Abstract
Purpose
The endoplasmic reticulum-associated degradation (ERAD) pathway is responsible for the translocation of misfolded proteins across the ER membrane into the cytosol for subsequent degradation by the proteasome. In order to understand the spectrum of clinical and molecular findings in a complex neurological syndrome, we studied a series of eight patients with inherited deficiency of N-glycanase 1 (NGLY1), a novel disorder of cytosolic ERAD dysfunction.
Methods
Whole-genome, whole-exome or standard Sanger sequencing techniques were employed. Retrospective chart reviews were performed in order to obtain clinical data.
Results
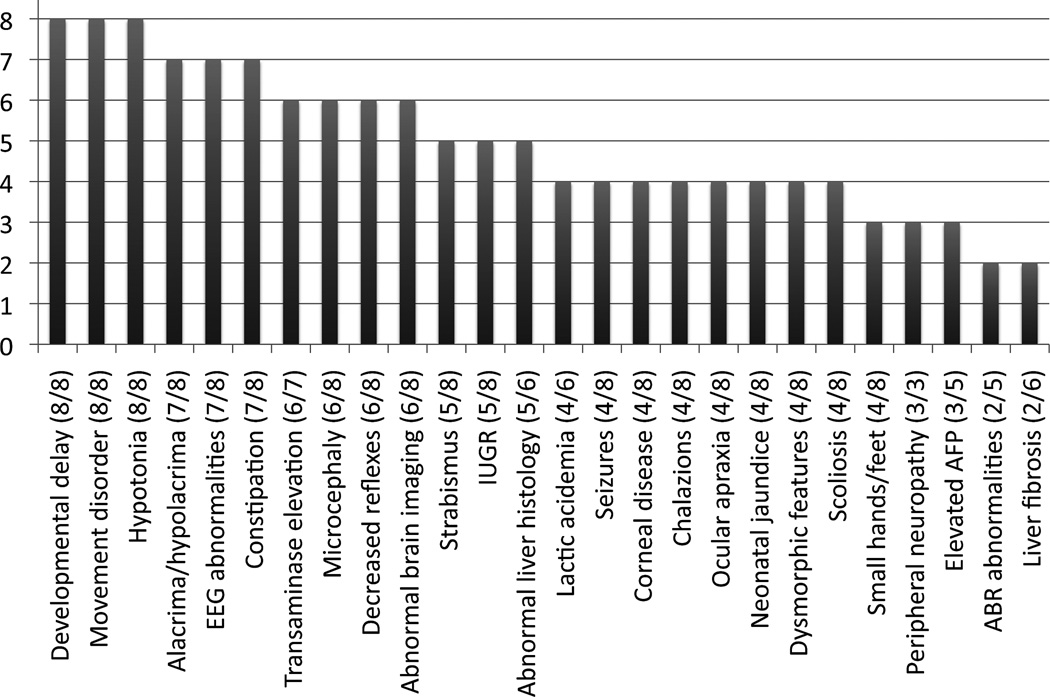
All patients had global developmental delay, a movement disorder, and hypotonia. Other common findings included hypo- or alacrima (7/8), elevated liver transaminases (6/7), microcephaly (6/8), diminished reflexes (6/8), hepatocyte cytoplasmic storage material or vacuolization (5/6), and seizures (4/8). The nonsense mutation c.1201A>T (p.R401X) was the most common deleterious allele.
Conclusions
NGLY1 deficiency is a novel autosomal recessive disorder of the ERAD pathway associated with neurological dysfunction, abnormal tear production, and liver disease. The majority of patients detected to date carry a specific nonsense mutation that appears to be associated with severe disease. The phenotypic spectrum is likely to enlarge as cases with a more broad range of mutations are detected.
Keywords: NGLY1, alacrima, choreoathetosis, seizures, liver disease
INTRODUCTION
The enzyme N-glycanase 1 (NGLY1), also known as peptide:N-glycanase (PNGase, EC 3.5.1.52), catalyzes protein deglycosylation by cleaving the -aspartyl glycosylamine bond of N-linked glycoproteins with the subsequent release of intact N-glycan species [1]. NGLY1 activity was first described in almond seeds [1], but is ubiquitously present across a wide range of species, including bacteria [2], yeast [3–5], amoeba [6], fish [7], and mammals [8,9]. Evidence suggests that NGLY1 participates as a key cytoplasmic component of the endoplasmic reticulum-associated degradation (ERAD) machinery along with the AAA ATPase complex p97 [10,11]. The ERAD pathway is a mechanism for identifying and degrading misfolded glycoproteins. N-glycans that are high in mannose content act as quality control tags for proteins in the early stages of the secretory pathway [12,13]. Misfolded glycoproteins are detected by ER luminal lectins and are then translocated to the cytosol via the ERAD machinery, to be subsequently degraded by cytosolic enzymes including NGLY1 [14].
To date, a single patient with NGLY1 deficiency has been reported as part of a whole-exome sequencing (WES) study focusing on the utility of this technology to detect the underlying genetic etiology of disorders affecting patients with previously undiagnosed or unresolved genetic conditions [15]. Need et al. described a 3-year-old boy with compound heterozygous inactivating mutations in NGLY1 and suggested that this could be a new disorder. The initial patient had a clinical phenotype suggestive of a congenital disorder of glycosylation, although repeated transferrin isoelectric focusing and N-glycan analyses were normal. Liver biopsy showed accumulation of an amorphous unidentified substance throughout the cytoplasm, a finding likely consistent with NGLY1 dysfunction, which would be expected to result in abnormal accumulation of misfolded glycoproteins because of impaired cytosolic degradation [15]. We now report clinical and molecular findings present in seven newly diagnosed patients with mutations in NGLY1, and further details related to the original case. These observations confirm NGLY1 deficiency as an inherited disorder associated with the ERAD process and document its clinical presentation.
