Abstract
The synthesis of fluorine-18 labeled 3-fluoro-5-[(pyridin-3-yl)ethynyl] benzonitrile ([18F]FPEB) for imaging metabotropic glutamate receptor subtype type 5 (mGluR5) was achieved with a commercial continuous-flow microfluidics device. This work represents the first positron emission tomography (PET) radiopharmaceutical that is suitable for human use with this technology. We also describe a validated synthesis of [18F]FPEB with a commercial reactor-based system.
Continuous-flow microfluidics (a synergy between microfluidics and continuous flow chemistry) has exciting advantages over traditional radiochemical synthesis.1–4 Such microfluidic technologies can offer low sample and reagent consumption, faster kinetics, high throughput screening of reaction conditions, high reproducibility, enhanced product yields and facile automation. Implementation of such a method has significantly accelerated the process of synthesizing labeled compounds and positron emission tomography (PET) radiopharmaceuticals.1 To date, over fifty radiolabeled compounds have been synthesized in continuous-flow microfluidic devices1 for PET applications including [18F]SFB, [18F]FMISO, [124I]doxorubicin, [64Cu]DOTA-cyclo(RGDfK) and [68Ga]NOTA/DOTA-cyclo(RGDfK). Our group has recently demonstrated “microfluidic flow hydrogenation” i.e., 18F-labeling and subsequent hydrogenation to efficiently synthesize 18F-labeled compounds by integrating two commercially available continuous-flow microfluidic systems.5
The first reported syntheses PET radiotracers in a microfluidic device were published nearly a decade ago and demonstrated that this technology is capable of providing multi-doses of fluorine-18 (18F; t½ = 109.7 min) labeled compounds.6,7 A recent report described a prototypic batch-reactor microfluidic device to prepare [18F]fallypride for application in a human PET study.8 Despite extensive preclinical imaging with radiotracers prepared by continuous-flow microfluidics, there is still no published PET radiopharmaceutical prepared for human use by flow chemistry.
The goal of the present work was to synthesize 18F-labeled 3-fluoro-5-[(pyridin-3-yl)ethynyl] benzonitrile ([18F]FPEB; 2, Scheme 1) suitable for clinical studies by the most commonly used microfluidic flow platform in radiochemistry (NanoTek®; Advion, Inc.). Fluorine-18 labeled FPEB is a metabotropic glutamate receptor subtype 5 (mGluR5) antagonist with high potency, selectivity and brain penetration in rodents, nonhuman primates and human subjects.9–13 Our preliminary PET imaging studies with this radiopharmaceutical, as prepared using a commercial automated radiosynthesis unit (GE Tracerlab FXFN; see ESI), have focused on well characterized patients suffering from Alzheimer’s disease (AD). Several lines of evidence point to a critical role for mGluR5 in the pathologic process of AD. Oligomeric Aβ reliably induces abnormal accumulation and over-stabilization of mGluR5 receptors, which is up-regulated in AD, and co-localizes with Aβ in AD.14 Figure 1C shows [18F]FPEB binding observed in default network cortices and in medial temporal cortex and hippocampus, in a subject with early mild cognitive impairment.
Fig. 1.
Preliminary PET data from a 64 year-old subject with evidence of mild memory impairment: (A) [11C]PiB, (B) [18F]FDG, (C) [18F]FPEB and (D) MRI showing hyperactivity in the hippocampus.15
While several precursors have been developed for the radiosynthesis of [18F]FPEB,9a,10a,12,16–18 our laboratory has validated the production of this radiotracer using a commercially available precursor, 3-nitro-5-[(pyridin-3-yl)ethynyl] benzonitrile (1; ABX GmbH), and optimized its production (see ESI). Radiochemical yields for this reaction are low (1–5% uncorrected, relative to starting 18F-fluoride) as this nucleophilic substitution reaction at the meta-position occurs on a non-activated aromatic ring. It is noteworthy that improved conversions have been noted with diaryliodonium precursors in a continuous flow microfluidic reactor.19 In the present work, we utilized a NanoTek® system to perform 18F-labeling of [18F]FPEB. For the proof of concept this system was integrated to a GE Tracerlab FXFN unit to carry out semi-preparative HPLC purification and formulation.
