Abstract
An investigation was made of the number of preformed organs in winter buds of 3‐year‐old reiterated complexes of the ‘Granny Smith’ cultivar. Winter bud content was studied with respect to bud position: terminal buds were compared on both long shoots and spurs according to branching order and shoot age, while axillary buds were compared between three zones (distal, median and proximal) along 1‐year‐old annual shoots in order 1. The percentage of winter buds that differentiated into inflorescences was determined and the flowers in each bud were counted for each bud category. The other organ categories considered were scales and leaf primordia. The results confirmed that a certain number of organs must be initiated before floral differentiation occurred. The minimum limit was estimated at about 15 organs on average, including scales. Total number of lateral organs formed was shown to vary with both bud position and meristem age, increasing from newly formed meristems to 1‐ and 2‐year‐old meristems on different shoot types. These differences in bud organogenesis depending on bud position, were consistent with the morphogenetic gradients observed in apple tree architecture. Axillary buds did not contain more than 15 organs on average and this low organogenetic activity of the meristems was related to a low number of flowers per bud. In contrast, the other bud categories contained more than 15 differentiated organs on average and a trade‐off was observed between leaf and flower primordia. The ratio between the number of leaf and flower primordia per bud varied with shoot type. When the terminal buds on long shoots and spurs were compared, those on long shoots showed more flowers and a higher ratio of leaf to flower primordia.
Key words: Preformation, neoformation, primordia, floral differentiation, architecture
INTRODUCTION
Because they represent the plant’s potential for both growth and branching, buds are essential organs in the biology of perennial plants. In most temperate species buds are protected by scales, which in fact are modified leaves, covered by a thick cuticle. Scales are often hairy; hairs allow air to be maintained around the bud and thus temper major temperature fluctuations. Scaly buds contain an embryonic stem, terminated by an apex, and organ primordia which originate from the outer cell layers of the meristematic zone (Camefort, 1977). These organ primordia, which develop after a period of time corresponding to winter in temperate regions, are called preformed organs. In contrast, when organogenesis and elongation occur without any resting period, the resulting organs are called neoformed organs (Rivals, 1965).
Scaly bud formation was described many years ago in the apple tree. One of the first descriptions of winter bud content was proposed by Abbott (1970): ‘These consist of nine budscales, three transition leaves, six true leaves and three bracts. The axis is terminated by a flower primordium (the ‘King’ flower), and lateral flower primordia are formed in the axils of the three bracts and the three distal leaves.’ It is noteworthy that this very precise definition did not include any quantitative variation in bud constitution. This study, like most of the others dealing with the winter bud content of apple trees (Fulford, 1965, 1966; Luckwill, 1974; Abbott, 1977), mainly described buds which were terminal on spurs, and focused particularly on the dynamics of fruit‐bud organogenesis. The main objective of these studies was to understand fruit‐bud formation and its correlation with leaves. These studies led to precise descriptions of the floral bud formation as reported by Verheij (1996): ‘the first leaf primordia that differentiate transform into scales while the following ones transform into transition leaves and finally into ‘true’ leaves. Soon after bud burst, scales and transition leaves abscise while the ‘true’ leaves primordia give rise to leaves photosynthetically active.’
The idea that winter bud content could vary as a function of within‐tree bud position was first suggested by Critchfield (1960) and Rivals (1965). This latter investigated the number of scales and primordia in a large number of fruit tree species, with buds being sampled in equivalent positions. This sampling method led to almost identical bud contents expressed as a percentage (not a constant value). More recently, winter bud content was explored in relation to within‐tree bud position and the vigour of the parent shoot, in several species: Larix laricina (Remphrey and Powell, 1984), Fraxinus pennsylvanica (Remphrey and Davidson, 1994), Persea sp. (Thorp et al., 1994), Juglans regia (Sabatier and Barthélémy, 2000) and Nothofagus sp. (Souza et al., 2000; Puntieri et al., 2002). These studies showed that winter bud content varied with the within‐tree bud position and highlighted a relationship between bud dimensions and (a) the number of primordia and (b) the parent shoot vigour and further shoot development. When annual shoots are composed of preformed organs only the number of preformed primordia can be an efficient predictor of annual shoot length, even though several growth cycles can be observed during a growing season. This is the case for Pinus pinaster Ait. (Kremer, 1981). However, in many species, shoots can develop a neoformed part after expansion of preformed organs, and this capability, which also depends on within‐tree position, has been interpreted as a feature that provides plasticity in shoot development (Hallé et al., 1978; Puntieri et al., 2002).