MATERIALS AND METHODS
The Stanford Institutional Review Board approved this study. Clinical data were abstracted from the medical records by retrospective chart review. Further data were collected by direct interaction with the families of participating subjects.
Exome sequencing was performed in Patient 1 (Duke University), Patients 5 and 6 (University of British Columbia) and parents using the Illumina HiSeq2000 platform and the Agilent SureSelect Human All Exon 50 Mb Kit. For Patient 3 (Stanford University), the patient and parents were sequenced using both Illumina HiSeq2000 and Complete Genomics platforms. Variants in Illumina-sequenced reads were called using both the Hugeseq and Real Time Genomics pipelines and Complete Genomics variants were identified by their own variant callers. For Patient 3 (Baylor College of Medicine) DNA was capture-sequenced using a commercially developed capture reagent (VCRome2). Sequence data were generated on an Illumina HiSeq2000 producing an average coverage of 80× with >90% of targeted bases at 20× coverage or higher. WES was performed on a clinical basis in three patients (Patient 2 [Baylor College of Medicine Whole Genome Laboratory, Houston, Texas], and Patients 6 and 7 [Emory Genetics Laboratory, Atlanta, Georgia]). Sanger sequencing of NGLY1 was performed in Patient 4 at Duke University and results were confirmed by a clinical laboratory (GeneDx, Gaithersburg, Maryland).
RESULTS
Case reports
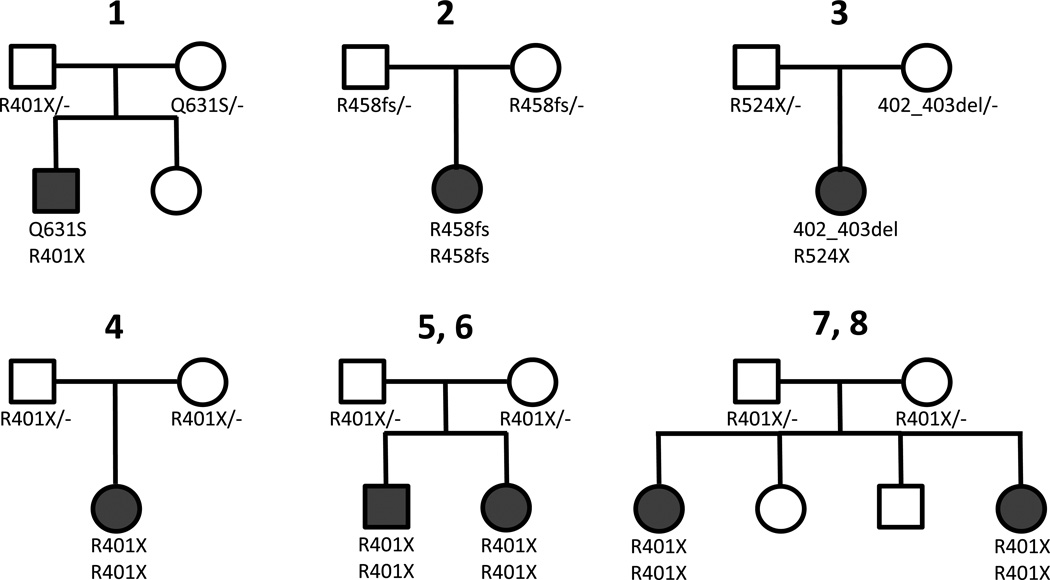
The clinical and molecular findings demonstrate clear clinical similarities of patients with NYGL1 deficiency, although some variation in overall severity was observed, with the most common mutation being associated with more severe outcomes (Table 1 and Figure 1). All patients had global developmental delay, a movement disorder, and hypotonia. Other common findings included hypo- or alacrima (7/8), abnormal brain imaging (7/8), EEG abnormalities (7/8), elevated liver transaminases (6/7), microcephaly (6/8), diminished reflexes (6/8), hepatocyte cytoplasmic storage material or vacuolization (5/6), seizures (4/8), and abnormal nerve conduction (3/3). Two of the patients died prematurely at 9 months and 5 years of age. Pedigrees for all patients are shown in Figure 2. Short clinical summaries are provided below. Further details may be found in Supplementary Materials and Methods.
Table 1.
