Abstract
Quercus seedlings have hypogeal cotyledons and tap roots, both of which act as storage organs. The importance of the storage function in the two organs may change as the seedling develops. Therefore, changes in carbohydrate reserves in cotyledons and roots of Q. crispula grown under 40 % and 3 % of full light from shoot emergence to the completion of the first leaf flush were monitored. In addition, a shoot‐clipping treatment was performed to examine the relative contribution of the cotyledons and tap roots to resprouting. Cotyledons maintained large amounts of nonstructural carbohydrates during shoot development, and carbohydrates were still present in the cotyledons during the final phase of leaf flush. In addition, a notable increase in the amount of carbohydrates was observed in tap roots before leaf flush at both light levels. Since root development occurred before leaf flush, even in plants grown under 3 % light, the carbohydrate found in them presumably originated from seed reserves and was translocated to roots as storage reserves. When shoots were clipped at the leaf flushing stage, the amount of carbohydrate decreased only in the cotyledons after resprouting, suggesting that cotyledons act as the main storage organs during shoot development stages. However, it could be advantageous as a ‘risk avoidance strategy’ for the seedlings to store reserves in both cotyledons and roots, since cotyledons may be removed by predators during shoot development.
Key words: Quercuscrispula Blume, Mizunara oak, seedling, carbohydrate storage, germination stage, hypogeous plant, resprouting, shoot destruction, TNC, seed reserves
INTRODUCTION
Quercus species have well‐developed tap roots at the seedling stage (Abrams, 1996; Larsen and Johnson, 1998), and this feature first appears in the germination‐establishment stages (Jones, 1959; Crow, 1988). In addition to helping withstand drought (Rao, 1988; Callaway, 1992; Canham et al., 1996), storage has been regarded as an important function of the tap roots in Quercus species (Jones, 1959; Matsubara and Hiroki, 1985; Crow, 1988; Larsen and Johnson, 1998). Storage functions in tap roots have been shown in seedlings older than 1 year, and saplings (Kruger and Reich, 1993; Sakai et al., 1997; Canham et al., 1999), but there have been few studies concerning the importance of the tap roots as storage organs during germination to establishment stages.
Quercus species generally have large seeds. In several large‐seeded species, a large amount of seed biomass remains in cotyledons even after the development of functional shoots. Several ecologists have argued that the biomass remaining in cotyledons provides a reserve that can be used for growth and survival of seedlings in the presence of stress factors, such as low light, frequent shoot destruction, and strong competition (Grime and Jeffrey, 1965; Janzen, 1976; Brookes et al., 1980; Westoby et al., 1992; Leishman and Westoby, 1994; Kitajima, 1996; Dalling et al., 1997; Frost and Rydin, 1997; Hoshizaki et al., 1997; Bonfil, 1998; Dalling and Harms, 1999). However, the importance of the storage function of cotyledons remains controversial. To evaluate the importance of the biomass remaining in the cotyledons several studies have been carried out, in which cotyledons were removed when seedlings had almost developed functional shoots. Some of these studies have suggested that the residual cotyledon biomass may be beneficial for the growth and survival of seedlings growing under stressful conditions (Bonfil, 1998; Dalling and Harms, 1999). In addition, Bonfil (1998) showed that, in Quercus laurina, cotyledon removal can severely reduce the survival rates of seedlings, even under moderate conditions. In studies of Q. robur, however, the results are mixed; in some cases cotyledon removal has not affected seedling growth and survival, nor regrowth after defoliation (Sonesson, 1994; Andersson and Frost, 1996), but in an investigation by Frost and Rydin (1997) cotyledon removal did reduce the growth both of seedlings subjected to defoliation and seedlings that were adversely affected by root competition.
This study is based on the hypothesis that the importance of storage in cotyledons varies, depending on the availability of storage substances in the tap roots. Therefore, to evaluate the relative importance of the storage function in cotyledons and tap roots, changes in carbohydrate storage levels in cotyledons and roots of Quercus crispula were monitored from germination to the development of a functional shoot. A shoot clipping experiment to examine the relative contributions of the reserves in cotyledons and tap roots during resprouting was also performed.
MATERIALS AND METHODS
Plant material
Quercuscrispula is a common deciduous broad‐leaved tree species in the cool temperate forests of Japan. The species shows a flush‐type shoot elongation pattern (Kikuzawa, 1983), and the seedlings’ shoots usually elongate once in spring on natural forest floors. However, amongst seedlings growing under favourable light conditions (e.g. in a gap created by a tree fall), there is often a further period of shoot elongation at some stage during the growing season. Considerable numbers of seedlings lose their shoots through herbivory and shoot dieback, especially during the autumn and the following early spring (Kabeya, 2001). Like other Quercus species, the species has hypogeous cotyledons, and the seedlings produce at least two lateral buds around the root collar (i.e. below ground). In addition, they have four or five scaly leaves with lateral buds on their epicotyl. Following shoot destruction, they can resprout from these dormant buds. Thus, above‐ground damage usually causes very little meristematic limitation in this species, although Hasegawa (1984) reported that Q. crispula seedlings could not resprout when they were clipped below the root collar.
