Abstract
An analysis was carried out on the length, diameter and number of leaves, and the ratios between these variables for current‐year growth units (sibling growth units) derived from different nodes of previous‐year growth units (parent growth units) of young Nothofagus dombeyi and Nothofagus pumilio trees. Changes in sibling growth unit length, diameter, and number of leaves with position on the parent growth unit were assessed. In both species, sibling‐growth unit morphology varied according to both the axis type of the parent growth unit and the position of the sibling growth unit on its parent growth unit. For the largest parent growth units, the length, diameter and number of leaves of their sibling growth units decreased from distal to proximal positions on the parent growth unit. Distal sibling growth units had a more slender stem and longer internodes than proximal sibling growth units. Sibling growth units in equivalent positions tended to have a more slender stem for N. dombeyi than for N. pumilio. Long main‐branch growth units of N. pumilio had longer internodes than those of N. dombeyi; the converse was true for shorter growth units. The growth unit diameter/leaf number ratio was consistently higher for N. pumilio than for N. dombeyi. Nothofagus pumilio axes would go through a faster transition from an ‘exploring’ morphology to an ‘exploiting’ morphology than N. dombeyi axes. Within‐ and between‐species variations in growth unit morphology should be considered when assessing the adaptive value of the branching pattern of plants.
Key words: Architecture, axis differentiation, branching pattern, growth unit, morphogenetic gradients, Nothofagus dombeyi, Nothofagus pumilio, tree
INTRODUCTION
Plants may be regarded as modular organisms consisting of linear sequences of nodes and internodes. Much of the inter‐specific variation in the aerial structure of plants relates to the branching pattern resulting from the specific way in which old structural units generate new ones. Because of its involvement in the display and function of leaves, the branching pattern is regarded as relevant for the adaptation of plants to different conditions (Horn, 1971; Brunig, 1976; Pickett and Kempf, 1980; Watkinson, 1988). Most studies on branching patterns have focused on the role of environmental factors on these patterns. A number of studies on the branching pattern of perennial herbs have dealt with the morphological features of their component structural units, such as branching frequency, number of leaves and internode length (Sutherland and Stillman, 1988; Watkinson, 1988, and references therein). On the contrary, branching patterns of woody plants have mostly been described, partly because of their complexity, from a broader scale, without delving into the morphological features of their structural units (e.g. Pickett and Kempf, 1980; Steingraeber and Waller, 1986; Bertram, 1989; Harris and Bassuk, 1993).
A more botanically minded perspective of branching patterns integrates plant morphology and ontogeny (Hallé et al., 1978; Barthélémy et al., 1989, 1997). In this approach, structural units derived from the activity of primary meristems are identified and differentiated according to the position on the plant of the meristem concerned and the ontogenetic stage of the plant. In many plants with rhythmic growth each structural unit resulting from an uninterrupted event of primary growth may be termed ‘growth unit’ (GU; Caraglio and Barthélémy, 1997). An annual shoot, i.e. the axis portion extended in 1 year, may consist of one or more than one GU. Under this perspective, the most important sources of inter‐species differences in branching pattern are the time at which GUs develop from a particular portion of an axis, and the relationships between the position of a GU and its size and form (Caraglio and Barthélémy, 1997). Systematic variations in the size of GUs simultaneously formed from a common parent GU (hereafter referred to as ‘sibling GUs’) have been described either qualitatively (e.g. Parker and Johnson, 1987; Caraglio and Barthélémy, 1997; Thiébaut et al., 1997) or quantitatively (e.g. McCurdy and Powell, 1987; Costes et al., 1992; Honda et al., 1997; Hatta et al., 1999; Stecconi et al., 2000; Sabatier and Barthélémy, 2001) for some woody species. Within‐species variations in the morphology of GUs depending on their architectural position and the developmental stage of their bearing plant have led to the idea that plants consist of different types of axes and then to the concept of ‘axis differentiation’ (Hallé et al., 1978). The more differentiated an axis, the more limited the morphology of the GUs it would be able to develop. The effects of environmental factors on GU morphology would be limited by the differentiation level of the axis concerned (see Remphrey and Powell, 1984; de Reffye et al., 1991a, b, 1997, 1999). For a small number of tree species, the endogenous rules of the growth and branching of different axis types have been deeply investigated, enabling the development of the precise mathematical models and computer simulations of trees now being applied in forestry, agronomy and fruit production (Godin et al., 1999; Godin, 2000; Seleznyova et al., 2002; Heuret et al., 2003). The application of this approach to the study of branching patterns may help to relate axis differentiation to the functional properties of GUs. Moreover, our understanding of the adaptive value and evolution of branching patterns may be largely improved by comparing the branching patterns of related species. The latter may only be achieved with the support of previous knowledge of the types of axes each of the species to be compared is able to develop (e.g. Grosfeld, 2002; Heuret, 2002).
The basic morphology of GUs of Nothofagus species has been investigated in recent years (Puntieri et al., 1998, 2000, 2002; Souza et al., 2000; Stecconi et al., 2000). However, within‐ and between‐species variations in the morphology and branching pattern of GUs of saplings of these species had not been reported so far. In the present study the branching patterns of two Nothofagus (Nothofagaceae) species are compared from this perspective. For sibling GUs, the length, basal diameter and number of leaves (i.e. size descriptors) and the ratios between these parameters (i.e. form descriptors) after extension were analysed. The effects are assessed of (a) the size and position on the tree of the parent GUs and (b) the position on their parent GUs of the sibling GUs on sibling‐GU size and form.
MATERIALS AND METHODS
Study species and sampling sites
Nothofagus dombeyi (Mirb.) Oersted and N. pumilio (Poepp. et Endl.) Krasser are among the most important tree species of southern South America in terms of forest area (Veblen et al., 1996). The evergreen N. dombeyi is a tall tree (up to 40 m high) which forms dense stands in the most humid areas of the northern‐central Andes of Patagonia, between 400 and 1200 m altitude and between 39°25′ and 45°40′S (Correa, 1984). Nothofagus pumilio is a winter‐deciduous species which may reach 35 m in height at low altitude (between sea level and ∼1600 m, depending on the latitude). It adopts a bushy form at the high‐altitude timberline along the Andes from northern (38°S) to southern Patagonia (55°S). Extensive abutting between N. dombeyi and N. pumilio forests occurs but mixed stands with both species are uncommon (Donoso, 1994).
Plants of both species have a stage of fast height growth between about 5 and 30 years after emergence (Barthélémy et al., 1999), with a well‐defined, vigorous vertical axis or trunk (Fig. 1). Axes derived from the trunk show a high degree of variation in size. In order to analyse the architecture of these species at this stage of development, a categorization of axes other than the trunk has been proposed (Barthélémy et al., 1999; Puntieri et al., 1999; Fig. 1): (a) main branches are slanted or horizontal axes formed at the distal end of trunk GUs; (b) secondary branches are horizontal axes which derive from the distal end of main‐branch GUs as well as from intermediate nodes of trunk GUs; (c) short branches are horizontal or slanted axes derived from the proximal end of trunk and main‐branch GUs and from any positions on secondary‐branch GUs. All of these types of axes extend by means of the production of an annual shoot consisting on one GU. The production of a second GU in an annual shoot of these axes may occur after the traumatic death of the apex (Puntieri et al., 1998). The apex of a GU may persist after GU extension. Far more commonly, apex death occurs after GU extension; axis growth in the following year takes place through the development of a relay GU derived from one of the most distal axillary buds of the previous year’s GU. On each GU of both species, each of the one to four most proximal nodes bears a cataphyll and each of the other nodes (which vary in number from 2 up to about 30) bears a green leaf. Internodes between cataphylls are usually very short (<1 mm), whereas those between green leaves vary in length from <1 mm to 30 mm. Most usually a GU only develops branches 1 year after its extension and these branches derive from the axillary buds of green leaves. The present study concerned main branches (MB), secondary branches (SCB) and short branches (SHB). Trunk GUs were not included in the present study because of their high morphological variability in N. dombeyi (Puntieri et al., 1998).
