Abstract
Background
Allogeneic mesenchymal precursor cells (MPC) injected during left ventricular assist device (LVAD) implantation may contribute to myocardial recovery. This trial explores the safety and efficacy of this strategy.
Methods and Results
In this multi-center, double-blind, sham-procedure controlled trial, 30 patients were randomized (2:1) to intramyocardial injection of 25M MPCs or medium during LVAD implantation. The primary safety endpoint was incidence of infectious myocarditis, myocardial rupture, neoplasm, hypersensitivity reaction, and immune sensitization (90 days post-randomization). Key efficacy endpoints were functional status and ventricular function, while temporarily weaned from LVAD support (90 days post-randomization). Patients were followed until transplant or 12 months post-randomization, whichever came first. Mean age was 57.4 (±13.6) years, mean LVEF 18.1%, and 66.7% were destination therapy LVADs. No safety events were observed. Successful temporary LVAD weaning was achieved in 50% of MPC and 20% of control patients at 90 days (p=0.24); the posterior probability that MPCs increased the likelihood of successful weaning is 93%. At 90 days, 3 deaths occurred in control and none in MPC patients. Mean LVEF following successful wean was 24.0% (MPC=10) and 22.5% (Control=2) (p=0.56). At 12 months, 30% of MPC and 40% of control patients were successfully temporarily weaned from LVAD support (p=0.69) and 6 deaths occurred in MPC patients. Donor-specific HLA sensitization developed in 2 MPC and 3 control patients and resolved by 12 months.
Conclusions
In this preliminary trial, administration of MPCs appeared to be safe and there was a potential signal of efficacy. Future studies will evaluate the potential for higher or additional doses to enhance the ability to wean LVAD recipients off support.
Clinical Trial Registration Information
ClinicalTrials.gov. Identifier: NCT01442129.
Keywords: Left Ventricular Assist Device, Heart Failure, Mesenchymal precursor cell, Stem cells, Placebo, Randomized controlled trial
Left ventricular assist devices (LVADs) have well-documented survival and quality of life benefits in patients with advanced heart failure both as a bridge to cardiac transplantation and as a long-term therapy in patients who are not transplant candidates.1–4 Reports of improved myocardial function have motivated investigation of the use of LVADs as a bridge to recovery. While most LVAD recipients do show some indications of reverse remodeling of the left ventricle as evidenced by salutary changes in ventricular structure, myocyte contractile strength5, normalization of extracellular matrix and tissue and circulating neurohormones6, and programs of gene expression7–10 these improvements are rarely sufficient to allow removal of the device.11 The disconnect between reverse remodeling and recovery of cardiac function have prompted efforts to investigate adjunctive therapies to LVAD support, including novel pharmacotherapies12 and stem cells as potential interventions to augment ventricular recovery.
Recent pre-clinical and clinical evidence suggests that myocardial transplantation of allogeneic mesenchymal stem cells, in particular, can enhance cardiac performance in settings of acute and chronic functional impairment.13–15 Unlike whole organ transplantation or many other allogeneic cell transplants, mesenchymal stem cell transplants do not appear to cause rejection and instead may be associated with evidence of induced tolerance to the donor.16, 17
We have, therefore, begun investigation of allogeneic mesenchymal precursor cell transplantation concomitant with LVAD placement in patients with advanced heart disease. While our ultimate goal is the achievement of robust bridging to recovery, allosensitization could adversely impact donor suitability in LVAD recipients who are transplant candidates. Accordingly, the primary goal of the initial trial reported here was exploration of the safety of intramyocardial implantation of a single low dose of allogeneic mesenchymal precursor cells (MPCs) together with assessment of left ventricular performance during short intervals of temporary reduction of LVAD support, over 1 year of observation after the implants, to assess safety and any impact on reverse remodeling.
METHODS
STUDY DESIGN AND TRIAL OVERSIGHT
This early phase, randomized trial was designed to enroll 30 patients, and if safety would be established, a larger follow-up trial will be conducted. Patients were randomly assigned in a 2:1 ratio to 25 million MPCs (Mesoblast, Inc.) or control, comprised of cryoprotective medium alone (50% Alpha-MEM/42.5% ProFreeze NAO Freezing Medium/7.5% DMSO). Randomization was blocked to ensure equivalence of group size. All investigators and patients were masked to treatment intervention and overall outcomes data. Endpoints were measured monthly until 90 days, and every 60 days thereafter until 12 months after randomization. All patients were followed until cardiac transplantation (for bridge to transplant [BTT]) or until 12 months following randomization (for BTT and Destination Therapy [DT]), whichever came first.
The trial was conducted in 11 U.S. centers with a Data and Clinical Coordinating Center (DCC; International Center for Health Outcomes and Innovation Research [InCHOIR], Icahn School of Medicine at Mount Sinai) under an investigational new drug application. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) definitions were utilized for all relevant adverse events; bleeding events were defined by transfusion of ≥ 4 units of packed cells within any 24 hour period during the first 7 days post LVAD implantation, and any transfusion of packed cells within any 24 hour period thereafter. An independent Clinical Events Committee adjudicated adverse events and causes of death. An NIH-appointed protocol review committee (PRC) reviewed the trial design and data and safety monitoring board (DSMB) reviewed the trial progress. Institutional review boards of participating centers and the DCC approved the protocol, and all patients provided written informed consent.
PATIENTS AND INTERVENTIONS
The target population was adults with end-stage heart failure, of either ischemic or non-ischemic etiology, who had a planned, clinically indicated LVAD implantation for BTT or DT. Assist devices were required to be FDA approved, contemporary, implantable, continuous flow LVADs; selection of the specific device was left to the discretion of the surgeon. Patients were ineligible if percutaneous LVAD or biventricular mechanical support was anticipated, if they had cardiothoracic surgery or myocardial infarction within 30 days prior to randomization, had undergone prior cardiac transplantation, LV reduction surgery, or cardiomyoplasty, or were considered to have an acute reversible cause of heart failure. Other selected exclusion criteria included the presence of >10% anti-HLA antibody titers with known specificity to MPC donor HLA antigens, active systemic infection within 48 hours prior to randomization, history of cancer prior to screening (excluding basal cell carcinoma), or stroke within 30 days prior to randomization. Patients were ineligible if they had received prior stem cell therapy for cardiac repair, or any investigational cell based therapy within 6-months prior to randomization. (Supplemental Materials include complete eligibility criteria).