Plant cultivation
Experiments were conducted in the Hakkoda Botanical Laboratory (40°38′N, 140°51′E, altitude 900 m) in 1999 and 2001. Acorns used in these experiments were collected from the eastern side of lake Towada (40°26′N, 140°56′E, altitude 400 m) in the autumns of 1998 and 2000 for the 1999 and 2001 experiments, respectively. Collected acorns were washed and stored in a dark cold‐room (<5 °C) until sowing. Before sowing, the fresh mass of each acorn was measured. The mean fresh mass of the acorns was 3·8 and 2·2 g in 1999 and 2001, respectively, and there was a significant difference between these years (ANOVA, F = 51·9, P < 0·001). In 1999, to estimate the contribution of the initial cotyledon dry mass to the fresh mass of the acorn, the fresh mass of 12 acorns was measured, they were then dried in an oven at 70 °C for 3 d, and re‐weighed. The relationship between the fresh mass of the acorn (including the seed coat) and the cotyledon dry mass was found to be:
cotyledon dry mass (mg) = 392·5 × acorn fresh mass (g) – 233·4 (R2 = 0·96).
One acorn was sown per pot (12 × 14 cm) filled with AKADAMA soil : sand (2 : 1, v/v). No fertilizer was supplied during the experiment.
In 1999, approx. 500 acorns were sown. To evaluate the effect of photosynthesis on the growth and amount of storage resources, pots were divided into two groups, one of which was placed in 40 % of full light, and the other in 3 % of full light using shade cloths to set the light levels. Seedlings were checked every day to determine the date of shoot emergence (when the tip of the hypocotyl emerged from the seed coat). In 2001, approx. 150 acorns were sown on 14 June 2001. To minimize the effect of the seedlings’ photosynthesis on carbohydrate storage (see Results), all of these seedlings were subjected to 3 % light.
Harvest of control seedlings
To evaluate changes in biomass distribution and the levels of carbohydrate reserves in roots and cotyledons, unclipped seedlings were harvested six times in 1999 and twice in 2001. Hereafter, these seedlings are referred to as control seedlings to distinguish them from the clipped seedlings (see below).
In 1999, most of the seedlings’ shoots emerged 10–22 d after sowing. Here, to reduce the effects of ontogenical differences, seedlings that emerged on similar dates were collected. Since the numbers of single cohort seedlings (i.e. seedlings that sprouted within a few days of each other) were insufficient for the experiment, two seedling cohorts were used. Cohorts 1 and 2 consisted of seedlings with shoots that emerged between 29 June and 2 July, and 5 July and 10 July, respectively. Seven seedlings growing under each of the two light conditions were collected in each harvest. The cohort 1 seedlings were collected on 7 July, 21 July and 12 Aug. (1, 3 and 6 weeks after shoot emergence, respectively), while the cohort 2 seedlings were harvested on 7 July, 21 July and 5 Aug. (0, 2 and 4 weeks after shoot emergence, respectively). Seedlings were selected randomly from each cohort.
At each harvest, seedlings were collected in the morning, brought back to the laboratory, separated into leaves, stems, roots, cotyledons and seed coats, and placed in an oven within 6 h of collection. The seedlings were dried at 70 °C for 3 d then weighed. After weighing, roots and cotyledons of each seedling were used for carbohydrate analysis (see below).
In 2001, shoot elongation started in almost all of the seedlings 11–13 d after sowing (25–27 June); these seedlings were regarded as a single cohort. Six control seedlings from each light treatment were collected on 19 July and 8 Aug. (3 and 6 weeks after shoot emergence, respectively). In both harvests, the same protocols were used as in 1999.
Clipping experiment
To examine the relative contribution of the reserves in the cotyledons and roots to resprouting, shoot clipping experiments were carried out in both 1999 and 2001.
In 1999, 20 seedlings of cohort 1 were selected randomly from each light treatment and their epicotyls were clipped 1·5 cm above the cotyledonary node (root collar) on 22 July (3 weeks after shoot emergence). Leaf expansion of each seedling was approx. 50 % complete at this time. Clipped seedlings were checked daily until 12 Aug. to observe whether they had resprouted. The date of bud break and the date that leaf flush began were recorded. On 12 Aug., seedlings that had resprouting shoots exceeding 0·5 cm in length, or in which leaf flush had started, were harvested. Harvested seedlings were separated into roots, cotyledons, stems, resprouting stems and resprouting leaves. They were then dried and weighed. Weighed roots and cotyledons of seven randomly selected seedlings in each light environment were used for carbohydrate analysis. The seedlings that did not resprout by 12 Aug. were checked for resprouting every 2 weeks until the end of the growing season (4 Oct.).
In 2001, clipping treatments were applied on two different dates: 19 July (the 3‐week clipping) and 8 Aug. (the 6‐week clipping). Mice gnawed the shoots of some seedlings and/or removed their cotyledons during the experimental period. For this reason, only 14 and 15 seedlings were subjected to the 3‐week clipping and 6‐week clipping treatments, respectively. On 14 Aug. (4 weeks after the 3‐week clipping) and 30 Aug. (3 weeks after the 6‐week clipping), seedlings with resprouting shoots that exceeded 0·5 cm in length or in which leaf flush had started were harvested. All seedlings that had not resprouted by the harvest times were re‐examined to see if they had begun to resprout at the end of the growing season (27 Sept.). The harvested seedlings were treated in the same way as those in the 1999 experiment. In this case, however, the roots and cotyledons of all harvested seedlings were used for carbohydrate analysis.