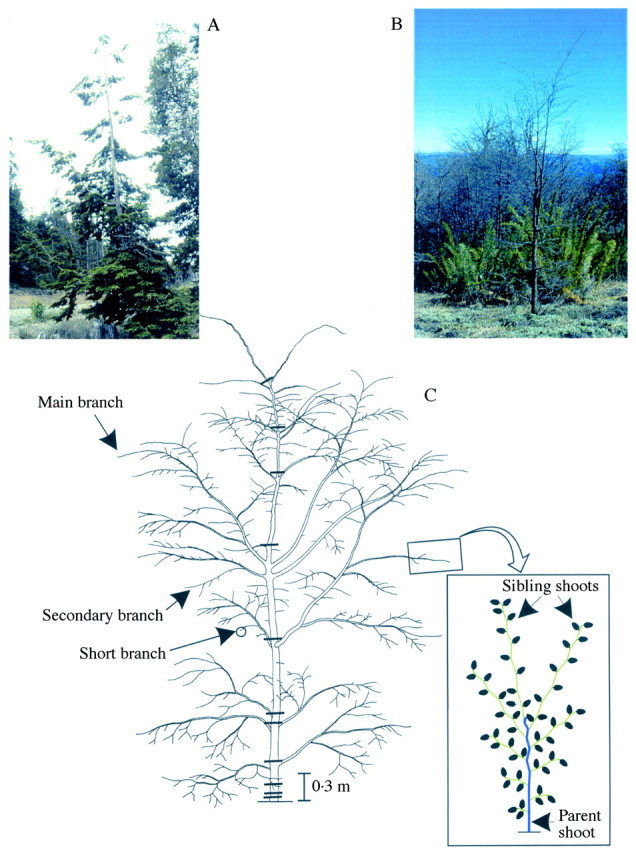
Fig. 1. Young trees of N. dombeyi (A) and N. pumilio (B) such as those selected for the present study. C, Diagrammatic representation of a Nothofagus tree in which main branches, secondary branches and short branches are indicated. Thick horizontal lines on the tree trunk indicate limits between two successive annual growth units. A parent growth unit (green stem) and its sibling growth units (green stem with black leaves) are shown in the detail.
For each species, 52 trees were randomly selected from a population of homogeneous, healthy and unshaded trees in north‐western Patagonia, Argentina. Tree height, basal diameter and age (determined by counting annual shoots along the trunk) are described in Table 1. In this region, precipitation is mainly in autumn and winter (Conti, 1998). Soils derive mostly from volcanic ash (Scoppa, 1998). Nothofagus dombeyi trees were sampled from a population located along 1000 m of roadside between the localities of San Carlos de Bariloche and Villa Mascardi (41°10′S, 71°10′W, 850 m altitude), where mean annual precipitation reaches about 1000 mm (Conti, 1998). The population sampled is a natural regeneration of the mixed Austrocedrus chilensis (Cupressaceae)–N. dombeyi forest present in this area before road construction. The N. pumilio trees selected were growing in large gaps within a 10 ha site at Cerro Otto, San Carlos de Bariloche (41°09′S, 71°10′W, 1350 m altitude), where adult N. pumilio trees were scattered. Precipitation in this area reaches 800 mm (Conti, 1998). The sampling sites were about 20 km apart. It was considered that the sampled trees were, for each species, representative of young trees growing in full‐sun conditions.
Table 1.
Mean, standard error (s.e.), maximum (max.) and minimum (min.) height, basal diameter (diameter) and age of Nothofagus dombeyi and N. pumilio trees selected for the present study
| Mean | s.e. | Max. | Min. | |
| N. dombeyi | ||||
| Height (m) | 4·2 | 0·1 | 5·3 | 3·4 |
| Diameter (mm) | 66·3 | 2·0 | 95 | 37 |
| Age (years) | 12·6 | 0·2 | 15 | 10 |
| N. pumilio | ||||
| Height (m) | 4·1 | 0·1 | 5·7 | 3·0 |
| Diameter (mm) | 69·8 | 1·8 | 103 | 33 |
| Age (years) | 14·9 | 0·2 | 20 | 12 |
n = 52 for each species.
For each axis type (i.e. MB, SCB and SHB) of each tree, one annual shoot consisting of one GU extended in the 1996–97 growing season was collected in April 1998 (Fig. 1). By the time of sampling, each of these GUs (parent GUs) was bearing a set of sibling GUs extended in the 1997–98 growing season. In order to homogenize the sample, only parent GUs derived from axillary buds, with a dead apex and devoid of intra‐annual branches were included in this study. Only GUs positioned between 1 and 2 m high in the trees and without evident damage by herbivores were chosen. These restrictions in GU selection were considered necessary in view of previous results on GU growth and size variability (see Puntieri et al., 1998).
By the time of sampling, the size of each parent GU and each of their sibling GUs was measured in terms of stem length, basal stem diameter and number of leaves. Length was measured to the nearest millimetre with a measuring tape and diameter was measured to the nearest 0·1 mm with digital callipers. Because of the relatively invariable number of cataphylls per GU (two or three for N. dombeyi and four for N. pumilio; Barthélémy et al., 1999; Puntieri et al., 2000, 2002; Souza et al., 2000) and the consistently short internodes separating them, irrespective of GU size, only green leaves were considered in the number of leaves per GU. The ratios length : diameter (= stem slenderness), length : number of leaves (= internode length) and diameter : number of leaves were computed as measures of GU form.
Statistical analyses
Parent‐GU size and form descriptors were compared between species for each axis type by means of one‐way ANOVA on log‐transformed data (Sokal and Rohlf, 1981). To compare the size and form of sibling GUs, a mean value for each variable was obtained for the sibling GUs in ranges of five node ranks counted from the 6th node (on a proximal‐to‐distal basis) on the parent GU (the five most proximal nodes usually do not develop branches in these species). The resulting ranges of node ranks and their representation for each axis type of each species were as follows: 6–10, all axis types of both species; 11–15, MB and SCB of both species, SHB of N. dombeyi; 16–20, MB and SCB of both species; 21–25, MB of both species, SCB of N. dombeyi; 26–30, MB of N. dombeyi. Comparisons among sibling GUs in different positions within each species and axis type were performed with one‐way ANOVA (Sokal and Rohlf, 1981).
To quantify the rate of change in sibling GU length, diameter and number of leaves along each parent GU, the slope of the least‐squares regression line relating the magnitude of each of these variables (dependent variable) to the rank number of the sibling GU (independent variable; rank number = 1 for the most proximal branch) was computed for each parent GU with at least three sibling GUs. All sibling GUs developed from node rank 6 upwards were considered in line‐fitting. In some cases somewhat better fittings were reached with second‐ or third‐order polynomials or power functions, but it was considered that the low degree of improvement achieved did not justify the subsequent increase in the number of terms and/or the complexity of the function. Linear regressions were always significant and judged appropriate given the wide range of sizes of the parent GUs. Regression slopes were averaged for each axis type of each species, separating parent GUs according to their size assessed by their number of nodes (6–10, 11–15, 16–20, 21–25 and 26–30 nodes). Mean slopes were compared between parent GUs of different size for each species and each axis type with one‐way ANOVA followed by a posteriori Tukey–Kramer tests. Comparisons between species and parent GU sizes were performed for each axis type by means of two‐way ANOVA (GLM procedure for unbalanced designs; Sokal and Rohlf, 1981). In the latter case, only parent‐GU sizes represented in both species were compared for each axis type.
