Abstract
Asymmetric cell division of neural progenitors, which involves the segregation of distinct differentiation factors in daughter cells, is a crucial event in the generation of neuronal diversity. In this issue of Science Signaling, Bhat reports a novel role for Notch in promoting asymmetric cell division. While it was previously thought that Notch acts post mitotically in response to its negative regulator, Numb to promote cell fate, Notch in fact appears to act before cell division to promote asymmetric cytokinesis and differentiation by regulating Numb localization in precursor cells. As Numb also regulates Notch activity, this forms a regulatory feedback loop.
A fundamental evolutionary step that allowed the development and evolution of multicellular organisms was the acquisition of the capacity of a cell to divide asymmetrically: The mother cell generates two distinct progeny, or stem cells self-renew and generate a progeny with a distinct fate. It is then not surprising that cell division and cellular differentiation are tightly coupled processes, although we know little about how they are molecularly linked(1). The development of the Central Nervous System of the Drosophila embryo has historically served as a powerful model to study the molecular basis of asymmetric cell division(2–6). In the Drosophila embryo, neuronal precursors (neuroblasts, NB), divide asymmetrically, self renewing and producing a smaller ganglion mother-cell (GMC), which undergoes a terminal asymmetric division producing two distinct neurons(7). NB asymmetric division invariably shows asymmetric cytokinesis, with the largest daughter cell maintaining the NB identity. Interestingly some GMCs seem to have maintained this characteristic, also exhibiting asymmetric cytokinesis. Notch, Numb and Inscuteable (Insc) play a central role in the generation of asymmetric cytokinesis of GMCs and asymmetric differentiation of daughter neurons. However the details of how these cellular and molecular events interact are not known(2, 3, 5). In this issue of Science Signaling, Bhat(8) addresses this question and reports that Notch, previously believed to act post-mitotically in one of the neuronal progeny, in fact acts in the GMC to coordinate cytokinesis and asymmetric differentiation by regulating Numb localization.
The NB4-2 lineage is a well-studied example in the fly embryo where the first GMC (GMC-1) shows asymmetric cytokinesis, producing a larger sized motor neuron (RP2) and a smaller sibling (‘sib’) cell of unknown fate(2, 3, 5), a difference in fate that is due to different Notch activity in the two daughter cells. As in other lineages, Insc and Numb initially show a uniform distribution in GMC-1. However, just before cytokinesis, Insc and Numb show opposite localizations in an axis perpendicular to the plane of cytokinesis: Insc is localized to the apical pole and Numb to the basal pole. The asymmetric division and specification is tightly related to the asymmetric segregation of Insc and Numb in GMC-1: Upon division, the smaller apical daughter cell, where Insc accumulates, is specified as ‘sib’ by Notch activity; the basal daughter cell inherits Numb, which specifies the RP2 fate by inhibiting Notch activity(4, 5). This suggests a possible link between Notch and Insc, leaving open the question of how Insc and Numb asymmetric distributions are established in the GMC-1 before division.
