Abstract
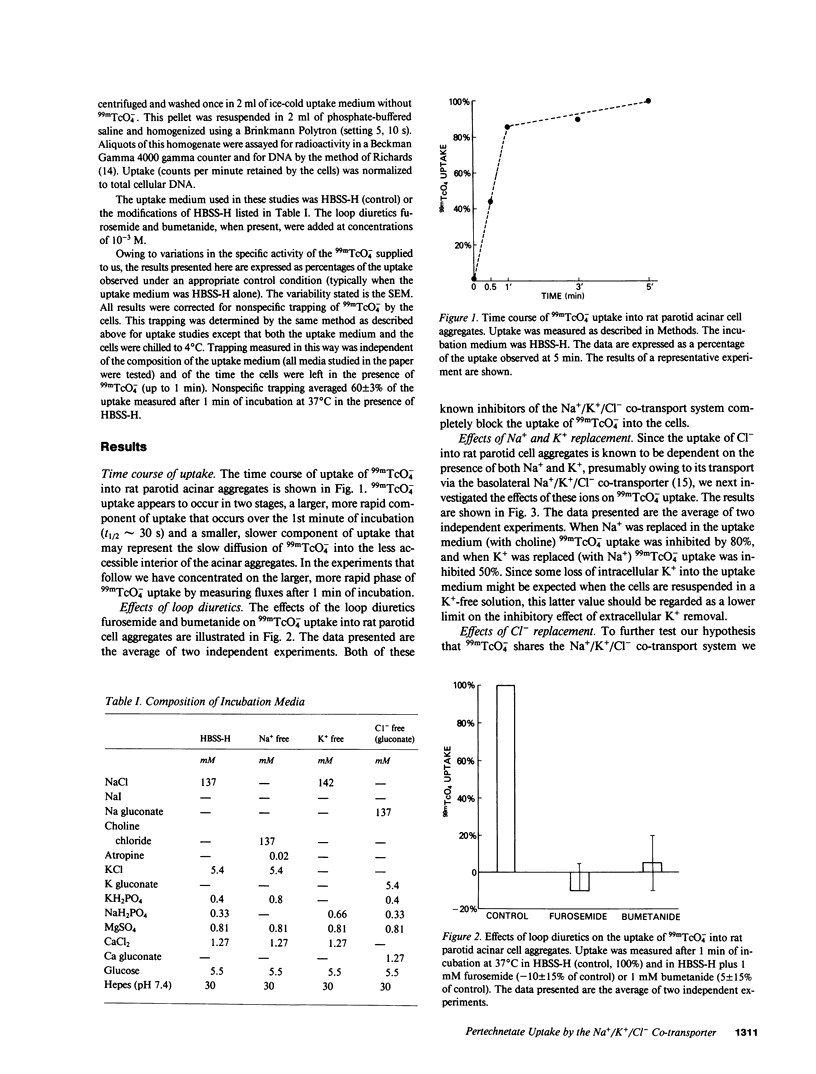
99mTc-Pertechnetate (99mTcO4-) has widespread clinical use in the diagnosis and evaluation of dysfunctions in many different tissues. However, despite the broad clinical application of this radionuclide, very little is known about the mechanism by which 99mTcO4- enters a cell. We report evidence here that 99mTcO4- shares the Na+/K+/Cl- co-transport system localized to the basolateral membrane of rat parotid acinar cells. 99mTcO4- uptake by these cells was quite rapid (t1/2 approximately 30 s), was completely inhibited by the loop diuretics furosemide and bumetanide, and was markedly dependent on the presence of Na+, K+, and Cl- in the extracellular medium. Relative to uptake measured in the presence of physiological extracellular salt concentrations (Hanks' salts), 99mTcO4- uptake was inhibited 80% by sodium replacement and 50% by potassium replacement. When Cl- was replaced with the physiologically inert anion gluconate a threefold stimulation in 99mTcO4- uptake resulted. These observations provide strong evidence that 99mTcO4- can substitute for Cl- as a substrate for the Na+/K+/Cl- co-transporter and indicate that 99mTcO4- uptake by salivary glands (e.g., as seen with salivary scintiscans), and possibly by a variety of other tissues, reflects the functional activity of this co-transport mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDROS G., HARPER P. V., LATHROP K. A. PERTECHNETATE-99M LOCALIZATION IN MAN WITH APPLICATIONS TO THYROID SCANNING AND THE STUDY OF THYROID PHYSIOLOGY. J Clin Endocrinol Metab. 1965 Aug;25:1067–1076. doi: 10.1210/jcem-25-8-1067. [DOI] [PubMed] [Google Scholar]
- Alexander W. D., Harden R. M., Mason D. K., Shimmins J., Kostalas H. Comparison of the concentrating ability of the human salivary gland for bromine, iodine and technetium. Arch Oral Biol. 1966 Nov;11(11):1205–1207. doi: 10.1016/0003-9969(66)90180-4. [DOI] [PubMed] [Google Scholar]
- BAUMANN E. J., SEARLE N. Z., YALOW A. A., SIEGEL E., SEIDLIN S. M. Behavior of the thyroid toward elements of the seventh periodic group. Am J Physiol. 1956 Apr;185(1):71–76. doi: 10.1152/ajplegacy.1956.185.1.71. [DOI] [PubMed] [Google Scholar]
- Fox P. C., Bodner L., Bowers M. R., Baum B. J. Uptake and secretion of technetium pertechnetate by the rat parotid gland. Comp Biochem Physiol A Comp Physiol. 1986;83(3):579–584. doi: 10.1016/0300-9629(86)90149-0. [DOI] [PubMed] [Google Scholar]