The investigational agent was allogeneic mesenchymal precursor cells (MPCs), which are a STRO-3 immuno-selected, culture-expanded, immature sub-fraction of adult bone marrow-derived mononuclear cells.18 The allogeneic MPCs are formulated and cryopreserved in 7.5% DMSO/50% Alpha Modified Eagle’s Medium (MEM) and 42.5% ProFreeze® and stored in the vapor phase of liquid nitrogen until use. Cell procurement, processing, cryopreservation, and storage procedures were performed by a contract manufacturing facility under cGMP conditions. Donor and process testing were conducted for transmissible infectious diseases, karyotype, tumorigenicity, sterility, endotoxins, and mycoplasma. The product is characterized by cell count, viability, surface antigen expression of STRO-1, CC-9, and HLA class I and II. Cryopreserved products were shipped to sites for local storage, and cells were thawed and injected according to study procedures.
Intramyocardial injections of MPCs or control were performed at the time of LVAD implantation. Injection procedures were protocol-defined, providing standardization of the intervention across sites and designed to maximize injections across as much of the left ventricular (LV) myocardium as possible. LVAD implantation and management were performed in accordance with the Directions for Use, and the protocol provided guidance with respect to long-term management, including optimization of hemodynamic off-loading of the LV, reduction of mitral regurgitation when present, and optimization of mean blood pressure.
LVAD weaning was defined as a transient reduction in pump speed to minimize forward flow through the pump in order to assess native myocardial function. Protocol-specified guidelines for weaning were adopted from the Harefield Hospital protocol, and included guidance for antithrombotic regimens, incremental speed reductions to a “low speed” target of 6,000 rpm, and monitoring of patients during the wean. The 6000 rpm target was chosen as this is the minimum speed necessary to prevent retrograde flow through the pump into the LV.19 Weans were performed in patients deemed to be clinically stable by the clinical team and were terminated if patients developed signs or symptoms of low output or vascular congestion, such as light headedness, dyspnea, fatigue, chest pain or pulmonary edema. Patients who completed weaning but developed transient symptoms at any point during “low speed” were categorized as “wean failures” for that particular wean. A six minute walk (6MW) was performed after 20 minutes of “low speed”. A comprehensive echocardiogram was performed at full support prior to the wean, at 15 minutes of “low speed” and again following the 6MW. The LVAD was reprogrammed to full support thereafter.
ENDPOINTS
The primary endpoint was safety, defined by the incidence of potential study intervention-related adverse events within 90 days post-randomization, including infectious myocarditis, myocardial rupture, neoplasm, hypersensitivity reaction, and immune sensitization (defined as a clinical syndrome accompanied by detection of donor specific antibodies within 30 days of onset of the syndrome). The key efficacy endpoints were functional status, defined by the ability to tolerate wean from LVAD support for 30 minutes without signs or symptoms of hypoperfusion, and left ventricular ejection fraction (LVEF) while weaned from LVAD support at 90 days post-randomization. Ventricular function was quantified by LVEF assessed by transthoracic echocardiogram (TTE) while weaned from LVAD support, in those patients able to be weaned for 30 minutes.
Secondary endpoints for patients who tolerated weaning included echocardiographic assessments of myocardial size and function, 6MW, and duration of wean, all assessed while weaned from support, at multiple time points (30, 60 and 90 days post-randomization, and every 60 days thereafter until cardiac transplantation or 12 months, whichever comes first) over the 12 months of the trial follow-up. Additional endpoints included the incidence of serious adverse events, anti-HLA antibody sensitization, neurocognitive outcomes, survival to transplantation, and exploratory mechanistic assessments including histological assessments of myocardium at explant (at cardiac transplantation, LVAD replacement or autopsy), peripheral blood cell phenotypic and functional analyses, and plasma chemo/cytokine analyses at multiple time points over the 12 months of trial follow-up.
STATISTICAL ANALYSIS
Safety
A safety monitoring plan was based on pre-specified rare events and mortality. Enrollment would be halted if any of the pre-specified events associated with experimental treatment (infectious myocarditis, myocardial rupture, neoplasm, hypersensitivity reaction, or immune sensitization) were observed within 90 days post-randomization. Similarly, randomization would be halted if, after 10 patients were randomized, the posterior probability that mortality on active therapy was increased compared to control exceeded 80%. Rates of adverse events were compared using Poisson regression.
Efficacy
Superiority of MPC compared to control was assessed using a Bayesian approach. The posterior probability that the proportion of successes (ability to tolerate LVAD wean for 30 minutes at 90 days post-randomization) in the MPC group was greater than the proportion of successes in the control group was calculated based on the observed proportions of successes in the two groups. A non-informative prior distribution, beta (1,1), was assumed for the true success probabilities in the two treatment groups.
Categorical variables were summarized as frequencies, continuous variables as means and standard deviations. As per the protocol, LVEF was evaluated only in patients who tolerated the LVAD wean. Fisher’s Exact Test and Wilcoxon sum-rank test were used to compare the two groups when the sample size permitted. Kaplan-Meier curves and the log-rank test were used to assess survival in the two groups.
Sample size was determined based on simulations. A sample size of 30 patients was chosen to allow us to detect an approximate tripling of the odds that active therapy is superior (i.e., from 50% probability of active therapy’s superiority, or 1:1 odds, to 75% or 3:1 odds) with probability 75% or more if the absolute probability of a successful outcome with active therapy is about 10–15% higher than for control.
RESULTS
PATIENTS
Eighty one patients were screened, 47 eligible, and 30 were randomized (Figure 1); 20 patients to intramyocardial MPC administration and 10 to intramyocardial injection of cryoprotective medium (control). Treatment intervention was withheld from one MPC patient who was randomized prior to obtaining core lab results of the panel of reactive antibodies (PRA) to exclude pre-existing donor-specific antibodies.