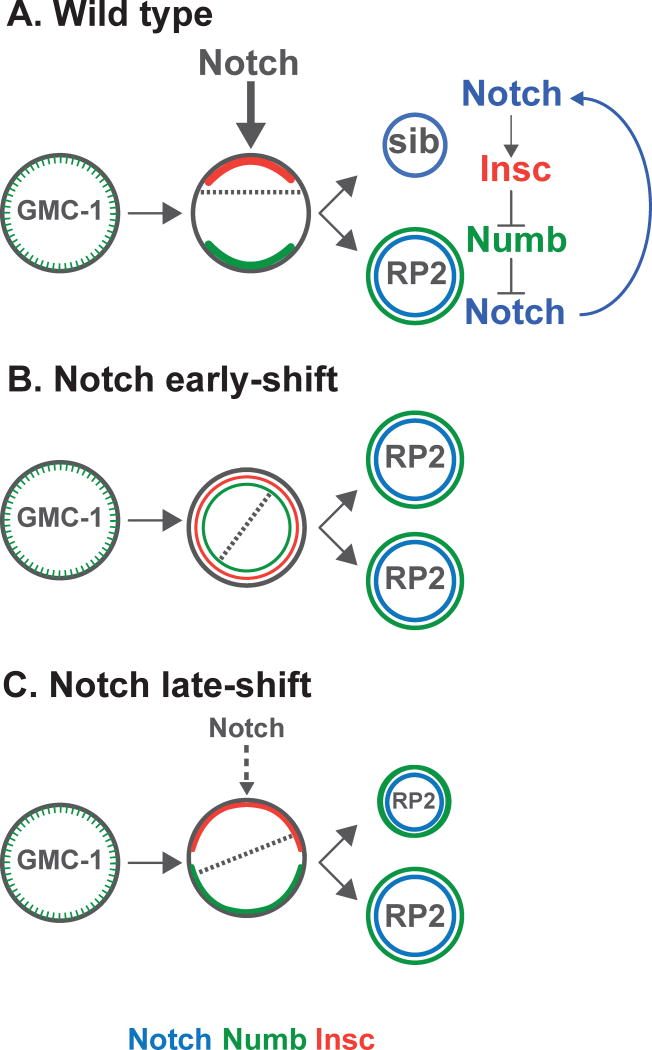
Bhat analyzed the problem by looking at the NB4-2→GMC-1→RP2/sib lineage and using a Notch temperature sensitive mutant (Notchts). When the temperature shift occurred just after GMC-1 formation (early loss of Notch function), the sister cells showed symmetric cytokinesis, producing two daughters cells of identical size. However, when Notch was inhibited just before the division of GMC-1 (late loss of Notch function), the basal cell was larger (Fig. 1). In the two conditions, both cells were specified as RP2, confirming that the sib identity is defined by Notch before cytokinesis. In a newly formed GMC-1, Numb is initially distributed uniformly and later accumulates near the basal cortex where it forms a crescent just before division. Surprisingly, when the author studied Numb localization when Notch is inhibited, he discovered that, after early shift of Notchts mutants, no crescent formed and Numb remained symmetrical with both progeny inheriting Numb, hence leading to two identical-sized cells that became RP2 (Fig. 1). Similar results were observed in mastermind (mam) mutants, an essential component of Notch signaling(9). These observations indicate that Notch signaling mediates Numb localization in GMC-1 via Mam before division. They challenge the established belief that in GMC-1, it is Numb that controls asymmetric Notch activity, as it does in the daughter cells. In Notchts mutants shifted late, Numb forms a crescent that is still localized (although larger), thus explaining the production of cells of different sizes. However, the lack of Notch activity in the smaller cell prevents its further development as sib. This shows that asymmetric cytokinesis and cell fate are tightly linked via Notch activity in the pre-mitotic GMC, forming an autocatalytic regulatory loop; Notch controls Numb localization and Numb controls Notch activity(5, 6, 10)(Fig. 1).
Figure 1.
The localization patterns of Numb and Insc are shown with blue or red crescents, respectively. The size of the progeny reflects the asymmetric/symmetric-sized division patterns induced by the Numb crescent. Dashed line on the GMCs represents the axis of cell division. The early distribution of Numb is punctate throughout the cortex of GMC-1, perhaps allowing for restricted Notch signaling. In Wild type (A), before cytokinesis, Notch activity localizes Insc and Numb to opposite locations in an axis perpendicular to the plane of cytokinesis. As a consequence the smaller apical daughter cell accumulates Insc and is specified as ‘sib’ via Notch activity; the basal daughter cell, inherits Numb, which specifies the RP2 fate by inhibiting Notch activity. In Notch mutant embryos from early (B) and late shifts (C), both daughter cells are specified as RP2. Asymmetrical cytokinesis in C is due to earlier Notch activity in GMC-1 that allows partial localization of Insc/Numb.
Both in NBs and in GMCs, loss of Insc affects the axis of cell division (3, 11). Additionally, Insc controls asymmetric Numb distribution (3). The author thus went to investigate Insc distribution when Notch activity is removed at different times: Early Notch also regulates the localization of Insc and, as a consequence, both daughter cells show homogeneous Numb distribution and are specified as RP2. Furthermore, asymmetric localization of Numb correlates with asymmetric cleavage when Notch function is removed at different times: The bigger the Numb crescent is (less localized), the more symmetric cytokinesis is.