Carbohydrate analysis
To determine the amount of carbohydrate reserves, the concentrations of total nonstructural carbohydrate (TNC) in cotyledons and roots were analysed by enzymatic hydrolysis methods similar to those of Ono et al. (1996). Roots and cotyledons were ground using a Wiley mill, passed through a 65‐mesh screen and dried in the oven for 24 h. Samples (2–3 mg) were homogenized with 80 % ethanol. After the ethanol had been removed by evaporation, they were suspended in 0·5 ml of 0·2 m KOH and placed in boiling water for 30 min. After cooling, 2·00 ml of 1·0 m acetic acid was added. To digest starch to glucose, 0·5 ml of Rhizopus amyloglucosidase (Sigma, St Louis, USA) solution (35 units ml–1 in 50 mm Na acetate buffer, pH 4·5) was added, and the tubes were incubated at 55 °C for 30 min. After digestion, the tubes were placed in boiling water for 1 min and then centrifuged. The supernatant was transferred to another tube, and its total sugar content was determined by the phenol–sulfuric acid method (Dubois et al., 1956). Absorbance was measured at 490 nm in a spectrophotometer (UV‐16A; Shimadzu Co., Japan), using a glucose solution as a calibration standard. TNC pools, i.e. the actual amounts of TNC in the cotyledons and roots, were calculated by multiplying the concentration of TNC by the respective dry masses of these organs.
In control seedlings harvested 3 weeks after shoot emergence (and also 6 weeks after shoot emergence in 2001), and resprouting seedlings in both years, the starch concentrations of cotyledons and roots were determined as follows:
starch concentration (glucose equivalent) = glucose concentration after starch hydrolysis – glucose concentration before starch hydrolysis
where the glucose concentration before starch hydrolysis was determined from the subsamples collected from the supernatant of sample liquid before the addition of amyloglucosidase solution in the method described above. For the determination of glucose concentrations, a glucose‐oxidase kit (Glucose B test; Wako Co., Osaka, Japan) was used. Starch pools in the various organs were calculated by multiplying the concentration of starch by the respective dry masses.
Statistical analysis
The effects of light conditions and harvest time (i.e. seedling development stages) were tested on plant dry masses, TNC concentrations in cotyledons and roots, and TNC pools in cotyledons and roots. For this, two‐way ANOVA was used, with light environment (40 % and 3 %) and harvest time (0, 1, 2, 3, 4 and 6 weeks after shoot emergence) as the main effects, with subsequent Games–Howell post hoc test. In the 1999 clipping experiment, to assess the contribution of carbohydrate storage in cotyledons and roots to resprouting, two comparisons were performed. In the first, TNC (starch) concentrations and pools were measured in the two organs of the seedlings at two developmental stages: the levels at the time of clipping, and levels after resprouting (designated ‘initial’ and ‘resprouting’ levels, respectively). In the second comparison, TNC concentrations and pools were compared between treatments (i.e. between the resprouting seedlings and the unclipped (control) seedlings, both of which were harvested at the same time). For this, two‐way ANOVA was used, with light environment and seedling status (initial, resprouting and control) as the main effects, and each comparison between seedlings of differing types was tested by Dunnett post hoc tests. In the 3‐week clipping experiment in 2001, the results were also evaluated by one‐way ANOVA, with seedlings status as the main effect, to clarify the effect of resprouting on the carbohydrate stored in the cotyledons and roots. To analyse differences between TNC concentrations and pools in roots and cotyledons, the Wilcoxon sign rank test (Sokal and Rohlf, 1995) was used. To analyse differences in resprouting shoot mass between light conditions (in 1999) and clipping periods (in 2001), the Mann–Whitney U‐test was applied. SAS software (SAS 1989) was used for all statistical analyses except the Games–Howell post hoc tests, which were calculated by StatView software (SAS, 1998), and the Wilcoxon sign rank tests.
RESULTS
Control seedling development after shoot emergence
The total dry mass of the control seedlings (i.e. shoot and root dry mass, excluding cotyledons) increased as seedling development progressed, under both 40 and 3 % of full light (Fig. 1A). Leaf flushing started 2 weeks after shoot emergence and was complete within 4 weeks of shoot emergence. From 0 to 3 weeks after shoot emergence, the total dry mass of the control seedlings grown under 40 and 3 % light was similar. From 4 weeks after shoot emergence, the total dry mass of the control seedlings growing in 40 % light tended to be greater than that of seedlings in 3 % light, although there was only marginal interaction between harvest time and light (Table 1). In 3 % light, root dry mass did not increase after 2 weeks from shoot emergence (Fig. 1C). In addition, the root dry mass was greater in 40 % light than in 3 % light at 4 weeks after shoot emergence, but the shoot dry mass did not differ between the environments during the developmental period (Fig. 1B and Table 1).
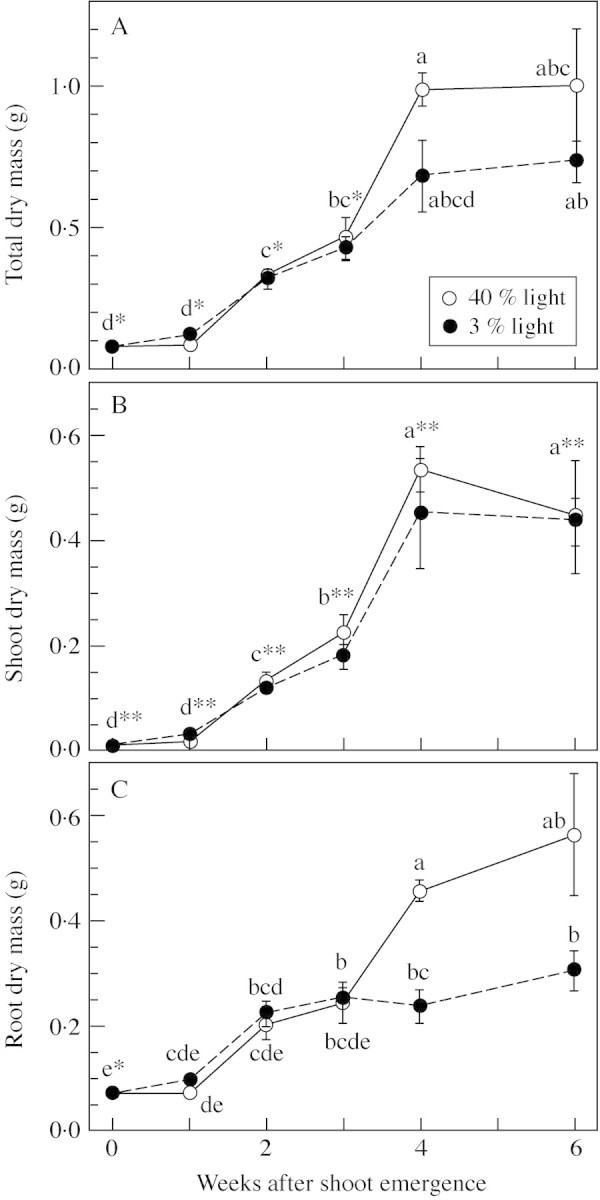
Fig. 1. Total, shoot and root dry mass of the Q. crispula seedlings growing in 40 and 3 % of full light in 1999 (mean ± s.e., n = 7) (A, B and C, respectively). Symbols with different letters are significantly different at the P < 0·05 level according to the Games–Howell test. * The same letters apply to both light symbols; ** results from the two light treatments were pooled.