- Hannafin J., Kinne-Saffran E., Friedman D., Kinne R. Presence of a sodium-potassium chloride cotransport system in the rectal gland of Squalus acanthias. J Membr Biol. 1983;75(1):73–83. doi: 10.1007/BF01870801. [DOI] [PubMed] [Google Scholar]
- Harden R. M., Alexander W. D. The relation between the clearance of iodide and pertechnetate in human parotid saliva and salivary flow rate. Clin Sci. 1967 Oct;33(2):425–431. [PubMed] [Google Scholar]
- Hays M. T., Green F. A. In vitro studies of 99m Tc-pertechnetate binding by human serum and tissues. J Nucl Med. 1973 Mar;14(3):149–158. [PubMed] [Google Scholar]
- Ito H., Baum B. J., Roth G. S. Beta-adrenergic regulation of rat parotid gland exocrine protein secretion during aging. Mech Ageing Dev. 1981 Feb;15(2):177–188. doi: 10.1016/0047-6374(81)90073-7. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M., Turner R. J., Baum B. J. 36Cl- and 86Rb+ uptake in rat parotid acinar cells. Arch Oral Biol. 1986;31(10):679–683. doi: 10.1016/0003-9969(86)90097-x. [DOI] [PubMed] [Google Scholar]
- Koenig B., Ricapito S., Kinne R. Chloride transport in the thick ascending limb of Henle's loop: potassium dependence and stoichiometry of the NaCl cotransport system in plasma membrane vesicles. Pflugers Arch. 1983 Nov;399(3):173–179. doi: 10.1007/BF00656711. [DOI] [PubMed] [Google Scholar]
- Man S. F., Ahmed I. H., Man G. C., Nguyen A. Characteristics of pertechnetate movement across the canine tracheal epithelium. Am Rev Respir Dis. 1985 Jan;131(1):90–93. doi: 10.1164/arrd.1985.131.1.90. [DOI] [PubMed] [Google Scholar]
- Martinez J. R., Cassity N. Cl- requirement for saliva secretion in the isolated, perfused rat submandibular gland. Am J Physiol. 1985 Oct;249(4 Pt 1):G464–G469. doi: 10.1152/ajpgi.1985.249.4.G464. [DOI] [PubMed] [Google Scholar]
- Palfrey H. C., Silva P., Epstein F. H. Sensitivity of cAMP-stimulated salt secretion in shark rectal gland to "loop" diuretics. Am J Physiol. 1984 Mar;246(3 Pt 1):C242–C246. doi: 10.1152/ajpcell.1984.246.3.C242. [DOI] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Silva P., Stoff J., Field M., Fine L., Forrest J. N., Epstein F. H. Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol. 1977 Oct;233(4):F298–F306. doi: 10.1152/ajprenal.1977.233.4.F298. [DOI] [PubMed] [Google Scholar]
- Turner R. J., George J. N., Baum B. J. Evidence for a Na+/K+/Cl- cotransport system in basolateral membrane vesicles from the rabbit parotid. J Membr Biol. 1986;94(2):143–152. doi: 10.1007/BF01871194. [DOI] [PubMed] [Google Scholar]



