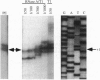
Abstract
Cytotactin/tenascin is an extracellular matrix glycoprotein expressed in a restricted anteroposterior pattern during vertebrate development and is reexpressed in the adult during wound healing, tumorigenesis, and nerve regeneration. Previously, we have characterized the chicken cytotactin promoter and have shown its regulation by homeobox gene products in vitro. We have now isolated the promoter for the mouse tenascin gene in order to determine whether common or different DNA regulatory elements control the expression of this gene in these two species. Like the chicken cytotactin gene, the mouse tenascin gene has a single RNA start site that lies 27 bp downstream of a TATA box. A 4028-bp region of DNA upstream of the mouse tenascin gene was sequenced and examined for regulatory motifs in common with the upstream sequence from the chicken cytotactin promoter. Two hundred thirty base pairs of the proximal promoter regions from both genes had an extended sequence similarity and contained common regulatory motifs such as two tracts of homopolymeric dA.dT sequence, an octamer motif, an ATTA (TAAT) motif which is a common core sequence for binding of homeodomain transcription factors, and a TATA-box/cap-site region. Reporter gene constructs with various 5' deletions of the mouse tenascin upstream sequence were tested in transient transfections of mouse NIH 3T3 and chicken embryo fibroblasts. The conserved proximal promoter region of tenascin was responsible for most of the positive regulatory activity. In addition, an upstream region (-2478 to -247) repressed proximal promoter activity in mouse fibroblasts and also in chicken embryo fibroblasts. These data indicate that both the structure and function of the cytotactin/tenascin proximal promoters have remained conserved over 250 million years.
Full text
PDF
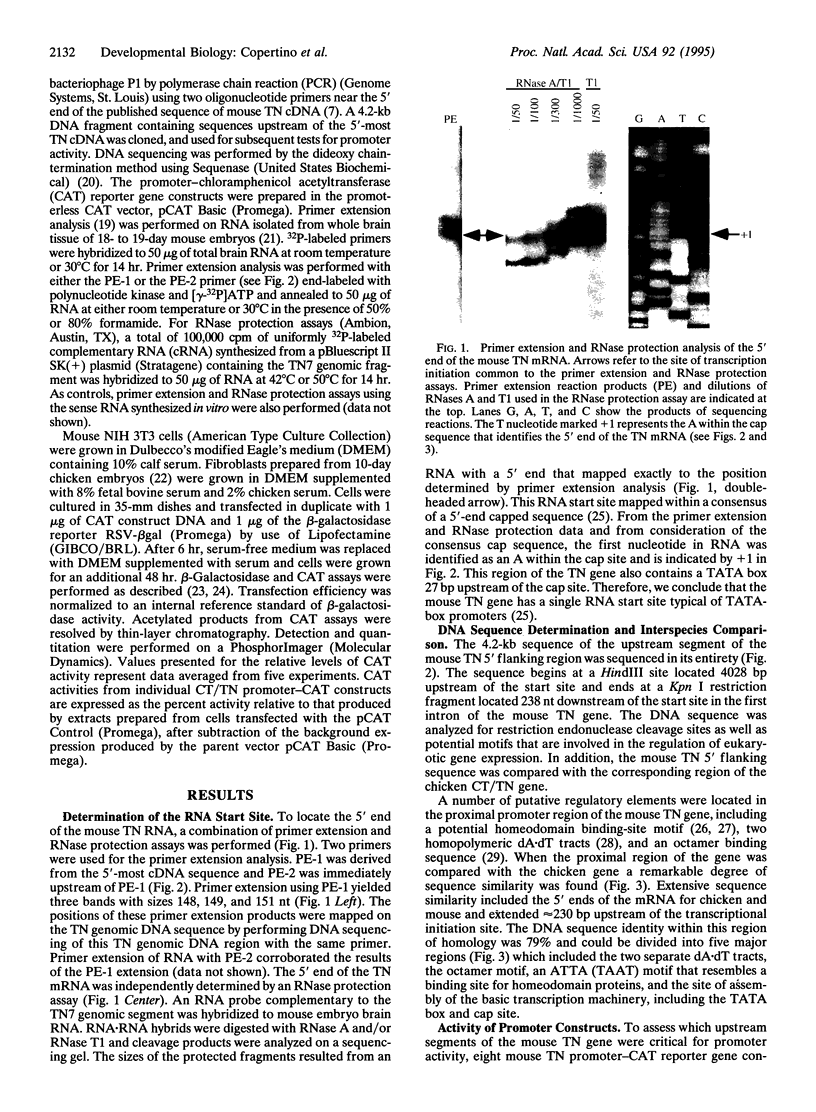
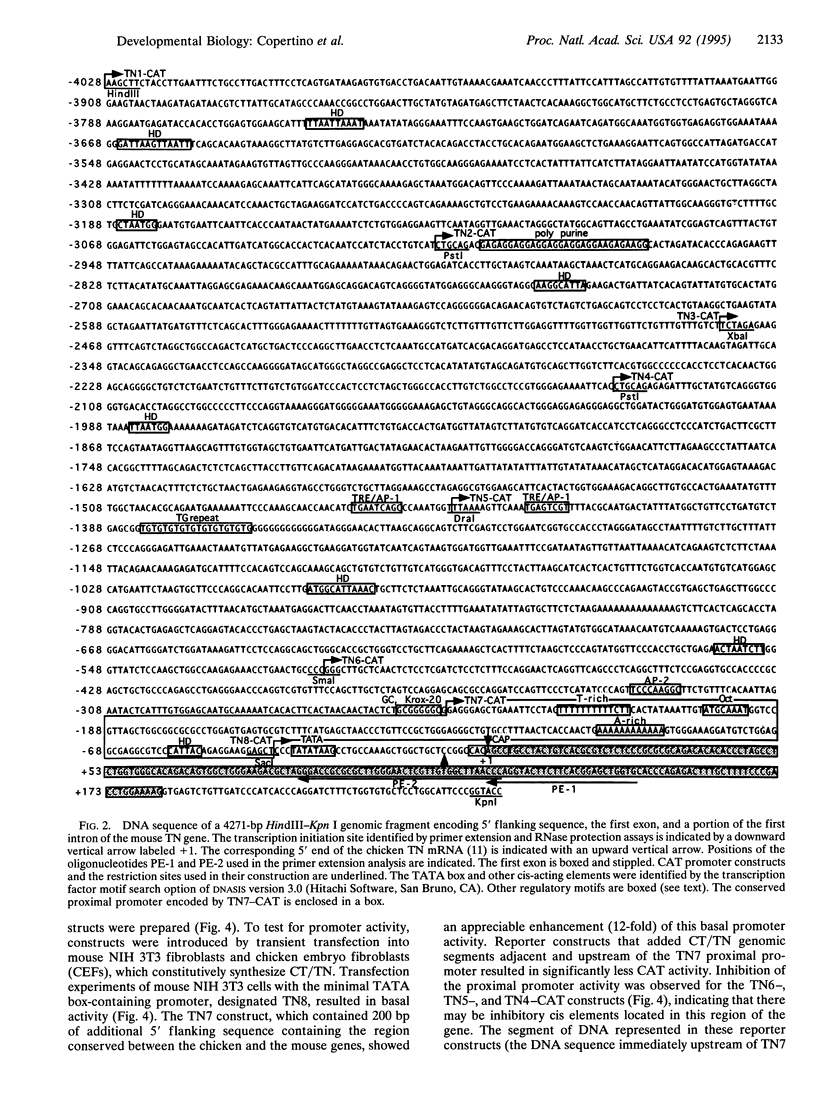
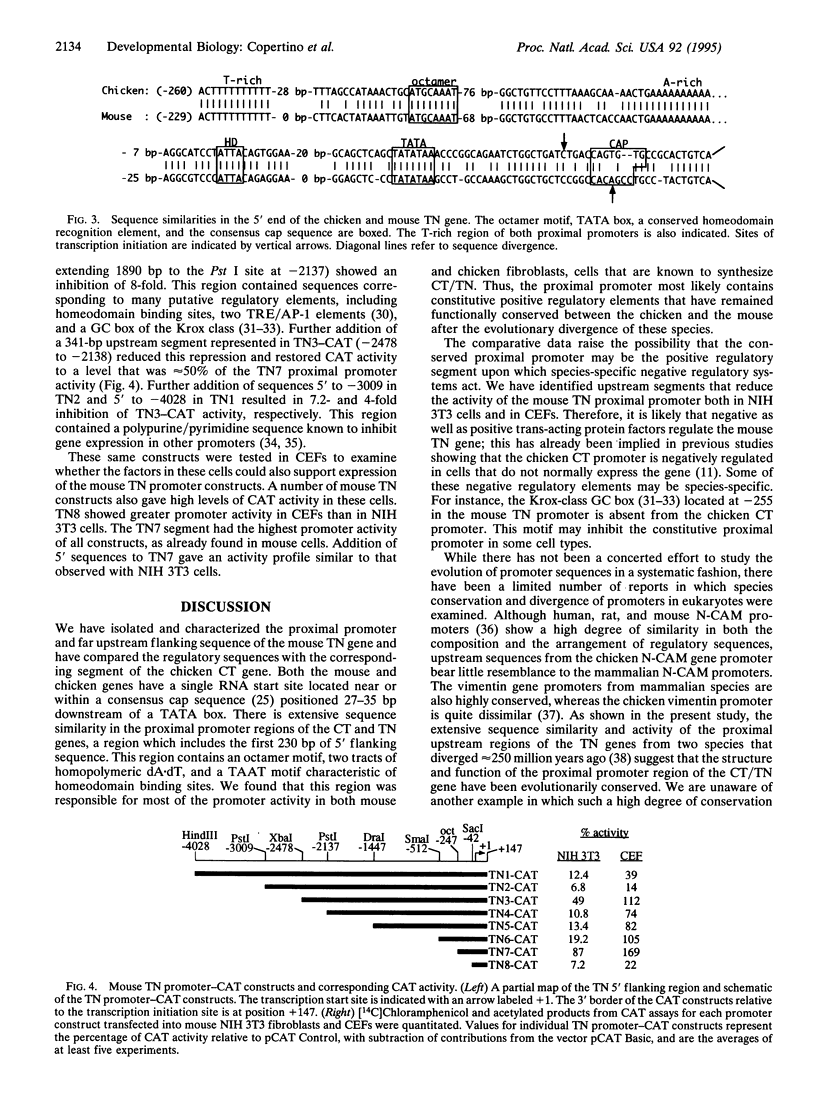