Figure 1.
CONSORT Diagram.
The treatment groups were similar with respect to baseline characteristics (Table 1). The mean age was 57.4 years (±13.6) and 83% were male. The mean LVEF was 18% (± 4.3), 37% had ischemic cardiomyopathy, and all patients were implanted with Heart Mate II® LVADs (Thoratec Corp.), 67% were implanted for DT indication.
Table 1.
Baseline Characteristics of the Patients*
| Characteristic | MPC (N=20) |
Control (N=10) |
|---|---|---|
| Male | 17 (85) | 8 (80) |
| Age (yr) | 55.1 ± 15.4 | 62.2 ± 7.8 |
| Race | ||
| White | 14 (70) | 8 (80) |
| Black or African American | 6 (30) | 2 (20) |
| Cardiomyopathy | ||
| Ischemic | 7 (35) | 4 (40) |
| Non Ischemic | 13 (65) | 6 (60) |
| Biventricular Pacemaker | 11 (55) | 7 (70) |
| Pre-op IABP | 3 (15) | 0 (0) |
| Creatinine (mg/dL) | 1.3 ± 0.4 | 1.2 ± 0.3 |
| Cardiac Index (I/min/m2) | 1.8 ± 0.6 | 1.8 ± 0.5 |
| PVR (Wood Units) | 2.7 ± 2.0 | 3.2 ± 1.8 |
| LVEF (%) | 17.5 ±3.9 | 19.3 ± 5.1 |
| NYHA | ||
| Class I & II† | 0 (0) | 1 (10) |
| Class III | 3 (15) | 2 (20) |
| Class IV | 17 (85) | 7 (70) |
| Medications | ||
| Beta Blocker | 14 (70) | 5 (50) |
| Aldosterone Receptor Antagonist | 11 (55) | 5 (50) |
| ACEi/ARB | 7 (35) | 7 (70) |
| Intropic Therapy | ||
| 1 Agent | 10 (50) | 7 (70) |
| >1 Agent | 4 (20) | 2 (20) |
| Vasoactive Therapy | 4 (20) | 0 (0) |
| Indication for LVAD | ||
| Bridge to Transplantation | 7 (35) | 3 (30) |
| Destination Therapy | 13 (65) | 7 (70) |
Plus-minus values are means ± SD, categorical values are n (%)
One patient classified as NYHA II heart failure
SAFETY AND MORTALITY
No patients developed a primary safety event within 90 days following randomization (the primary endpoint), or during the 12-month follow-up period. At 90 days, there were three deaths (30%) in the control group and none in the MPC group. In a post-hoc analysis, the posterior probability that mortality at 90 days is reduced in the MPC group exceeded 80%. Six MPC patients (30%) died over the 12 months, with no additional deaths occurring in the control group. Figure 2 depicts the Kaplan-Meier survival curves. The most frequent primary causes of death in this patient population were LVAD failure (22.2%) and multi-system organ failure (22.2%); and the most frequent underlying causes of death were pump thrombus (33.3%) and sepsis (22.2%). Causes of death were similar between the groups, and no deaths were classified as related to the study intervention.
Figure 2.
Survival. Crosses depict censored patients.
Serious adverse event rates were similar between the two groups (Table 2). At 90 days the serious adverse event rate was 1.30 per patient-month in the treatment group and 1.16 in the control group. The overall rate was 6.95 per patient-year in the treatment group and 6.89 in the control group. The most prevalent serious adverse events over the course of the trial were major bleeding, respiratory failure, right heart failure, and localized non-device infection. The major bleeding event rate per patient-year at 12 months was 3.88 in the treatment group and 3.97 in the control group; major bleeding requiring surgery or re-hospitalization is depicted in Table 2. Of note, one trial patient, who did not receive intramyocardial injections at the time of LVAD implantation (described above), had multiple bleeding events and received 32 units of packed red cells over the course of the trial. Donor-specific HLA sensitization developed post-randomization in two MPC and in three control patients. By one year, one sensitized patient in each arm died and all donor-specific antibodies had resolved in surviving patients.