A schematic of the radiosynthesis of [18F]FPEB using the Nanotek® is shown in Figure 2, A–F. Fluorine-18 labeled fluoride was produced by the 18O(p,n)18F nuclear reaction using a cyclotron (GE PETtrace, 16.5 MeV) and delivered to the microfluidic module. As previously described,5 the [18F]fluoride was trapped on an ion exchange resin, eluted using a solution of tetra-n-butylammonium bicarbonate (nBu4NHCO3, 0.075M, 0.6 mL) and delivered to the [18F]fluoride drying concentrator (A). The reaction mixture was dried azeotropically three times by addition of anhydrous CH3CN under N2 flow and vacuum over 2 min. The precursor 1 and dried [18F]nBu4NF (re-solubilized in DMSO) were loaded into storage loop (C) and dispensed into the microfluidic flow reactor (D) at various temperatures and flow rates. The ensuing reaction mixture was transferred into dilution vial (E, pre-loaded with 25 mL of H2O) and mixed under a stream of nitrogen. The diluted crude mixture was pre-purified on a solid phase extraction cartridge (Waters Oasis HLB Light; F) to remove DMSO and subsequently transferred to a GE TracerLab FXFN for HPLC purification and formulation (see Fig 2 and ESI).
Fig. 2.
Schematics of microfluidic flow platform and parameters for the radiosynthesis of [18F]FPEB: (A) [18F]fluoride drying concentrator, (B) high resolution syringe pump, (C) storage loop (polyether ether ketone), (D) microfluidic flow reactor, (E) dilution vial, (F) solid phase exchange cartridge. Specifications of the microfluidic flow reactor : volume 8–64 µL, reaction path from 1–8 meter, I.D. 100 µm, temperature range from 25 °C to 220 °C, pressure up to 3.45MPa (500 psi).
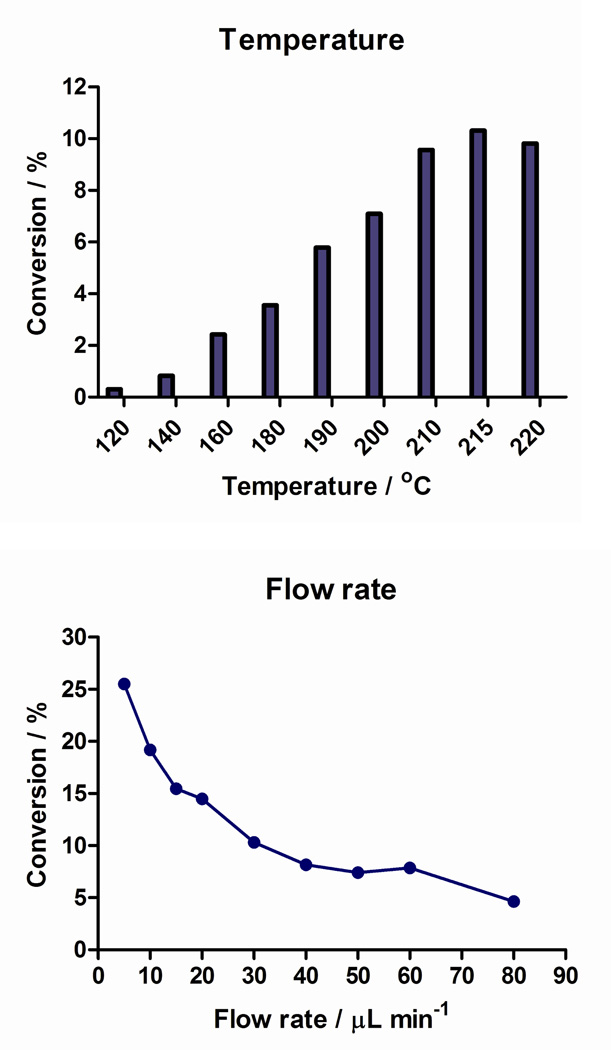
The microfluidic flow platform enabled rapid optimization of radiolabeling conditions by dispensing a small aliquot of reagent and isotope and performing reaction in a continuous-flow microfluidic reactor. Reaction parameters including temperatures, flow rates and precursor/18F isotope ratios were varied via two syringe pumps (Figure 2,B). Flow reactions occurred inside coiled silica glass capillary tube (16 µL internal volume; 2 meter length) encased in a brass housing (Figure 2,D). Fluorine-18 incorporation in DMSO gradually increased from 120 °C and reached the maximum radiochemical conversion (10%) at 215 °C (Figure 3A). As shown in Figure 3B, flow rate also played an important role in the production of [18F]FPEB. The conversion increased from 5% to 26% when the flow rate of the reaction was decreased from 80 µL/min to 5 µL/min. Although the slowest flow rate gave the highest conversion, considering that the volume of our starting 18F-fluoride stock solution is 250 µL, we determined that 10 µL/min (per syringe pump) was the most practical flow rate for this reaction (total flow rate is 20 µL/min) and offers a suitable compromise of radioactive decay relative to reaction time (50 min vs. 25 min). In our initial screening, we also determined that the optimal ratio of flow rates (precursor to 18F isotope) was 1:1.