In apple trees, the different shoot types that can develop from winter buds have been classified according to the length of the neoformation period and their growth rhythm (Crabbe, 1984). Shoot development and the trade‐off between preformed and neoformed organs have been shown to depend on within‐tree bud position, and this both at annual shoot and whole tree scales (Costes and Guédon, 2002; Costes et al., 2003). As in other species, neoformation has been shown to develop mostly during early tree development, on the trunk, main branches and vigorous shoots (Remphrey and Powell, 1984; Costes, 1993), and the progressive reduction in neoformation has been shown to be identical for all branches within a tree (Costes et al., 2003). Since bourses (or floral units) are entirely preformed (Crabbe and Escobedo‐Alvarez, 1991), the question of the winter bud content is a key feature in better understanding the within‐tree distribution of fruiting potential. Moreover, the early development of fruit, including cell divisions and fruit set, has been shown to depend on spur leaf photosynthesis (Hansen, 1971; Lakso, 1984). During the first 3 weeks after full bloom, fruitlet and bourse shoot development are competing for current assimilates (Abbott, 1970; Ferree and Palmer, 1982). Thus, the variation in the number of preformed leaves is of particular interest, especially for spurs which are the most numerous shoot type within a canopy (Lakso, 1984, Wünsche et al., 1996). Previous studies demonstrated that both the number of flowers per spur and fruit set depend on their within‐tree position, considered as height from the soil (Barritt and Konishi, 1993; (Barritt et al., 1987). Recently, the number of flowers per spur was evaluated for a range of apple cultivars and climatic conditions (Ferree et al., 2001) but not with respect to within‐tree variation.
The study described here examined the variation in the number of preformed organs, including scales, leaves and flower primordia, in the winter buds of the ‘Granny Smith’ apple cultivar. Winter bud content was studied with respect to bud position on annual shoots, and according to branching order and shoot age in 3‐year‐old branching systems. Terminal buds were compared for both long shoots and spurs. In addition, the percentage of winter buds that differentiated into inflorescence was determined for buds in different within‐tree positions. The insertion rank of the axillary flowers and the number of flowers per bud were also investigated and the results expressed according to bud position.
PLANT MATERIAL AND METHODS
Observations were carried out on 80 8‐year‐old apple trees (Malus × domestica Borkh), cultivar ‘Granny Smith’ grafted on M9. The trees were planted at the Centre Experimental en Horticulture de Marsillargues (CEHM, South of France) and were organized in two rows with tree spacing of 4·5 × 1·5 m. The Solaxe training system (Lespinasse and Lauri, 1999) was used and the classical agronomic practices of commercial orchards were applied for irrigation, fertilization and thinning.
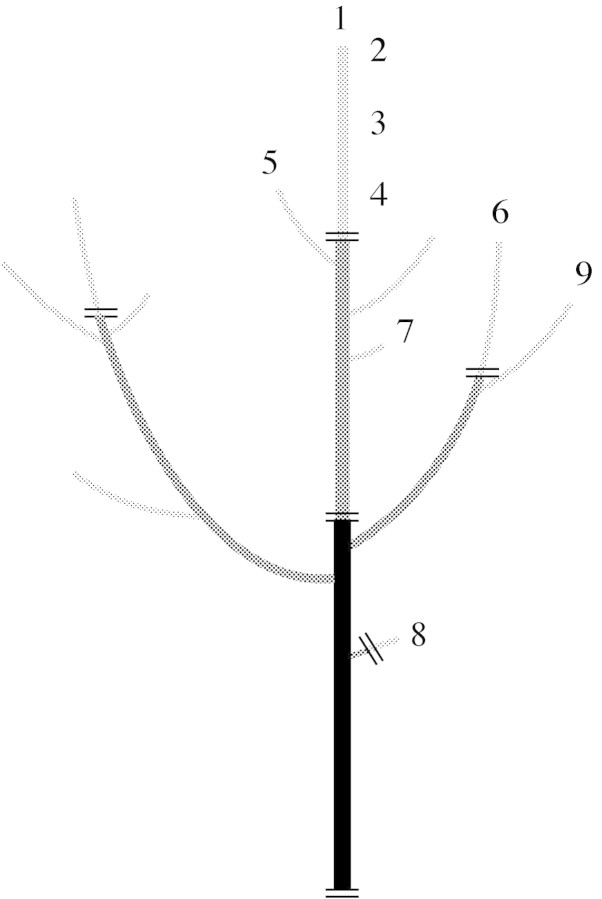
On each tree, a single branching system developing at trunk curvature, i.e. corresponding to a reiterated complex, was selected for bud examination on the basis of two criteria: (1) all branching systems were 3 years old; and (2) they had not been pruned since the start of their development. Because they were all located on the upper part of the trees, the selected branching systems were assumed to be comparable regarding their environmental conditions, particularly as concerns their light environment. Since reiterated complexes are assumed to be similar in structure to the young tree (Oldeman, 1974), the relative branching orders used in the complexes are similar to those usually defined at the whole tree level: order 1 represents the trunk, order 2 the branches borne along the trunk and so on (Fig. 1).
Fig. 1. Positions of the buds sampled in the branching system. 1, Apical buds of 3‐year‐old long shoots in order 1; 2, 3 and 4: axillary buds, respectively, in distal, median and proximal positions; 5 and 6, apical buds on 1‐ and 2‐year‐old long shoots in order 2; 7 and 8, apical buds on 1‐ and 2‐year‐old spurs in order 2; 9, apical buds on long shoots in order 3.
In January, after the reiterated complexes had been collected, all the buds were cut and sorted into nine categories as indicated in Fig. 1.
1: apical buds in order 1
2, 3 and 4: axillary buds on 1‐year‐old annual shoots in order 1, in the upper third, median and proximal third
5 and 6: apical buds on 1‐year‐old and 2‐year‐old long shoots, in order 2
7 and 8: apical buds on 1‐year‐old and 2‐year‐old spurs, in order 2
9: apical buds on 1‐year‐old long shoots in order 3
Some reiterated complexes did not show any order 3 branches and consequently the total number of observed buds was less for this category (55 buds).