Table 2.
Serious Adverse Events
| 90 Days | 12 Months | |||||
|---|---|---|---|---|---|---|
| MPC (N=20) (Person Month=59.2) |
Control (N=10) (Person Month=25) |
P- value |
MPC (N=20) (Person Year=18.3) |
Control (N=10) (Person Year=7.6) |
P- value |
|
| No. of Events (Rate/Pt Month) | No. of Events (Rate/Pt Year) | |||||
| Bleeding | ||||||
| Intra-operative Bleeding | 2 (0.03) | 1 (0.04) | 2 (0.11) | 1 (0.13) | ||
| Major Bleeding Requiring Surgery | 7 (0.12) | 1 (0.04) | 7 (0.38) | 1 (0.13) | ||
| Major Bleeding Requiring Re-Hosp | 0 (0.00) | 4 (0.16) | 10 (0.55) | 7 (0.93) | ||
| Cardiac Arrhythmias | ||||||
| Cardiac arrest | 0 (0.00) | 0 (0.00) | 1 (0.05) | 0 (0.00) | ||
| Sustained ventricular arrhythmia* | 2 (0.03) | 2 (0.08) | 2 (0.11) | 2 (0.26) | ||
| Sustained supraventricular arrhythmia† | 1 (0.02) | 2 (0.08) | 2 (0.11) | 2 (0.26) | ||
| Pericardial Effusion‡ | 3 (0.05) | 0 (0.00) | 3 (0.16) | 0 (0.00) | ||
| Device Malfunction | ||||||
| Pump Failure | 0 (0.00) | 0 (0.00) | 1 (0.05) | 0 (0.00) | ||
| Non-Pump Failure | 0 (0.00) | 0 (0.00) | 3 (0.16) | 0 (0.00) | ||
| Pump Thrombus suspected | 0 (0.00) | 1 (0.04) | 1 (0.05) | 1 (0.13) | ||
| Pump Thrombus confirmed | 2 (0.03) | 1 (0.04) | 4 (0.22) | 1 (0.13) | ||
| Hemolysis | 1 (0.02) | 1 (0.04) | 1 (0.05) | 2 (0.26) | ||
| Hepatic Dysfunction | 0 (0.00) | 0 (0.00) | 1 (0.05) | 0 (0.00) | ||
| Major Infection | ||||||
| Localized Non-Device Infection | 3 (0.05) | 2 (0.08) | 4 (0.22) | 2 (0.26) | ||
| Internal Pump Component Inflow or Outflow Tract Infection | 0 (0.00) | 0 (0.00) | 1 (0.05) | 0 (0.00) | ||
| Sepsis | 2 (0.03) | 0 (0.00) | 3 (0.16) | 1 (0.13) | ||
| Unspecified | 0 (0.00) | 0 (0.00) | 1 (0.05) | 0 (0.00) | ||
| Neurological Dysfunction | ||||||
| TIA | 0 (0.00) | 0 (0.00) | 1 (0.05) | 0 (0.00) | ||
| Ischemic Stroke | 0 (0.00) | 0 (0.00) | 1 (0.05) | 0 (0.00) | ||
| Hemorrhagic Stroke | 0 (0.00) | 1 (0.04) | 0 (0.00) | 1 (0.13) | ||
| Toxic Metabolic Encephalopathy | 2 (0.03) | 0 (0.00) | 2 (0.11) | 0 (0.00) | ||
| Other | 0 (0.00) | 0 (0.00) | 1 (0.05) | 0 (0.00) | ||
| Renal Dysfunction | ||||||
| Acute | 4 (0.07) | 1 (0.04) | 4 (0.22) | 1 (0.13) | ||
| Chronic | 1 (0.02) | 0 (0.00) | 1 (0.05) | 0 (0.00) | ||
| Respiratory Failure | 5 (0.08) | 2 (0.08) | 5 (0.27) | 2 (0.26) | ||
| Right Heart Failure | 3 (0.05) | 1 (0.04) | 3 (0.16) | 3 (0.40) | ||
| Venous Thromboembolism | 1 (0.02) | 1 (0.04) | 1 (0.05) | 1 (0.13) | ||
| Other | 38 (0.64) | 8 (0.32) | 61 (3.34) | 24 (3.18) | ||
| Total | 77 (1.30) | 29 (1.16) | 0.60 | 127 (6.95) | 52 (6.89) | 0.96 |
Sustained ventricular arrhythmia requiring defibrillation or cardioversion
Sustained supraventricular arrhythmia requiring drug treatment or cardioversion
Fluid or clot in the pericardial space that requires surgical intervention or percutaneous catheter drainage
EFFICACY
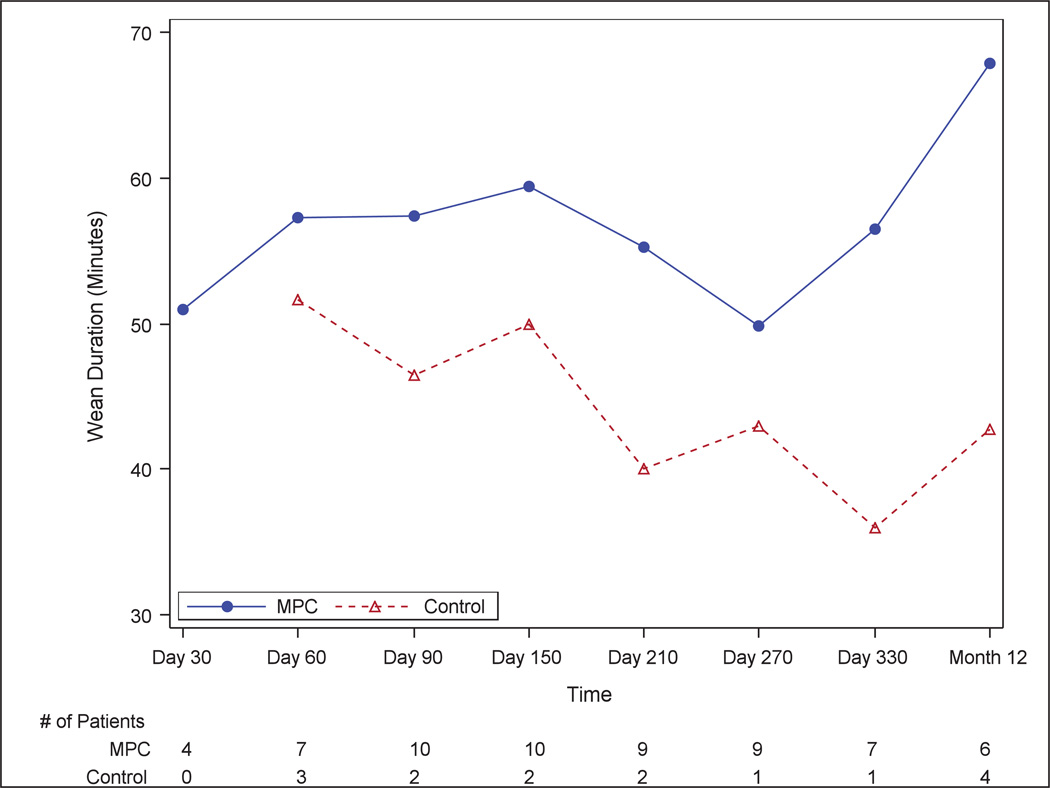
In the MPC group, 50% of patients were able to successfully tolerate the wean from LVAD support for 30 minutes at 90 days, compared to 20% in the control group (p=0.24). Based on these results, the posterior probability that MPCs increase the likelihood of successful weaning is 93% (Figure 3). The duration of temporary LVAD wean, for those who tolerated it, was greater in MPC than control patients at each time point (Figure 4). None of the control patients and four (20%) of the MPC treated patients were able to tolerate the LVAD wean at the 30-day time point.
Figure 3.
