Abstract
Objectives
To assess and to compare the effects of Gluma® Desensitizer (GDL) with an experimental glutaraldehyde and HEMA containing fumed silica dispersion (GDG) on dentin permeability using a chemiluminous tracer penetration test.
Material and Methods
Twenty disc-shaped dentin specimens were dissected from extracted human third molars. The dentin specimens were mounted in a split chamber device for determination of permeability under liquid pressure using a photochemical method. Ten specimens were randomly selected and allocated to the evaluation groups Gluma® Desensitizer as aqueous solution and glutaraldehyde/HEMA as fumed silica dispersion, respectively. Dentin disc permeability was determined at two pressure levels after removal of smear with EDTA, after albumin soaking, and after application of the desensitizing agents. Two desensitizer-treated and rinsed specimens of each group were examined by scanning electron microscopy (SEM) for surface remnants.
Results
Comparatively large standard deviations of the mean EDTA reference and albumin soaked samples permeability values reflected the differences of the dentin substrates. The mean chemiluminescence values of specimen treated with GDL and GDG, respectively, were significantly reduced after topical application of the desensitizing agents on albumin-soaked dentin. The effects of GDL and GDG on permeability were not significantly different. Treated specimens showed no surface remnants after rinsing.
Conclusions
The experimental desensitizer gel formulation reduced dentin permeability as effectively as the original Gluma® Desensitizer solution.
Keywords: Denting desensitizing agents, Gluma Desensitizer, Glutaraldehyde, Dentinal fluid, Dentin permeability, Luminescence
INTRODUCTION
Gluma® Desensitizer (GDL - Heraeus Kulzer, Hanau, Germany) has been introduced to the dental market more than a decade ago. During this period the product has gained considerable market share and seems to be well accepted by dental practitioners.
Gluma® Desensitizer is a spin-off from the original Gluma Bonding system. Since the Gluma Primer contains glutaraldehyde (GA) it was reasonable to assume that this fixative might react with and precipitate plasma proteins from the dentin tubular liquid by coagulation inside the tubules. According to Brännström’s hydrodynamic theory3-5 hypersensitivity of dentin occurs when dentin with orally patent tubular orifices is exposed to tactile, thermal or osmotic stimuli, causing minute movement of fluid within the tubules, acting on mechanoreceptors of odontoblasts and provoking pain19-21. Consequently, anything reducing fluid flow in such patent tubules should result in decrease of dentin sensitivity.
This anticipated intratubular blocking of fluid flow by protein coagulation was verified in a morphological study, using scanning electron microscopy (SEM), transmission electron microscopy (TEM), and confocal laser scanning microscopy (CLSM)26. A number of laboratory and clinical trials proved the immediate and long-term high efficacy of the desensitizing effect of GDL2,8-10,12. Two published in vitro studies, performed to visualize tubular occlusion of dentin specimens after application of Gluma® Desensitizer using SEM, have to be interpreted critically, since the dentin specimens were thoroughly rinsed with water, thus eliminating dentinal tubular fluid including the proteins, supposed to react with glutaraldehyde for precipitation the of the obstructing coagulates1,22.
Adverse effects such as burning of adjacent gingival areas or even ulceration of the gingiva after application of the desensitizing GDL solution may occur when the liquid is inappropriately applied and unintentionally stays in prolonged contact with gingival tissue. However, when applied professionally according to the manufacturer’s instructions such adverse effects can readily be avoided.
In order to facilitate application of this topical desensitizer and to prevent contamination of adjacent gingival areas the manufacturer has developed a gel as a new experimental application form (GDG). This gel is applied to the target area from a syringe fitted with a blunt needle, where it stays during the prescribed dwell time without running. Apart from the original GDL components HEMA, GA and water, the formulation includes fumed silica as a thickening agent.
Since the reaction with the protein-containing tubular liquid depends upon transportation of a sufficient amount of the coagulating agent during the dwell time from the dentin surface into the liquid-filled tubules, a suitable in vitro screening test would be highly desirable, predictive to the intratubular occlusion or reduction of liquid fluid flow. Although in vitro investigations of the efficiency of desensitizing agents cannot fully simulate the complexity of vital dentin, many attempts have been made to determine in vitro fluid flow through dentin as a result of topical application of desensitizing compounds. Common tests are based on the dentin disc model for assessment of hydraulic conductance of dentin6,11,13,14,17,21,23 and SEM investigation of tubular occlusion capability of such agents1,17,18,20,22. Ishihata, et al.16 (2003) have described a permeability test by a tracer penetrating method using approximately one millimeter thick occlusal dentin discs, soaked with albumin solution for simulation of the dentinal fluid and subsequent treatment with desensitizing agents. They evaluated dentin permeability quantitatively in a split-chamber device, using a chemiluminescence signal as target parameter16. This test is considered a suitable and reliable screening method for assessment of topical desensitizing agents efficacy in reducing or eliminating dentin permeability, irrespective of the tubular blocking mechanism used.