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Bastian H., Gruss P. A murine even-skipped homologue, Evx 1, is expressed during early embryogenesis and neurogenesis in a biphasic manner. EMBO J. 1990 Jun;9(6):1839–1852. doi: 10.1002/j.1460-2075.1990.tb08309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S., Martin D., Hagios C., Chiquet-Ehrismann R. Tenm, a Drosophila gene related to tenascin, is a new pair-rule gene. EMBO J. 1994 Aug 15;13(16):3728–3740. doi: 10.1002/j.1460-2075.1994.tb06682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon M. A., Ruoslahti E. Tenascin mediates cell attachment through an RGD-dependent receptor. J Cell Biol. 1989 Mar;108(3):1149–1155. doi: 10.1083/jcb.108.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher P. Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J Mol Biol. 1990 Apr 20;212(4):563–578. doi: 10.1016/0022-2836(90)90223-9. [DOI] [PubMed] [Google Scholar]
- Catron K. M., Iler N., Abate C. Nucleotides flanking a conserved TAAT core dictate the DNA binding specificity of three murine homeodomain proteins. Mol Cell Biol. 1993 Apr;13(4):2354–2365. doi: 10.1128/mcb.13.4.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Zerial M., Lemaire P., Almendral J., Bravo R., Charnay P. A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells. EMBO J. 1988 Jan;7(1):29–35. doi: 10.1002/j.1460-2075.1988.tb02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A., Reyes A., Akeson R. A homopurine:homopyrimidine sequence derived from the rat neuronal cell adhesion molecule-encoding gene alters expression in transient transfections. Gene. 1993 Jun 30;128(2):211–218. doi: 10.1016/0378-1119(93)90565-k. [DOI] [PubMed] [Google Scholar]
- Chen W., Tabor S., Struhl K. Distinguishing between mechanisms of eukaryotic transcriptional activation with bacteriophage T7 RNA polymerase. Cell. 1987 Sep 25;50(7):1047–1055. doi: 10.1016/0092-8674(87)90171-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colwell G., Li B., Forrest D., Brackenbury R. Conserved regulatory elements in the promoter region of the N-CAM gene. Genomics. 1992 Dec;14(4):875–882. doi: 10.1016/s0888-7543(05)80108-9. [DOI] [PubMed] [Google Scholar]