Posterior Distribution Analysis Curve. Difference = Difference in response-rates (MPC-Placebo); 93% of the area under the curve is between 0–1, corresponding to the probability that MPCs provide better response than placebo.
Figure 4.
Duration of LVAD Wean.
The mean LVEF at the conclusion of the temporary wean, for those who tolerated LVAD turn down, was 24% (MPC; n=10) and 22.5% (Control; n=2) at 90 days (p=0.56), and the median 6MW was 883 (Q1, Q3 [first and third quartiles] 750, 1042) feet in the treatment arm and 1080 (Q1, Q3 871, 1289) feet in the control arm (p=0.35).
At 12 months, there was no difference between groups in the ability to tolerate temporary weaning; 30% of MPC and 40% of control patients (p=0.69) weaned from LVAD support. Eighty-five percent (85%) of MPC patients tolerated one or more temporary LVAD weans over the 12 month follow-up period, compared to 40% of control patients (p=0.03) (Figure 5). Importantly, heart failure therapy, including angiotensin receptor and aldosterone antagonists, beta blockers, diuretics, and inotropic therapy, was similar between the two groups at 90 days. At one year, the regimens remained similar across groups with the exception of angiotensin antagonists (MPC: n=14 [100%]; control: n=4 [57%]).
Figure 5.
Number of Successful Weans.
HOSPITALIZATIONS
There was no difference between groups with respect to hospitalizations. The median length of stay of index hospitalization was 29.5 days in the MPC and 35.0 days in the control group (p= 0.91). By 90 days post-randomization, 26 patients had been discharged from the index hospitalization; of those 22% (4/18) of MPC patients and 38% (3/8) of control were readmitted. The rate of re-hospitalization per person-year was 2.15 (MPC) and 2.14 (control). The median time to first readmission was 91 days (Q1, Q3 44, 263) in the MPC group and 51 days (Q1, Q3 10, 150) in the control group. The most frequent reasons for readmission were non-cardiovascular in both groups, driven by infection (8.8%) and bleeding (70.6%); 96% of the latter were gastrointestinal in origin (Table 3).
Table 3.
Hospitalization Experience
| MPC (N=20) |
Control (N=10) |
P Value | |
|---|---|---|---|
| Index Hospitalization | |||
| Median Length of stay (Days) | 29.5 (20.5, 43) | 35 (17, 45) | 0.91 |
| Readmissions at One Year | |||
| No. of patients with ≥ 1 readmissions | 12 (67) | 6 (75) | |
| Median time to first readmission (days) | 91 (44, 263) | 51 (10, 150) | |
| Total No. of readmissions (rate per patient year) | 34 (2.1) | 14 (2.1) | |
| Reasons for readmission (%) | |||
| LVAD related | 8 (24) | 1 (7) | |
| Cardiovascular Non-LVAD related | 3 (9) | 2 (14) | |
| Non-CV | 23 (68) | 11 (79) |
DISCUSSION
This is the first randomized trial of allogeneic MPCs in patients undergoing LVAD implantation for the management of advanced heart failure.20–22 Early experience with mesenchymal stem cells, or their subpopulations, suggests that they may be more effective than unfractionated bone marrow mononuclear cells in clinical applications. MPCs are multipotent cells with extensive proliferative potential that secrete numerous antiapoptotic, angiogenic factors, and growth factors.23–24 Since MPCs are immune privileged, they can be transplanted into unrelated recipients without the need for HLA matching or immunosupression, thereby creating the possibility of an allogeneic, off-the-shelf cell product, readily available for administration.13,15,25 The predominant mechanism of MPC therapy in cardiovascular disease is generally considered to be mediated by the paracrine effects of the cells, since both long-term engraftment and trans-differentiation into cardiomyocytes are unlikely based on previous studies; neither mechanism can account for the biological activity demonstrated in numerous studies.15,26–28 Indeed, MPCs are known to secrete significant amounts of potentially relevant growth and angiogenic factors, such as stromal cell–derived factor-1, hepatocyte growth factor-1, insulin-like growth factor-1, vascular endothelial growth factor, and interleukin-6.25,28 Mechanistic effects of the MPCs employed in this trial will be examined in further biospecimen analyses.
The majority of patients enrolled in this trial had non-ischemic cardiomyopathy, which, although atypical for the epidemiology of the broader heart failure population, is representative of the breakdown by etiology of the advanced heart failure LVAD subgroup.4,29 Nearly 70% of trial patients received an LVAD for long term use, with approximately a third of the patients receiving LVAD support for BTT. The BTT population in particular, influenced the dose selection for this trial, since these patients are in general younger and may be more likely to experience myocardial recovery, but also are at unique risk of jeopardizing their transplant eligibility if they become immune sensitized. For this reason, we selected a dose of 25M cells, a comparatively low dose relative to other trials of cell based therapies.
This early trial demonstrates that MPCs are safe; no primary safety endpoint adverse events occurred within 90 days after randomization or over the course of the 12 month follow-up. As observed, one of the major safety concerns in the BTT LVAD population is HLA sensitization. Interestingly, more control patients developed donor-specific antibodies (DSA) within the first 90 days post-randomization than those who received MPCs, and by one year all donor-specific sensitization had resolved. All three control patients who developed DSA received transfusions following randomization, perhaps contributing to the sensitization. No sensitization that developed during the trial was associated with any clinical findings. These data provide sufficient safety experience to explore higher doses of MPCs in this vulnerable patient population.
Serious adverse event rates at 90 days and over one year were similar between MPC and control groups. Furthermore, adverse event rates generally were similar, except for bleeding, to those previously reported for the LVAD population.2,4,30 Major bleeding was adjudicated more conservatively in this trial than is current practice. In this trial bleeding events were defined by transfusion requirements at 24 hour increments, regardless of the presence of a clinical bleeding episode, and an ongoing bleed over time was captured as multiple events based on transfusions per time period. This categorization may have contributed to the higher rate of major bleeding observed in this trial relative to the published literature. Nearly 75% of bleeding events occurred more than 30 days following LVAD surgery and intramyocardial injections. Respiratory failure, although theorized to be associated with trapping of cells in the lungs in the setting of cell-based therapies, was experienced at similar rates across the treatment groups in this trial, and was consistent with existing benchmarks in LVAD patients.30–31 Direct comparison of suspected and confirmed device thrombus rates seen in this small population with those in the literature is challenged by differences in definitions and duration of LVAD support. That said, the 90 day and 12 month rates observed in the trial population are not dissimilar to those recently reported,32 and are the same between treatment groups. (See Supplemental Table 1 for 30 day AE rates).
