Abstract
Purpose
In this study we sought to: (1) objectively assess the risk related to various pre-transplant recipient and donor characteristics; (2) devise a preoperative risk stratification score (RSS) based on pre-transplant recipient and donor characteristics predicting graft loss at 1-year; and (3) define different risk strata based on RSS.
Methods
UNOS provided de-identified patient-level data. Analysis included 11,703 orthotopic heart transplant recipients aged ≥ 18 years and transplanted between 1/1/01-12/31/07. The primary outcome was 1-year graft failure. Multivariable logistic regression analysis (backward p-value < 0.20) was used to determine the relationship between pre-transplant characteristics and 1-year graft failure. Using the odds ratio for each identified variable, a RSS was devised. RSS strata were defined by calculating ROC curves and stratum specific likelihood ratios.
Results
The strongest negative predictors of 1-year graft failure included: RVAD-only, ECMO, renal failure, extracorporeal LVAD, total artificial heart, and advanced age. Threshold analysis identified five discrete RSS strata: low risk (LR, RSS: <2.55; n=3242, 27.7%), intermediate risk (IR, RSS: 2.55-5.72; n=6347, 54.2%), moderate risk (MR, RSS: 5.73-8.13; n=1543, 13.2%), elevated risk (ER, RSS: 8.14-9.48; n=310, 2.6%), and high risk (HR, RSS: >9.48; n=261, 2.2%). The 1-year actuarial survival (%) in the LR, IR, MR, ER, and HR groups were 93.8, 89.2, 81.3, 67.0, and 47.0, respectively.
Conclusion
Pre-transplant recipient variables significantly influence early and late graft failure following heart transplantation. RSS may improve organ allocation strategies by reducing the potential negative impact of transplanting candidates who are at a high risk for poor postoperative outcomes.
Keywords: health outcomes, heart transplantation, survival
INTRODUCTION
With median post-transplant survival approaching ten years [1-3], heart transplantation remains the gold standard in the treatment of end-stage heart failure patients. However, transplantation is limited by a scarcity of donor organs, which number under 2,500 per year [4] compared to the approximately 60,000 potential recipients who could benefit from transplantation [5]. Therefore, achieving maximal benefit from transplantation is predicated on improved recipient selection and recipient and donor matching. To this end, the risks and benefits associated with transplanting various types of heart failure patients must be better understood.
The purpose of this study is: (1) to objectively define pre-transplant recipient and donor characteristics associated with graft failure at 1-year; (2) to devise a preoperative risk stratification score (RSS) based on these pre-transplant characteristics predicting graft failure at 1-year; and (3) to define different risk strata based on the RSS.
MATERIAL AND METHODS
Data Collection
Approval for this study was granted by Columbia University's Institutional Review Board, and use of this data is consistent with the United Network for Organ Sharing (UNOS) Data Use Agreement. The Standard Transplant Analysis and Research Dataset was provided by UNOS (data source # 061809-6).
Study Population
All individuals aged 18 years and older undergoing heart transplantation between January 1, 2001 and December 31, 2007 in the United States were included in the study population. Patients were excluded if they underwent other simultaneous organ transplant (n=319) and if they lacked follow-up data (n=57). Follow-up data was provided through June 18, 2009. Patients were followed from the date of transplant until death, retransplantation, or date of last known follow-up, which was the last day of follow-up data provided by UNOS.
Data Analysis
All data were analyzed using a standard statistical software package, Stata 9 (Stata Corp, College Station, TX). Continuous variables were reported as mean ± standard deviation and compared using the Student's T-test. To compare categorical variables, the chi-squared test was used. Kaplan-Meier analysis with log-rank test was used for time to event analysis. The conventional p-value of 0.05 or less was used to determine the level of statistical significance. All reported p-values are two-sided.