Table 1.
F statistics (with probabilities) for a two‐way ANOVA testing the effects of the number of days from the start of shoot emergence to harvesting (Harvest, H), light conditions (Light, L), and their interaction, on dry mass (total, shoot and root), the percentage of cotyledon dry mass in dry mass at sowing (estimated from fresh mass), TNC concentrations in cotyledons and roots, and TNC pools in cotyledons and roots of the seedlings in 1999
| Harvest (5)* | ||||||
| F | P | Light (1)* | H × L (5)* | |||
| Dry mass | ||||||
| Total | 45·8 | <0·001 | 5·7 | 0·019 | 2·3 | 0·051 |
| Shoot | 40·2 | <0·001 | 0·9 | 0·346 | 0·3 | 0·928 |
| Root | 31·3 | <0·001 | 12·5 | 0·001 | 6·7 | <0·001 |
| Percentage of cotyledon mass | 58·7 | <0·001 | 2·1 | 0·154 | 1·6 | 0·176 |
| TNC concentrations | ||||||
| Cotyledon | 47·7 | <0·001 | 3·6 | 0·062 | 2·4 | 0·045 |
| Root | 2·7 | 0·029 | 2·9 | 0·095 | 2·8 | 0·024 |
| TNC pools | ||||||
| Cotyledon | 30·5 | <0·001 | 2·0 | 0·157 | 2·4 | 0·042 |
| Root | 16·1 | <0·001 | 12·1 | 0·001 | 5·9 | <0·001 |
* Degrees of freedom.
The dry mass of the cotyledons decreased as the seedlings developed (Fig. 2), and the rate of reduction was similar in both 40 and 3 % light (Table 1). However, 30 % of the initial dry mass remained in cotyledons 6 weeks after shoot emergence, by which time shoot development was already complete (Fig. 2).
Fig. 2. Changes in the percentage of dry mass remaining in the cotyledons (mean ± s.e., n = 7). Seedlings were grown in 40 % and 3 % of full light. The initial dry mass of the cotyledons (cotyledon dry mass at sowing) was calculated from the regression of dry mass of cotyledon on fresh mass of the acorn. Symbols with different letters are significantly different at the P < 0·05 level according to the Games–Howell test (both light conditions pooled).
Substantial concentrations of TNC were observed in the roots of the control seedlings throughout the experiment period in both 40 and 3 % light (Fig. 3). We found significant effects of harvest time on the root TNC concentrations in 40 % light (one‐way ANOVA, P = 0·013). but not in 3 % light (P = 0·124); i.e. root TNC concentrations tended to increase slightly throughout the experimental period in 40 % light, but not in 3 % light (Fig. 3). TNC concentrations in cotyledons decreased during the experimental period in both 40 and 3 % light (Fig. 3; Table 1), but the relative difference between the light environments varied at different harvest times (as shown by the significant interaction in Table 1). TNC concentrations in cotyledons were as low as those in the roots 6 weeks after shoot emergence (Wilcoxon sign rank test, P = 0·13 and 0·08 in 40 and 3 % light, respectively).
Fig. 3. TNC concentrations in cotyledons and roots of the seedlings growing in 40 and 3 % of full light in 1999 (mean ± s.e., n = 7).
The TNC pools (i.e. the amounts rather than concentrations of TNC) in the roots of the control seedlings in both 40 and 3 % light increased at similar rates for the first 2–3 weeks from shoot emergence (Fig. 4). However, after 4 weeks in 40 % light the TNC pools in the roots had continued to increase, whereas those in 3 % light grew much more slowly. The TNC pools in the cotyledons declined throughout the developmental process in both light treatments (Fig. 4), although the relative difference between the light environments varied at different harvest times (Table 1). Six weeks after shoot emergence, more than 90 % of the initial TNC pool (i.e. the TNC pool at 0 weeks) in the cotyledons had been consumed in both 40 % and 3 % light, and there was no significant difference between the TNC pools in the cotyledons and roots in the two light treatments at that time (Wilcoxon sign rank test, P = 0·08 and 0·11 in 40 and 3 % light, respectively).
Fig. 4. TNC pools (log‐scale) in cotyledons and roots of the seedlings growing in 40 and 3 % of full light in 1999 (mean ± s.e., n = 7).