The median length of stay for the index hospitalization was similar between the two groups, and although somewhat prolonged, was not dissimilar to length of stay data previously reported in the LVAD population.33 The frequency of readmissions by both 90 days and one year post-randomization for patients discharged after their index hospitalization was also similar between the treatment groups, and consistent with the expected range for the LVAD population.34 Interestingly, although the rate of readmissions was similar between the treatment groups, the median time to first readmission was earlier in the control group (51 days [10, 150]) than in the treatment group (91 days [44, 263]). The most common cause of readmission, similar to what has been shown previously in continuous flow device trials, was gastrointestinal bleeding.35–41
The 90-day mortality rate was 30% in the control group and zero in the MPC group. In a post-hoc analysis, the posterior probability that mortality at 90 days is reduced in the MPC group exceeded 80%. At one year, the mortality rates were the same for both groups (30%). Factors known to increase mortality in LVAD recipients, such as prior cardiac surgery, history of stroke, diabetes, and dialysis, were balanced across the treatment groups and within the expected range for the advanced heart failure population, and no patient received a right ventricular assist device implantation at the time of surgery.30,38–41
Despite the low dose of cells deployed in this trial, a likely efficacy signal was observed; a greater proportion of MPC patients experienced successful temporary weans at 90 days. Moreover, the total number of temporary weans tolerated by MPC patients was double that of the control group. Similarly, the significantly lower early mortality rate and fewer hospitalizations in MPC patients compared to control are promising. However, the treatment effect was not seen at one-year. This argues for evaluating higher doses in this population, especially since sensitization concerns were addressed at the lower dose. Consideration also should be given to re-dosing after 90 days to determine whether this might improve the durability of a treatment effect. Re-dosing by systemic intravenous infusion is being employed in another trial of the same MPCs in a non-cardiovascular application.
This trial has several limitations. It is a small, exploratory trial; the efficacy endpoints such as weaning, LVEF, and 6MW at 90 days are based on a comparison of 10 patients in the MPC and 2 patients in the control group, limiting the insight that can be drawn. A larger follow-up trial is being planned. In addition, efficacy endpoints, such as functional status and re-hospitalizations, as often used in other heart failure trials, are not straightforward within the context of LVAD support.42–43 We selected an efficacy endpoint that combines tolerance of LVAD weaning (without symptoms of cardiovascular compromise) with additional assessment of functional status. However, the ability to wean also is affected by non-cardiovascular factors, such as debilitation secondary to co-morbidities or inability to optimize anticoagulation, which, in turn, is critical to safely turning down the pump speed. These factors are unrelated to the intervention and in a small trial may impact a potential efficacy signal.
LVADs offer a unique “clinical laboratory” for evaluation of adjunctive cardiac regenerative therapies. The cells used in this trial have many potential advantages including, “off the shelf” availability and the potential for immunologic privilege. This exploratory trial confirms feasibility and safety, and suggests early efficacy of MPCs, opening up the field for further clinical investigation.
Supplementary Material
Acknowledgments
The investigators acknowledge the scientific leadership of Dr. Sonia Skarlatos whose contributions to this trial and the clinical investigation of cell-based therapies were substantial.
Funding Sources: Trial support was through cooperative agreements (U01 HL088942, HL088957, HL088951, HL088955, HL088939, HL088953) funded by: National Heart Lung and Blood Institute (NHLBI), the National Institute of Neurological Diseases and Stroke of the NIH, Bethesda, MD, and the Canadian Institutes for Health Research. This trial was conducted by the Cardiothoracic Surgical Trials Network (CTSN) in collaboration with the Cardiovascular Cell Therapy Research Network. Preliminary development of the MPC-cell line was supported by an NHLBI grant (HL077096). Investigational product (for investigational use) was provided by Mesoblast, Inc.