Clinical and molecular findings in NGLY1 deficiency
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Totals | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 5 y | 20 y | 4 y | 2 y | d.5 y | d.9 m | 3 y | 16 y | |
| Gender | M | F | F | M | M | F | F | F | |
| Ethnicity | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | |
| Countries of Origin mother/father |
Puerto Rico, South Europe/ North Europe |
Italy/Italy | Germany, Ireland, Scotland, Sweden/ Holland, Ireland, Italy, Germany |
Germany/ Germany |
England, Finland Ukraine/ England |
England, Finland, Ukraine/ England |
Unknown | Unknown | |
| Consanguinity | − | + | − | − | − | − | − | − | 1/8 |
| Mutations (maternal/paternal allele) |
c.C1891del (p.Q631S)/ c.1201A>T (p.R401X) |
c.1370dupG (p.R458fs)/ c.1370dupG (p.R458fs) |
c.1205_1207del (p.402_403del)/ c.1570C> (p.R524X) |
c.1201A>T (p.R401X) c.1201A>T (p.R401X) |
c.1201A>T (p.R401X)/ c.1201A>T (p.R401X) |
c.1201A>T (p.R401X)/ c.1201A>T (p.R401X) |
c.1201A>Y (p.R401X)/ c.1201A>T (p.R401X) |
c1201A>T (p.R401X)/ c.1201A>T (p.R401X) |
|
| IUGR | − | + | − | + | + | + | − | + | 5/8 |
| Brain imaging abnormalities | +a | −b | +c | +d | +e | +f | − | +g | 6/8 |
| Global developmental delay | + | + | + | + | + | + | + | + | 8/8 |
| Microcephalyh | − | + | + | − | + | + | + | + | 6/8 |
| Hypotonia | + | + | + | + | + | + | + | + | 8/8 |
| Movement disorder | + | + | + | + | + | + | + | + | 8/8 |
| EEG abnormalities | + | + | + | + | + | + | − | + | 7/8 |
| ↓DTRs | + | + | − | + | + | − | + | + | 6/8 |
| Seizures | + | − | − | + | + | − | − | + | 4/8 |
| Ocular apraxia | − | + | + | − | − | − | + | + | 4/8 |
| Alacrima/hypolacrima | + | + | + | + | + | − | + | + | 7/8 |
| Corneal ulcerations/scarring | + | + | − | + | − | − | − | + | 4/8 |
| Chalazions | + | − | + | + | − | − | + | − | 4/8 |
| Strabismus | − | − | + | + | − | − | + | + | 5/8 |
| ABR abnormalities | − | − | + | + | − | ND | ND | ND | 2/5 |
| Lactic acidosis | − | + | + | + | −i | ND | + | ND | 4/6 |
| Neonatal jaundice | + | − | + | + | − | − | + | − | 4/8 |
| Elevated liver transaminases | + | + | + | + | + | ND | + | − | 6/7 |
| Elevated AFP | + | − | −j | + | + | ND | ND | ND | 3/5 |
| Liver fibrosis | + | − | − | + | − | − | ND | ND | 2/6 |
| Liver storage or vacuolization | + | + | + | − | +k | +l | ND | ND | 5/6 |
| Constipation | + | + | + | + | + | − | + | + | 7/8 |
| Dysmorphic features | − | − | − | − | +m | +n | +o | +p | 4/8 |
| Scoliosis | − | + | − | + | + | − | − | + | 4/8 |
| Small hands/feet | + | − | + | + | − | − | − | + | 4/8 |
| Peripheral neuropathyq | + | + | ND | ND | + | ND | ND | ND | 3/3 |
Normal (MRI 8 months), prominent perivascular spaces and periatrial white matter gliosis (MRI 15 months);
Slightly dilated lateral ventricles (MRI 4 months);
Mild global reduction in cerebral hemisphere volume and thin corpus callosum and anterior commissure (MRI 17 months), MRS normal;
Delayed myelination (MRI 6 months), delayed myelination, supratentorial atrophy considered secondary to steroid therapy (MRI 10 months), delayed myelination, recovery from supratentorial atrophy (MRI 19 months);
Mild ventriculomegaly, small pons and midbrain (CT 10 months), normal (MRI 2 and 4 years);
Prominent lateral ventricles (CT 9 months);
Subtle increased T2 signal in periventricular white matter (MRI 9 years);
Information on birth head circumference was not available for Patient 2, and microcephaly was present at birth for Patient 8, but for all others microcephaly was acquired;
Blood lactate was mildly elevated on two occasions, but thought to be secondary to difficult blood draws, but repeat testing was normal;
Not tested in infancy;
Hepatocytes showed marked vacuolization consistent with storage;
Hepatocytes showed nonspecific vacuolization that was considered to be secondary to agonal hypoxia/ischemia, but such findings may also be consistent with early storage;
Mild ptosis, hypoplastic supraorbital ridges, epicanthic folds, long eyelashes, short nose, thick alveolar ridges, high-arched palate, and mild micrognathia;
Epicanthic folds, epicanthic folds, and short nose;
Frontal bossing, metopic ridge, epicanthic folds, tented upper lip, hypoplastic left nipple, tapered fingers and mild 4th and 5th toe clinodactyly;
Long eyelashes, arched eyebrows, decreased facial subcutaneous fat, mild proptosis, broad nasal bridge, and crowded dentition;
As determined by nerve conduction velocity testing.
Abbreviations: ABR=auditory brainstem response hearing testing; AFP=alpha-fetoprotein; DTRs= deep tendon reflexes; EEG=electroencephalography; IUGR=intrauterine growth restriction.
Figure 1.
Relative frequency of clinical findings in NGLY1 deficiency.
Figure 2.
Pedigrees of NGLY1-deficienct patients.
Patient 1, a now 5-year-old male, presented in the neonatal period with involuntary movements, including athetosis involving the trunk and extremities and constant lip smacking and pursing while awake. Pregnancy and birth history were unremarkable. He had mild neonatal jaundice requiring phototherapy, but otherwise appeared well. Global developmental delay, hypotonia, intractable multifocal epilepsy, consisting of myoclonic seizures, drop attacks, and staring or tonic episodes, and liver disease were present in infancy. He has cortical vision loss and congenital alacrima and corneal ulcerations with scarring were noted at age 4 years. Now, at age 5 years, the movement disorder has not abated and he has central hypotonia and global developmental delay.
Patient 2, a now 20-year-old female, was born at 39 weeks of gestation via Cesarean section because of intrauterine growth retardation and an abnormal appearing placenta. At four months of age, hypotonia, developmental delay and elevated liver transaminases were noted. At approximately 4 years of age, a slight intention tremor and frequent involuntary movements of her neck, hands and arm were observed. At 5 years of age, she was noted to have ocular apraxia, distal tapering of hands and feet, and diminished deep tendon reflexes. She has cortical vision impairment, as well as alacrima and dry eyes that require lubrication, but has not developed corneal scarring. Presently, she has marked intellectual disabilities and requires total care. She has very little expressive speech and communicates through an electronic speech-generating device. She continues to ambulate with a walker.