Risk Score
Logistic regression was used to develop a model to predict 1-year graft failure in order to assess the simultaneous effect of multiple variables. Graft failure was defined as death or retransplantation occurring within one year of transplant. Variables included in the model are summarized in the caption of Table 1. All variables significant in univariate analysis were included in the regression analysis, and backward selection (p < 0.20) was used to construct the model. The odds ratio and 95% confidence interval are reported for each factor and represent the risk of graft failure at one year. Factors with a statistically significant association with graft failure at 1 year were used to develop the risk score. The odds ratios calculated in regression analysis were used to assign weights for each of these risk factors. Model discrimination between the two groups of interest (graft survival and graft failure) was assessed by calculating the area under the receiver operating characteristic (ROC) curve.
TABLE 1.
Pre-transplant high risk factors
| Risk Factors | Weight | Odds Ratio | Upper 95% CI | Lower 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Comorbities | Recipient Age: >70yo | 2.1 | 2.069 | 1.387 | 3.086 | 0.000 |
| Recipient Age: 55-70yo | 1.2 | 1.216 | 1.075 | 1.375 | 0.002 | |
| Previous Cardiac Surgery | 1.3 | 1.269 | 1.117 | 1.442 | 0.000 | |
| Etiology: Congential | 2.3 | 2.319 | 1.754 | 3.068 | 0.000 | |
| Etiology: Amyloidosis | 1.8 | 1.755 | 0.811 | 3.798 | 0.154 | |
| Diabetes complicated by CVA | 1.4 | 1.354 | 0.907 | 2.022 | 0.138 | |
| eGFR < 33 | 2.8 | 2.813 | 2.374 | 3.333 | 0.000 | |
| eGFR 33-53 | 1.4 | 1.380 | 1.202 | 1.584 | 0.000 | |
| Total Bilirubin > 2 | 1.7 | 1.745 | 1.508 | 2.020 | 0.000 | |
| Acuity | Intubated | 1.8 | 1.784 | 1.338 | 2.379 | 0.000 |
| Hospitalized | 1.2 | 1.172 | 1.038 | 1.323 | 0.011 | |
| Mechanical Supprt | RVAD-only | 4.7 | 4.739 | 1.982 | 11.331 | 0.000 |
| ECMO | 3.9 | 3.852 | 1.993 | 7.446 | 0.000 | |
| Extracorporeal LVAD | 2.7 | 2.748 | 1.836 | 4.114 | 0.000 | |
| Total Artifical Heart | 2.4 | 2.400 | 1.311 | 4.391 | 0.005 | |
| Paracorporeal LVAD | 1.2 | 1.250 | 0.969 | 1.612 | 0.086 | |
| Donors | Hepatitis C (+) donor | 2.1 | 2.127 | 1.124 | 4.026 | 0.020 |
| Insulin dependent donor | 1.8 | 1.795 | 1.010 | 3.191 | 0.046 | |
| Donor Age: 50 - 59yo | 1.7 | 1.686 | 1.391 | 2.044 | 0.000 | |
| Donor Age: 40 - 49yo | 1.5 | 1.464 | 1.258 | 1.703 | 0.000 | |
| Donor Age: 30 - 39yo | 1.3 | 1.265 | 1.086 | 1.473 | 0.003 | |
| Pairing | Ischemic Time >6 hrs | 1.7 | 1.699 | 1.041 | 2.771 | 0.034 |
| Ischemic Time 4-6 hrs | 1.4 | 1.436 | 1.255 | 1.644 | 0.000 | |
| Female donor : Male recipient | 1.2 | 1.230 | 1.032 | 1.467 | 0.021 | |
| Female donor : Female recipient | 1.2 | 1.189 | 0.997 | 1.419 | 0.054 |
Regression analysis included the following variables:
Recipient: Intubated at the time of transplant, UNOS Status 1A, UNOS Status 1B, Hospitalized in Intensive Care Unit at time of transplant, Hospitalized at the time of transplant, Hepatitis C+ Recipient, Previous Cardiac Surgery, BMI: <18.5 (underweight), BMI >35, Recipient eGRF (ml/min) < 33, Recipient eGRF (ml/min) 33 – 53, Diabetes complicated by PVD, Diabetes complicated by CVA, Etiology: Ischemic Cardiomyopathy, Etiology: Congential, Etiology: Restrictive, Etiology: Hypertropic, Etiology: Valvular, Etiology: Sarcoidosis, Etiology: Amyloid, Etiology: other, ECMO at time of transplant Total bilirubin > 2.0 mg/dl, Recipient Age (yo): 55 – 70, Recipient Age (yo): ≥ 70, RVAD at the time of transplant, BVAD at the time of transplant, implantable VAD at the time of transplant, extracorporeal LVAD at the time of transplant, paracorporeal LVAD at the time of transplant, total artificial heart at the time of transplant, number of previous heart transplants, transplant in the past 90 days, mean center OHT volume (tx/year), Transplant Year.
