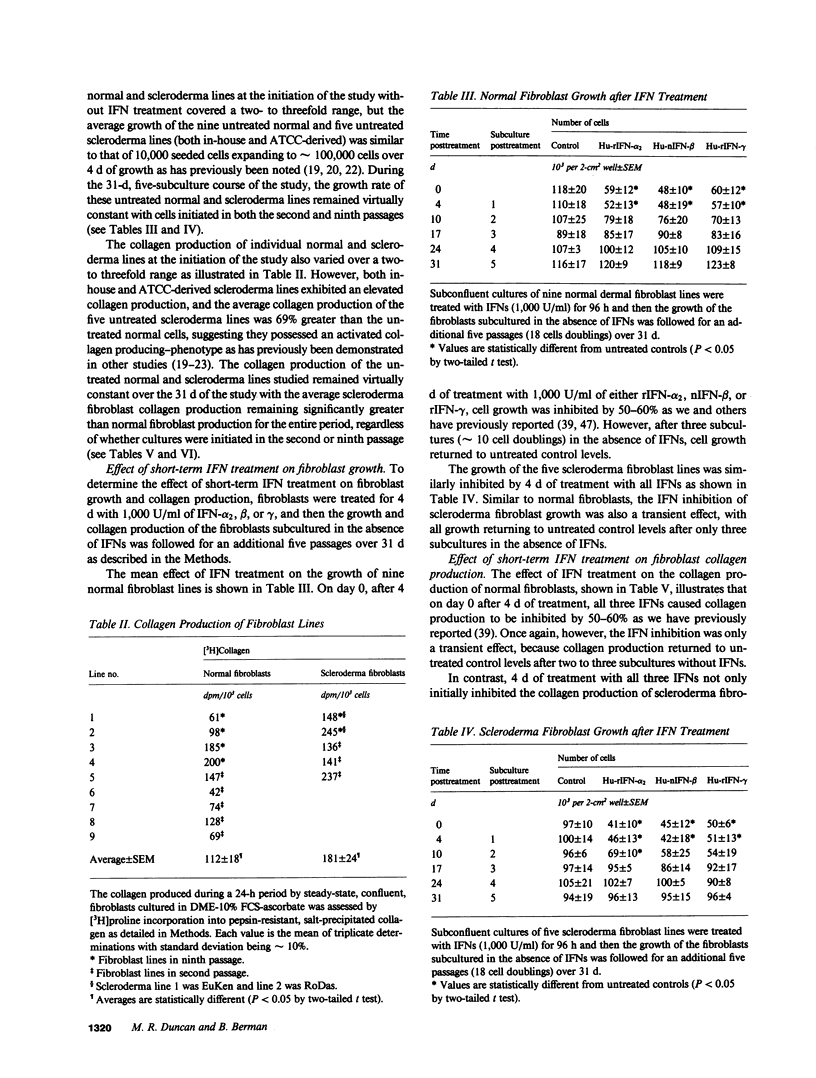
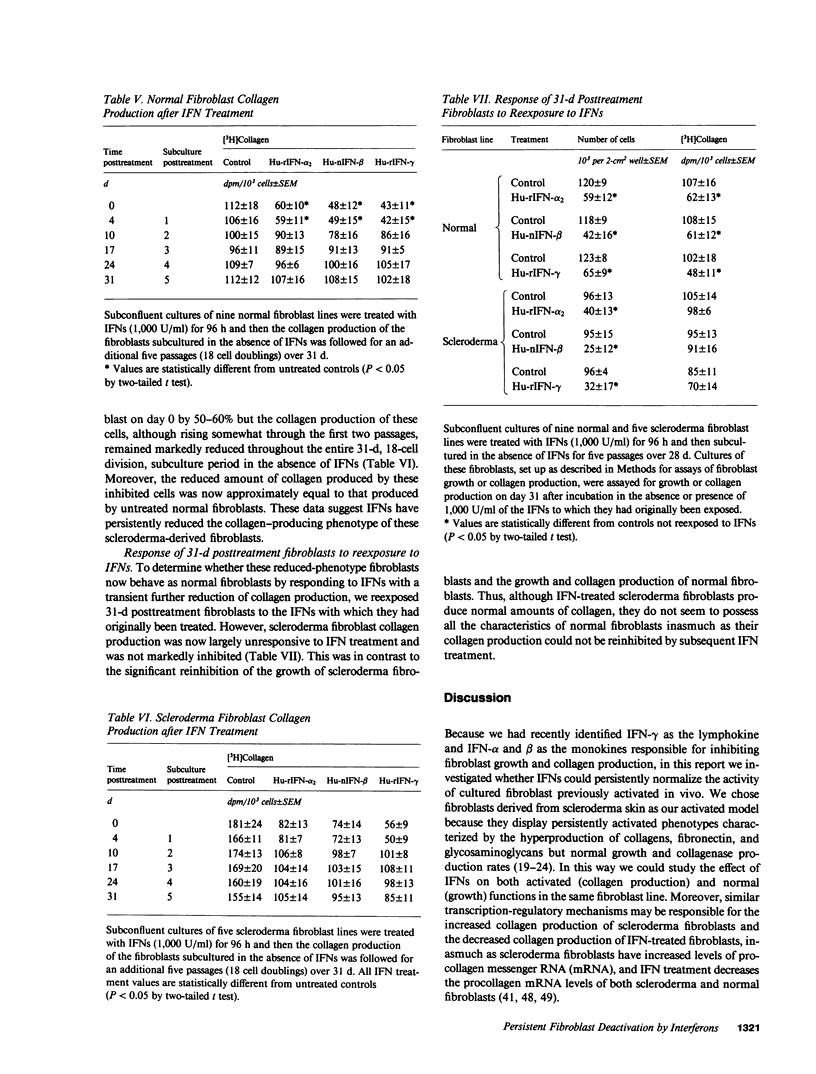
Abstract
Transient exposure to inflammation-associated, fibroblast-stimulatory factors appears to initiate fibrosis by inducing the persistently activated phenotypes displayed by fibroblast cultures derived from scleroderma skin and other fibrotic tissues. To determine whether one class of fibroblast-inhibitory factors, the interferons (IFNs), plays a role in terminating fibrosis by acting as persistent fibroblast deactivators, we inhibited (40-60%) the growth and collagen production of normal dermal fibroblasts and hypercollagen-producing scleroderma fibroblasts by short-term exposure to IFN-alpha, beta, or gamma. During subsequent subculture in the absence of IFNs, the growth and collagen production of normal fibroblasts and the growth of scleroderma fibroblasts increased to untreated control levels after two to three passages. In contrast, collagen production by scleroderma fibroblasts remained inhibited for at least five passages (18 cell doublings) and was not further suppressed by subsequent IFN exposure. These data suggest that IFNs may help terminate fibrosis by suppressing persistently activated fibroblast functions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abergel R. P., Pizzurro D., Meeker C. A., Lask G., Matsuoka L. Y., Minor R. R., Chu M. L., Uitto J. Biochemical composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. J Invest Dermatol. 1985 May;84(5):384–390. doi: 10.1111/1523-1747.ep12265471. [DOI] [PubMed] [Google Scholar]
- Amento E. P., Bhan A. K., McCullagh K. G., Krane S. M. Influences of gamma interferon on synovial fibroblast-like cells. Ia induction and inhibition of collagen synthesis. J Clin Invest. 1985 Aug;76(2):837–848. doi: 10.1172/JCI112041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashey R. I., Jimenez S. A. Increased sensitivity of scleroderma fibroblasts in culture to stimulation of protein and collagen synthesis by serum. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1214–1222. doi: 10.1016/0006-291x(77)90985-8. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Wewers M. D., Rennard S. I., Adelberg S., Crystal R. G. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986 Mar;77(3):700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. A., Polak K. L., Uitto J. Collagen biosynthesis by human skin fibroblasts. I. Optimization of the culture conditions for synthesis of type I and type III procollagens. Biochim Biophys Acta. 1980 Mar 28;607(1):145–160. doi: 10.1016/0005-2787(80)90228-2. [DOI] [PubMed] [Google Scholar]