Patient 3, a now 4-year-old girl, was born via Cesarean section at term for a non-reassuring fetal heart tracing and was monitored in the NICU because of poor feeding and lethargy. The pregnancy was complicated by a positive second trimester screen noting increased risk for Smith-Lemli Opitz syndrome (SLOS) and trisomy 18 (AFP 1.63 MoM, uE3 0.26 MoM, hCG 0.54 MoM, inhibin 1.02 MoM), but karyotype on amniocentesis was normal. As a neonate, she had hyperbilirubinemia that was treated with phototherapy and was noted to have elevated liver transaminases and transient thrombocytopenia.
In infancy, she was noted to have global developmental delay, acquired microcephaly, bilateral exotropia, hypotonia, constipation, and intermittent mild lactic acidemia (3.7 to 7.5 mM, normal <3). At approximately age 1 year, the parents noticed that she did not make tears when crying, although she had adequate tear production to keep her eyes moist. She has had intermittent chalazions, but no corneal scarring. She also developed staring spells, lasting up to 15 seconds, at approximately age 1 year; these episodes occur about once daily and can be interrupted by gentle contact. By age 17 months she had developed an extrapyramidal movement disorder consisting of asynchronous myoclonic jerks of the limbs and shoulders and subtle choreoathetotic movements of the hands and fingers. At 4 years she can ambulate unassisted, although her gait is unsteady, and communicates with vocalizations, gestures and use of a speech-generating device.
Patient 4, a now 2-year-old boy, was delivered by Cesarean section at 38 weeks of gestation after fetal distress was noted on cardiotocography. Pregnancy history was positive for intrauterine growth restriction (IUGR) and oligohydramnios. He had mild hyperbilirubinemia, but otherwise his neonatal course was unremarkable and he was discharged on day of life three. Intermittent head flexion was noted at 6 months, and an EEG at 8 months showed generalized poly-spike discharges. Soon thereafter, mild tonic seizures with head and body flexion started, and evolved to single, symmetric spasms with bilateral arm extension. Involuntary movements of the upper extremities were also noted at this time. In addition, global developmental delay, bilateral ptosis, abnormal tear production, elevated liver transaminases (3 to 4 times upper limit of normal), and constipation were noted in infancy. He has had recurrent episodes of keratoconjunctivitis and poor lid closure during sleep with resultant corneal scarring.
Patient 5, a boy who died at the age of 5 years, was born at term following a pregnancy that was complicated by a positive second trimester serum screening for trisomy 18 and Smith-Lemli-Opitz syndrome (SLOS) (AFP 1.97 MoM, uE3 0.24 MoM and hCG 0.48 MoM). Cytogenetic analysis of cultured amniocytes showed a normal male karyotype and measurement of 7-dehydrocholesterol in amniotic fluid excluded SLOS. Because of concerns for IUGR and a non-reassuring stress test, he was delivered by Cesarean section at 36 weeks of gestation. He was noted to have mild flexion contractures of both knees, but had an uneventful neonatal period. He had global developmental delay and constant movements of his arms and legs since early infancy and developed head bobbing at 7 months. At 8 months, liver transaminase elevations (approximately 1.5 times the upper limit of normal) were noted, and the elevations persisted until age 3 ½ years. Although his reflexes appeared normal in infancy, they were diminished by age 2 years and at 38 months could no longer be elicited. During the second year of life, he was noted to have dry eyes that were treated with lubricant drops at bedtime, and microcephaly was present by 16 months. At 2 ½ years, he developed myoclonic seizures that became intractable despite numerous therapeutic trials. Between the ages of 10 months and five years, he showed slow developmental progress, but regressed during the last year. He died at age 5 years following a viral illness and a prolonged seizure.
Patient 6 is the younger sister of Subject 5. The pregnancy was also complicated by a positive second trimester serum screen for trisomy 18 and SLOS (AFP 0.87 MoM, uE3 0.31 MoM, hCG 0.57 MoM). Cytogenetic analysis of cultured amniocytes showed a normal female karyotype. She was delivered by Cesarean section at 35 weeks of gestation following an ultrasound that was concerning for IUGR. She had jaundice requiring phototherapy, but her course in the nursery was otherwise uneventful. By age 9 months, developmental delay was apparent and she had developed hypotonia, microcephaly, a mildly myopathic-appearing face and constant involuntary movements with a tendency to hyperextend her arms and close her fists. At 9 ½ months of age, she died unexpectedly in her sleep and the cause of death remains unknown.
Patient 7, a now 3-year-old girl, was born at term via scheduled Cesarean section and, other than mild jaundice, did not have any neonatal complications. Prenatal screen showed an increased risk for trisomy 21. In infancy, she was noted to have strabismus, hypotonia, athetoid arm and hand movements, clasped hands, and elevated liver transaminases (approximately 3 times upper limit of normal). Microcephaly was present by age 2 years. In addition, severe developmental delay was present, but she has shown slow progress without regression. She learned to sit alone and started to crawl at 2 years, but had no words.
Patient 8, a now 16-year-old girl, is the older sister of Subject 7. She was born at term via Cesarean section for decreased fetal movement and bradycardia. Prenatal history was significant for IUGR. She had cyanosis at birth and required resuscitation. Anal stenosis requiring dilatation was noted in the first week of life, but she did not have further neonatal problems and was discharged home on day of life 7. She was noted to have hypotonia, a movement disorder consisting of head bobbing and extremity athetosis, and developmental delay in early infancy. At 3 years, she could stand unsupported, ambulate with a walker, and started to babble. Seizures developed at age 11 years and she lost all mobility. Other medical history includes acquired microcephaly, an inability to close her eyes completely during sleep, chronic conjunctivitis, corneal clouding, hypolacrima, strabismus, hearing impairment, gastro-esophageal reflux, chronic constipation, severe scoliosis and talipes equinovarus.