Donor: Ischemic Time 2 – 4 1 hrs, Ischemic Time 4 – 6hrs, Ischemic Time > 6 hrs, Female Donor to 2M ale Recipient, Female Donor to Female Recipient, Male Donor to Female Recipient, Donor 3e GRF (ml/min): 30-60, Donor eGRF (ml/min): < 33, Donor Age (yo): 30 – 39, Donor Age (yo): 40 4– 49, Donor Age (yo): 50 – 59, Donor Insulin Dependence, Hepatitis C+ Donor.
* The regression model accounted for center volume (OR=0.995, 0.992-0.999; p=0.01) and transplant year (OR=0.97, 0.94-1.00, p=0.09). However, because these variables were not donor or recipient characteristics they were not included as part of the risk score.
Stratum-Specific Likelihood Ratios
Risk stratification scores (RSS) were calculated using threshold analysis with ROC curves and stratum-specific likelihood ratios (SSLR). ROC curves were generated by plotting sensitivity on the ordinate and 1 - specificity on the abscissa with RSS as a continuous variable and 1-year graft failure as a binary outcome [6]. SSLRs and 95% confidence intervals for 1-year graft failure were generated for regular intervals (0.01) of RSS (eg. RSS=0-0.01, RSS=0.01-0.002, RSS=0.02-0.03) as previously described [7, 8]. SSLRs were defined with the use of the following formula:
where χ1g = the number of episodes of graft failure at less than 1 year in the gth stratum of the given RSS interval, η1 = the total number of grafts lost at less than 1 year follow-up after transplantation, χ0g = the number of grafts surviving at 1 year follow-up or more after transplantation in the gth stratum of the given RSS interval, η0 = the total number of grafts surviving at 1 year follow-up or more after transplantation, and where χ1g/η1 = the probability of being in the gth stratum of a given RSS interval given that a graft has failed at less than 1 year follow-up after transplantation, and χ0g/η0 = the probability of being in the gth stratum of a given RSS interval given that one has survived at 1 year follow-up or more after transplantation. Ninety-five percent confidence intervals were calculated as follows:
where
RSS Strata were generated by combining adjacent RSS intervals in a stepwise fashion with other statistically indistinct RSS intervals based on the presence of SSLRs with overlapping 95% confidence intervals. A new strata was formed when the next RSS interval was statistically different based on the presence of SSLRs with non-overlapping 95% confidence intervals. Survival analysis, ROC curve areas, and SSLR computations were performed with Stata 9.
Survival Analysis
Kaplan-Meier analysis with log-rank test was used for time to event analysis for actuarial survival. For survival analysis, the outcome of interest was graft failure. Graft failure was defined as death or retransplantation occurring during the study period. In order to assess the impact of RSS on early and late mortality, the incidence rate of graft failure per 100 patient-years was calculated at multiple time intervals (<90 days, 90 days - 1 year, 1 - 2 years, 2 - 5 years, > 5 years).
RESULTS
Study population
Analysis included 11,703 heart transplant recipients with a mean follow-up time of 3.33 ± 2.22 years (range: 0 – 8.28 years). Due to missing data, 837 individuals (6.67%) were excluded from the logistic regression analysis. In graft survival analysis, the outcome of interest was death (n=2,722, 23.26%) or retransplantation (n=120, 1.03%). Patients lost to follow-up (n=277, 2.37%) or alive at last known follow-up (n=8,584, 73.35%) were censored at the date of last known follow-up.