RESULTS
Size and form of parent GUs
For both species, parent GU length, diameter and number of leaves decreased following the sequence: MB, SCB and SHB (Table 2). Parent GU length and number of leaves were higher for N. dombeyi than for N. pumilio for each axis type. Parent GU diameter was similar for both species in the case of MB and SCB. SHB were significantly thicker for N. pumilio than for N. dombeyi. For each axis type, the stem of N. dombeyi GUs was more slender than that of N. pumilio GUs (more notably so in the case of SHB). Internode length was significantly higher for N. dombeyi than for N. pumilio in the case of SCB and SHB and similar for both species in the case of MB. The diameter : number of leaves ratio was significantly higher for N. pumilio than for N. dombeyi for all three axis types (Table 2).
Table 2.
Morphological traits of parent growth units of main branches (MB), secondary branches (SCB) and short branches (SHB) of Nothofagus dombeyi and N. pumilio trees.
| Nothofagus dombeyi | Nothofagus pumilio | ||||||
| Mean | s.e. | n | Mean | s.e. | n | F‐test | |
| Length (mm) | |||||||
| MB | 241·1 | 18·4 | 42 | 143·9 | 8·8 | 51 | 25·4*** |
| SCB | 100·8 | 5·5 | 50 | 53·1 | 5·1 | 52 | 42·3*** |
| SHB | 26·4 | 1·8 | 50 | 3·4 | 0·3 | 51 | 158·1*** |
| Diameter (mm) | |||||||
| MB | 4·42 | 0·20 | 42 | 4·71 | 0·18 | 51 | 0·8 ns |
| SCB | 2·48 | 0·07 | 52 | 2·63 | 0·08 | 52 | 0·2 ns |
| SHB | 1·47 | 0·03 | 50 | 1·65 | 0·04 | 51 | 17·4*** |
| No. of leaves | |||||||
| MB | 18·1 | 1·0 | 42 | 11·8 | 0·6 | 51 | 32·0*** |
| SCB | 11·9 | 0·4 | 50 | 6·9 | 0·4 | 52 | 74·3*** |
| SHB | 6·4 | 0·3 | 50 | 3·0 | 0·0 | 51 | 125·9*** |
| Stem slenderness (length: diameter) | |||||||
| MB | 5·27 | 0·28 | 42 | 3·03 | 0·11 | 51 | 63·2*** |
| SCB | 3·96 | 0·16 | 50 | 1·90 | 0·15 | 52 | 92·3*** |
| SHB | 1·72 | 0·08 | 50 | 0·20 | 0·01 | 51 | 343·1*** |
| Internode length (length: no. of leaves) | |||||||
| MB | 1·30 | 0·05 | 42 | 1·22 | 0·04 | 51 | 2·0 ns |
| SCB | 0·84 | 0·03 | 50 | 0·71 | 0·04 | 52 | 5·5* |
| SHB | 0·40 | 0·01 | 50 | 0·11 | 0·01 | 51 | 253·7*** |
| Diameter: no. of leaves | |||||||
| MB | 0·26 | 0·01 | 42 | 0·42 | 0·02 | 51 | 72·1*** |
| SCB | 0·22 | 0·01 | 50 | 0·39 | 0·01 | 52 | 131·0*** |
| SHB | 0·24 | 0·01 | 50 | 0·57 | 0·01 | 51 | 823·0*** |
Mean, standard error (s.e.) and sample size (n) are indicated.
The results of inter‐species comparisons are indicated (F‐tests).
***, P < 0·001; *, P < 0·05; ns, P > 0·05.
Size and form of sibling GUs
Variation according to position on the parent GU.
In the case of MB of both species and SCB of N. dombeyi, the length, diameter and number of leaves of sibling GUs increased from proximal (6–10) to intermediate (16–20) positions on the parent GU and tended to be constant between intermediate and distal positions (Fig. 2; Table 3). A similar pattern of variation was found for stem slenderness and internode length of the same GUs (Fig. 3A and B; Table 3). The ratio between stem diameter and number of leaves of sibling GUs tended to decrease from proximal to distal positions on the parent GUs for MB and SCB of N. dombeyi and MB of N. pumilio (Fig. 3C; Table 3)
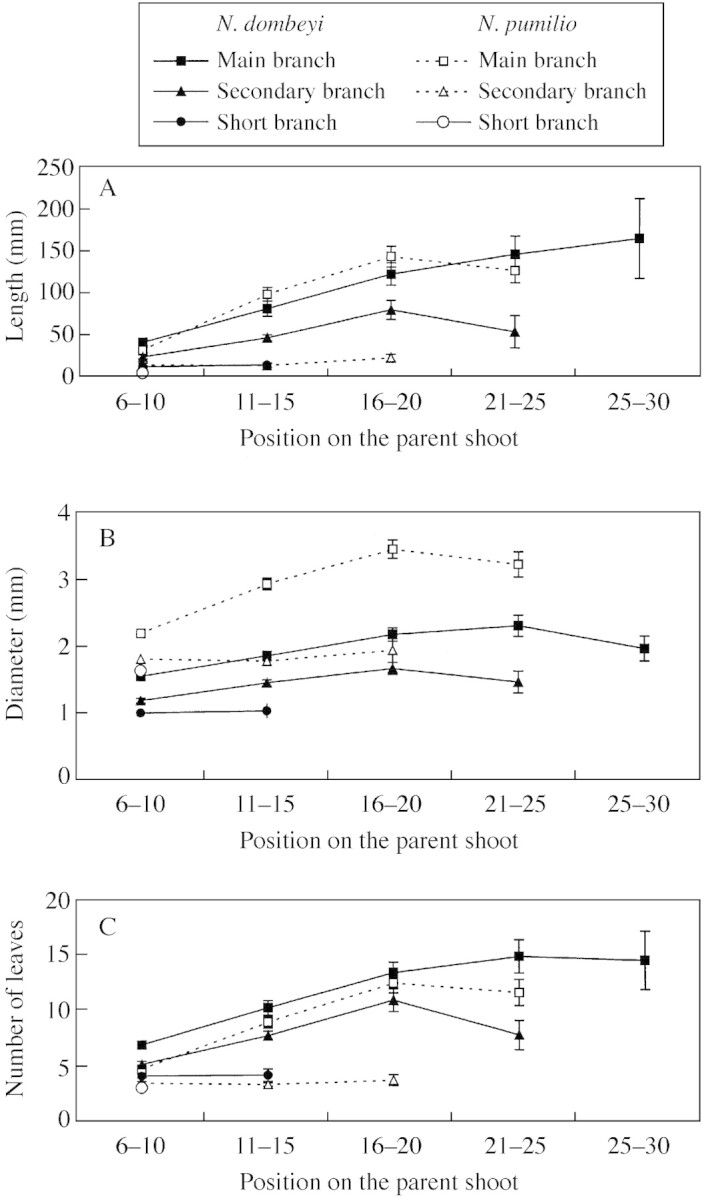
Fig. 2. Mean (±1 s.e.) length (A), basal diameter (B) and number of leaves (C) of sibling growth units of N. dombeyi (closed symbols) and N. pumilio (open symbols) developed in different positions on their parent growth units and grouped according to the rank number of the parent growth unit nodes (counted from the parent growth unit’s proximal end) from which sibling growth units derived. Data corresponding to parent growth units of main branches, secondary branches and short branches are shown separately.