Taken together, these results suggest that apical-basal polarity inherited from the NBs is maintained in the GMCs under the regulation of Notch signaling. In the absence of either Insc or Numb, both daughter cells adopt the ‘sib’ fate via Notch (8). Overexpression of the Notch intra cellular domain, circumventing Notch repression by Numb, gives rise to two ‘sib’ cells (8). Because Notch/Numb double mutants generate two RP2 cells(2), this means that asymmetric differentiation is entirely dependent on Numb localization repressing Notch.
This hints on how Notch couples asymmetric cell division and differentiation: Notch localizes Insc to the apical pole of the GMC-1. Insc represses Numb, which accumulates at the basal pole of the cell and this asymmetry promote cytokinesis perpendicular to the apical basal. Numb localization also represses Notch and allows RP2 fate and this is regulated by the Numb-Notch positive feedback loop (Fig. 1).
How does Notch signaling become asymmetrically active in GMC-1? Bhat shows that the earlier distribution of symmetric Numb is in fact not uniform but is punctate throughout the cortex. The author suggests that this allows for Numb to partially reduce Notch signaling, allowing enough Notch activity to promote the asymmetric localization of Insc and Numb, but not enough to induce the premature “sib” identity of the GMC itself. It remains to be answered how the apical/basal axis is defined in the GMCs and/or imposed by its NB, and what are the molecular mechanism allowing asymmetric Notch activity to localize Insc and Numb.
While lineages exist where Insc or Numb is not involved, Bhat shows that in many of them, Notch regulates the distribution of Insc or Numb already in precursor cells. This forms a positive feedback circuit that coordinates cytokinesis and asymmetric differentiation even before division and suggests a universal strategy in coordinating these events.
References
- 1.Knoblich J. Mechanisms of asymmetric stem cell division. Cell. 2008;132(4):583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Wai P, Truong B, Bhat KM. Cell division genes promote asymmetric interaction between Numb and Notch in the Drosophila CNS. Development. 1999;126:2759. doi: 10.1242/dev.126.12.2759. [DOI] [PubMed] [Google Scholar]
- 3.Buescher M, et al. Binary sibling neuronal cell fate decisions in the Drosophila embryonic central nervous system are nonstochastic and require inscuteable-mediated asymmetry of ganglion mother cells. Genes & development. 1998;12(12):1858–1870. doi: 10.1101/gad.12.12.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skeath JB, Doe CQ. Sanpodo and Notch act in opposition to Numb to distinguish sibling neuron fates in the Drosophila CNS. Development. 1998;125(10):1857–1865. doi: 10.1242/dev.125.10.1857. [DOI] [PubMed] [Google Scholar]
- 5.Gaziova I, Bhat KM. Generating asymmetry: with and without self-renewal. Prog Mol Subcell Biol. 2007;45:143. doi: 10.1007/978-3-540-69161-7_7. [DOI] [PubMed] [Google Scholar]
- 6.Spana EP, Doe CQ. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron. 1996;17(1):21–26. doi: 10.1016/s0896-6273(00)80277-9. [DOI] [PubMed] [Google Scholar]
- 7.Hartenstein V, Campos-Ortega JA. Early neurogenesis in wild type melanogaster. Wilhelm Roux’s Arch Dev Biol. 1984;193:308–325. doi: 10.1007/BF00848159. [DOI] [PubMed] [Google Scholar]
- 8.Bhat K. Notch specifies asymmetric cleavage and cell fate at the pre- mitotic precursor stage. Science Signaling 2014 doi: 10.1126/scisignal.2005317. ?(?):? [DOI] [PubMed] [Google Scholar]
- 9.Guruharsha KG, K M, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012 Sep;13(9):654–66. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17(1):27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- 11.Kraut R, Chia W, Jan LY, Jan YN, Knoblich JA. Role of inscuteable in orienting asymmetric cell divisions in Drosophila. Nature. 1996;383(6595):50–55. doi: 10.1038/383050a0. [DOI] [PubMed] [Google Scholar]