Risk Factors
The logistic regression model of graft failure based on preoperative recipient and donor characteristics is shown in Table 1. Using the odds ratio calculated in regression analysis, weights were assigned to each risk factor. The maximum possible score was 23.2 based on recipient characteristics, and 8.5 based on donor characteristics, for an overall maximum score of 31.7. The model, with an area under the receiver operating characteristics curve of 0.666 (0.651 - 0.681), had moderate ability to discriminate between graft survival and graft failure.
Risk Groups
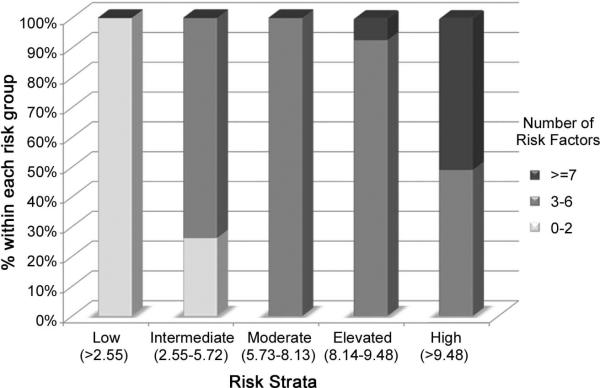
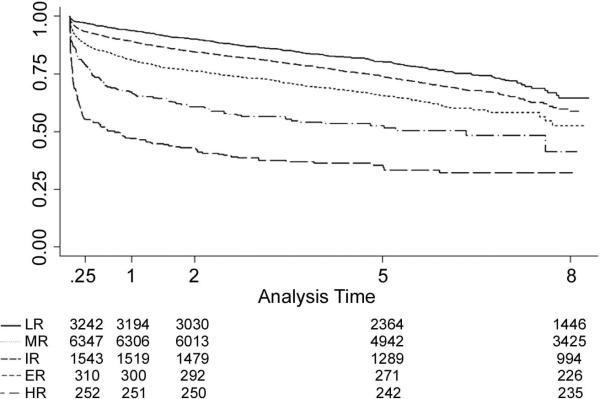
Threshold analysis identified five discrete risk groups: low risk (LR), intermediate risk (IR), moderate risk (MR), elevated risk (ER), and high risk (HR) [Table 2]. The number of risk factors per group are shown in Figure 1. Patients with scores > 9.48 were in the HR group (n=261; 2.2%). All of the patients in the HR group had at least four risk factors. No single HR characteristic was sufficient to place recipients in the HR group. Nearly 93.5% (n=243) of recipients in the HR group possessed ≥ 6 risk factors. This pattern of stacked risk factors persisted in the ER group, with 97.1% (n=301) of this group having ≥ 5 risk factors. In contrast, all of the recipients in the LR had between 0 - 2 risk factors. The HR group had a 1-year actuarial graft survival of 47.0%, and a median survival of 0.79 years. Graft survival functions and statistics can be found in Figure 2.
TABLE 2.
Risk group strata thresholds and outcomes
| Risk | Low | Intermediate | Moderate | Elevated | High | Total |
|---|---|---|---|---|---|---|
| Risk Score | <2.55 | 2.55-5.72 | 5.73-8.13 | 8.14-9.48 | >9.48 | |
| SSLR Lower Limit | 0.47 | 0.88 | 1.70 | 3.72 | 8.20 | |
| 95% CI Upper Limit | 0.41 | 0.84 | 1.52 | 2.96 | 6.44 | |
| 95% CI | 0.54 | 0.93 | 1.90 | 4.67 | 10.43 | |
| n | 3242 | 6347 | 1543 | 310 | 261 | 11703 |
| % | 27.7% | 54.2% | 13.2% | 2.6% | 2.2% | |
SSLR = stratum specific likelihood ratio
95% CI = 95% confidence interval
Figure 1.
Number of risk factors by risk strata
Figure 2.