The buds were then fixed in 70 % alcohol and dissected under a 10–50 binocular microscope (aus JENA GSZ). The proportion of buds differentiating into a floral bud was first determined for each bud position. During bud dissection, the scales and transition leaves were grouped together and were considered as scales. ‘True’ leaf primordia were counted for each bud. Scales were brownish and hard, and made up of a single structure. ‘True’ leaf primordia were more flexible and hairy, consisting of three parts, i.e. the future blade and the two lateral stipules.
As previously described by several authors (Abbott, 1970; Luckwill, 1974; Escobedo Alvarez, 1990), flowers were located in axils of primordia and the king flower was located terminally. Even though a continuum was observed between the ‘true’ leaves and bracts, for simplicity all primordia bearing an axillary flower were considered as a bract. Total flowers and their insertion rank from the basis were counted for each dissected bud. Since the bracts in some buds did not bear flowers, the number of bracts did not correspond to the number of flowers. Consequently, the total number of preformed organs was considered as the sum of the scales, leaf primordia initiated before the first axillary flower and bracts. Even though the leaves of the future bourse shoot were sometimes already visible, they were distinguishable in very few buds and were not taken into account in what follows.
All statistical analyses were performed using Statistica software (Kernel version 6.0; StatSoft, Inc.). Proportions of floral buds per bud category were compared using a χ2‐test. Because of the low number of vegetative buds in some positions, the investigation of bud constitution was performed mainly on floral buds. When the number of buds per category was more than 30 and the number of organs followed normal distributions, Newman–Keuls test was performed to compare mean values between the different bud positions. This was observed for all buds in terminal positions and for axillary buds when their nature (floral vs. vegetative) was not considered. When floral and vegetative axillary buds were considered separately, the number of buds per position was not constant and was low in some positions. In these cases, non‐parametric Mann–Whitney U tests were performed for comparing the insertion zones. Correlations between the number of flowers and the other organs (total number of preformed organs, scales and leaf primordia) were investigated for all buds grouped and for each bud category.
RESULTS
Proportion of floral differentiation in winter buds
Buds in terminal position either on long shoots or spurs differentiated into floral clusters in similar proportions (Table 1). Indeed, regardless of the branching order and shoot age, 90–100 % of buds differentiated into floral buds. In contrast, buds in axillary positions on 1‐year‐old long shoots differentiated less often into a floral bud. In addition, a marked gradient was expressed depending on the insertion zone: in the upper zone, 87·5 % of buds differentiated into floral clusters vs. 62·5 % in the median zone and 13·75 % in the proximal zone. Thus, the higher the zone, the most frequently buds differentiated into inflorescences. Because of these differences in the proportion of floral differentiation, and therefore in the number of floral/vegetative buds observed in the different bud positions, further analyses mainly focused on floral buds. In addition, axillary floral and vegetative buds were considered separately and different statistical tests were performed.
Table 1.
Percentage of floral buds at different bud positions within 3‐year‐old branching systems of ‘Granny Smith’
| Shoot age | |||
| Branching order | 1‐year‐old | 2‐year‐old | 3‐year‐old |
| 1 Long shoots | 90 (72/8)a | ||
| 2 Long shoots | 92 (74/6)a | 95 (76/4)a | |
| 2 Spurs | 100 (80/0)a | 98 (78/2)a | |
| 3 Long shoots | 100 (55/0)a | ||
| Axillary buds | Distal | Median | Proximal |
| 87·5 (70/10)a | 62·5 (50/30)b | 13·75 (11/69)c | |
Numbers of floral/vegetative buds are indicated in brackets.
Values with the same superscript letters are not significantly different according to a χ2 test (P < 0·01).
Total number of organs per bud
The total mean number of organs per floral bud, i.e. all scales, leaf primordia and bracts, was quite similar for all buds in the terminal position of shoots, ranging from 18 on 1‐year‐old spurs to 21·6 on long shoots in order 1 (Table 2A). Only these two extreme categories were significantly different from the others, while the four other shoot categories exhibited similar mean numbers of primordia (about 20) in terminal buds. An increase in the total number of organs was observed between the 1‐year‐old and 2‐year‐old shoots, particularly in spurs.
Table 2.