- Buckingham R. B., Prince R. K., Rodnan G. P. Progressive systemic sclerosis (PSS, scleroderma) dermal fibroblasts synthesize increased amounts of glycosaminoglycan. J Lab Clin Med. 1983 May;101(5):659–669. [PubMed] [Google Scholar]
- Buckingham R. B., Prince R. K., Rodnan G. P., Taylor F. Increased collagen accumulation in dermal fibroblast cultures from patients with progressive systemic sclerosis (scleroderma). J Lab Clin Med. 1978 Jul;92(1):5–21. [PubMed] [Google Scholar]
- Castor C. W., Dorstewitz E. L., Rowe K., Ritchie J. C. Abnormalities of connective tissue cells cultured from patients with rheumatoid arthritis. II. Defective regulation of hyaluronate and collagen formation. J Lab Clin Med. 1971 Jan;77(1):65–75. [PubMed] [Google Scholar]
- Castor C. W., Miller J. W., Walz D. A. Structural and biological characteristics of connective tissue activating peptide (CTAP-III), a major human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1983 Feb;80(3):765–769. doi: 10.1073/pnas.80.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart M. K., Krakauer R. S. Immunologic enhancement of collagen accumulation in progressive systemic sclerosis (PSS). Clin Immunol Immunopathol. 1981 Oct;21(1):128–133. doi: 10.1016/0090-1229(81)90201-4. [DOI] [PubMed] [Google Scholar]
- Clark R. A. Cutaneous tissue repair: basic biologic considerations. I. J Am Acad Dermatol. 1985 Nov;13(5 Pt 1):701–725. doi: 10.1016/s0190-9622(85)70213-7. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Van Wyk J. J. Somatomedin-C and platelet-derived growth factor stimulate human fibroblast replication. J Cell Physiol. 1981 Mar;106(3):361–367. doi: 10.1002/jcp.1041060305. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'orco R. T. Maintenance of human diploid fibroblasts as arrested populations. Fed Proc. 1974 Aug;33(8):1969–1972. [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S. Platelet-derived growth factor. Structure, function, and roles in normal and transformed cells. J Clin Invest. 1984 Sep;74(3):669–676. doi: 10.1172/JCI111482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. R., Berman B. Gamma interferon is the lymphokine and beta interferon the monokine responsible for inhibition of fibroblast collagen production and late but not early fibroblast proliferation. J Exp Med. 1985 Aug 1;162(2):516–527. doi: 10.1084/jem.162.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. R., Perlish J. S., Fleischmajer R. Lymphokine/monokine inhibition of fibroblast proliferation and collagen production: role in progressive systemic sclerosis (PSS). J Invest Dermatol. 1984 Nov;83(5):377–384. doi: 10.1111/1523-1747.ep12264686. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Krieg T., Timpl R. Variability in collagen and fibronectin synthesis by scleroderma fibroblasts in primary culture. J Invest Dermatol. 1981 May;76(5):400–403. doi: 10.1111/1523-1747.ep12520933. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., West W. P. Ultrastructure of cutaneous cellular infiltrates in scleroderma. Arch Dermatol. 1977 Dec;113(12):1661–1666. [PubMed] [Google Scholar]
- Giri S. N., Hyde D. M., Marafino B. J., Jr Ameliorating effect of murine interferon gamma on bleomycin-induced lung collagen fibrosis in mice. Biochem Med Metab Biol. 1986 Oct;36(2):194–197. doi: 10.1016/0885-4505(86)90124-6. [DOI] [PubMed] [Google Scholar]
- Graves P. N., Weiss I. K., Perlish J. S., Fleischmajer R. Increased procollagen mRNA levels in scleroderma skin fibroblasts. J Invest Dermatol. 1983 Feb;80(2):130–132. doi: 10.1111/1523-1747.ep12532908. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Jimenez S. A., Freundlich B., Rosenbloom J. Selective inhibition of human diploid fibroblast collagen synthesis by interferons. J Clin Invest. 1984 Sep;74(3):1112–1116. doi: 10.1172/JCI111480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez S. A., McArthur W. M., Bashey R. I., Rosenbloom J. Selective inhibition of excessive scleroderma fibroblast collagen production by lymphokines from normal human mononuclear cells. Arthritis Rheum. 1985 May;28(5):502–510. doi: 10.1002/art.1780280506. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., McArthur W., Rosenbloom J. Inhibition of collagen synthesis by mononuclear cell supernates. J Exp Med. 1979 Dec 1;150(6):1421–1431. doi: 10.1084/jem.150.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahaleh M. B., Osborn I., Leroy E. C. Elevated levels of circulating platelet aggregates and beta-thromboglobulin in scleroderma. Ann Intern Med. 1982 May;96(5):610–613. doi: 10.7326/0003-4819-96-5-610. [DOI] [PubMed] [Google Scholar]
- Kenyon A. J. Delayed wound healing in mice associated with viral alteration of macrophages. Am J Vet Res. 1983 Apr;44(4):652–656. [PubMed] [Google Scholar]
- Korn J. H. Fibroblast prostaglandin E2 synthesis. Persistence of an abnormal phenotype after short-term exposure to mononuclear cell products. J Clin Invest. 1983 May;71(5):1240–1246. doi: 10.1172/JCI110873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn J. H., Halushka P. V., LeRoy E. C. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest. 1980 Feb;65(2):543–554. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotzer T. I., Clagett J. A., Kolb W. P., Page R. C. Complement-dependent induction of DNA synthesis and proliferation of human diploid fibroblasts. J Cell Physiol. 1980 Dec;105(3):503–512. doi: 10.1002/jcp.1041050314. [DOI] [PubMed] [Google Scholar]