Table 3.
Results of one‐way ANOVA comparisons (F, Fisher’s statistic; P, probability of error) of morphological traits between sibling growth units in different positions on their parent growth units, for each species (N. dombeyi and N. pumilio) and axis type
| N. dombeyi | N. pumilio | |||
| Axis type | F | P | F | P |
| Length | ||||
| MB | 13·4 | *** | 38·3 | *** |
| SCB | 21·9 | *** | 0·7 | ns |
| SHB | 0·5 | ns | ‐ | ‐ |
| Diameter | ||||
| MB | 10·4 | *** | 34·3 | *** |
| SCB | 13·3 | *** | 0·5 | ns |
| SHB | 0·2 | ns | ‐ | ‐ |
| No. of leaves | ||||
| MB | 14·7 | *** | 38·1 | *** |
| SCB | 22·2 | *** | 0·3 | ns |
| SHB | 0·0 | ns | ‐ | ‐ |
| Stem slenderness | ||||
| MB | 15·2 | *** | 42·8 | *** |
| SCB | 30·1 | *** | 1·2 | ns |
| SHB | 0·5 | ns | ‐ | ‐ |
| Internode length | ||||
| MB | 9·0 | *** | 40·7 | *** |
| SCB | 16·2 | *** | 2·3 | ns |
| SHB | 1·2 | ns | ‐ | ‐ |
| Diameter: no. of leaves | ||||
| MB | 8·2 | *** | 28·5 | *** |
| SCB | 4·9 | ** | 0·2 | ns |
| SHB | 0·0 | ns | ‐ | ‐ |
MB, main branches; SCB secondary branches; SHB, short branches.
***, P < 0·001; **, P < 0·01; ns, P > 0·05.
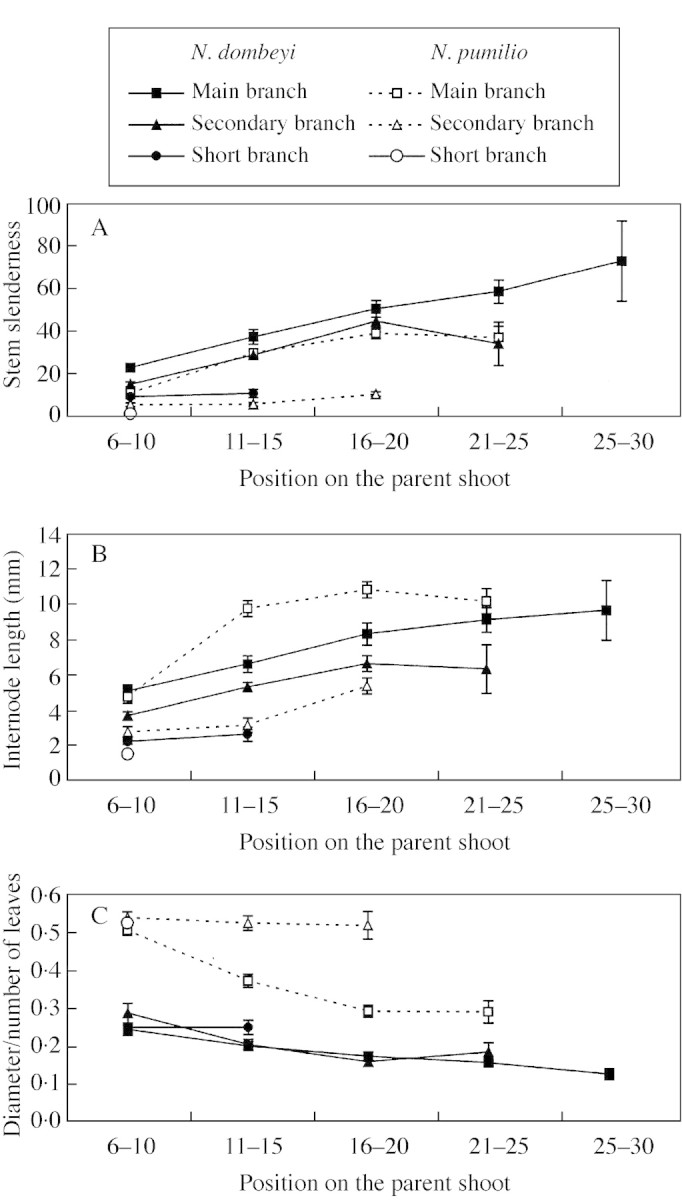
Fig. 3. Mean (±1 s.e.) stem slenderness (length : diameter; A), internode length (length : number of leaves; B) and diameter : leaf number ratio (C) for sibling growth units of N. dombeyi (closed symbols) and N. pumilio (open symbols) developed in different positions on their parent growth units. Other details as in Fig. 2.
For SCB of N. pumilio and SHB of N. dombeyi, sibling GU length, diameter and number of leaves did not vary with sibling GU position on the parent GU. Stem slenderness, internode length and the diameter/number of leaves ratio did not vary significantly for SCB sibling GUs of N. pumilio (Fig. 3; Table 3).
All parent GUs of SHB of N. pumilio developed only one sibling GU derived from their most distal node.
Inter‐species differences in sibling‐GU size and form.
MB sibling GUs of both species in similar position on the parent GU were similar in terms of length; those of N. pumilio tended to be thicker and to have fewer leaves than those of N. dombeyi (Fig. 2). MB sibling GUs of N. dombeyi were more slender than those of N. pumilio, whereas internode length (except in the most proximal positions) and the diameter : number of leaves ratio were higher for N. pumilio than for N. dombeyi (Fig. 3).
In the case of SCB and SHB, the length and number of leaves of sibling GUs were higher for N. dombeyi than for N. pumilio for all positions; the converse was true for the diameter (Fig. 2). Sibling GUs of SCB and SHB of N. dombeyi were more slender and had longer internodes than their respective counterparts of N. pumilio in similar parent‐GU positions (Fig. 3A and B). The diameter : number of leaves ratios of SCB and SHB were notably higher for N. pumilio than for N. dombeyi (Fig. 3C).
Slope of sibling‐GU size variation with position on the parent GU.
In the case of MB of each species, the slope of change in sibling‐GU length was significantly higher for parent GUs with less leaves than for those with more leaves (Fig. 4; Table 4). Similar tendencies were found for the diameter and number of leaves of MB, but differences among means were not significant. For MB, between‐species differences in the slope of change among sibling GUs (when considering the effect of parent GU size) were significant only in the case of diameter (N. pumilio > N. dombeyi; Fig. 4; Table 4). With regards to SCB, the slopes of change in length, diameter and number of leaves were significantly higher for smaller than for larger parent GUs of N. pumilio and did not vary significantly with parent GU size in the case of N. dombeyi. The slopes of change in length, diameter and, more notably, in number of leaves of SCB sibling GUs were, on average, higher for N. dombeyi than for N. pumilio. For SHB of N. dombeyi, the slopes of change in length, diameter and number of leaves did not vary with parent GU size.