Sequencing results
The nonsense mutation c.1201A>T (p.R401X) was the most common deleterious allele identified, present in homozygous state in 5 of 8 cases and in compound heterozygous state in one case. Patients 2 and 3 did not carry the c.1201A>T (p.R401X) mutation and their clinical phenotype was relatively mild in comparison (Table 1).
For Patient 1, whole exome sequencing (WES) performed as part of a research protocol detected putative knock out mutations forming a compound heterozygote genotype in the NGLY1 gene (Maternal frameshift: Q631S. at cDNA level: C1891del in transcript ENST00000280700. EXON 12. Paternal nonsense: 3_25750426_A, which causes a nonsense mutation, R401X, in transcript ENST00000280700. At the cDNA level this is A1201T EXON 8) [15].
For Patient 2, WES (Baylor College of Medicine Whole Genome Laboratory) revealed a homozygous mutation in exon 9 of the NGLY1 gene denoted as c.1370dupG or p.R458fs. Both parents were confirmed to be heterozygous carriers by Sanger sequencing. The mutation causes a frame shift in codon 458, causing insertion of 13 incorrect residues before a stop codon is introduced towards the end of exon 9. The mutation was not seen in any of 3321 other subjects sequenced at Duke, nor was it seen in 6503 subjects on the Exome Variant Server (NHLBI GO Exome Sequencing Project (ESP), Seattle, WA (URL: http://evs.gs.washington.edu/EVS/) [accessed June 2013]).
For Patient 3, WES and whole-genome sequencing were performed using research protocols at Baylor College of Medicine and Stanford University. Mutations in NGLY1 that followed a compound heterozygous inheritance pattern were identified by at least 2 of the variant calling approaches at each site (see Supplemental Materials and Methods). A stop gain mutation caused by a G>A mutation at position 3:25761670 (hg19) resulting in p.R542X was identified in both the father and daughter. Secondly, a 3 base pair in-frame deletion TCC> beginning at position 3:25775416 (hg19) was identified in both the mother and daughter. At additional G>T mutation resulting in a heterozygous SMP at position 3:25777564 was identified in the daughter, mother and father. This mutation was called by two programs in the daughter and one program in the mother and father. This mutation was not previously observed in 1000 genomes and is a coding region; however, it is present in heterozygous form in all three individuals.
For Patient 4, Sanger sequencing (Duke University) detected a homozygous nonsense mutation, p.R401X, at position 3:25775422 (hg19) in transcript ENST00000280700. At the cDNA level this is c.1201A>T in exon 8 of NGLY1. This finding was confirmed in a CLIA-certified laboratory (GeneDx).
For Patients 5 and 6, the variant in NGLY1, single nucleotide variant T >A at position 3:25775422 (hg19), which was called to be homozygous in both children, was present in the mother’s exome as a heterozygous call (see Supplementary Materials and Methods). The NGLY1 variant was then independently validated by Sanger sequencing in both patients and both parents. This base pair substitution causes a nonsense mutation, R401X.
For Patients 7 and 8, WES (performed commercially at Emory University) detected a homozygous nonsense mutation, R401X, at position 3:25775422 (hg19) in transcript ENST00000280700 in both siblings. At the cDNA level this is c.1201A>T in exon 8 of NGLY1.
DISCUSSION
The finding of compound heterozygous knockout mutations in NGLY1 in the original case suggested that NGLY1 deficiency (OMIM 615273) represents a new disorder [15]. Soon after the initial report, we were able to identify the seven additional cases reported herein, therefore confirming NGLY1 deficiency as an inherited disorder of the ERAD pathway and the first to be identified that involves the cytosolic proteasome. The rapid ascertainment of cases occurred over a period of several months and was assisted by strong advocacy from families and connections made possible by the internet and social media (see Editorial Commentary). NGLY1-deficient patients have a striking clinical triad consisting of abnormal tear production, choreoathetosis and liver disease. In addition, global developmental delay, acquired microcephaly, hypotonia, EEG abnormalities with or without overt seizures, brain imaging abnormalities, a peripheral neuropathy, constipation and a history of IUGR were common findings. Some patients were noted to have dysmorphic features, but overall these were not considered to be particularly prominent (Table 1). Interestingly, low uE3 was present in three patients, including in two children who died and were found to have significant adrenal cortex vacuolation. Although adrenal function was not specifically evaluated in our patients, some degree of dysfunction remains possible. Further research will be required in order to determine if low uE3 might provide a clue to underlying NGLY1 deficiency.
The constellation of features present in our cohort is in some ways reminiscent of the clinical features present in the congenital disorders of glycosylation (CDGs) [16]. CDGs feature multisystem disease involving particularly the central nervous system, heart, liver and gastrointestinal tract, and endocrine system. Indeed, the possibility of an underlying CDG was high in the differential diagnosis for all cases. Based on the initial report of a single NGLY1-deficient patient, a recent CDG review considered that NGLY1 deficiency may even be considered to be the first “congenital disorder of deglycosylation” [17]. Although there are clearly similarities between NGLY1 deficiency and the CDGs as a whole, such as IUGR, failure to thrive, global developmental delay, and liver impairment, these features are non-specific and certain differences also are apparent. Unlike CDGs, NGLY1 deficiency does not appear to be associated with cerebellar atrophy, lipodystrophy, or significant heart manifestations. Our patients commonly had abnormalities detected on brain imaging, but findings were typically mild and non-specific (Table 1). In addition, at this early point of delineation of the clinical phenotypic spectrum, the combination of hypo- or alacrima and a movement disorder consisting of tremulousness and varying degrees of choreoathetosis appear to be pathognomonic for NGLY1 deficiency, but are not particularly associated with CDGs.