Fig. 3.
Temperature (A) and flow rate (B) effect of [18F]FPEB synthesis. (a) Conditions: [18F]fluoride was trapped on an anion exchange resin (MP1, ORTG, Inc., USA), eluted with nBu4NHCO3 (0.075M aqueous solution) and azeotropically dried using standard NanoTek drying macro sequence (see ESI). The temperature effect was screened at a total flow rate of 60 µL/ min and the flow rate was evaluated at 210°C. (b) Radiochemical conversion of [18F]FPEB (n = 1 per data point) was determined with radio-HPLC (tR (FPEB) = 7.0 min, tR (impurity) = 6.2 min, mobile phase: 60/40 CH3CN/H2O + 0.1N ammonium formate; Luna 5µ C18 100 Å, λ = 254 nm, 250 × 4.6 mm; flow rate: 1 mL/min).
Our radiochemistry optimization was achieved in one day using one batch of [18F]fluoride (ca. 20 mCi, 740 MBq) and a small amount (2 mg in 200 µL of DMSO) of 1 (Fig. 3).
Following the optimization process of [18F]FPEB, the reaction was seamlessly extrapolated to a production level. In order to scale up the reaction, we doubled the flow rate as well as the reactor length and internal volume. The system was set up with a 4 meter reactor (32 µL internal volume) and the radiosynthesis was carried out at 215 ± 5 °C with a flow rate of 20 µL/min per syringe pump (total flow rate is 40 µL/min). To our knowledge, we tested the maximum amount of radioactivity (> 4.5 Ci or 166 GBq of starting 18F), representing the highest radioactivity concentration (>10 Ci/mL) on a continuous-flow microfluidic system, and no radiolysis was observed by HPLC.
Three consecutive productions of [18F]FPEB were carried out to validate this radiopharmaceutical for human use (Table 1). Uncorrected radiochemical yields of [18F]FPEB (2.1 ± 0.4% relative to starting [18F]fluoride) by this method are consistent with those achieved previously,9a,10a and high specific activities were obtained in the final formulation (4.4 Ci/µmol) within 75 minutes. HPLC analysis of the formulated [18F]FPEB product in 10% EtOH in 0.9% sodium chloride revealed high radiochemical purity (>95%). The formulated [18F]FPEB PET radiopharmaceutical maintained was found to be stable over a period of 6 h. No long-lived isotopes were observed in the final product, as determined by the long lived impurity analysis on a HPGE detector after 18F-decay. The formulated product had no detectable endotoxin and was sterile. Volatile organic compound analysis was carried out by GC-FID showing residual acetone, CH3CN, and DMSO below the lower limit of detection, thereby exceeding the International Conference on Harmonisation of Technical Requirement of Pharmaceuticals for Human Use requirements.