The purpose of this study was to evaluate and to compare the effects of GDL liquid and GDG gel on permeability of freshly cut human dentin discs. The null hypothesis to be tested was that application of GDL is more effective in reducing dentin permeability than GDG.
MATERIAL AND METHODS
Test device
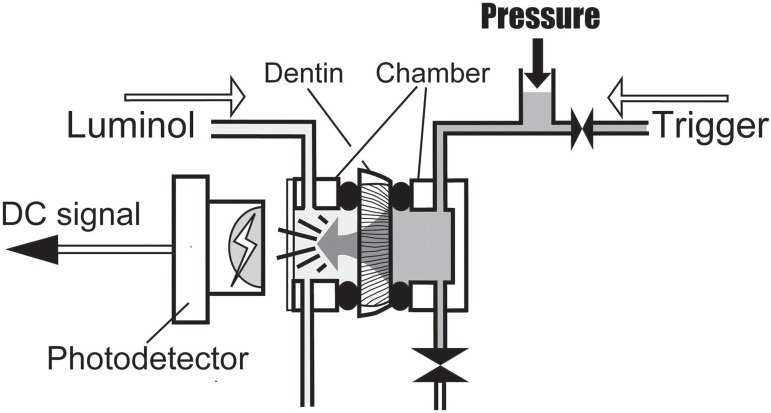
For determination of dentin permeability a split chamber column was used. Two cylindrical acrylic chambers are sealed with O-rings on each side of a dentin slice and clamped in a metal frame. Each chamber has a liquid intake and a drainage hole. The chamber, fitting the occlusal side of the sandwiched dentin slice is sealed with a clear glass cover slip and filled with a chemical illuminant reagent, an aqueous solution of 0.02% luminol (5-amino-2,3-dihydro-1,4-phtalazinedione) and 1% sodium hydroxide. The opposite chamber is filled with an activator liquid, an aqueous solution of 1% potassium ferricyanide and 0.3% hydrogen peroxide. When the activator containing side of the cell is pressurized, the liquid passes through the dentin tubules and produces a luminescence reaction upon mixing with the illuminant reagent. This luminescence signal is recorded with a photodiode (S 9295; Hamamatsu Photonics, Hamamatsu City, Japan), installed 5 mm from the cover slip on the occlusal chamber (Figure 1). The entire equipment is set up in a lightproof box to prevent any outer light signal to interfere with the luminescence signal. The output voltage of the photodiode is recorded with an AD converter at 1 kHz and stored in a CPU unit controlling the system, from where the data at the end of the experimentation are transferred to a PC for further processing and analysis. The entire procedure is automated in a programmable sequencer.
Figure 1.
Measuring device for determination of dentin permeability. The activator solution (trigger) is enclosed in the chamber on the pulpal side of the specimen. Upon start of measurement, the trigger is pressurized to 2.5 and 13 kPa respectively, while the luminol remains at atmospheric pressure. The trigger penetrates through the dentin specimen, and a photochemical reaction is generated upon contact with the luminol. Light emission is detected with a photodetector through a window of the chamber on the occlusal side and outputted as DC signal
Specimen preparation and application of desensitizing agents
The Research Ethics Committee of the Dental Faculty of Tohoku University, Japan approved the present investigation. Twenty human third molars, frozen immediately after extraction, were used. All teeth were free of decay and restorations. Coronal dentin slices, 1.3 mm in thickness, were cut with a diamond wafer saw microtome (Model SP 1600; Leica Microsystems Nusssloch GmbH, Nussloch, Germany) under copious water-cooling perpendicular to the vertical tooth axis between the occlusal enamel portion and the pulp horns. Each slice was cleaned from both sides for 60 s with neutralized 0.5 M EDTA solution (pH 7.4), using a slight dabbing action with a soaked microbrush, to remove the cutting smear and to open the dentin tubules. After thorough rinsing with deionized water the slices were slightly dried with compressed air.
The EDTA-treated and rinsed dentin specimens were mounted between the split chambers for determination of baseline permeability. The illuminant reagent was injected into the occlusal chamber and the activating solution into the opposite chamber. Then, the activator liquid was pressurized with 2.5 kPa for 2 min followed by a pressure-free interval of 2 min. When the activator solution reached the luminal-containing side a photochemical signal was generated, recorded with the photodiode, and registered as output voltage. Following this first run, the illuminant reagent in the occlusal chamber was discharged and re-injected. Then the same pressurizing and recording cycles described above were performed as the second run. A wash cycle with water in both chambers was automatically initiated before the chambers were refilled with fresh reagent solutions, pressurized with 13 kPa for 1 min, left without pressure for 1 min, and finally flushed with water as above. This entire procedure for determination of baseline permeability was repeated on each dentin specimen. The area under the output voltage line during the pressurizing period was integrated (mV•s). The mean value of the first and the second run was considered a measure of the specimen’s permeability.