- Crosby S. D., Puetz J. J., Simburger K. S., Fahrner T. J., Milbrandt J. The early response gene NGFI-C encodes a zinc finger transcriptional activator and is a member of the GCGGGGGCG (GSG) element-binding protein family. Mol Cell Biol. 1991 Aug;11(8):3835–3841. doi: 10.1128/mcb.11.8.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Hoffman S., Grumet M., Thiery J. P., Edelman G. M. Site-restricted expression of cytotactin during development of the chicken embryo. J Cell Biol. 1986 May;102(5):1917–1930. doi: 10.1083/jcb.102.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Hoffman S., Tan S. S., Edelman G. M. Cytotactin and its proteoglycan ligand mark structural and functional boundaries in somatosensory cortex of the early postnatal mouse. Dev Biol. 1989 Dec;136(2):381–392. doi: 10.1016/0012-1606(89)90264-9. [DOI] [PubMed] [Google Scholar]
- Crossin K. L., Prieto A. L., Hoffman S., Jones F. S., Friedlander D. R. Expression of adhesion molecules and the establishment of boundaries during embryonic and neural development. Exp Neurol. 1990 Jul;109(1):6–18. doi: 10.1016/s0014-4886(05)80004-4. [DOI] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The sequence specificity of homeodomain-DNA interaction. Cell. 1988 Sep 23;54(7):1081–1090. doi: 10.1016/0092-8674(88)90123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dush M. K., Martin G. R. Analysis of mouse Evx genes: Evx-1 displays graded expression in the primitive streak. Dev Biol. 1992 May;151(1):273–287. doi: 10.1016/0012-1606(92)90232-6. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Jones F. S. Cytotactin: a morphoregulatory molecule and a target for regulation by homeobox gene products. Trends Biochem Sci. 1992 Jun;17(6):228–232. doi: 10.1016/0968-0004(92)90383-k. [DOI] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Crossin K. L., Edelman G. M. Cytotactin, an extracellular matrix protein of neural and non-neural tissues that mediates glia-neuron interaction. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8075–8079. doi: 10.1073/pnas.82.23.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. R., Gaugler L., Deagostini-Bazin H., Bally-Cuif L., Goridis C. Identification of positive and negative regulatory elements governing cell-type-specific expression of the neural cell adhesion molecule gene. Mol Cell Biol. 1990 May;10(5):1959–1968. doi: 10.1128/mcb.10.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst B. D., Goomer R. S., Wood I. C., Edelman G. M., Jones F. S. Binding and activation of the promoter for the neural cell adhesion molecule by Pax-8. J Biol Chem. 1994 Sep 2;269(35):22245–22252. [PubMed] [Google Scholar]