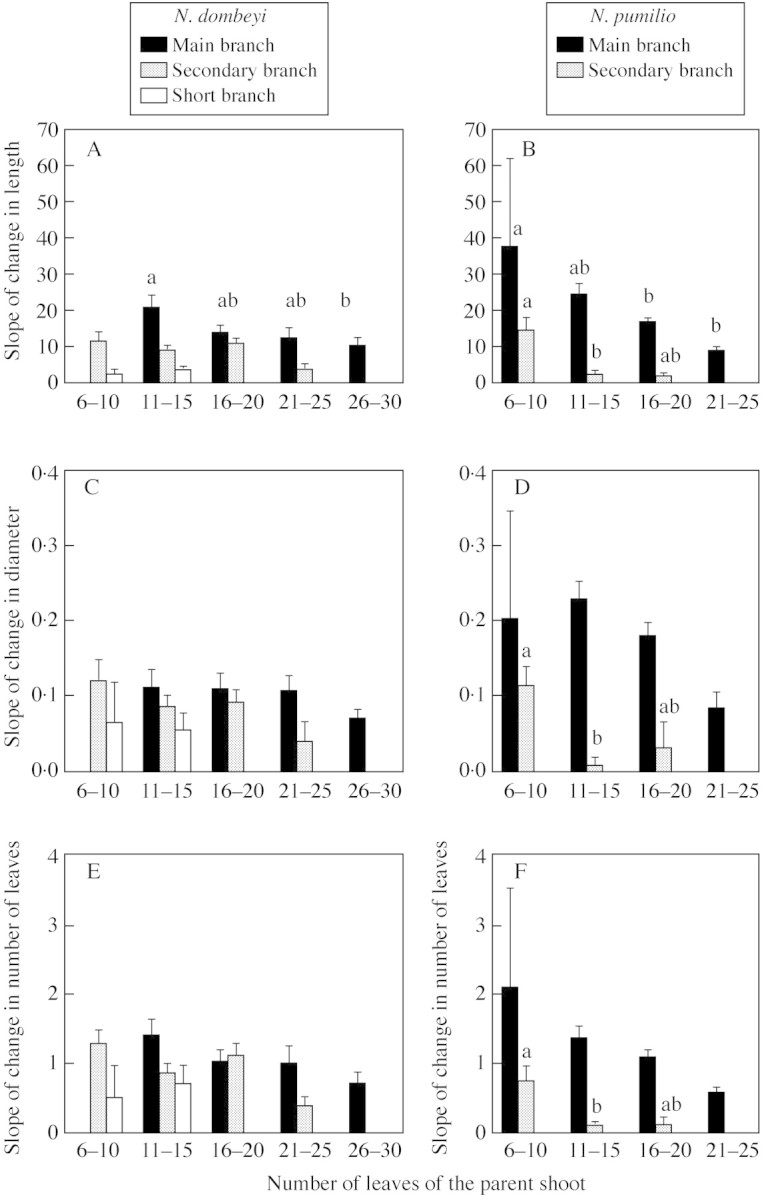
Fig. 4. Mean (+ 1 s.e.) slope of change in sibling‐growth unit length (A and B), diameter (C and D) and number of leaves (E and F) for sibling growth units developed from main‐branch (black bars), secondary‐branch (shaded bars) and short‐branch (white bars) parent growth units of N. dombeyi (A, C and E) and main‐branch (black bars) and secondary‐branch (shaded bars) parent growth units of N. pumilio (B, D and F). Results for parent growth units with different number of nodes (6–10 nodes, 11–15 nodes, 16–20 nodes, 21–25 nodes and 26–29 nodes) are shown separately. The means of bars of the same species and axis type with different letters on top differ significantly (P < 0·05) from each other (Tukey–Kramer tests; see Table 4 for ANOVA comparisons).
Table 4.
Results of comparisons of the slope of change in length, diameter and number of nodes between parent growth units of different size for each axis type of N. dombeyi and N. pumilio by means of one‐way ANOVAs, and between species and parent growth unit sizes (in terms of number of nodes) for each axis type by means of two‐way ANOVAs (species × size : species × parent growth unit size interaction)
| One‐way ANOVA | Two‐way ANOVA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| N. dombeyi | N. pumilio | Species | GU size | Species × size | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Slope of change in | Branch type | F | P | F | P | F | P | F | P | F | P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Length | MB | 3·6 | * | 3·8 | * | 0·0 | ns | 6·9 | ** | 0·4 | ns | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SCB | 1·8 | ns | 6·8 | ** | 4·4 | * | 6·9 | ** | 3·0 | ns | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHB | 1·0 | ns | – | – | – | – | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diameter | MB | 0·8 | ns | 1·8 | ns | 5·2 | * | 2·5 | ns | 2·2 | ns | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SCB | 1·1 | ns | 9·7 | *** | 5·0 | * | 7·5 | ** | 2·0 | ns | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHB | 0·0 | ns | – | – | – | – | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. of leaves | MB | 2·5 | ns | 2·7 | ns | 0·9 | ns | 4·8 | * | 0·7 | ns | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SCB | 2·5 | ns | 5·1 | * | 20·6 | *** | 6·0 | ** | 0·5 | ns | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHB | 0·1 | ns | – | – | – | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
For each comparison, the value of Fisher’s statistic (F) and the probability of error (P) are indicated.
MB, main branches; SC, secondary branches; SHB short branches.
*** P < 0·001; ** P < 0·01; * P < 0·05; ns, P > 0·05.
The slope of change in sibling GU length differed among axis types for both species (N. dombeyi: MB > SC > SHB, F = 13·6, P < 0·001; N. pumilio: MB > SC, F = 22·3, P < 0·001); similar differences were found for the slopes of change in diameter and number of leaves for N. pumilio (F = 38·5, P < 0·001 and F = 28·3, P < 0·001, respectively) but not for N. dombeyi (F = 1·5, P > 0·2 and F = 2·5, P > 0·05, respectively).
DISCUSSION
GU size and size variation in Nothofagus trees
In young N. dombeyi and N. pumilio trees the size (in terms of length, diameter and number of leaves) of a GU relates to the type of parent axis and the position on the parent GU of the node of origin of the sibling GU. In the case of MB of both species and SCB of N. dombeyi, the size of sibling GUs tends to increase from proximal to intermediate and distal positions along parent GUs (Fig. 5; see also following section). On the contrary, hardly any variation in sibling GU size is found for SCB of N. pumilio and SHB of N. dombeyi. SHB of N. pumilio develop only one distal sibling GU (see following section).
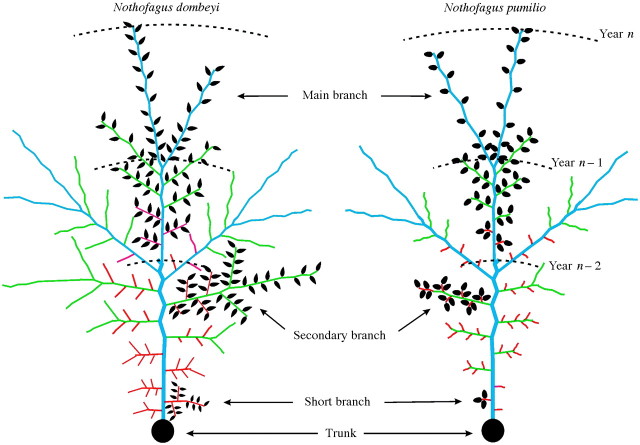
Fig. 5. Diagrammatic representation of a plan view of a 3‐year‐old main branch of N. dombeyi and N. pumilio and all growth units derived from them. Growth units of different axis types are differentiated by colour: main branch, blue; secondary branch, green; short branch, red. Leaves have been drawn on those growth units derived from a main branch, a secondary branch and a short branch of each species. Limits between main branch annual growth units are indicated by dashed arcs. See text for details.