Footnotes
Conflict of Interest Disclosures: Authors report the following: D.D. Ascheim, none; E. Bagiella, none; T. Dewey, none; E. Eichhorn, none; D. Feldman, Thoratec Inc., HeartWare, Inc. consultant; A.C. Gelijns, none; D. Goldstein, Medical advisory board, Thoratec Inc.; Consultant, Heartware Inc.; N. Jeffries, none; C.T. Klodell, none; S. Lee, none; J. Lynch, none; C. Milano, Thoratec Inc., HeartWare Inc. consultant; M.A. Miller, none; Y. Naka, Thoratec Inc. consultant; P.T. O’Gara, none; J.E. Rame, Thoratec, Inc., Heartware Inc. research funding; J.G. Rogers, Thoratec consultant; E.A. Rose, Board of Directors, Mesoblast, Inc.; R. Simari, none; N. Smedira, none; L.A. Moye, none; M.K. Parides, none; D. Skerrett, Chief Medical Officer, Mesoblast, Inc.; B. Sun, Thoratec Inc., Sunshine Heart consultant; A. Szady, none; W. Taddei-Peters, none; D.A. Taylor, Miromatrix Medical Inc, founder; Y.J. Woo, none
References
- 1.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH Heart Mate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Russo MJ, Hong KN, Davies RR, Chen JM, Sorabella RA, Ascheim DD, Williams MR, Gelijns AC, Stewart AS, Argenziano M, Naka Y. Post transplant survival is not diminished in heart transplant recipients bridged with implantable left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;138:1425–1432. doi: 10.1016/j.jtcvs.2009.07.034. e1–3. [DOI] [PubMed] [Google Scholar]
- 4.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH Heart Mate II Clinical Investigators. Use of continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 5.Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor down regulation in the failing human heart. Circulation. 2001;104:881–886. doi: 10.1161/hc3301.094911. [DOI] [PubMed] [Google Scholar]
- 6.Thompson LO, Skrabal CA, Loebe M, Lafuente JA, Roberts RR, Akgul A, Jones V, Bruckner BA, Thohan V, Noon GP, Youker KA. Plasma neurohormone levels correlate with left ventricular functional and morphological improvement in LVAD patients. J Surg Res. 2005;123:25–32. doi: 10.1016/j.jss.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Felkin LE, Lara-Pezzi EA, Hall JL, Birks EJ, Barton PJ. Reverseremodelingandrecoveryfromheartfailureareassociatedwithcomplexpatternsofgeneexpression. J Cardiovasc Transl Res. 2011;4:321–331. doi: 10.1007/s12265-011-9267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall JL, Fermin DR, Birks EJ, Barton PJ, Slaughter M, Eckman P, Baba HA, Wohlschlaeger J, Miller LW. Clinical, molecular, and genomic changes in response to a left ventricular assist device. J Am Coll Cardiol. 2011;57:641–652. doi: 10.1016/j.jacc.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heerdt PM, Klotz S, Burkhoff D. Cardiomyopathic etiology and SERCA2a reverse remodeling during mechanical support of the failing human heart. Anesth Analg. 2006;102:32–37. doi: 10.1213/01.ane.0000183642.09435.ad. [DOI] [PubMed] [Google Scholar]
- 10.Birks EJ. Molecularchangesafterleftventricularassistdevicesupportforheartfailure. Circ Res. 2013;113:777–791. doi: 10.1161/CIRCRESAHA.113.301413. [DOI] [PubMed] [Google Scholar]
- 11.Birks EJ, George RS, Firouzi A, Wright G, Bahrami T, Yacoub MH, Khaghani A. Long-termoutcomesofpatientsbridgedtorecoveryversuspatientsbridgedtotransplantation. J Thorac Cardiovasc Surg. 2012;144:190–196. doi: 10.1016/j.jtcvs.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, Banner NR, Khaghani A, Yacoub MH. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 13.Houtgraaf JH, de Jong R, Kazemi K, de Groot D, van der Spoel TI, Arslan F, Hoefer I, Pasterkamp G, Itescu S, Zijlstra F, Geleijnse ML, Serruys PW, Duckers HJ. Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ Res. 2013;113:153–166. doi: 10.1161/CIRCRESAHA.112.300730. [DOI] [PubMed] [Google Scholar]
- 14.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.See F, Seki T, Psaltis PJ, Sondermeijer HP, Gronthos S, Zannettino AC, Govaert KM, Schuster MD, Kurlansky PA, Kelly DJ, Krum H, Itescu S. Therapeutic effects of human STRO-3-selected mesenchymal precursor cells and their soluble factors in experimental myocardial ischemia. J Cell Mol Med. 2011;15:2117–2129. doi: 10.1111/j.1582-4934.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jellema RK, Wolfs TG, Lima Passos V, Zwanenburg A, Ophelders DR, Kuypers E, Hopman AH, Dudink J, Steinbusch HW, Andriessen P, Germeraad WT, Vanderlocht J, Kramer BW. Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS One. 2013;8:e73031. doi: 10.1371/journal.pone.0073031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Liu J, Xu C, Zhang W, Bai L, Li N, Liu Y, Wang Y, Su Y, Hu D. Bone marrow transplantation combined with mesenchymal stem cells induces immune tolerance without cytotoxic conditioning. J Surg Res. 2011;171:e123–e131. doi: 10.1016/j.jss.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Gronthos S, Fitter S, Diamond P, Simmons PJ, Itescu S, Zannettino AC. A novel monoclonal antibody (STRO-3) identifies an isoform of tissue nonspecific alkaline phosphatase expressed by multipotent bone marrow stromal stem cells. Stem Cells Dev. 2007;16:953–963. doi: 10.1089/scd.2007.0069. [DOI] [PubMed] [Google Scholar]
- 19.George RS, Sabharwal NK, Webb C, Yacoub MH, Bowles CT, Hedger M, Khaghani A, Birks EJ. Echocardiographic assessment of flow across continuous-flow ventricular assist devices at low speeds. J Heart Lung Transplant. 2010;29:1245–1252. doi: 10.1016/j.healun.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Gojo S, Kyo S, Nishimura S, Komiyama N, Kawai N, Bessho M, Sato H, Asakura T, Nishimura M, Ikebuchi K. Cardiac resurrection after bone-marrow-derived mononuclear cell transplantation during left ventricular assist device support. Ann Thorac Surg. 2007;83:661–662. doi: 10.1016/j.athoracsur.2006.06.074. [DOI] [PubMed] [Google Scholar]
- 21.Miyagawa S, Matsumiya G, Funatsu T, Yoshitatsu M, Sekiya N, Fukui S, Hoashi T, Hori M, Yoshikawa H, Kanakura Y, Ishikawa J, Aozasa K, Kawaguchi N, Matsuura N, Myoui A, Matsuyama A, Ezoe S, Iida H, Matsuda H, Sawa Y. Combined autologous cellular cardiomyoplasty using skeletal myoblasts and bone marrow cells for human ischemic cardiomyopathy with left ventricular assist system implantation: report of a case. Surg Today. 2009;39:133–136. doi: 10.1007/s00595-008-3803-x. [DOI] [PubMed] [Google Scholar]