Mean number of (A) total preformed organs (including scales, leaf primordia and bracts), (B) scales and (C) leaf primordia (initiated before the first axillary flower) contained in buds at different positions in 3‐year‐old branching systems of ‘Granny Smith’
| Shoot age | |||
| Branching order | 1‐year‐old | 2‐year‐old | 3‐year‐old |
| (A) Total preformed organs | |||
| Terminal buds | |||
| 1 Long shoots | 21·62 (2·49)a | ||
| 2 Long shoots | 20·32 (1·61)b | 20·63 (2·06)ab | |
| 2 Spurs | 17·99 (1·77)c | 20·79 (2·06)ab | |
| 3 Long shoots | 20·29 (2·44)b | ||
| Axillary buds | Distal | Median | Proximal |
| 15·04 (3·23)d | 15·30 (2·73)d | 11·38 (2·42)e | |
| *Floral buds | 15·51 (2·93)d | 16·62 (2·33)c | 13·82 (2·68)d |
| *Vegetative buds | 11·70 (3·43)ef | 13·10 (1·77)e | 10·99 (2·15)f |
| (B) Scales | |||
| Terminal buds | 1‐year‐old | 2‐year‐old | 3‐year‐old |
| 1 Long shoots | 5·57 (1·66)e | ||
| 2 Long shoots | 7·18 (1·10)c | 5·87 (1·53)e | |
| 2 Spurs | 7·91 (1·06)b | 9·90 (1·61)a | |
| 3 Long shoots | 6·53 (1·14)d | ||
| Axillary buds | Distal | Median | Proximal |
| 3·73 (0·98)g | 4·16 (0·74)f | 3·04 (0·86)h | |
| *Floral buds | 3·80 (0·96)f | 4·2 (0·67)f | 3·6 (0·92)f |
| *Vegetative buds | 3·20 (1·03)fg | 4·1 (0·84)f | 2·94 (0·82)g |
| (C) Leaf primordia | |||
| Terminal buds | 1‐year‐old | 2‐year‐old | 3‐year‐old |
| 1 Long shoots | 11·19 (1·93)a | ||
| 2 Long shoots | 8·07 (1·83)c | 9·55 (2·06)b | |
| 2 Spurs | 4·64 (2·01)d | 5·33 (1·20)d | |
| 3 Long shoots | 7·98 (1·67)c | ||
| Axillary buds | Distal | Median | Proximal |
| 7·96 (2·35)c | 8·50 (1·86)c | 7·86 (1·58)c | |
| *Floral buds | 7·88 (2·30)c | 8·20 (1·99)c | 6·73 (1·49)c |
| *Vegetative buds | 8·50 (2·72)bc | 9·0 (1·53)b | 8·04 (1·53)c |
Standard deviations are indicated in brackets.
For values with the same superscript letters no significant difference was obtained between the corresponding mean values by a Newman–Keuls test (P < 0·01) or * with a Mann–Whitney U test (P < 0·01).
In axillary buds, the number of organs in the floral buds was significantly less than in the terminal position and varied from 14 to 17. When all axillary buds were considered together, those in distal and median zones contained about 15 organs while those in the proximal zone developed only 11 organs. The difference was significant between the proximal and the two other zones. When floral and vegetative buds were considered separately, the buds located in the median zone contained slightly more organs than those in distal and proximal zones. However, this difference did not follow either an acrotonic or a basitonic gradient and was less that the difference observed between floral and vegetative axillary buds. Indeed, the vegetative buds in axillary positions contained significantly fewer preformed organs than all the other buds, irrespective of position.
Number of scales and leaf primordia at different bud positions
The mean number of scales and leaf primordia initiated before the first axillary flower in terminal buds varied, depending on bud position and, to a greater extent, than the total number of organs (Table 2B and C). However, the standard deviations were always smaller than the corresponding mean values, indicating only small variations occurred within each bud category. The mean number of scales and leaf primordia varied in opposite directions: the long shoots in order 1 showed the highest number of leaves and the lowest number of scales per bud, while spurs possessed the highest number of scales and the lowest number of leaves. More generally, regardless of shoot age, the mean number of leaf primordia was higher for buds terminating long shoots than those terminating spurs, while the reverse was observed for the mean number of scales.
The mean number of leaf primordia was affected by branching order: the greater the branching order the fewer the leaf primordia. However, in order 2, the mean number of leaf primordia increased with shoot age for both long shoots and spurs (even though the difference between 1‐year‐old and 2‐year‐old spurs was not significant). The older the shoot, the higher the number of leaf primordia. In order 3, the mean number of leaf primordia was comparable to that of buds of the same age in order 2, but was less than for 2‐year‐old buds in order 2. This suggests that shoot age may have more impact than branching order.
In contrast, the number of scales did not vary with shoot age in the same direction in terminal buds on long shoots and spurs: terminal buds on long shoots contained fewer scales and more leaf primordia when on 2‐year‐old shoots than on 1‐year‐old shoots, while the reverse was observed between 1‐ and 2‐year‐old spurs. Consequently, the increase observed in the total number of organs on 2‐year‐old spurs mainly resulted from the increase in the number of scales.
Axillary buds on 1‐year‐old long shoots showed the lowest mean number of scales. When the nature of the bud (floral vs. vegetative) was ignored, the buds located in the median zone had the highest number of scales. However, no significant difference was found between the three zones for either floral or vegetative buds, or when comparing these two bud categories. Less contrast was observed between axillary and terminal buds regarding the mean number of leaf primordia, since axillary buds did not significantly differ from 1‐year‐old long shoots in either order 2 or 3. In addition, axillary buds contained similar mean numbers of leaf primordia in both floral and vegetative buds. Finally, the lower number of total organs contained in axillary buds compared with terminal buds resulted from a lower number of scales, not a lower number of leaf primordia.
Mean number of preformed flowers per cluster
The number of flowers per inflorescence also varied according to bud position (Table 3). Mean values varied from 5·18 to 3·45, i.e. by a factor of 1·5. The highest mean number of flowers per bud was found on the terminal buds of long shoots, particularly in orders 2 and 3. Fewer flowers were found in order 1 but the difference was not significant when compared with 1‐year‐old long shoots. In the same manner as the number of leaves, the mean number of flowers increased slightly from 1‐year‐old to 2‐year‐old spurs and long shoots in order 2 (even though the difference was not significant for long shoots). Regardless of shoot age, spurs had a significantly lower mean number of flowers per cluster than long shoots at the same order.