For both species, the length, diameter and number of leaves of sibling GUs at any given position on the parent GU decrease from MB to SCB and SHB. Likewise, stem slenderness and internode length of sibling GUs are higher for MB than for SCB and higher for these than for SHB. These results comply with those on other tree species studied so far, for which stem slenderness and internode length were found to be positively correlated to GU length (Costes et al., 1992; Costes and Guédon, 1997; Nicolini, 1998). The diameter : number of leaves ratio decreased as the diameter and number of leaves of the sibling GU increased, but its variation with respect to the type of axis depended on the species considered (see below).
The close relationship between axis type and the size and form of sibling GUs supports the idea of axis differentiation in trees (Sabatier and Barthélémy, 1995, 1999; Barthélémy et al., 1997). Among the axis types included in the present study, MB could be considered the least specialized type of axis for both species, since GUs within a broad range of sizes may derive from a MB. On the same grounds, SHB may be described as the most specialized type of axis since their production is limited to short GUs (Figs 2 and 3).
The slope of change in sibling GU size along a parent GU depends, for each species, on both the axis type concerned and the size of the parent GU. MB GUs exhibit, on average, sharper differences in sibling GU size than SCB GUs (Fig. 4). For MB, the slope of change in sibling GU length tends to be inversely related to the size of the parent GU from which the sibling GUs derived. Differences in the size of GUs derived simultaneously from a common parent GU have been explained by alluding to patterns of resource allocation (Küppers, 1994; Pallardy et al., 1995), sometimes shown to be controlled by the parent GU’s distal end (Brown et al., 1967; Cline, 1997; Cook et al., 1999; Wilson, 2000). Under this perspective, in young N. dombeyi and N. pumilio trees, the proportional allocation of resources to distal rather than proximal sibling GUs would be higher for MB than for SCB and would tend to be higher for smaller than for larger parent GUs of the same axis type (especially for N. pumilio).
Variation in the pattern of branch development according to axis type and size may have evolved as a way of achieving high levels of light interception in relation to the biomass of the GU. This may be exemplified by comparing the branching patterns of a long GU and a short GU of a MB. The long GU may be assumed to reach a more peripheral position within the crown than the short GU (Fig. 5). By developing several long sibling GUs with long internodes and a slender stem close to its distal end (i.e. a low slope of variation in sibling GU length, as observed here for Nothofagus), the long MB GU would increase the volume reached by the tree crown without extensive overlapping with other MB. The short MB GU, on the other hand, would be more benefited by developing a long sibling GU only at its distal end (resulting in a higher slope of change in sibling GU length), as long proximal sibling GUs would be likely to overlap with other GUs. In the case of even less peripheral (shorter) SCB and SHB, light interception in relation to the biomass of the GUs produced would be increased by decreasing the proportional investment in support organs (i.e. a stem short and narrow relative to its number of leaves) in all positions (Fig. 5).
Differences between species
Young trees of N. pumilio and N. dombeyi differ with regards to some morphological features of their GUs. The dimension of GUs which differs most notably between these species, irrespective of the type of axis considered, is stem diameter after extension: it is notably higher (40 % on average) for N. pumilio than for N. dombeyi for GUs of any given length or number of leaves. The difference in GU diameter between species determines the higher GU slenderness and lower diameter : number of leaves ratio for N. dombeyi than for N. pumilio for each axis type and position of the sibling GU on its parent GU. This difference between species could be related to different cross‐sectional areas of transport tissues. The higher average leaf size (J. Puntieri, unpublished data), nutrient content and nitro gen‐resorption (Mazzarino et al., 1998; Diehl et al., 2003) for N. pumilio than for N. dombeyi might also imply higher metabolic requirements per leaf for the former species which would demand a larger area of conducting tissues. Anatomical and physiological comparative studies would be needed to assess these hypotheses.
Although N. pumilio forests are usually at higher altitudes than those of N. dombeyi, both species are subjected to snowfalls in winter. Stem flexibility is one of the morphological features which increase the capacity of stems to withstand snow weight without breaking (Givnish, 1995; Payette et al., 1996; Valinger and Fridman, 1997). Having a flexible stem in a region with winter snowfalls would represent a more beneficial trait in an evergreen species than in a winter‐deciduous species. The development of a more slender stem in GUs of the evergreen N. dombeyi than in the winter‐deciduous N. pumilio would support this idea, in case a positive relationship between stem slenderness and flexibility were demonstrated for these species.
Nothofagus pumilio and N. dombeyi differ in several aspects of their GU morphology relative to the type of axis from which the GU derives: (a) internodes are longer for N. pumilio than for N. dombeyi in the case of MB GUs but the opposite is true for SCB and SHB; (b) SCB of N. pumilio resemble SHB rather than SCB of N. dombeyi with respect to length and number of leaves; (c) the differences in the slope of change in length, diameter and number of leaves among sibling GUs both between MB and SCB parent GUs with a similar number of nodes and between SCB parent GUs with a different number of nodes, are more notable in N. pumilio than in N. dombeyi (Figs 4 and 5); (d) morpho logical variation among SHB GUs of N. pumilio is very low (i.e. these GUs consist of three leaves, very short internodes and develop a single distal branch; Figs 2, 3 and 5). In addition, SHB of N. pumilio have a turned‐up distal end and their leaves re‐arranged in a tristichous‐like display (Puntieri et al., 1999). This specialized axis type of N. pumilio trees, also observed in the related species Betula papyrifera (Macdonald and Mothersill, 1983) and Fagus sylvatica (Nicolini and Chanson, 1999), seems not to have an equivalent axis type in young N. dombeyi trees. These results suggest a sharper gradient from less differentiated to more differentiated axes in N. pumilio than in N. dombeyi. The development of a specialized SHB in N. pumilio trees would allow light exploitation with low investment in support tissues and low overlapping among leaves. On the other hand, the less specialized, relatively long SHB of N. dombeyi seems more suitable for light exploration, at the cost of increasing the extent of overlapping between neighbour branches (see Küppers, 1994; Cao, 1995). Since the leaves of N. dombeyi are retained at least for 2 years, the development at the centre of the crown of SHB with internodes as short as those of SHB of the deciduous N. pumilio would imply very high levels of leaf overlapping.
CONCLUSIONS
The identification of biologically meaningful structural units of each plant species is fundamental for the study of their branching patterns. A deep knowledge of the restrictions imposed by the species’ endogenous developmental rules on the size and form of such units is necessary before assessing the role of environmental factors on the branching pattern of a species or comparing branching patterns between species or between populations of the same species (see Watson et al., 1995). The differences pointed out here between the size and form of GUs and the branching patterns of N. dombeyi and N. pumilio could be considered valid for young individuals growing under optimal conditions. Similar information is not available for other populations of the same species. However, partial data sets from other studies on N. pumilio (Passo et al., 2002) and N. dombeyi (Puntieri et al., 2002) suggest that the main results would have been similar had the study been performed on other populations of young trees. Variations in the branching pattern of these species for older trees or trees growing under stressful conditions ought to be addressed.
ACKNOWLEDGEMENTS
We thank Segundo Beccar Varela for his help in field work and measurements, Marina Stecconi, Eric Nicolini and Yves Caraglio for their support and discussions on the subject. The Research Project in which this study is included is supported by CIRAD and INRA (France), and by the Universidad Nacional del Comahue (Argentina; project B704) and CONICET (Argentina, PEI No. 0800/98).