Clipping treatment
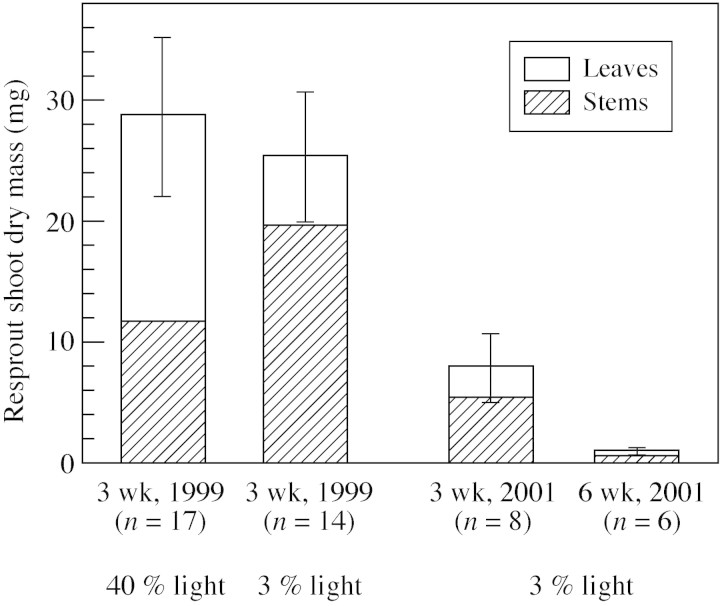
In 1999, 17 out of 20 seedlings had resprouted in 40 % light and 14 out of 20 had resprouted in 3 % light, 3 weeks after shoot clipping (12 Aug.). By the end of the growing season (4 Oct. 2001), 19 seedlings had resprouted in both 40 % and 3 % light. In 2001, eight out of 14 and five out of 15 seedlings from the 3‐week and 6‐week clipping treatments, respectively, had resprouted. At the end of the growing season (27 Sep. 2001), bud swellings were observed in one and five seedlings that had not resprouted after the 3‐week and 6‐week clippings, respectively. In 1999, there was no significant difference between the mean dry masses of resprouting shoots in the 40 % and 3 % light treatments (Mann–Whitney U‐test; P = 0·88, Fig. 5). In 2001, the dry mass of the resprouted shoots in 3 % light that had been clipped at 6 weeks was lower than that of the seedlings clipped at 3 weeks (P = 0·05, Fig. 5).
Fig. 5. Dry mass of the resprouted shoot (leaves + stems) of the seedlings in shoot clipping treatments in 1999 and 2001. Means and s.e. are shown as columns with error bars. In 1999, all seedlings were clipped 3 weeks after shoot emergence, and in 2001 seedlings growing under 3 % of full light were clipped 3 and 6 weeks after shoot emergence.
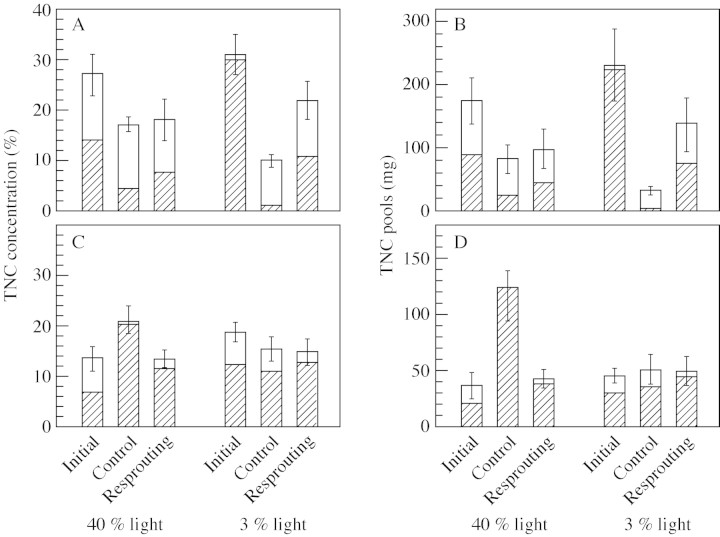
In 1999, the concentration of TNC in cotyledons of the resprouting seedlings was significantly lower than initial levels (obtained from control seedlings harvested 3 weeks after emergence) when data from seedlings in both light environments were pooled (Table 2; Fig. 6A). Although the TNC concentration in the cotyledons tended to be higher in the resprouting seedlings than the control seedlings (i.e. those harvested 6 weeks after emergence) particularly in 3 % light, this difference was not statistically significant. The TNC pools in the cotyledons showed the same trends as the TNC concentrations in the cotyledons (Fig. 6B). Although the trends shown by starch concentrations and pools in cotyledons were the same as those of TNC concentrations and pools in the cotyledons, the light environment and seedling status showed significant interactions in this case (Table 2). Furthermore, the differences between initial starch concentration and pools in cotyledons and those found in resprouting seedlings were statistically significant only for seedlings grown in 3 % light (Dunnett test, P < 0·05). In contrast, the concentrations and pools of TNC and starch in roots were not lower than initial levels at the time of resprouting in either 40 or 3 % light (Fig. 6C and D). Furthermore, in 40 % light, TNC pools, starch concentrations and starch pools in roots of resprouting seedlings were lower than those of control seedlings (P < 0·05). The results of the 3‐week clipping treatment in 2001 were similar to those obtained in 1999 (Fig. 7), but significant differences were found only in the comparison between TNC pools in cotyledons of resprouting seedlings and initial levels (Dunnett test P < 0·05).
Table 2.