Table 3.
Mean number of flowers per floral bud at different bud positions in 3‐year‐old branching systems of ‘Granny Smith’
| Shoot age | |||
| Branching order | 1‐year‐old | 2‐year‐old | 3‐year‐old |
| Terminal buds | |||
| 1 Long shoots | 4·68 (1·16)b | ||
| 2 Long shoots | 5·00 (0·88)ab | 5·18 (0·89)a | |
| 2. Spurs | 4·08 (0·62)c | 4·58 (0·57)b | |
| 3 Long shoots | 5·11 (0·53)a | ||
| Axillary buds | Distal | Median | Proximal |
| 3·60 (0·91)d | 3·88 (0·96)cd | 3·45 (1·51)d | |
Standard deviations are indicated in brackets.
For values with the same superscript letters no significant difference was obtained between the mean values by a Newman–Keuls test (P < 0·01).
Axillary buds on 1‐year‐old long shoots possessed the lowest mean number of flowers, i.e. about three or four flowers per cluster. These means were significantly lower than those on terminal buds but were not significantly different from one zone to another.
Relative number of flowers and other organs in buds
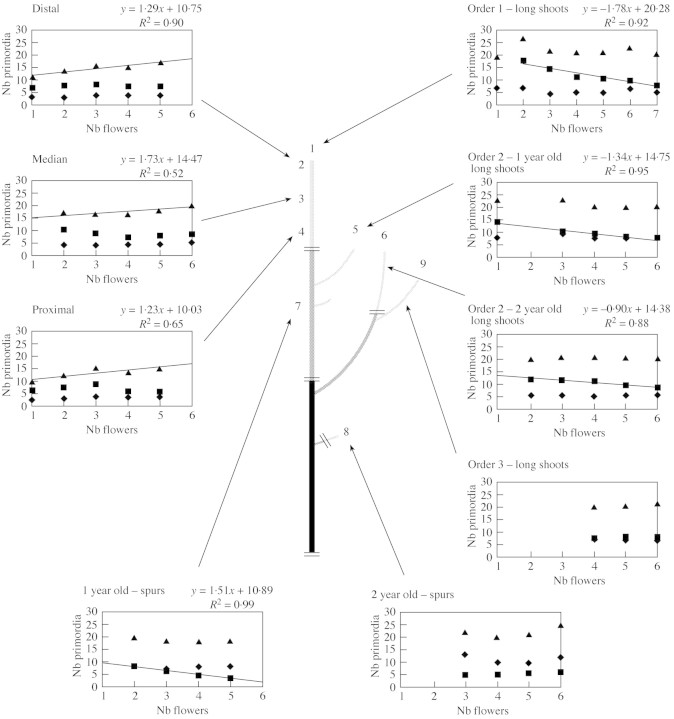
In floral buds, all the flowers except the king flower differentiate in the axils of bracts. A correlation was sought between the number of flowers per bud and the other organ categories (total number of preformed organs, scales and leaf primordia). No correlation was found when all bud positions were considered together. A possible relationship was therefore investigated for each bud category, gathering buds per class of similar number of flowers. The three mean numbers of preformed organs (total, scales and leaves) were plotted for each class of flower number (Fig. 2).
Fig. 2. Mean number of total preformed organs (▴), leaf primordia (▪) and scales (♦) according to the number of flowers per bud and at different bud positions in 3‐year‐old branching system of ‘Granny Smith’.
In axillary buds, the mean values of total number of preformed organs increased linearly with the number of flowers, while the mean number of scales and leaf primordia remained constant. This was observed in distal positions and to a lesser extent in median and proximal positions. In these bud categories, mean values of total organs varied from 10 to 20. In the other bud categories, the mean values of total organs was higher (varying from 17 to 27, Fig. 2 and Table 2A) and remained constant in each bud category with increasing number of flowers. In contrast, in four bud categories, the mean number of leaf primordia decreased linearly when the number of flowers increased: (1) terminal on long shoots in order 1; (2 and 3) terminal on 1‐year‐old and 2‐year‐old long shoots in order 2; and (4) 1‐year‐old spurs. No relationship was detected for buds in order 3 or for 2‐years old spurs.
The ratio of the number of leaf primordia to the number of flowers per floral cluster was calculated and compared according to floral bud position (Table 4). This showed a gradient in the number of leaves per flower. The ratio was highest for buds in the terminal position in order 1 (more than two leaves per flower) and for axillary buds in distal and median positions. However, the high value obtained in this latter case resulted from the small number of flowers per bud. Buds in terminal positions on long shoots in order 2 possessed fewer than two leaves per flower. Those in order 3, with a mean ratio of 1·5 leaves per flower, showed intermediate values not significantly different from those on long shoots in order 2 or from spurs. Buds terminal on spurs, regardless of age, possessed the lowest number of leaves per flower (about 1·2). In both spurs and long shoots in order 2, standard deviation values decreased from 1‐year‐old to 2‐year‐old shoots. Finally, the ratio between the number of leaf primordia and the number of flowers per bud depended on both branching order and shoot type, but remained constant with shoot age.
Table 4.