- 22.Nasseri BA, Kukucka M, Dandel M, Knosalla C, Potapov E, Lehmkuhl HB, Meyer R, Ebell W, Stamm C, Hetzer R. Intramyocardial delivery of bone marrow mononuclear cells and mechanical assist device implantation in patients with end-stage cardiomyopathy. Cell Transplant. 2007;16:941–949. doi: 10.3727/096368907783338235. [DOI] [PubMed] [Google Scholar]
- 23.Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, Yang YZ, Pan C, Ge J, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Martens TP, See F, Schuster MD, Sondermeijer HP, Hefti MM, Zannettino A, Gronthos S, Seki T, Itescu S. Mesenchymal lineage precursor cells induce vascular network formation in ischemic myocardium. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S18–S22. doi: 10.1038/ncpcardio0404. [DOI] [PubMed] [Google Scholar]
- 25.Psaltis PJ, Paton S, See F, Arthur A, Martin S, Itescu S, Worthley SG, Gronthos S, Zannettino AC. Enrichment for STRO-1 expression enhances the cardiovascular paracrine activity of human bone marrow-derived mesenchymal cell populations. J Cell Physiol. 2010;223:530–540. doi: 10.1002/jcp.22081. [DOI] [PubMed] [Google Scholar]
- 26.Psaltis PJ, Carbone A, Nelson AJ, Lau DH, Jantzen T, Manavis J, Williams K, Itescu S, Sanders P, Gronthos S, Zannettino AC, Worthley SG. Reparative effects of allogeneic mesenchymal precursor cells delivered transendocardially in experimental nonischemic cardiomyopathy. JACC Cardiovasc Interv. 2010;3:974–983. doi: 10.1016/j.jcin.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Dixon JA, Gorman RC, Stroud RE, Bouges S, Hirotsugu H, Gorman JH, 3rd, Martens TP, Itescu S, Schuster MD, Plappert T, St John-Sutton MG, Spinale FG. Mesenchymal cell transplantation and myocardial remodeling after myocardial infarction. Circulation. 2009;120:S220–S229. doi: 10.1161/CIRCULATIONAHA.108.842302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slaughter MS, Pagani FD, McGee EC, Birks EJ, Cotts WG, Gregoric I, Howard Frazier O, Icenogle T, Najjar SS, Boyce SW, Acker MA, John R, Hathaway DR, Najarian KB, Aaronson KD Heart Ware Bridge to Transplant ADVANCE Trial Investigators. Heart Ware ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013;32:675–683. doi: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Timothy Baldwin J, Young JB. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Everaert BR, Bergwerf I, De Vocht N, Ponsaerts P, Van Der Linden A, Timmermans JP, Vrints CJ. Multimodal in vivo imaging reveals limited allograft survival, intrapulmonary cell trapping and minimal evidence for ischemia-directed BMSC homing. BMC Biotechnol. 2012;12:93. doi: 10.1186/1472-6750-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, Rame JE, Acker MA, Blackstone EH, Ehrlinger J, Thuita L, Mountis MM, Soltesz EG, Lytle BW, Smedira NG. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 33.Slaughter MS, Bostic R, Tong K, Russo M, Rogers JG. Temporal changes in hospital costs for left ventricular assist device implantation. J Card Surg. 2011;26:535–541. doi: 10.1111/j.1540-8191.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 34.Smedira NG, Hoercher KJ, Lima B, Mountis MM, Starling RC, Thuita L, Schmuhl DM, Blackstone EH. Unplanned hospital readmissions after Heart Mate II implantation. JACC Heart Fail. 2013;1:31–39. doi: 10.1016/j.jchf.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Forest SJ, Bello R, Friedmann P, Casazza D, Nucci C, Shin JJ, D'Alessandro D, Stevens G, Goldstein DJ. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg. 2013;95:1276–1281. doi: 10.1016/j.athoracsur.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 36.Hasin T, Marmor Y, Kremers W, Topilsky Y, Severson CJ, Schirger JA, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Stulak JM, Joyce L, Daly R, Park SJ, Kushwaha SS. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61:153–163. doi: 10.1016/j.jacc.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 37.Stulak JM, Lee D, Haft JW, Romano MA, Cowger J, Park SJ, Aaronson KD, Pagani FD. Gastrointestinal bleeding and subsequent risk of thromboembolic events during support with a left ventricular assist device. J Heart Lung Transplant. 2013 Sep 7; doi: 10.1016/j.healun.2013.07.020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38.Lazar JF, Swartz MF, Schiralli MP, Schneider M, Pisula B, Hallinan W, Hicks GL, Jr, Massey HT. Survival after left ventricular assist device with and without temporary right ventricular support. Ann Thorac Surg. 2013 Sep 12; doi: 10.1016/j.athoracsur.2013.07.008. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 39.Atluri P, Goldstone AB, Fairman AS, MacArthur JW, Shudo Y, Cohen JE, Acker AL, Hiesinger W, Howard JL, Acker MA, Woo YJ. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann Thorac Surg. 2013;96:857–863. doi: 10.1016/j.athoracsur.2013.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, Jaski B, Farrar DJ, Slaughter MS. Predicting survival in patients receiving continuous flow left ventricular assist devices: the Heart Mate II risk score. J Am Coll Cardiol. 2013;61:313–321. doi: 10.1016/j.jacc.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 14.Wever-Pinzon O, Drakos SG, Kfoury AG, Nativi JN, Gilbert EM, Everitt M, Alharethi R, Brunisholz K, Bader FM, Li DY, Selzman CH, Stehlik J. Morbidity and mortality in heart transplant candidates supported with mechanical circulatory support: is reappraisal of the current United network for organ sharing thoracic organ allocation policy justified? Circulation. 2013;127:452–462. doi: 10.1161/CIRCULATIONAHA.112.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drakos SG, Wever-Pinzon O, Selzman CH, Gilbert EM, Alharethi R, Reid BB, Saidi A, Diakos NA, Stoker S, Davis ES, Movsesian M, Li DY, Stehlik J, Kfoury AG UCAR (Utah Cardiac Recovery Program) Investigators. Magnitude and time course of changes induced by continuous-flow left ventricular assist device unloading in chronic heart failure: insights into cardiac recovery. J Am Coll Cardiol. 2013;61:1985–1994. doi: 10.1016/j.jacc.2013.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George RS, Yacoub MH, Tasca G, Webb C, Bowles CT, Tansley P, Hardy JP, Dreyfus G, Khaghani A, Birks EJ. Hemodynamic and echocardiographic responses to acute interruption of left ventricular assist device support: relevance to assessment of myocardial recovery. J Heart Lung Transplant. 2007;26:967–973. doi: 10.1016/j.healun.2007.07.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.