Mitochondrial disorders are also associated with multi-system disease and prominent neuromuscular involvement and such disorders were also considered as diagnostic possibilities in many cases. Lactic acidemia was variably present, but tended to be mild; chronic elevations were not noted in any patient. Mitochondrial electron transport chain enzymology was also performed in skin fibroblasts and tissues (muscle or liver) when samples were available and no abnormalities were identified. A moderate reduction in mitochondrial DNA content was identified in a liver sample from a Patient 3 (see Supplemental Materials and Methods). Although NGLY1 deficiency clearly involves multiple systems, features more strongly associated with mitochondrial disease, such as basal ganglia involvement, retinitis pigmentosa, cardiomyopathy, liver failure, and renal tubular acidosis were absent [18]. Significant brain disease was noted on autopsy in two siblings (Patients 5 and 6) who were found to have pathological changes consistent with hypoxic-ischemic encephalopathy (HIE). HIE has been associated with secondary mitochondrial dysfunction, but is not a typical feature of inherited mitochondrial disorders [19]. Neuronal loss and gliosis may occur in both HIE and mitochondrial disorders, but spongiform degeneration is more typically seen in the latter and was not present in our cases [20]. In short, although mitochondrial disorders may be reasonably placed on the differential diagnosis, the findings in NGLY1 deficiency are not particularly suggestive of this category of disease.
Recently, disorders associated with components of the ERAD pathway involving the Golgi apparatus, ER lipid raft-associated protein 1/2 (erlin1/2) complex, and E3-ubiquitin ligase, have been identified. These conditions serve as interesting comparisons to deficiency of the cytosolic NGLY1. Mutations in MAN1B1, the gene coding for α-1,2-mannosidase, are associated with non-syndromic autosomal recessive intellectual disability and subtle dysmorphic features [21]. α-1,2-Mannosidase is a type II transmembrane protein that is primarily localized to the Golgi apparatus, where it undergoes O-glycosylation and participates in glycoprotein quality control [22,23]. Profound intellectual disability, developmental regression and multiple contractures are features associated with autosomal recessive mutations in ERLIN2. In this case, abnormal erlin2 causes impaired ERAD of activated inositol 1,4,5-triphosphate receptors (IP3) and other substrates by compromising the structure of the erlin1/2 complex [24,25]. Despite the severity of the intellectual disability and neuromuscular findings, the results of brain imaging, electromyography and muscle biopsy appeared normal in the initial erlin2-deficient patients [24]. Another family was found to have a homozygous null mutation in ERLIN2, with affected individuals presenting with a hereditary spastic paraplegia phenotype [26].
IP3 receptors form calcium channels in ER membranes and play an important role in mammalian cell signaling. Ultimately, IP3 receptors undergo rapid degradation via the ubiquitin-proteasome pathway in response to cell stimulation. Evidence suggests that RNF170, an E3-ubiquitin ligase, mediates ubiquitination and processing of the IP3 receptor via interaction involving the erlin1/2 complex [25,27]. Mutations in RNF170 cause a late-onset autosomal dominant sensory ataxia characterized by distal sensory loss, diminished to absent reflexes, wide-based gait, and normal brain imaging [28–30]. Abnormal E3-ubiquitin ligase activity has also been postulated to play a role in the pathogenesis of an autosomal dominant form of Charcot-Marie-Tooth disease [31]. In short, different steps of the quality control system for targeting and degrading misfolded proteins have been implicated in a number of neurological conditions with varying severity and either autosomal recessive or dominant inheritance.
NGLY1 is a cytoplasmic enzyme that participates in the proteasomal degradation of aberrant glycoproteins that are synthesized in the ER and subsequently translocated to the cytoplasm [1,10,32,33]. By performing the initial cleavage of bulky N-glycan chains on misfolded glycoproteins, NGLY1 makes further proteolysis possible; glycoproteins thus trimmed can enter the cylinder of the 20S proteasome to be acted upon by proteases [32]. Therefore, NGLY1 deficiency would be expected to result in accumulation of intact glycoproteins in the cytoplasm. The accumulation of an amorphous substance in the liver of three patients, as well as the vacuolization consistent with storage in two others, is supportive of this role of NGLY1 and may help explain the liver disease noted in our patients. The undefined stored substance likely represents accumulation of misfolded glycoproteins in the cytoplasm that have been retrotranslocated from the ER but cannot undergo further processing. Transferrin isoelectric focusing or mass spectrometry studies in NGLY1-deficient patients have been normal or only subtly abnormal. This is not particularly surprising, because these methods detect the absence or structural alterations of N-glycan chains [34], which would not be expected to accumulate in NGLY1 deficiency.
The presence of axonal loss and gliosis in the brains suggestive of HIE suggests that NGLY1 plays a role in maintaining central nervous system integrity. A study in Caenorhabditis elegans detected peripheral nervous system defects, including aberrant neuronal branching, in animals with loss-of-function mutations in png-1, the ortholog of NGLY1 [35]. Neuronal branching abnormalities possibly relate to the peripheral neuropathy that seems to be relatively common in NGLY1 deficiency, but further studies are needed to determine the underlying pathogenesis of both central and peripheral nervous system abnormalities found in our patients.
The nonsense mutation c.1201A>T (p.R401X) was the most commonly detected deleterious allele in NGLY1, present in homozygous state in five of eight cases (Patients 4 to 8) and in compound heterozygous state in one case (Patient 1). Clearly, homozygous mutations associated with p.R401X cause a severe phenotype. However, the range of outcomes is variable, with some patients having an early demise, and others living at least into their teenage years. Patients 2 and 3 appear to have a relatively mild phenotype and are the only two individuals in the current cohort who do not carry the c.1201A>T (p.R401X) mutation. Aside from these broad considerations, more detailed genotype-phenotype correlations will be possible only with the detection of further patients. Consistent with the severe presentation of NGLY1 deficiency, NGLY1 is amongst the 20th percentile of genes in the human genome that are the most intolerant of functional genetic variation in the human population [36], and we may expect that less extreme mutations than those seen here may also be associated with disease.