Mean ratio of the number of leaf to flower primordia per floral bud at different bud positions in 3‐year‐old branching systems of ‘Granny Smith’
| Shoot age | |||
| Branching order | 1‐year‐old | 2‐year‐old | 3‐year‐old |
| Terminal buds | |||
| 1 Long shoots | 2·76 (1·87)a | ||
| 2 Long shoots | 1·92 (2·08)bc | 1·97 (0·86)bc | |
| 2 Spurs | 1·21 (0·66)d | 1·18 (0·30)d | |
| 3 Long shoots | 1·58 (0·37)cd | ||
| Axillary buds | Distal | Median | Proximal |
| 2·42 (1·21)ab | 2·33 (1·16)ab | 2·73 (2·35)a | |
Standard deviations are indicated in brackets.
For values with the same superscript letters no significant difference was obtained between the mean values by a Newman–Keuls test (P < 0·01).
DISCUSSION
A particularly high proportion of terminal buds differentiated into floral buds on both long shoots and spurs. This is in agreement with the terminal and regular bearing behaviour of ‘Granny Smith’ (Lespinasse, 1977).
The mean total number of organs reflected the organogenetic activity of the corresponding meristem before it entered into dormancy. The mean values obtained from about 20 organs, are consistent with those previously reported (Abbott, 1970). The main differences observed between bud positions concerned axillary vs. terminal buds and to a lesser extent the bud positions within branching systems. The differences found between bud positions resulted from as least two factors: branching order and shoot age. Since shoot age in perennial plants is strongly correlated with shoot position, the sampling method did not allow us to compare shoots of similar age at different branching orders. Although these two factors could not be clearly separated in this study, shoot age seemed to have a greater effect than branching order.
The differences in the total number of organs between bud positions did not correspond to the growth period of the corresponding shoots since the largest numbers of organs were found in the most apical buds (i.e. in order 1) which are supposed to continue growing for the longest period. In other words, the buds which contained the most organs differentiated the latest. This suggests that no simple relationship exists between the period of bud formation and its organogenesis, at least regarding the number of differentiated organs, contrary to the suggestion of Luckwill (1974). In contrast, the differences observed were in agreement with the morphogenetic gradients in plant architecture as demonstrated previously in several species by Barthélémy et al. (1997) and in apple trees by Costes et al. (2003): buds in terminal positions on long shoots possessed more organs than those on spurs at the same age, themselves possessing more organs than axillary buds. These results are consistent with those of other studies conducted in different species and demonstrating a decrease in the number of preformed leaves with branching order (Remphrey and Davidson, 1994). However, the results obtained in the present study suggest that an additional effect may result from meristem age. Indeed, axillary buds—the first development stage of a newly formed meristem—differentiated fewer organs that all other bud categories. When axillary buds were compared with 1‐year‐old and 2‐year‐old spurs which correspond to subsequent stages in meristem life, this showed a progressive increase in organogenesis. A similar though less marked increase was also observed from 1‐year‐old to 3‐year‐old long shoots.
A clear basipetal gradient in floral differentiation was observed for axillary buds according to their insertion zone along the parent shoot. No gradient was found in the axillary buds for either the number of organs or the number of scales or leaf primordia between the three zones along the parent shoot. The lower number of organs observed in the proximal zone resulted from the higher proportion of vegetative buds in this zone. Different results have been found in different species regarding the existence of a basipetal gradient. Remphrey and Davidson (1994) and Puntieri et al. (2002) showed a basipetal decrease in Fraxinus pennsylvanica and Nothofagus antarctica, respectively, whereas the number of leaf primordia was shown to be constant in Persea (Thorp, 1994) and Juglans regia (Sabatier and Barthélémy, 2000). The results presented here show that the number of leaf primordia in the ‘Granny Smith’ apple trees studied is constant in axillary winter buds along a parent shoot. The difference in subsequent shoot development leading to the acrotonic gradient of laterals observed on apple tree annual shoots (Champagnat, 1947; Cook et al., 1998) may result from differences in neoformation development or might occur during the last stages of bud development, just before bud break, as observed in Persea (Thorp, 1994).
Vegetative buds, which were studied solely in axillary positions, differentiated fewer organs than floral buds in similar positions. But the difference did not depend on either the number of scales or the number of leaf primordia. This suggests that organogenesis stopped in these buds before they possessed the minimum number of organs which must be initiated before flowers differentiate, as suggested by previous authors (Abbott, 1970; Luckwill, 1974).
Floral bud constitution clearly depended on bud position in the branching systems. When bud position was not taken into account, no significant correlation was found in floral buds between organ categories, including between the number of flowers per bud and either the number of leaf primordia or the total number of organs. This agrees with the results obtained by Verheij (1996) under different experimental conditions, and particularly different temperatures.
Floral buds in axillary positions significantly differed from those in terminal positions: they contained fewer scales and flowers but a similar number of leaves when compared with terminal buds. Regarding the constitution of floral buds in terminal positions, those on spurs contained fewer leaf primordia and flowers than the mean values cited in previous studies. Fulford (1966) counted about eight leaf primordia before flower occurrence in spurs. Similarly, Ferree et al. (2001) found a higher mean number of flowers per spurs in different cultivars and climatic conditions, even though their study did not include ‘Granny Smith’. This could result from the absence of pruning on the studied branching systems which may enhance competition between the developing buds. The organogenesis occurring within buds before they enter into dormancy could thus respond to source/sink relationships between other organs developing during the growing season, such as leaves, fruits, shoots and roots. Another reason could be that ‘Granny Smith’ is a relatively old cultivar and more modern apple cultivars could have a different bud constitution.