F statistics (with probabilities) for a two‐way ANOVA of the effects of seedling status (Status, S) (i.e. initial, control or resprouting, described in Fig. 6), light conditions (Light, L), and their interaction, on TNC concentrations and pools in cotyledons and roots of the seedlings in 1999
| Status (2)* | ||||||
| F | P | Light (1)* | S × L (2)* | |||
| TNC concentrations | ||||||
| Cotyledon | 10·2 | <0·001 | 5·7 | 0·960 | 2·3 | 0·185 |
| Root | 1·6 | 0·224 | 11·5 | 0·888 | 6·1 | 0·081 |
| TNC pools | ||||||
| Cotyledon | 8·4 | 0·001 | 2·1 | 0·588 | 1·6 | 0·283 |
| Root | 6·8 | 0·003 | 3·8 | 0·125 | 2·3 | 0·012 |
| Starch concentrations | ||||||
| Cotyledon | 18·7 | <0·001 | 2·2 | 0·054 | 2·7 | 0·015 |
| Root | 4·6 | 0·017 | 1·8 | 0·530 | 2·4 | 0·003 |
| Starch pools | ||||||
| Cotyledon | 11·9 | <0·001 | 11·1 | 0·058 | 5·8 | 0·041 |
| Root | 9·2 | 0·001 | 0·031 | 0·001 | ||
* Degrees of freedom.
Fig. 6. TNC concentrations and pools in cotyledons and roots of the seedlings at the time of shoot clipping (initial), seedlings after resprouting (resprouting) and control (unclipped) seedlings harvested at the same time as resprouting (control) seedlings given the clipping treatment in 1999 (mean ± s.e., n = 7). Seedlings were grown under 40 and 3 % of full light. TNC is divided into starch (shaded) and other sugars (open). The graphs show TNC concentration in cotyledons (A), TNC pools in cotyledons (B), TNC concentrations in roots (C) and TNC pools in roots (D).
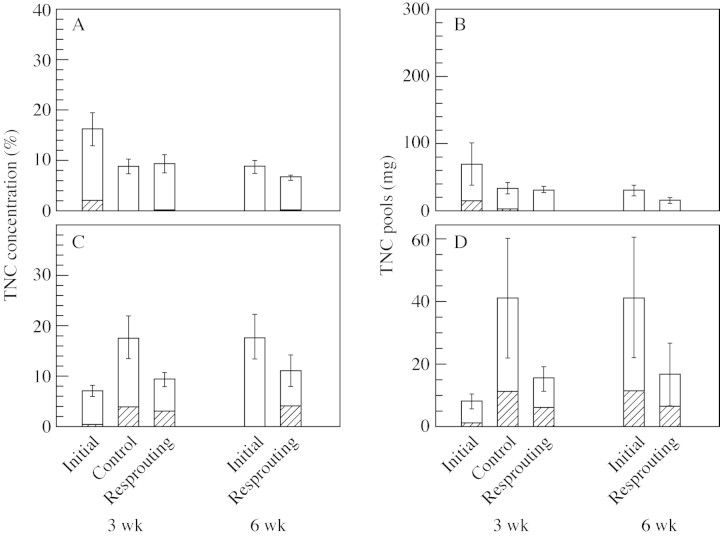
Fig. 7. TNC concentrations and pools in cotyledons and roots of the initial, control and resprouting seedlings given the clipping treatment in 2001. Means and s.e. are shown as columns with error bars. Sample sizes were six in initial and control, eight in resprouting at 3‐week clipping and five in 6‐week clipping. Seedlings were grown under 3 % of full light, and their shoots were clipped 3 and 6 weeks after shoot emergence. TNC is divided into starch (shaded) and other sugars (open). The graphs show TNC concentration in cotyledons (A), TNC pools in cotyledons (B), TNC concentrations in roots (C) and TNC pools in roots (D).
Following the 6‐week clipping, TNC concentrations in both roots and cotyledons tended to decrease after resprouting (Fig. 7). However, the detected changes were not statistically significant (Mann–Whitney U‐test, P = 0·14 and 0·18 for cotyledons and roots, respectively). Similar trends were found in TNC pools in both roots and cotyledons (P = 0·12 and 0·24 in cotyledons and roots, respectively).
DISCUSSION
Importance of the cotyledons as storage organs in the early stages of seedling development
Considerable amounts of nonstructural carbohydrate were shown to be present in the cotyledons and roots of Q. crispula during the final phase of shoot development (3–4 weeks after shoot emergence, Fig. 4). Of these carbohydrates, only those in the cotyledons decreased after resprouting (Fig. 6). This suggests that the carbohydrate reserves in the hypogeal cotyledons, rather than the roots, were used for resprouting in Q. crispula seedlings, at least when their shoots were destroyed during the developmental period. The amount of carbohydrate in cotyledons of control (unclipped) seedlings also decreased during this period (Fig. 6), but this decrease is considered to have been due to consumption during the growth of the original shoots (see Fig. 1B). This hypothesis is supported by the finding that carbohydrates in cotyledons of unclipped seedlings tended to decrease more than those of resprouting seedlings, particularly in 3 % light (Fig. 6): larger shoots of unclipped seedlings would require more reserves for development than the shoots of resprouting seedlings.
It is still unclear when the storage function of roots replaces that of cotyledons, but as much carbohydrate was present in the roots as in the cotyledons from 4 weeks after shoot emergence onwards. In the 6‐week clipping experiment, only a weak tendency was found for the carbohydrate reserves to decline, in both cotyledons and roots after resprouting, but this was probably mainly because the samples were too small to yield statistically significant results (Fig. 7). Since cotyledons of Q. crispula have withered and fallen by the second growing season (D. Kabeya, pers. obs.), the storage role of cotyledons is presumably replaced by roots within 1 year after germination.