Survival function by risk strata
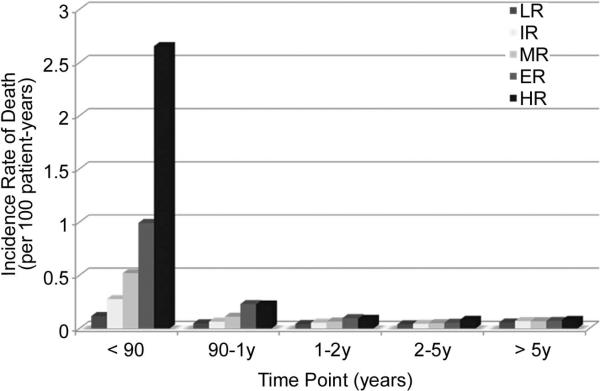
Figure 3 illustrates the incidence rate of graft failure during various time periods following transplantation by risk group. At 0 - 90 days and 90 days - 1 year, differences in the incidence rate existed across group. However, after the first year post-transplantation, differences in the incidence rate only existed between the low and high risk groups; there were no differences in the other groups at 1 - 2 years, 2 - 5 years, or > 5 years.
Figure 3.
Incidence rate of death by risk strata at different time points.
At < 90 and 90 days - 1 year, significant differences existed across all groups. After one year, differences existed only between low and high risk groups.
COMMENT
Heart transplantation remains the gold standard in the treatment of end-stage heart failure. Unfortunately, as organs available for transplant remain critically scarce, achieving maximal benefit from transplantation is predicated upon improved recipient selection and matching of recipient-donor pairs. This analysis provides further evidence that it is possible to risk stratify heart transplant candidates based on pretransplant characteristics.
Risk Factors
Recipient risk factors which correlate with graft failure include: poor renal function, poor liver function, advanced age, congenital etiology, and amyloidosis. In addition, markers of patient acuity which correlate with graft failure include ventilator dependence as well as hospitalization at the time of transplant. A recent study from our group [9] demonstrated that pre-transplant implantable LVAD dependence is not associated with decreased graft survival. The current analysis, however, demonstrates that bridging with other types of mechanical circulatory support, including RVAD-only, ECMO, extracorporeal LVAD, and total artificial heart are associated with poor graft survival. Donor risk factors which correlate with graft failure include hepatitis C seropositivity, insulin dependent diabetes mellitus, advanced donor age, prolonged ischemic time, and female gender (regardless of recipient gender).
Due to limitations of UNOS data, some characteristics likely to be associated with diminished graft survival were not statistically significant. For example, peripheral vascular disease occurs across a spectrum of varying severity and anatomical manifestations. This is not reflected in the UNOS data. Rather, it is a data point collected as a binary categorical variable.
Hemodynamic measurements such as pulmonary pressures, may be confounded by a number of factors. Given the available data, it is not possible to know the precise conditions or timing of the measurements. For example it is not possible to know whether or not the patient was under anesthesia or clinically decompensated (or optimized) at the time of the measurements; information regarding vasoactive agents at the time of the measurements was not known; and it is no clear when the measurements were taken relative to the transplantation. Other potentially important characteristics were excluded from the analysis because of poor characterization in the UNOS database. These included high vasopressor or inotrope requirements in donors, coronary artery disease in donors, panel reactive antibodies in recipients, and alcohol, intravenous drug use, or smoking in recipients and donors. Nevertheless, therefore these parameters should not be overlooked in decisions regarding recipient-donor matching.
Risk Strata
As depicted in Figure 1, more than half of all recipients in the HR group possessed ≥ 7 risk factors, and nearly all recipients in the ER group had ≥ 5 risk factors. In contrast, all of the recipients in the LR group had between 0 - 2 risk factors. This supports the concept that stacking risk factors presages poor outcome.
While no single risk factor was sufficient to place a recipient in the highest risk category, some risk factors were more frequently associated with the HR group than others. For example, greater than half of all extracorporeal LVAD patients, two-thirds of ECMO patients, and four-fifths of RVAD-only patients were in the HR group. This is in part explained by the other risk factors associated with these characteristics, including hospitalization at the time of transplant, ventilator dependence, and various degrees of multi-system organ failure.