Floral buds in terminal positions on long shoots contained proportionally fewer scales and more leaves than those on spurs. They also contained more flowers, particularly when located in orders 2 and 3.
The consideration of bud position highlighted two main situations regarding bud constitution. In axillary buds, an increase in the number of flowers corresponded to an increase in the mean values of the total number of primordia, suggesting that the organogenetic activity of the meristems could be a limiting factor for flower differentiation. Similar assumptions have been proposed in previous studies, particularly regarding the role of scales in floral differentiation (Crabbe and Escobedo‐Alvarez, 1991; Fulford, 1965, 1966; Abbott, 1970; Luckwill, 1974) but without any mention of bud position. The second situation corresponds to most of the other bud positions, in which more primordia differentiated. In this case, a trade‐off between leaf and flower primordia was observed: the more flowers the fewer leaves. This suggests that the length of time between floral initiation and organogenesis cessation affects the number of differentiated flowers. However, no significant relationships were observed in bud positions corresponding to the periphery of the branching systems. These locations may correspond to ‘stable’ situations where fewer fluctuations in the timing of bud formation and thereafter in bud constitution occur. This is supported by the lower standard deviations observed with shoot age in both long shoots and spurs.
The ratio of the number of leaves to flowers could be used to define ‘floral bud quality’. Indeed, bourse leaves provide most of the assimilates to the young flowers, at least during fruit set and in the early stages of development in young fruits (Hansen, 1971; Lakso, 1984), while bourse shoot leaves have been shown to be involved in return bloom phenomenon on spurs (Proctor and Palmer, 1991). It can thus be assumed that a high ratio is a first step to obtain a high fruit yield. The results presented here showed quite a high ratio in axillary buds, regardless of their insertion zone. However, this resulted from a very low number of flowers and from their immaturity since they did not develop more than 15 organs on average. In fact, these lateral flowers are known to be poorly productive and are usually removed in conventional conditions. The ratio for terminal buds on long shoots decreased with branching order but was always higher than for terminal buds on spurs, whose significantly lower ratio was mainly due to a small number of leaf primordia and a relatively high number of scales. In addition, the number of scales increased with spur age, resulting in a lower ratio. This suggests that floral buds on spurs are of poorer quality, at least in the experimental conditions presented here. Again, this situation could result from the absence of pruning on the branching systems studied and consequent competition between the developing buds. This can be different in orchards where long shoots are removed because tree production is considered to rely quantitatively on fruiting spurs (Lakso, 1984; Wünsche et al., 1996).
ACKNOWLEDGEMENTS
I am most grateful to G. Pena for his technical assistance in bud dissection and CEHM (Centre Experimental en Horticulture de Marsillargues) for authorizing samples of branching systems in an experimental orchard.
Supplementary Material
Received: 28 March 2003; Returned for revision: 2 June 2003; Accepted: 11 June 2003 Published electronically: 15 August 2003
References
- AbbottDL.1970. The role of budscales in the morphogenesis and dormancy of the apple fruit bud. In: Luckwill LC, Cutting CV, eds. Physiology of tree crops London: Academic Press, 65–81. [Google Scholar]
- AbbottDL.1977. Fruit‐bud formation in Cox’s Orange Pippin. Long Ashton Research Station Annual Report 1976–1977: 167–176. [Google Scholar]
- BarrittBH, Konishi BJ.1993. Influence of apple cultivar and canopy position on fruit spur leaf development within a season. Fruit Varieties Journal 47: 5–12. [Google Scholar]
- BarrittBH, Rom C, Guelich K, Drake S, Dilley M.1987. Canopy position and light effects on spur, leaf, and fruit characteristics of ‘Delicious’ apple. Hortscience 22: 402–405. [Google Scholar]
- BarthélémyD, Caraglio Y, Costes E.1997. Architecture, gradients morphogénétiques et âge physiologique chez les végétaux. In: Bouchon J, de Reffye P, Barthélémy D, eds. Modélisation et simulation de l’architecture des végétaux Science UpDate Paris: INRA Editions, 89–136. [Google Scholar]
- CamefortH.1977.Morphologie des végétaux vasculaires: cytologie, anatomie, adaptations. Paris: Doin Editions. [Google Scholar]
- ChampagnatP.1947. Les principes généraux de la ramification des végétaux ligneux. Revue Horticole 2143: 335–341. [Google Scholar]
- CookNC, Rabe E, Keulemans J, Jacobs G.1998. The expression of acrotony in deciduous fruit trees: a study of the apple rootstock M.9. Journal of the American Society for Horticultural Science 123: 30–34. [Google Scholar]
- CostesE.1993. Architecture aérienne de l’abricotier en développement libre. Acta Botanica Gallica 140: 249–261. [Google Scholar]