- Krane S. M., Dayer J. M., Simon L. S., Byrne M. S. Mononuclear cell-conditioned medium containing mononuclear cell factor (MCF), homologous with interleukin 1, stimulates collagen and fibronectin synthesis by adherent rheumatoid synovial cells: effects of prostaglandin E2 and indomethacin. Coll Relat Res. 1985 Mar;5(2):99–117. doi: 10.1016/s0174-173x(85)80033-9. [DOI] [PubMed] [Google Scholar]
- LeRoy E. C. Increased collagen synthesis by scleroderma skin fibroblasts in vitro: a possible defect in the regulation or activation of the scleroderma fibroblast. J Clin Invest. 1974 Oct;54(4):880–889. doi: 10.1172/JCI107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornex J. F., Martinet Y., Yamauchi K., Bitterman P. B., Grotendorst G. R., Chytil-Weir A., Martin G. R., Crystal R. G. Spontaneous expression of the c-sis gene and release of a platelet-derived growth factorlike molecule by human alveolar macrophages. J Clin Invest. 1986 Jul;78(1):61–66. doi: 10.1172/JCI112574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers S. L., Castor C. W. Connective tissue activation. XV. Stimulation of glycosaminoglycan and DNA synthesis by a polymorphonuclear leukocyte factor. Arthritis Rheum. 1980 May;23(5):556–563. doi: 10.1002/art.1780230506. [DOI] [PubMed] [Google Scholar]
- Oka Y., Orth D. N. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J Clin Invest. 1983 Jul;72(1):249–259. doi: 10.1172/JCI110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer L. M., Murphy J. S., Tamm I. Interferon effects on the growth and division of human fibroblasts. Exp Cell Res. 1979 Jun;121(1):111–120. doi: 10.1016/0014-4827(79)90450-6. [DOI] [PubMed] [Google Scholar]
- Pohjanpelto P. Stimulation of DNA synthesis in human fibroblasts by thrombin. J Cell Physiol. 1978 May;95(2):189–194. doi: 10.1002/jcp.1040950208. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Smith G. N., Mainardi C. L., Seyer J. M., Kang A. H. Lymphocyte modulation of fibroblast function in vitro: stimulation and inhibition of collagen production by different effector molecules. J Immunol. 1984 May;132(5):2470–2477. [PubMed] [Google Scholar]
- Rosenbloom J., Feldman G., Freundlich B., Jimenez S. A. Inhibition of excessive scleroderma fibroblast collagen production by recombinant gamma-interferon. Association with a coordinate decrease in types I and III procollagen messenger RNA levels. Arthritis Rheum. 1986 Jul;29(7):851–856. doi: 10.1002/art.1780290706. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J., Feldman G., Freundlich B., Jimenez S. A. Transcriptional control of human diploid fibroblast collagen synthesis by gamma-interferon. Biochem Biophys Res Commun. 1984 Aug 30;123(1):365–372. doi: 10.1016/0006-291x(84)90422-4. [DOI] [PubMed] [Google Scholar]