Storage in roots during shoot development and its ecological significance
Regardless of the light environment, there were already notable amounts of carbohydrates in roots of Q. crispula seedlings when their leaf expansion phase started (Fig. 3). Since the root growth was virtually complete at that time, particularly in 3 % light (Fig. 1), this nonstructural carbohydrate is considered to be a reserve substance rather than a resource used for immediate root growth. Further more, most of these carbohydrates are believed to originate from seed resources. This is because it is unlikely that seedlings will be able to generate sufficient amounts of photosynthates to support growth and development until their shoot becomes functional and, even if they can photosynthesize, almost all of the photosynthates are used in the developing shoots (Dickson et al., 2000). Therefore, we conclude that some of the seed resources were translocated to roots and stored as reserves in an early period of seedling development.
Translocation of reserve substances from seeds to roots could have an adaptive value as a means of hedging risks. Hypogeous cotyledons of various species are often eaten by predators, such as jays and rodents (Bossema, 1979; Forget, 1992; Hoshizaki et al., 1997; Sork, 1987), and this kind of predation also occurs in Q. crispula (pers. obs.). In addition, loss of cotyledons often occurs during seedling development. Thus, the risk of seedlings losing carbohydrate reserves would be likely to increase cumulatively with time, if all the resources were contained in cotyledons. Therefore, translocation of some of these resources to roots in early periods of establishment may reduce the risk of the plants losing all their reserves through loss of their cotyledons.
A previous study showed that 80 % of Q. crispula seedlings, which stored about 20 mg of carbohydrates in their roots, successfully resprouted (Kabeya et al., 2003). In this study, 20 % of the carbohydrates, in terms of concentration (Fig. 3), and 20–50 mg in terms of pools (Fig. 4), were found in roots of the seedlings that had just started their leaf expansion phase (2 weeks after shoot emergence). This finding indicates that Q. crispula seedlings, whose shoots are destroyed after they have started leaf expansion, are able to resprout even if their cotyledons are removed. This hypothesis should be tested in cotyledon removal experiments in the future.
The decline in carbohydrate reserves in the seedlings’ cotyledons during shoot development showed very similar trends in both 40 and 3 % light (Figs 3 and 4). If reserves in cotyledons were needed for growth or survival under low light, they would be consumed in large amounts and/or exhausted earlier in seedlings growing under low light conditions than in counterparts growing under favourable light conditions, as reported by Jones (1959). Hence, the results presented here suggest that, in Q. crispula seedlings, cotyledon reserves do not contribute greatly to survival under low light conditions. This seems to conflict with the prediction that the reserves in large seeds are likely to be more important for the survival of seedlings growing under low light conditions than for similar seedlings growing in more favourable light (Fenner, 1987; Crow, 1988; Leishman and Westoby, 1994). However, it is likely that some seed resources that are translocated to roots play an important role in survival under such conditions. Quercus crispula seedlings appear to survive under heavily shaded conditions by consuming carbohydrate reserves in their roots (Kabeya et al., 2003). Since they cannot photosynthesize sufficiently to maintain themselves under such conditions (Hashimoto and Shirahata, 1995), seed reserves are expected to act as an important source of carbohydrates in the roots of established seedlings in shaded conditions.
CONCLUSIONS
The hypogeal cotyledons of Quercus crispula act as storage organs during the developmental stage, and the carbohydrate reserves stored in the cotyledons are important for resprouting after shoot removal. The roots of Q. crispula seedlings also have carbohydrate reserves, which are translocated from the cotyledons in the early phase of development. The amount of carbohydrate stored in roots increases with growth, and overtakes that in the cotyledons 2 weeks after the growth of functional shoots. However, no conclusive evidence was found for the hypothesis that carbohydrate reserves in roots play an important role in resprouting during the shoot developmental period.
ACKNOWLEDGEMENTS
We thank Akiko Sakai and Kiyoshi Matsui for helpful suggestions during this study, Drs Elizabeth A. Newell and Consuelo Bonfil for their constructive comments, Kenichi Sato for plant cultivation, and Koji Yonekura for providing us with facilities in the Hakkoda Botanical laboratory.
Supplementary Material
Received: 20 January 2003; Returned for revision: 23 April 2003; Accepted: 10 June 2003 Published electronically: 7 August 2003
References
- AbramsMD.1996. Distribution, historical development and ecophysiological attributes of oak species in the eastern United States. Annales des Sciences Forestieres 53: 487–512. [Google Scholar]
- AnderssonC, Frost I.1996. Growth of Quercus robur seedlings after experimental grazing and cotyledon removal. Acta Botanica Neerlandica 45: 85–94. [Google Scholar]
- BonfilC.1998. The effects of seed size, cotyledon reserves, and herbivory on seedling survival and growth in Quercus rugosa and Q laurina (Fagaceae). American Journal of Botany 85: 79–87. [PubMed] [Google Scholar]
- BossemaI.1979. Jays and oaks: an eco‐ethological study of a symbiosis. Behaviour 70: 1–117. [Google Scholar]