Survival
Figure 3 illustrates that at 0 - 90 days and 90 days - 1 year, differences in the incidence rate of graft failure existed across group. However, not surprisingly, after the first year post-transplant, differences in the incidence rate of graft failure only existed between the LR and HR groups; there were no differences in the other groups at 1 - 2 years, 2 - 5 years, or > 5 years. Given the focus of 1 year survival in this analysis, this observed trend is not surprising. Furthermore, this trend is a common finding in survival analysis of transplant recipients - differences in outcomes are largely seen in early follow-up, with risk of death usually normalizing across groups with time [1, 9]. This is likely in part because patient acuity at transplant is most highly associated with short-term survival. Further, certain recipients are selected out by death. Finally, post-transplant events (not accounted for in this model) likely have a significant influence on longer-term outcomes. These include rejection, infection, stroke, medical non-compliance, renal failure, and transplant coronary artery disease. Future studies should be undertaken to assess the impact of these events on long-term survival to help direct clinical care. In addition, these studies would provide recipients and their families with important information regarding long term prognosis.
Who is Too High Risk?
When comparing the HR group with other groups, the risk of graft failure in the first year post-transplant was several-fold higher. Furthermore, long-term graft survival was diminished, with median graft survival in the HR group significantly less than one year. For the HR group, transplantation has limited clinical effectiveness. This raises questions regarding the appropriateness of allocating organs to such high-risk patients.
Conversely, the LR and IR groups all achieved acceptable short- and long-term outcomes; these groups comprised approximately 80% of all recipient-donor pairs transplanted during the study period. Though reduced compared with the LR and IR, the median survival in the MR group exceeded 8 years.
The appropriateness of transplanting the ER group, however, is debatable. Short-term graft survival was relatively poor—with one-third of grafts failing at one year. And while median survival exceeded six years, this is significantly less than the other lower-risk groups. Nevertheless, in order to determine the appropriateness of transplanting this group, additional information including quality of life, costs, as well as a better understanding of this cohort's survival in the absence of transplantation is needed.
Organ allocation to high risk donors
Alternate list transplantation has been applied by an increasing number of centers in order to improve donor organ allocation to “high-risk” recipients. Under alternate lists, high-risk recipients are only eligible for donor organs not suitable for any potential standard recipient. Because donor organs utilized by alternate list recipients would otherwise be discarded, this strategy may expand the donor pool and offer the benefits of transplantation to recipients who otherwise may not benefit from this therapy. However, the criteria used for alternate list transplantation have not been studied prospectively and have not been standardized across centers [5, 10-15].
While the current analysis confirmed many commonly used alternate list criteria including recipient advanced age, amyloidosis, and renal dysfunction as well as donor advanced age, hepatitis C seropositivity, and diabetes mellitus, several limitations with this strategy were also highlighted. First, a number of additional risk factors that correlate with poor outcomes but are not commonly used in alternate list criteria were identified. This includes recipient elevated bilirubin, RVAD-only, extracorporeal LVAD, and ECMO dependence. Conversely, retransplantation and recipient hepatitis C seropositivity, which some centers have used as criteria for alternate list, were not associated with significantly worse outcomes. Furthermore, this analysis supports the principle that the magnitude of risk related to a single factor is not sufficient to place a candidate in the high-risk category. In fact, among the commonly applied alternate list characteristics, poor renal function (eGFR < 33) was the highest weighted risk factor. And yet, a recipient with this and no other risk factor would remain in the low risk category. Furthermore, all recipients in the MR groups had at least 3 risk factors, and all recipients in the ER and HR groups had at least 4 risk factors.
In an effort to limit allocation of scare organs to candidates with a high likelihood of poor post-transplant outcomes, it may be prudent to modify the implementation of alternate list transplantation by utilizing a system such as risk stratification score that simultaneously considers multiple factors to estimate a candidate's post-transplant risk. For example, recipient/donor pairings with a risk score exceeding a predetermined threshold would be considered “High-Risk” and only allowed if an organ were declined for all potential non-“High-Risk” matches.