- Hunter E. Biological techniques for avian sarcoma viruses. Methods Enzymol. 1979;58:379–393. doi: 10.1016/s0076-6879(79)58153-1. [DOI] [PubMed] [Google Scholar]
- Jones F. S., Chalepakis G., Gruss P., Edelman G. M. Activation of the cytotactin promoter by the homeobox-containing gene Evx-1. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2091–2095. doi: 10.1073/pnas.89.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Crossin K. L., Cunningham B. A., Edelman G. M. Identification and characterization of the promoter for the cytotactin gene. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6497–6501. doi: 10.1073/pnas.87.17.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Hoffman S., Cunningham B. A., Edelman G. M. A detailed structural model of cytotactin: protein homologies, alternative RNA splicing, and binding regions. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1905–1909. doi: 10.1073/pnas.86.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Holst B. D., Minowa O., De Robertis E. M., Edelman G. M. Binding and transcriptional activation of the promoter for the neural cell adhesion molecule by HoxC6 (Hox-3.3). Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6557–6561. doi: 10.1073/pnas.90.14.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A., Bashan-Ahrend A., Budai-Hadrian O., Gartenberg D., Menasherow S., Wides R. Odd Oz: a novel Drosophila pair rule gene. Cell. 1994 May 20;77(4):587–598. doi: 10.1016/0092-8674(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Monuki E. S., Weinmaster G., Kuhn R., Lemke G. SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron. 1989 Dec;3(6):783–793. doi: 10.1016/0896-6273(89)90247-x. [DOI] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Prieto A. L., Edelman G. M., Crossin K. L. Multiple integrins mediate cell attachment to cytotactin/tenascin. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10154–10158. doi: 10.1073/pnas.90.21.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto A. L., Jones F. S., Cunningham B. A., Crossin K. L., Edelman G. M. Localization during development of alternatively spliced forms of cytotactin mRNA by in situ hybridization. J Cell Biol. 1990 Aug;111(2):685–698. doi: 10.1083/jcb.111.