Supplementary Material
Received: 14 March 2003 Revised: 20 May 2003; Accepted: 27 June 2003 Published electronically: 21 August 2003
References
- BarthélémyD, Caraglio Y, Costes E.1997. Architecture, gradients morphogénétiques et âge physiologique chez les végétaux. In: Bouchon J, de Reffye P, Barthélémy D, eds. Modélisation et simulation de l’architecture des végétaux Science Update. Paris: INRA Editions, 89–136. [Google Scholar]
- BarthélémyD, Edelin C, Hallé F.1989. Architectural concepts for tropical trees. In: Holm‐Nielsen LB, Baslev H, eds. Tropical forest: botanical dynamics, speciation and diversity London: American Press, 89–100. [Google Scholar]
- BarthélémyD, Puntieri JG, Brion C, Raffaele E.1999. Morfología de las unidades estructurales y modo de desarrollo básico de especies patagónicas de Nothofagus (Fagaceae). Boletín Sociedad Argentina de Botánica 34: 29–38. [Google Scholar]
- BertramJEA 1989. Size‐dependent differential scaling in branches: the mechanical design of trees revisited. Trees 4: 241–253. [Google Scholar]
- BrownCL, McAlpine RG, Kormanik PP.1967. Apical dominance and form in woody plants: a reappraisal. American Journal of Botany 54: 153–162. [Google Scholar]
- BrunigEF.1976. Tree forms in relation to environmental conditions: an ecological viewpoint. In: Cannell MGR, Last FT, eds. Tree physiology and yield improvement London: Academic Press, 139–156. [Google Scholar]
- CaoKF.1995.Fagus dominance in Chinese montane forests: natural regeneration of Fagus lucida and Fagus hayatae var. pashanica. PhD Thesis, University of Wageningen, Wageningen, The Netherlands. [Google Scholar]
- CaraglioY, Barthélémy D.1997. Revue critique des termes relatifs à la croissance et à la ramification des tiges des végétaux vasculaires. In: Bouchon J, de Reffye P, Barthélémy D, eds. Modélisation et simulation de l’architecture des végétaux Science Update. Paris: INRA Editions, 11–87. [Google Scholar]
- ClineMG.1997. Concepts and terminology of apical dominance. American Journal of Botany 84: 1064–1069. [PubMed] [Google Scholar]
- ContiHA.1998. Características climáticas de la Patagonia. In: Correa MN, ed. Flora Patagónica, Vol. VIII (I) Colección Científica. Buenos Aires: INTA, 31–47. [Google Scholar]
- CookN, Rabe E, Jacobs G.1999. Early expression of apical control regulates length and crotch angle of sylleptic shoots in peach and nectarine. HortScience 34: 604–606. [Google Scholar]
- CorreaMN.1984. Fagaceae. In: Correa MN, ed. Flora Patagónica, Vol. VIII (IVa) Colección Científica. Buenos Aires: INTA, 4–11. [Google Scholar]
- CostesE, Guédon Y.1997. Modeling the sylleptic branching on one‐year‐old trunks of apple cultivars. Journal of the American Society of Horticultural Science 122: 53–62. [Google Scholar]
- CostesE, de Reffye P, Lichou J, Guédon Y, Audubert A, Jay M.1992. Stochastic modelling of apricot growth units and branching. Acta Horticulturae 313: 89–98. [Google Scholar]
- de ReffyeP, Blaise F, Chemouny S, Jaffuel S, Fourcaud T, Houllier F.1999. Calibration of hydraulic architecture‐based growth models of cotton plants. Agronomie 19: 265–280. [Google Scholar]
- de ReffyeP, Dinouard P, Barthélémy D.1991a. Modélisation et simulation de l’architecture de l’Orme du Japon Zelkova serrata (Thumb.) Makino (Ulmaceae): la notion d’axe de reference. Naturalia Monspeliensia Suppl.: 251–266. [Google Scholar]
- de ReffyeP, Elguero E, Costes E.1991b. Growth units construction in trees: a stochastic approach. Acta Biotheoretica 39: 325–342 [Google Scholar]
- de ReffyeP, Houllier F, Blaise F, Fourcaud T.1997. Essai sur les relations entre l’architecture d’un arbre et la grosseur de ses axes végétatifs. In: Bouchon J, de Reffye P, Barthélémy D, eds. Modél isation et simulation de l’architecture des végétaux Science Update. Paris: INRA Editions, 255–423. [Google Scholar]
- DiehlP, Mazzarino MJ, Funes F, Fontenla S, Gobbi M, Ferrari J.2003. Nutrient conservation strategies in native Andean‐Patagonian forests. Journal of Vegetation Science 14: 63–70. [Google Scholar]
- DonosoC.1994.Bosques templados de Chile y Argentina. Variación, estructura y dinámica. Santiago de Chile: Editorial Universitaria. [Google Scholar]
- GivnishTJ.1995. Plant stems: biomechanical adaptation of energy capture and influence on species distributions. In: Gartner B, ed. Plant stems: physiology and functional ecology San Diego: Acad emic Press, 3–49. [Google Scholar]
- GodinC.2000. Representing and encoding plant architecture: a review. Annals of Forest Science 57: 413–438. [Google Scholar]
- GodinC, Guédon Y, Costes E.1999. Exploration of a plant architecture database with the AMAPmod software illustrated on an apple tree hybrid family. Agronomie 19: 163–184. [Google Scholar]
- GrosfeldJE.2002.Análisis de la variabilidad morfológica y arquitectural de Austrocedrus chilensis (D. Don) Pic. Serm. et Bizzarri, Fitzroya cupressoides (Molina) I. M. Johnst Pilgerodendron uviferum (D. Don) Florin y Cupressus sempervirens L. (Cupressaceae). PhD Thesis, Universidad Nacional del Comahue, Bariloche, Argentina. [Google Scholar]
- HalléF, Oldeman RAA, Tomlinson P.1978.Tropical trees and forests – an architectural analysis. Berlin: Springer‐Verlag. [Google Scholar]
- HarrisJR, Bassuk NL.1993. Adaptation of trees to low‐light environments: effects on branching pattern of Fraxinus americana Journal of Arboriculture 19: 339–343. [Google Scholar]
- HattaH, Honda H, Fisher JB.1999. Branching principles governing the architecture of Cornus kousa (Cornaceae). Annals of Botany 84: 183–193. [Google Scholar]
- HeuretP.2002.Analyse et modélisation de séquences d’événements botaniques: applications à la compréhension de la régularité d’expression des processus de croissance, de ramification et de floraison. PhD Thesis, Université Henri Poincaré, Nancy, France. [Google Scholar]
- HeuretP, Guédon Y, Guérard N, Barthélémy D.2003. Analysing branching pattern in plantations of young red oak trees (Quercus rubra L. Fagaceae). Annals of Botany 91: 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HondaH, Hatta H, Fisher JB.1997. Branch geometry in Cornus kousa (Cornaceae): computer simulations. American Journal of Botany 84: 745–755. [PubMed] [Google Scholar]
- HornHS.1971.Adaptive geometry of trees. Princeton: Princeton University Press. [Google Scholar]
- JonesCS.1999. An essay on juvenility, phase change, and heteroblasty in seed plants. International Journal of Plant Sciences 160 Suppl.: S105–S111. [DOI] [PubMed] [Google Scholar]
- KozlowskiTT.1971.Growth and development of trees. Vol. I. Seed germination, ontogeny and shoot growth. New York: Academic Press. [Google Scholar]
- KüppersM.1994. Canopy gaps: competitive light interception and economic space filling – a matter of whole‐plant allocation. In: Caldwell MM, Pearcy RW, eds. Exploitation of environmental heterogeneity by plants New York: Academic Press, 111–144. [Google Scholar]