The risk stratification system may have additional benefits. With increasing quality oversight and fixed reimbursement schemes, introduction of a risk stratification scheme may prevent penalizing centers that transplant a greater number of high-risk recipients by better accounting for case mix in quality measures and reimbursement determinations.
Future studies
Additional studies are needed to validate the model presented in this analysis. Future studies should consider quality of life and cost implications of transplanting various groups of heart transplant candidates. Furthermore, in order to improve organ allocation in heart transplantation, future studies should assess the competing outcomes related to organ allocation, including reducing deaths on the waiting list and maximizing post-transplant survival while ensuring efficient and equitable allocation of organs. In the context of this study, such an analysis of pre-transplant survival is complicated by the limitations of retrospective registry data. For example, any study of wait-list survival may be confounded by the non-random nature of patient censoring. Specifically, Cox modeling assumes patients are censored randomly. However, in the case of wait-list survival, this is not true because sicker patients may be preferentially transplanted. Furthermore, while candidates’ dependence on mechanical circulatory support at the time of listing was known, data regarding device implantation including date of implant and clinical status are not recorded in the UNOS database. Initiation of mechanical circulatory support during the waiting period is also not captured in the current data set. Analysis of waiting list survival would not account for initiation of mechanical circulatory support during the waiting period, which undoubtedly has an important impact on waiting list survival that cannot be accounted for. As a result, this analysis did not examine wait list survival nor did it attempt to draw conclusions regarding the net benefit of transplantation by risk strata. The UNOS data will soon capture more detailed information regarding the use of mechanical circulatory support, and therefore these analyses should be possible in the future.
Limitations
These data have several limitations. First, patient registries often suffer from variability in data entry. However, fields contained within this database were generally well populated with a 90-99% data entry rate for the majority of variables. Though the UNOS reporting system provided definitions for variables in data guidelines, definitions may vary by center. As the primary outcome measure was graft failure, it is unlikely that center variability would have significantly affected the final conclusions of the analysis, however center variability may have affected the point estimates of the risk factors. Second, although the data analysis supports associations between variables and outcomes, causal relationships cannot be determined. Many of the risk factors may simply be markers for poor clinical status before transplantation rather than a direct causal factor in poor survival. Third, for the purpose of simplifying the scoring scheme for clinical use, variables were assigned weights based on regression analysis and, continuous variables, such as recipient age and eGFR were converted to discrete categories. However, as a result, statistical power is lost. If applied for systematic organ allocation, a more sophisticated equation for generating scores could be developed. Fourth, the risk stratification score remains to be validated, and further studies are required to confirm its accuracy in predicting early post-transplant outcomes. Development of an accurate risk score involves a testing dataset, as used in our analysis to develop the model, and a training set used to test and further refine the model. Given that data from 2001-2008 was used to develop the model, opportunity now exist to validate and further refine the model using data from 2009-2011. This analysis would also ensure that the risk score is also relevant to the current population of recipients. Finally, future studies must also assess the ability of such an allocation scheme to address competing interests such as reducing deaths on the waiting-list while maximizing post-transplant survival.
CONCLUSION AND IMPLICATIONS
Pre-transplant recipient variables significantly influence early and late graft survival following heart transplantation. Thus, some patients face a higher than average risk of graft failure during the first year post-transplant, as well as severely diminished longer-term survival, such that the goal of equitable organ allocation may be compromised. A risk stratification score may improve organ allocation strategies by avoiding the potential negative impact of transplanting extremely high-risk candidates.
ACKNOWLEDGEMENTS
We thank UNOS for supplying this data, especially Jennifer L. Wainright, PhD, and Katarina Linden, PhD, for their assistance with our analysis. This work was supported in part by Health Resources and Services Administration contract 231-00-0115. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This work was supported in part by NIH Training Grant 5T32HL007854-13 (Dr. Iribarne).
