
- Peña‐MartínezJM.2002.El estudio del impacto de la contaminación atmosférica en los bosques. Organismo Autónomo de Parques Nacionales. Madrid: Ministerio de Medio Ambiente. [Google Scholar]
- PeñuelasJ, Filella I.2001. Phenology: responses to a warming world. Science 294: 793–795. [DOI] [PubMed] [Google Scholar]
- PeñuelasJ, Filella, I, Comas, Per E.2002. Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean Region. Global Change Biology 8: 531–544. [Google Scholar]
- RisserPG.1995. The status of the science examining ecotones. Bioscience 45: 318–325. [Google Scholar]
- Rivas‐MartínezS, Loidi J.1999. Bioclimatology of the Iberian Peninsula. Itinera Geobotanica 13: 41–47. [Google Scholar]
- Rivas‐MartínezS, Cantó P, Fernández‐González F, Molina JA, Pizarro JM, Puente E.1999. Synopsis of the Sierra de Guadarrama vegetation. Itinera Geobotanica 13: 189–260. [Google Scholar]
- ShawMR, Loik ME, Harte J.2000. Gas exchange and water relations of two Rocky Mountain shrub species exposed to a climate change manipulation. Plant Ecology 146: 197–206. [Google Scholar]
- SobrinoE, González A, Sanz‐Elorza M, Dana E, Sánchez‐Mata D, Gavilán R.2001. The expansion of thermophilic plants in the Iberian Peninsula as a sign of climatic change. In: Walther GR, Burga CA, Edwards PJ, eds. Fingerprints of climate change. Adapted behaviour and shifting species range New York: Kluwer Academic/Plenum Publishers, 163–184. [Google Scholar]
- TheurillatJP.1995. Climate change and the alpine flora: some perspectives. In: Guisan A, Holten JI, Spichiger R, Tessier L, eds. Potential ecological impacts of climate change in the Alps and Fennoscandian Mountains Geneva: Conservatoire et Jardin Botaniques de la ville de Genève, 121–127. [Google Scholar]
- ThornleyJHM, Cannell MGR.1997. Temperate grassland responses to climate change: an analysis using the Hurley pasture model. Annals of Botany 80: 205–221. [Google Scholar]
- VíasJ.2001.Memorias del Guadarrama: historia del descubrimiento de unas montañas. Madrid, Spain: Ediciones la Librería. [Google Scholar]
- WaynePM, Reekie EG, Bazzaz FA.1998. Elevated CO2 ameliorates birch response to high temperature and frost stress: implications for modeling climate‐induced geographic range shifts. Oecologia 114: 335–342. [DOI] [PubMed] [Google Scholar]
- ZarJH.1984.Biostatistical analysis. 2nd edn. Englewood Cliffs, New Jersey: Prentice‐Hall. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.