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon B. J., Winters R. S., Gordon D., Winter E. A peptide motif that recognizes A.T tracts in DNA. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11327–11331. doi: 10.1073/pnas.90.23.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig W. J., Hoffman S., Su S. L., Garin-Chesa P. Species diversity of neuronectin and cytotactin expression patterns in the vertebrate central nervous system. Brain Res. 1992 Sep 11;590(1-2):219–228. doi: 10.1016/0006-8993(92)91099-z. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Cromlish J. A., Gerster T., Kawakami K., Balmaceda C. G., Currie R. A., Roeder R. G. A human lymphoid-specific transcription factor that activates immunoglobulin genes is a homoeobox protein. Nature. 1988 Dec 8;336(6199):551–557. doi: 10.1038/336551a0. [DOI] [PubMed] [Google Scholar]
- Spring J., Beck K., Chiquet-Ehrismann R. Two contrary functions of tenascin: dissection of the active sites by recombinant tenascin fragments. Cell. 1989 Oct 20;59(2):325–334. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Spyropoulos D. D., Capecchi M. R. Targeted disruption of the even-skipped gene, evx1, causes early postimplantation lethality of the mouse conceptus. Genes Dev. 1994 Aug 15;8(16):1949–1961. doi: 10.1101/gad.8.16.1949. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R., Moir J. M. Wnt-1-inducing factor-1: a novel G/C box-binding transcription factor regulating the expression of Wnt-1 during neuroectodermal differentiation. Mol Cell Biol. 1993 Mar;13(3):1590–1598. doi: 10.1128/mcb.13.3.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller A., Beck S., Ekblom P. Amino acid sequence of mouse tenascin and differential expression of two tenascin isoforms during embryogenesis. J Cell Biol. 1991 Jan;112(2):355–362. doi: 10.1083/jcb.112.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Klundert F. A., van Eldik G. J., Pieper F. R., Jansen H. J., Bloemendal H. Identification of two silencers flanking an AP-1 enhancer in the vimentin promoter. Gene. 1992 Dec 15;122(2):337–343. doi: 10.1016/0378-1119(92)90223-c. [DOI] [PubMed] [Google Scholar]