Abbreviations and Acronyms
- ER
Elevated risk
- HR
High risk
- IR
Intermediate risk
- LR
Low risk
- MR
Moderate risk
- ROC
Receiver operating characteristic
- RSS
Risk Stratification Score
- SSLR
Stratum specific likelihood ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Russo MJ, Chen JM, Hong KN, et al. Survival After Heart Transplantation Is Not Diminished Among Recipients With Uncomplicated Diabetes Mellitus: An Analysis of the United Network of Organ Sharing Database. Circulation. 2006;114:2280–2287. doi: 10.1161/CIRCULATIONAHA.106.615708. [DOI] [PubMed] [Google Scholar]
- 2.Luckraz H, Sharples LD, Charman SC, et al. Does Heart Transplantation Confer Survival Benefit in All Risk Groups? J Heart Lung Transplant. 2005;24:1231–1234. doi: 10.1016/j.healun.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Russo MJ, Chen JM, Sorabella RA, et al. The effect of ischemic time on survival after heart transplantation varies by donor age: An analysis of the United Network of Organ Sharing Database. J Thorac Cardiovasc Surg. 2007;133:554–559. doi: 10.1016/j.jtcvs.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 4.OPTN/DRTR [January 27, 2010];Annual Report. 2008 Available at http://www.ustransplant.org/annual_reports/current/313_ord.htm.
- 5.Zaroff JG, Rosengard BR, Armstrong WF, et al. Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations, March 28-29, 2001, Crystal City, Va. Circulation. 2002;106:836–41. doi: 10.1161/01.cir.0000025587.40373.75. [DOI] [PubMed] [Google Scholar]
- 6.Hanley JA, McNeil BJ. A method of comparing the areas under the receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 7.Chen JM, Levin HR, Michler RE, Prusmack CJ, Rose EA, Aaronson KD. Reevaluating the significance of pulmonary hypertension before cardiac transplantation: determination of optimal thresholds and quantification of the effect of reversibility on perioperative mortality. J Thorac Cardiovasc Surg. 1997;114:627–634. doi: 10.1016/S0022-5223(97)70053-9. [DOI] [PubMed] [Google Scholar]
- 8.Pierce JC, Cornell RG. Integrating stratum-specific likelihood ratios with the analysis of ROC curves. Med Decis Making. 1993;13:141–151. doi: 10.1177/0272989X9301300208. [DOI] [PubMed] [Google Scholar]
- 9.Russo MJ, Hong KN, Davies RR, et al. Posttransplant survival is not diminished in heart transplant recipients bridged with implantable left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;138:1425–1432. doi: 10.1016/j.jtcvs.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Felker GM, Milano CA, Yager JE, et al. Outcomes with an alternate list strategy for heart transplantation. J Heart Lung Transplant. 2005;24:1781–1786. doi: 10.1016/j.healun.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Chen JM, Russo MJ, Hammond KM, et al. Alternate waiting list strategies for heart transplantation maximize donor organ utilization. Ann Thorac Surg. 2005;80:224–8. doi: 10.1016/j.athoracsur.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Laks H, Marelli D, Fonarow GC, et al. Use of two recipient lists for adults requiring heart transplantation. J Thorac Cardiovasc Surg. 2003;125:49–59. doi: 10.1067/mtc.2003.62. [DOI] [PubMed] [Google Scholar]
- 13.Russo MJ, Davies RR, Hong KN, et al. Transplanting high-risk recipients with marginal donor hearts is clinically effective. Ann Thor Surg. 2009;87:1066–70. doi: 10.1016/j.athoracsur.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller LW. Listing Criteria for Cardiac Transplantation: Result of an American Society of Transplant Physicians-National Institutes of Health Conference. Transplantation. 1998;106:947–951. doi: 10.1097/00007890-199810150-00032. [DOI] [PubMed] [Google Scholar]
- 15.Mehra MR, Kobashigawa J, Starling R, et al. Listing Criteria for Heart Transplantation: International Society for Heart and Lung Transplantation Guidelines for the Care of Cardiac Transplant Candidates—2006. J Heart Lung Transplant. 2006;24:1024–1042. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]