Table 1.
aSummary of [18F]FPEB specifications by continuous-flow microfluidics
| Parameters | Results (n = 3) |
|---|---|
| Synthesis time | 75 min (ready for injection) |
| Isolated product | 45 ± 10 mCi at end of synthesis |
| Visual inspection | Clear, absence of particulates |
| Radiochemical identity | ±10% of FPEB reference standard retention time |
| Radiochemical purity | ≥ 95% |
| Specific activity | 4.4 ± 0.3 Ci/µmol |
| Residual solvent analysis | DMSO < 5 mg/mL |
| Acetone < 5 mg/mL | |
| Acetonitrile < 0.4 mg/mL | |
| Ethanol < 10% v/v ±10% | |
| pH Assay | 5–5.5 |
| Sterile filter integrity test | ≥ 50 psi |
| Radionuclidic ID:photopeak | ≥ 99.5% emission @511 KeV, 1.022MeV, or Compton scatter peaks |
| Radionuclidic ID:Half-life | 105 – 115 minutes |
| Endotoxin analysis | Not greater than 17.5 EU per injected dose |
| Sterility testing | No evidence of growth at 14 days post inoculation |
Quality control was performed on an aliquot drawn from the product vial, and carried out according to an approved protocol at MGH; see ESI;
The radiochemical identity and purity were determined with radio-HPLC (tR = 4.97 min, mobile phase: 45:55 Ethanol/H2O; Waters Nova-Pak C18 4µm, λ = 254 nm, 150 × 4.6 mm; flow rate: 1 mL/min.).
At present, our clinical research studies are routinely carried with the optimized and validated GE Tracerlab FXFN conditions (see ESI) as higher radiochemical yields (7.0 ± 2.1%; uncorrected and relative to [18F]fluoride) and specific activities (7.07 ± 1.38 Ci/µmol) are achieved. The proof of concept microfluidic methodology demonstrates that [18F]FPEB can be validated for human use under an approved U.S. Food and Drug Administration (FDA) Investigational New Drug (IND) application, or equivalent regulatory submission, and offers an alternative technology to prepare PET radiopharmaceuticals.
Conclusions
The synthesis of the mGluR5 radiopharmaceutical [18F]FPEB was validated for routine human use with a commercial reactor-based platform. This synthesis was also achieved using a commercial continuous-flow microfluidic system. This proof of concept work demonstrates that microfluidics flow chemistry systems are capable of synthesizing PET radiopharmaceuticals that are suitable for human use.
Supplementary Material
Acknowledgement
We thank the Alzheimer’s Drug Discovery Foundation and Advion, Inc for generously providing funding and/or equipment for this research. We thank Kelvin Hammond for helpful discussions and David F. Lee Jr. and Brian Bradshaw for isotope production and support. We thank Hogger & Co. for graphical abstract design.
Footnotes
Electronic Supplementary Information (ESI) available: Automated synthesis of [18F]FPEB using GE Tracerlab FXFN; QC procedure; Nanotek plumbing diagram, production log, macro sequence and ICP-MS.
Notes and references
- 1.Pascali G, Watts P, Salvadori PA. Nucl Med Biol. 2013;40:776–787. doi: 10.1016/j.nucmedbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Miller PW. J Chem Technol Biotechnol. 2009;84:309–315. [Google Scholar]
- 3.Elizarov AM. Lab. Chip. 2009;9:1326–1333. doi: 10.1039/b820299k. [DOI] [PubMed] [Google Scholar]
- 4.Lu SY, Pike VW. PET Chemistry. Berlin: Springer; 2007. Micro-reactors for PET tracer labeling; pp. 271–287. [DOI] [PubMed] [Google Scholar]
- 5.Liang SH, Collier TL, Rotstein BH, Lewis R, Steck M, Vasdev N. Chem Commun. 2013;49:8755–8757. doi: 10.1039/c3cc45166f. [DOI] [PubMed] [Google Scholar]