In summary, NGLY1 deficiency is characterized by a constellation of unique features that should enable clinical recognition of this condition. The more salient features are elevation of AFP and liver enzymes in infancy with relative normalization in early childhood, accumulation of a substance with staining properties similar to glycogen (presumably misfolded glycoproteins) in hepatocyte cytoplasm, absent tears resulting in blepharitis and corneal ulceration, a movement disorder and peripheral neuropathy. However, the transient nature of the AFP and liver transaminase elevation may make older or more mildly affected individuals difficult to detect; therefore, affected older children and adults may remain undiagnosed. However, the widespread clinical availability of WES and whole genome sequencing will likely result in further cases being detected, including those with a more mild phenotype.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the generosity of the parents of all patients in providing samples and clinical data for this study. The Bertrand Might Research Fund (HHF) provided support for the study of subjects 1 and 2. Michael and Ellen Michelson, Tom and Diane Might, and Bobbie and Mike Wilsey provided additional support for this research. Christian Schaaf is a recipient of a Clinical Scientist Development Award by the Doris Duke Charitable Foundation. His work is generously supported by the Joan and Stanford Alexander Family. The sequencing work for subjects 5 and 6 was carried out through a research project (FORGE Canada) funded by the Government of Canada through Genome Canada, the Canadian Institutes of Health Research (CIHR) and the Ontario Genomics Institute. The FORGE Canada Steering Committee includes Kym Boycott (University of Ottawa), Jan Friedman (University of British Columbia, Jacques Michaud (Université de Montréal), Francois Bernier (University of Calgary), Michael Brudno (University of Toronto), Bridget Fernandez (Memorial University) Bartha Knoppers (McGill University), Mark Samuels (Université de Montréal), and Steve Scherer (University of Toronto).
Footnotes
Full list of steering committee members is provided in the acknowledgments
Conflicts of Interest
The authors declare no conflict of interest.
REFERENCES
- 1.Takahashi N. Demonstration of a new amidase acting on glycopeptides. Biochem Biophys Res Commun. 1977;76:1194–1201. doi: 10.1016/0006-291x(77)90982-2. [DOI] [PubMed] [Google Scholar]
- 2.Plummer TH, Jr, Elder JH, Alexander S, Phelan AW, Tarentino AL. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984;259:10700–10704. [PubMed] [Google Scholar]
- 3.Suzuki T, Park H, Hollingsworth NM, Sternglanz R, Lennarz WJ. PNG1, a yeast gene encoding a highly conserved peptide:N-glycanase. J Cell Biol. 2000;149:1039–1052. doi: 10.1083/jcb.149.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xin F, Wang S, Song L, Liang Q, Qi Q. Molecular identification and characterization of peptide: N-glycanase from Schizosaccharomyces pombe. Biochem Biophys Res Commun. 2008;368:907–912. doi: 10.1016/j.bbrc.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Masahara-Negishi Y, Hosomi A, Della Mea M, Serafini-Fracassini D, Suzuki T. A plant peptide: N-glycanase orthologue facilitates glycoprotein ER-associated degradation in yeast. Biochim Biophys Acta. 2012;1820:1457–1462. doi: 10.1016/j.bbagen.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Gosain A, Lohia R, Shrivastava A, Saran S. Identification and characterization of peptide: N-glycanase from Dictyostelium discoideum. BMC Biochem. 2012;13:9. doi: 10.1186/1471-2091-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seko A, Kitajima K, Inoue Y, Inoue S. Peptide:N-glycosidase activity found in the early embryos of Oryzias latipes (Medaka fish). The first demonstration of the occurrence of peptide:N-glycosidase in animal cells and its implication for the presence of a de-N-glycosylation system in living organisms. J Biol Chem. 1991;266:22110–22114. [PubMed] [Google Scholar]
- 8.Suzuki T, Seko A, Kitajima K, Inoue Y, Inoue S. Identification of peptide:N-glycanase activity in mammalian-derived cultured cells. Biochem Biophys Res Commun. 1993;194:1124–1130. doi: 10.1006/bbrc.1993.1938. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, Seko A, Kitajima K, Inoue Y, Inoue S. Purification and enzymatic properties of peptide:N-glycanase from C3H mouse-derived L-929 fibroblast cells. Possible widespread occurrence of post-translational remodification of proteins by N-deglycosylation. J Biol Chem. 1994;269:17611–17618. [PubMed] [Google Scholar]
- 10.Park H, Suzuki T, Lennarz WJ. Identification of proteins that interact with mammalian peptide:N-glycanase and implicate this hydrolase in the proteasome-dependent pathway for protein degradation. Proc Natl Acad Sci U S A. 2001;98:11163–11168. doi: 10.1073/pnas.201393498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, et al. Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat Cell Biol. 2002;4:134–139. doi: 10.1038/ncb746. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Kamiya Y. Structural views of glycoprotein-fate determination in cells. Glycobiology. 2007;17:1031–1044. doi: 10.1093/glycob/cwm046. [DOI] [PubMed] [Google Scholar]