- Roumm A. D., Whiteside T. L., Medsger T. A., Jr, Rodnan G. P. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984 Jun;27(6):645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Uitto J., Bauer E. A., Eisen A. Z. Scleroderma: increased biosynthesis of triple-helical type I and type III procollagens associated with unaltered expression of collagenase by skin fibroblasts in culture. J Clin Invest. 1979 Oct;64(4):921–930. doi: 10.1172/JCI109558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorio T. K., Kähäri V. M., Lehtonen A., Vuorio E. I. Fibroblast activation in scleroderma. Scand J Rheumatol. 1984;13(3):229–237. doi: 10.3109/03009748409100391. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Gately C. L. Modulation of fibroblast growth by a lymphokine of human T cell continuous T cell line origin. J Immunol. 1983 Mar;130(3):1226–1230. [PubMed] [Google Scholar]
- Wahl S. M., Malone D. G., Wilder R. L. Spontaneous production of fibroblast-activating factor(s) by synovial inflammatory cells. A potential mechanism for enhanced tissue destruction. J Exp Med. 1985 Jan 1;161(1):210–222. doi: 10.1084/jem.161.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster D. F., Harvey W. A quantitative assay for collagen synthesis in microwell fibroblast cultures. Anal Biochem. 1979 Jul 1;96(1):220–224. doi: 10.1016/0003-2697(79)90576-1. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Worrall J. G., Prince R. K., Buckingham R. B., Rodnan G. P. Soluble mediators from mononuclear cells increase the synthesis of glycosaminoglycan by dermal fibroblast cultures derived from normal subjects and progressive systemic sclerosis patients. Arthritis Rheum. 1985 Feb;28(2):188–197. doi: 10.1002/art.1780280214. [DOI] [PubMed] [Google Scholar]
- Wiestner M., Krieg T., Hörlein D., Glanville R. W., Fietzek P., Müller P. K. Inhibiting effect of procollagen peptides on collagen biosynthesis in fibroblast cultures. J Biol Chem. 1979 Aug 10;254(15):7016–7023. [PubMed] [Google Scholar]
- Worrall J. G., Whiteside T. L., Prince R. K., Buckingham R. B., Stachura I., Rodnan G. P. Persistence of scleroderma-like phenotype in normal fibroblasts after prolonged exposure to soluble mediators from mononuclear cells. Arthritis Rheum. 1986 Jan;29(1):54–64. doi: 10.1002/art.1780290108. [DOI] [PubMed] [Google Scholar]