- MacdonaldAD, Mothersill DH.1983. Shoot development in Betula papyrifera I. Short‐shoot organogenesis. Canadian Journal of Botany 61: 3049–3065. [Google Scholar]
- McCurdyWD, Powell GR.1987. Syllepsis in Larix laricina: association of sylleptic branching with cross‐sectional stem growth and stem form of saplings. Canadian Journal of Forest Research 17: 1609–1619. [Google Scholar]
- MazzarinoMJ, Bertiller M, Schlichter T, Gobbi M.1998. Nutrient cycling in Patagonian ecosystems. Ecología Austral 8: 167–181. [Google Scholar]
- NicoliniE.1998. Architecture et gradients morphogénétiques chez de jeunes hêtres (Fagus sylvatica L. Fagaceae) en milieu forestier. Canadian Journal of Botany 76: 1232–1244. [Google Scholar]
- NicoliniE, Chanson B.1999. La pousse courte, un indicateur du degré de maturation chez le hêtre (Fagus sylvatica L.). Canadian Journal of Botany 77: 1539–1550. [Google Scholar]
- OldemanRAA.1989. Biological implications of leguminous tree architecture. In: Stirton CH, Zarucchi JL, eds. Advances in legume biology Missouri: Missouri Botanic Gardens, 17–34. [Google Scholar]
- PallardyS, Cernák J, Ewers F, Kaufmann M, Parker W, Sperry JS.1995. Water transport dynamics in trees and stands. In: Smith W, Hinckley T, eds. Resource physiology of conifers. acquisition, allocation, and utilization London: Academic Press, 201–387. [Google Scholar]
- ParkerT, Johnson FD.1987. Branching and terminal growth of western red cedar. Northwest Science 61: 7–12. [Google Scholar]
- PassoA, Puntieri JG, Barthélémy D.2002. Trunk and main‐branch development in Nothofagus pumilio (Nothofagaceae): a retrospective analysis of tree growth. Canadian Journal of Botany 80: 763–772. [Google Scholar]
- PayetteS, Delwaide A, Morneau C, Lavoie C.1996. Pattern of tree stems decline along snow‐drift gradient at treeline: a case study using stem analysis. Canadian Journal of Botany 74: 1671–1683. [Google Scholar]
- PickettSTA, Kempf JS.1980. Branching patterns in forest shrubs and understorey trees in relation to habitat. New Phytologist 86: 219–228. [Google Scholar]
- PoorterL, Werger MJA.1999. Light environment, sapling architecture, and leaf display in six rain forest tree species. American Journal of Botany 86: 1464–1473. [PubMed] [Google Scholar]
- PuntieriJG, Barthélémy D, Martinez P, Raffaele E, Brion C.1998. Annual‐shoot growth and branching patterns in Nothofagus dombeyi (Mirb.) Blume (Fagaceae). Canadian Journal of Botany 76: 673–685. [Google Scholar]
- PuntieriJG, Barthélémy D, Mazzini C, Brion C.2002. Periods of organogenesis in shoots of Nothofagus dombeyi (Mirb.) Oersted (Nothofagaceae). Annals of Botany 89: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PuntieriJG, Raffaele E, Martinez P, Barthélémy D, Brion C.1999. Morphological and architectural features of young Nothofagus pumilio (Poepp. et Endl.) Krasser (Fagaceae). Botanical Journal of the Linnean Society 130: 395–410. [Google Scholar]
- PuntieriJG, Souza MS, Barthélémy D, Brion C, Nuñez M, Mazzini C.2000. Preformation, neoformation and shoot structure in Nothofagus dombeyi (Nothofagaceae). Canadian Journal of Botany 78: 1044–1054. [Google Scholar]
- RemphreyWR, Powell GR.1984. Crown architecture of Larix laricina saplings: shoot preformation and neoformation and their relation ships to shoot vigour. Canadian Journal of Botany 62: 2181–2192. [Google Scholar]
- RossignolM, Rossignol L, Oldeman RAA, Benzine‐Tizroutine S.1998.Struggle of life or the natural history of stress and adaptation. Treebook 1. Heelsum: Treemail Publishers. [Google Scholar]
- RustS, Hüttl RF.1999. The effect of shoot architecture on hydraulic conductance in beech (Fagus sylvatica L.). Trees 14: 39–42. [Google Scholar]
- SabatierS, Barthélémy D.1995. Architecture du cèdre de l’Atlas, Cedrus atlantica (Endl.) Manetti ex Carrière (Pinaceae). In: Bouchon J, ed. Architecture des arbres fruitiers et forestiers Paris: INRA Editions, Les Colloques, 157–173. [Google Scholar]
- SabatierS, Barthélémy D.1999. Growth dynamics and morphology of annual shoots according to their architectural position in young Cedrus atlantica (Endl.) Manetti ex Carrière (Pinaceae). Annals of Botany 84: 387–392. [Google Scholar]
- SabatierS, Barthélémy D.2001. Annual shoot morphology and architecture in Persian Walnut Juglans regia L. (Juglandaceae). Acta Horticulturae 544: 255–264. [Google Scholar]
- ScoppaCO.1998. Los suelos. In: Correa MN, ed. Flora Patagónica, Vol. VIII (I) Colección Científica. Buenos Aires: INTA, 15–30. [Google Scholar]
- SeleznyovaAN, Thorp G, Barnett AM, Costes E.2002. Quantitative analysis of shoot development and branching patterns in Actinidia Annals of Botany 89: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SokalRR, Rohlf FJ.1981.Biometry, 2nd edn. New York: W. H. Freeman and Co. [Google Scholar]
- SouzaMS, Puntieri JG, Barthélémy D, Brion C.2000. Bud content and its relation to shoot size and structure in Nothofagus pumilio (Poepp. et Endl.) Krasser (Nothofagaceae). Annals of Botany 85: 547–555. [Google Scholar]
- StecconiM, Puntieri JG, Barthélémy, D.2000. Annual shoot‐growth in Nothofagus antarctica (G. Forster) Oersted (Fagaceae) from northern Patagonia. Trees 14: 289–296. [Google Scholar]
- SteingraeberDA, Waller DM.1986. Non‐stationarity of tree branching patterns and bifurcation ratios. Proceeding of the Royal Society of London, Series B 228: 187–194. [Google Scholar]
- SutherlandWJ, Stillman RA.1988. The foraging tactics of plants. Oikos 52: 239–244. [Google Scholar]
- ThiébautB, Serey I, Druelle J‐L, Li J, Bodin A, Rechain J.1997. Forme de la plantule et architecture de quelques hêtres, chiliens (Nothofagus) et chinois (Fagus). Canadian Journal of Botany 75: 640–655. [Google Scholar]
- ValingerE, Fridman J.1997. Modelling probability of snow and wind damage in Scots pine stands using tree characteristics. Forest Ecology and Management 97: 215–222. [Google Scholar]
- VeblenTT, Donoso C, Kitzberger T, Rebertus AJ.1996.Ecology of southern Chilean and Argentinean Nothofagus forests. In: Veblen TT, Hill RS, Read J, eds. The ecology and biogeography of Nothofagus forests Yale: Yale University Press, 293–353. [Google Scholar]
- WatkinsonAR.1988. On the growth and reproductive schedules of plants: a modular viewpoint. Acta Oecologica Oecologia Plantarum 9: 67–81. [Google Scholar]
- WatsonMA, Geber MA, Jones CS.1995. Ontogenetic contingency and the expression of plant plasticity. Trends in Ecology and Evolution 12: 474–475. [DOI] [PubMed] [Google Scholar]
- WilsonBF.2000. Apical control of branch growth and angle in woody plants. American Journal of Botany 87: 601–607. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.