- BrookesPC, Wigston DL, Bourne WF.1980. The dependence of Quercus robur and Quercus petrata seedlings on cotyledon potassium, magnesium, calcium, and phosphorous during the first year of growth. Forestry 53: 167–177. [Google Scholar]
- CallawayRM.1992. Morphological and physiological responses of three California oak species to shade. International Journal of Plant Sciences 153: 434–441. [Google Scholar]
- CanhamCD, Berkowitz AR, Kelly VR, Lovett GM, Ollinger SV, Schnurr J.1996. Biomass allocation and multiple resource limitation in tree seedlings. Canadian Journal of Forest Research 26: 1521–1530. [Google Scholar]
- CanhamCD, Kobe RK, Latty EF, Chazdon RL.1999. Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia 121: 1–11. [DOI] [PubMed] [Google Scholar]
- CrowTR.1988. Reproductive mode and mechanisms for self‐replacement of northern red oak (Quercus rubra) – a review. Forest Science 34: 19–40. [Google Scholar]
- DallingJW, Harms KE.1999. Damage tolerance and cotyledonary resource use in the tropical tree Gustavia superba. Oikos 85: 257–264. [Google Scholar]
- DallingJW, Harms KE, Aizprua R.1997. Seed damage tolerance and seedling resprouting ability of Prioria copaifera in Panama. Journal of Tropical Ecology 13: 481–490. [Google Scholar]
- DicksonRE, Tomlinson PT, Isebrands JG.2000. Partitioning of current photosynthate to different chemical fractions in leaves, stems, and roots of northern red oak seedlings during episodic growth. Canadian Journal of Forest Research 30: 1308–1317. [Google Scholar]
- DuboisM, Gilles KA, Hamilton PA, Rebers PA, Smith F.1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28: 350–356. [Google Scholar]
- FennerM.1987. Seedling. New Phytologist 106 (Suppl.): 35–47. [Google Scholar]
- ForgetP‐M.1992. Seed removal and seed fate in Gustavia superba. (Lecythidaceae). Biotropica 24: 408–414. [Google Scholar]
- FrostI, Rydin H.1997. Effects of competition, grazing and cotyledon nutrient supply on growth of Quercus robur seedlings. Oikos 79: 53–58. [Google Scholar]
- GrimeJP, Jeffrey DW.1965. Seedling establishment in vertical gradients of sunlight. Journal of Ecology 53: 621–642. [Google Scholar]
- HasegawaS.1984. Basic studies on the conservation of natural coastal forests in Hokkaido. Research Bulletin of the College Experiment Forests, Hokkaido University 41: 312–422. [Google Scholar]
- HashimotoR, Shirahata M.1995. Comparative study of leaf carbon gain in saplings of Thujopsis dolabrata var. hondai and Quercus mongolica var. grosseserrata in a cool‐temperate deciduous forest. Ecological Research 10: 53–64. [Google Scholar]
- HoshizakiK, Suzuki W, Sakai S.1997. Impacts of secondary seed dispersal and herbivory on seedling survival in Aesculus turbinata Journal of Vegetation Science 8: 735–742. [Google Scholar]
- JanzenDH.1976. Reduction of Mucuna andreana (Legminosae) seedling fitness by artificial seed damage. Oecologia 94: 356–360. [Google Scholar]
- JonesEW.1959.Quercus L. Biological flora of the British Isles. Journal of Ecology 47: 169–222. [Google Scholar]
- KabeyaD.2001.Adaptive significance of carbohydrate storage on resprouting ability in seedlings of Quercus crispula, a deciduous broad‐leaved tree. PhD Dissertation, Tohoku University, Sendai. [Google Scholar]
- 23.KabeyaD, Sakai, A, Matsui, K, Sakai, S.2003. Resprouting ability of Quercus crispula seedlings depends on the vegetation cover of their microhabitats. Journal of Plant Research, 116: 207–216. [DOI] [PubMed] [Google Scholar]
- KikuzawaK.1983. Leaf survival of woody plants in deciduous broad‐leaved forests. 1. Tall trees. Canadian Journal of Botany 61: 2133–2139. [Google Scholar]
- KitajimaK.1996. Cotyledon functional morphology patterns of seed reserve utilization and regeneration niches of tropical tree seedlings. In: Swaine MD ed. The ecology of tropical forest tree seedlings. New York: Parthenon Publishing Group, 193–210. [Google Scholar]
- KrugerEL, Reich PB.1993. Coppicing affects growth, root: shoot relations and ecophysiology of potted Quercus rubra seedlings. Physiologia Plantarum 89: 751–760. [Google Scholar]
- LarsenDR, Johnson PS.1998. Linking the ecology of natural oak regeneration to silviculture. Forest Ecology and Management 106: 1–7. [Google Scholar]
- LeishmanMR,Westoby M.1994. The role of large seed size in shaded conditions: experimental evidence. Functional Ecology 8: 205–214. [Google Scholar]
- MatsubaraT, Hiroki S.1985. Ecological studies on the plants of Fagaceae. IV. Reserve materials in roots and growth until five years old in Quercus variabilis Japanese Journal of Ecology 35: 329–336. [Google Scholar]
- OnoK, Terashima I, Watanabe A.1996. Interaction between nitrogen deficit of a plant and nitrogen content in the old leaves. Plant and Cell Physiology 37: 1083–1089. [Google Scholar]
- RaoPB.1988. Effects of environmental factors on germination and seedling growth in Quercus floribunda and Cupressus torulosa tree species of central Himalaya India. Annals of Botany 61: 531–540. [Google Scholar]
- SakaiA, Sakai S, Akiyama F.1997. Do sprouting tree species on erosion‐prone sites carry large reserves of resources? Annals of Botany 79: 625–630. [Google Scholar]
- SAS1989SAS/STAT User’s guide (var. 6.0). Cary, NC: SAS Institute. [Google Scholar]
- SASStatView (var. 5·2). Cary, NC: SAS Institute. [Google Scholar]
- SokalRF, Rohlf FJ1995Biometry, 3rd edn. New York: Freeman. [Google Scholar]
- SonessonLK.1994. Growth and survival after cotyledon removal in Quercus robur seedlings, grown in different natural soil types. Oikos 69: 65–70 [Google Scholar]
- SorkVL.1987. Effects of predation and light on seedling establishment in Gustavia superba Ecology 68: 1341–1350 [Google Scholar]
- WestobyM, Jurado E,Leishman MR.1992. Comparative evolutionary ecology of seed size. Trends in Ecology and Evolution 7: 358–372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.