- CostesE, Guédon Y.2002. Modelling branching patterns in 1‐year‐old trunks of six apple cultivars. Annals of Botany 89: 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CostesE, Sinoquet H, Kelner JJ, Godin C.2003. Exploring within‐tree architectural development of two apple tree cultivars over 6 years. Annals of Botany 91: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CrabbeJ.1984. Vegetative vigor control over location and fate of flower buds, in fruit trees. Acta Horticulturae 149: 55–63. [Google Scholar]
- CrabbeJ, Escobedo‐Alvarez JA.1991. Activités méristématiques et cadre temporel assurant la transformation florale des bourgeons chez le Pommier (Malus × Domestica Borkh. cv. Golden Delicious). In: Edelin C, ed. L’arbre: biologie et developpement Naturalia Monspeliensia No Hors Série, 369–379. [Google Scholar]
- CritchfieldWB.1960. Leaf dimorphism in Populus trichocarpa American Journal of Botany, 47: 699–711. [Google Scholar]
- Escobedo‐AlvarezJA.1990.Etude de la transformation florale des bourgeons de la pousse de “bourse” du pommier. PhD Thesis, Faculté des Sciences Agronomiques de Gembloux. [Google Scholar]
- FerreeDC, Palmer JW.1982. Effect of spur defoliation and ringing during bloom on fruiting, fruit mineral level, and net photosynthesis of ‘Golden Delicious’ apple. Journal of the American Society for Horticultural Science 107: 1182–1186. [Google Scholar]
- FerreeDC, Bishop BL, Schupp JR, Tustin DS, Cashmore WM.2001. Influence of flower type, position in the cluster and spur characteristics on fruit set and growth of apple cultivars. Journal of Horticultural Science & Biotechnology 76: 1–8. [Google Scholar]
- FulfordRM.1965. The morphogenesis of apple buds. I. The activity of the apical meristem. Annals of Botany 29: 167–180. [Google Scholar]
- FulfordRM.1966. The morphogenesis of apple buds. II. The development of the bud. Annals of Botany 30: 27–38. [Google Scholar]
- HalléF, Oldeman RAA, Tomlinson PB.1978.Tropical trees and forests. Berlin: Springer‐Verlag. [Google Scholar]
- HansenP.1971. 14C studies on apple trees. VII. The early seasonal growth in leaves, flowers and shoots as dependent upon current photosynthates and existing reserves. Physiologia Plantarum 25: 469–473. [Google Scholar]
- KremerA.1981. Déterminisme génétique de la croissance en hauteur du Pin maritime (Pinus pinaster Ait.). 1. Rôle du polycyclisme. Annales des Sciences Forestières 38: 199–222. [Google Scholar]
- LaksoAN.1984. Leaf area development patterns in young pruned and unpruned apple trees. Journal of the American Society for Horticultural Science 109: 861–865. [Google Scholar]
- LespinasseJM.1977.La conduite du pommier (1ère partie): types de fructification – incidence sur la conduite de l’arbre. Paris: Invuflec. [Google Scholar]
- LespinasseJM, Lauri PE.1999. Intégration des nouveaux concepts de conduite dans le système Solaxe. Revue Suisse de Viticulture, Arboriculture, Horticulture 31: 167–171. [Google Scholar]
- LuckwillLC.1974. A new look at the process of fruit bud formation in Apple. Proceedings of the 19th International Horticultural Congress 3: 237–245. [Google Scholar]
- OldemanRAA.1974.L’architecture de la forêt guyanaise, Vol. Mémoire no. 73. Paris: ORSTOM. [Google Scholar]
- ProctorJTA, Palmer JW.1991. The role of spur and bourse leaves of three apple cultivars on fruit set and growth and calcium content. Journal of Horticultural Science 66: 275–282. [Google Scholar]
- PuntieriJG, Stecconi M, Barthélémy D.2002. Preformation and neoformation in shoots of Nothofagus antarctica (G. Forster) Oerst. (Nothofagaceae) shrubs from northern Patagonia. Annals of Botany 89: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RemphreyWR, Davidson CG.1994. Shoot preformation in clones of Fraxinus pennsylvanica in relation to site and year of bud formation. Trees: Structure and Function 8: 129–131. [Google Scholar]
- RemphreyWR, Powell GR.1984. Crown architecture of Larix laricina saplings: shoot preformation and neoformation and their relationships to shoot vigour. Canadian Journal of Botany 62: 2181–2192. [Google Scholar]
- RivalsP.1965. Essai sur la croissance des arbres et sur leurs systèmes de floraison (application aux espèces fruitières). I. Journal d’Agriculture Tropicale et de Botanique Appliquée XII: 655–686. [Google Scholar]
- SabatierS, Barthélémy D.2000. Bud structure in relation to shoot morphology and position on the vegetative annual shoots of Juglans regia L. (Juglandacea). Annals of Botany 87: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SouzaMS, Puntieri JG, Barthélémy D, Brion C.2000. Bud content and its relation to shoot size and structure in Nothofagus pumilio (Poepp. et Endl.) Krasser (Nothofagaceae). Annals of Botany 85: 547–555. [Google Scholar]
- ThorpTG, Aspinall D, Sedgley M.1994. Preformation of node number in vegetaive and reproductible proleptic shoot modules of Persea (Lauraceae). Annals of Botany 73: 13–22. [Google Scholar]
- VerheijFA.1996.Morphological and physiological aspects of the early phases of flower bud formation of apple. PhD Thesis, University of Wageningen. [Google Scholar]
- WünscheJN, Lakso AN, Robinson TL, Lenz F, Denning SS.1996. The bases of productivity in apple production systems: the role of light interception by different shoot types. Journal of the American Society for Horticultural Science 121: 886–893. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.