- 13.Kamiya Y, Satoh T, Kato K. Molecular and structural basis for N-glycan-dependent determination of glycoprotein fates in cells. Biochim Biophys Acta. 2012;1820:1327–1337. doi: 10.1016/j.bbagen.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Kamiya Y, Uekusa Y, Sumiyoshi A, Sasakawa H, Hirao T, et al. NMR characterization of the interaction between the PUB domain of peptide:N-glycanase and ubiquitin-like domain of HR23. FEBS Lett. 2012;586:1141–1146. doi: 10.1016/j.febslet.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Need AC, Shashi V, Hitomi Y, Schoch K, Shianna KV, et al. Clinical application of exome sequencing in undiagnosed genetic conditions. J Med Genet. 2012;49:353–361. doi: 10.1136/jmedgenet-2012-100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeze HH, Eklund EA, Ng BG, Patterson MC. Neurology of inherited glycosylation disorders. Lancet Neurol. 2012;11:453–466. doi: 10.1016/S1474-4422(12)70040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeze HH. Understanding human glycosylation disorders: biochemistry leads the charge. J Biol Chem. 2013;288:6936–6945. doi: 10.1074/jbc.R112.429274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 19.Gire C, Girard N, Nicaise C, Einaudi MA, Montfort MF, et al. Clinical features and neuroradiological findings of mitochondrial pathology in six neonates. Childs Nerv Syst. 2002;18:621–628. doi: 10.1007/s00381-002-0621-0. [DOI] [PubMed] [Google Scholar]
- 20.Brown GK, Squier MV. Neuropathology and pathogenesis of mitochondrial diseases. J Inherit Metab Dis. 1996;19:553–572. doi: 10.1007/BF01799116. [DOI] [PubMed] [Google Scholar]
- 21.Rafiq MA, Kuss AW, Puettmann L, Noor A, Ramiah A, et al. Mutations in the alpha 1,2-mannosidase gene, MAN1B1, cause autosomal-recessive intellectual disability. Am J Hum Genet. 2011;89:176–182. doi: 10.1016/j.ajhg.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan S, Wang S, Utama B, Huang L, Blok N, et al. Golgi localization of ERManI defines spatial separation of the mammalian glycoprotein quality control system. Mol Biol Cell. 2011;22:2810–2822. doi: 10.1091/mbc.E11-02-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan S, Cheng X, Sifers RN. Golgi-situated endoplasmic reticulum alpha-1, 2-mannosidase contributes to the retrieval of ERAD substrates through a direct interaction with gamma-COP. Mol Biol Cell. 2013;24:1111–1121. doi: 10.1091/mbc.E12-12-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yildirim Y, Orhan EK, Iseri SA, Serdaroglu-Oflazer P, Kara B, et al. A frameshift mutation of ERLIN2 in recessive intellectual disability, motor dysfunction and multiple joint contractures. Hum Mol Genet. 2011;20:1886–1892. doi: 10.1093/hmg/ddr070. [DOI] [PubMed] [Google Scholar]
- 25.Pearce MM, Wormer DB, Wilkens S, Wojcikiewicz RJ. An endoplasmic reticulum (ER) membrane complex composed of SPFH1 and SPFH2 mediates the ER-associated degradation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2009;284:10433–10445. doi: 10.1074/jbc.M809801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alazami AM, Adly N, Al Dhalaan H, Alkuraya FS. A nullimorphic ERLIN2 mutation defines a complicated hereditary spastic paraplegia locus (SPG18) Neurogenetics. 2011;12:333–336. doi: 10.1007/s10048-011-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu JP, Wang Y, Sliter DA, Pearce MM, Wojcikiewicz RJ. RNF170 protein, an endoplasmic reticulum membrane ubiquitin ligase, mediates inositol 1,4,5-trisphosphate receptor ubiquitination and degradation. J Biol Chem. 2011;286:24426–24433. doi: 10.1074/jbc.M111.251983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdmanis PN, Simoes Lopes AA, Gros-Louis F, Stewart JD, Rouleau GA, et al. A novel neurodegenerative disease characterised by posterior column ataxia and pyramidal tract involvement maps to chromosome 8p12-8q12.1. J Med Genet. 2004;41:634–639. doi: 10.1136/jmg.2004.019711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valdmanis PN, Brunet D, St-Onge J, Weston L, Rouleau GA, et al. A founder haplotype for autosomal dominant sensory ataxia in Eastern Canada. Neurology. 2006;67:2239–2242. doi: 10.1212/01.wnl.0000249314.96183.48. [DOI] [PubMed] [Google Scholar]
- 30.Valdmanis PN, Dupre N, Lachance M, Stochmanski SJ, Belzil VV, et al. A mutation in the RNF170 gene causes autosomal dominant sensory ataxia. Brain. 2011;134:602–607. doi: 10.1093/brain/awq329. [DOI] [PubMed] [Google Scholar]
- 31.Saifi GM, Szigeti K, Wiszniewski W, Shy ME, Krajewski K, et al. SIMPLE mutations in Charcot-Marie-Tooth disease and the potential role of its protein product in protein degradation. Hum Mutat. 2005;25:372–383. doi: 10.1002/humu.20153. [DOI] [PubMed] [Google Scholar]
- 32.Tanabe K, Lennarz WJ, Suzuki T. A cytoplasmic peptide: N-glycanase. Methods Enzymol. 2006;415:46–55. doi: 10.1016/S0076-6879(06)15004-1. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Park H, Lennarz WJ. Cytoplasmic peptide:N-glycanase (PNGase) in eukaryotic cells: occurrence, primary structure, and potential functions. FASEB J. 2002;16:635–641. doi: 10.1096/fj.01-0889rev. [DOI] [PubMed] [Google Scholar]
- 34.Freeze HH, Aebi M. Altered glycan structures: the molecular basis of congenital disorders of glycosylation. Curr Opin Struct Biol. 2005;15:490–498. doi: 10.1016/j.sbi.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Habibi-Babadi N, Su A, de Carvalho CE, Colavita A. The N-glycanase png-1 acts to limit axon branching during organ formation in Caenorhabditis elegans. J Neurosci. 2010;30:1766–1776. doi: 10.1523/JNEUROSCI.4962-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrovski S, Wang Q, Heinzen EL, Allen AS, Goldstein DB. Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet. 2013;9:e1003709. doi: 10.1371/journal.pgen.1003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.