- 6.Lee C-C, Sui G, Elizarov A, Shu CJ, Shin Y-S, Dooley AN, Huang J, Daridon A, Wyatt P, Stout D, Kolb HC, Witte ON, Satyamurthy N, Heath JR, Phelps ME, Quake SR, Tseng H-R. Science. 2005;310:1793–1796. doi: 10.1126/science.1118919. [DOI] [PubMed] [Google Scholar]
- 7.Lu SY, Watts P, Chin FT, Hong J, Musachio JL, Briard E, Pike VW, Lab Chip VW. 2004;4:523–525. doi: 10.1039/b407938h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebedev A, Miraghaie R, Kotta K, Ball CE, Zhang J, Buchsbaum MS, Kolb HC, Elizarov A. Lab Chip. 2013;13:136–145. doi: 10.1039/c2lc40853h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Hamill TG, Krause S, Ryan C, Bonnefous C, Govek S, Seiders TJ, Cosford ND, Roppe J, Kamenecka T, Patel S, Gibson RE, Sanabria S, Riffel K, Eng W, King C, Yang X, Green MD, O'Malley SS, Hargreaves R, Burns HD. Synapse. 2005;56:205–216. doi: 10.1002/syn.20147. [DOI] [PubMed] [Google Scholar]; (b) Patel S, Ndubizu O, Hamill T, Chaudhary A, Burns HD, Hargreaves R, Gibson R. Mol Imaging Biol. 2005;7:314–323. doi: 10.1007/s11307-005-0005-4. [DOI] [PubMed] [Google Scholar]
- 10.(a) Wang J-Q, Tueckmantel W, Zhu A, Pellegrino D, Brownell AL. Synapse. 2007;61:951–961. doi: 10.1002/syn.20445. [DOI] [PubMed] [Google Scholar]; (b) Pellegrino D, Cicchetti F, Wang X, Zhu A, Yu M, Saint-Pierre M, Brownell A-L. J Nucl Med. 2007;48:1147–1153. doi: 10.2967/jnumed.106.037796. [DOI] [PubMed] [Google Scholar]; (c) Wang J-Q, Brownell A-L. Curr Med Imag Rev. 2007;3:186–205. [Google Scholar]; (d) Zhu A, Wang X, Yu M, Wang J-Q, Brownell A-L. J Cereb Blood Flow Metab. 2007;27:1623–1631. doi: 10.1038/sj.jcbfm.9600461. [DOI] [PubMed] [Google Scholar]; (e) Sanchez-Pernaute R, Wang JQ, Kuruppu D, Cao L, Tueckmantel W, Kozikowski A, Isacson O, Brownell AL. NeuroImage. 2008;42:248–251. doi: 10.1016/j.neuroimage.2008.04.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alagille D, DaCosta H, Chen Y, Hemstapat K, Rodriguez A, Baldwin RM, Conn JP, Tamagnan GD. Bioorg Med Chem Lett. 2011;21:3243–3247. doi: 10.1016/j.bmcl.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong DF, Waterhouse R, Kuwabara H, Kim J, Brašić JR, Chamroonrat W, Stabins M, Holt DP, Dannals RF, Hamill TG, Mozley PD. J. Nucl Med. 2013;54:388–396. doi: 10.2967/jnumed.112.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan JM, Lim K, Labaree D, Lin S-f, McCarthy TJ, Seibyl JP, Tamagnan G, Huang Y, Carson RE, Ding Y-S, Morris ED. J Cereb Blood Flow Metab. 2013;33:532–541. doi: 10.1038/jcbfm.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Oka A, Takashima S. Acta Neuropathol. 1999;97:275–278. doi: 10.1007/s004010050985. [DOI] [PubMed] [Google Scholar]; (b) Casley CS, Lakics V, Lee HG, Broad LM, Day TA, Cluett T, Smith MA, O'Neill MJ, Kingston AE. Brain Res. 2009;17:65–75. doi: 10.1016/j.brainres.2008.12.082. [DOI] [PubMed] [Google Scholar]
- 15.The human imaging investigations were performed as part of Partners Human Research Committee and the New England Institutional Review Board (IRB) approved research protocols, for MGH and MNI respectively. Informed consent was obtained from all subjects
- 16.Lim K, Huang Y. J Labelled Compd Radiopharm. 2013;56:S150. [Google Scholar]
- 17.Moore TM, Akula MR, Collier TL, Tamagnan G, Papin C, Alagille D, Kabalka GW. J Labelled Compd Radiopharm. 2013;56:S130. [Google Scholar]
- 18.Cleij M, Fortt R, Gee A. J Labelled Compd Radiopharm. 2013;56:S450. [Google Scholar]
- 19.Telu S, Chun JH, Siméon FG, Lu S, Pike VW. Org Biomol Chem. 2011;9:6629–6638. doi: 10.1039/c1ob05555k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.