As some remaining chemiluminescence was present after repeated activator injections through the same specimen, minor baseline output shifts occurred between repeated tests. The mean values and standard deviations of total luminous output including the remaining stock luminescence were registered and characterized as "EDTA" reference.
In order to verify the results of the reference evaluation, the same specimens were evaluated once more following the cycles described above. Results were denominated "EDTA repetition".
The specimens were then removed from the device and the pulpal side was covered with a few droplets of 2% bovine albumin solution (Albumin, from Bovine Serum, Cohn Fraction V, pH 5.2; Wako Pure Chemical Industries, Osaka, Japan). On the opposite side of the sample a vacuum-connected chamber sealed with an O-ring was placed for 20 s to aspirate the albumin solution into the dentin tubules. The free dentin surfaces were then rinsed with deionized water for 5 s and re-mounted into the split-chamber device for the same duplicate loading and measuring cycles described above for baseline determination. The chemiluminescence results were denoted "albumin".
As a next step of the procedure the desensitizing agents shown in Figure 2 were either applied with a soaked microbrush (GDL), or in case of GDG directly delivered to the target surface from the syringe, and slightly agitated with a microbrush for 30 s followed by 30 s undisturbed dwell time. GDL was dried with a weak stream of compressed air for 5 s approximately. Finally, the air-dried GDL specimens and the dentin samples covered with GDG, respectively, were rinsed with deionized water for 5 s before the specimens were mounted for subsequent duplicate permeability measurement cycles as above. Results are referred to as "Gluma".
Figure 2.
Materials tested and application procedure
| Material | Code | LOT/Expiry | Composition/Instructions |
|---|---|---|---|
| Gluma® Desensitizer | GDL | 010071/2009-02 | 2-hydroxyethyl methacrylate, glutaraldehyde, water / a,b,c,d,e,f,g |
| Exp. Desensitizer Gel | GDG | VP280109B01/2009-07 | 2-hydroxyethyl methacrylate, glutaraldehyde, water, fumed silica / a,b,c,d,e,g |
a=rinse and air-dry dentin surfaces, b=60 s EDTA treatment of both disc sides, c=rinse and dry, d=apply desensitizing agent, e=30 s agitated and 30 s non-disturbed dwell time, f=5 s air-dry, g=10 s rinse and air-dry
Subsequently, the same specimens were investigated once more to evaluate the persistence of the permeability results obtained with the desensitizing agents during the first pressurizing cycles. Results are referred to as "Gluma repetition".
In a final test run on the same specimens, the desensitizing compounds GDL or GDG, respectively, were re-applied as above and evaluated following the same duplicate pressurizing procedures described above. Results are referred to as "Gluma re-application".
Statistical treatment of the data was done by Kruskal-Wallis ANOVA and Mann-Whitney’s post hoc test at a significance level of a=0.05 (SPSS 16.0 for Mac).
SEM analysis
In order to examine the completeness of removal of the thickening agent used in GDG from the dentin surface and the orifices of the dentinal tubules two dentin samples each were treated with the liquid (GDL) and the gel (GDG) respectively, and rinsed with deionized water for 5 s. The specimens were fractured perpendicular through the treated surfaces, mounted on metal stubs and sputter-coated with Pt (E 102 Ion Sputter; Hitachi Co. Ltd., Tokyo, Japan). The free surfaces of the treated and the fractured surfaces were inspected in the scanning electron microscope (Type VE-8800; Keyence Inc., Osaka, Japan) at 3000x magnification.
RESULTS
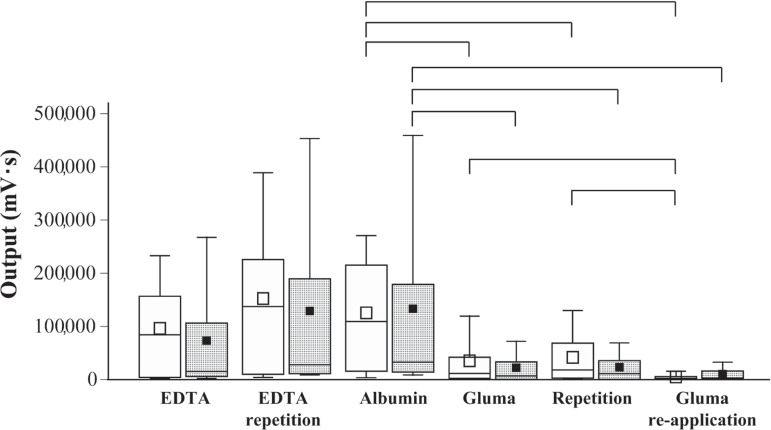
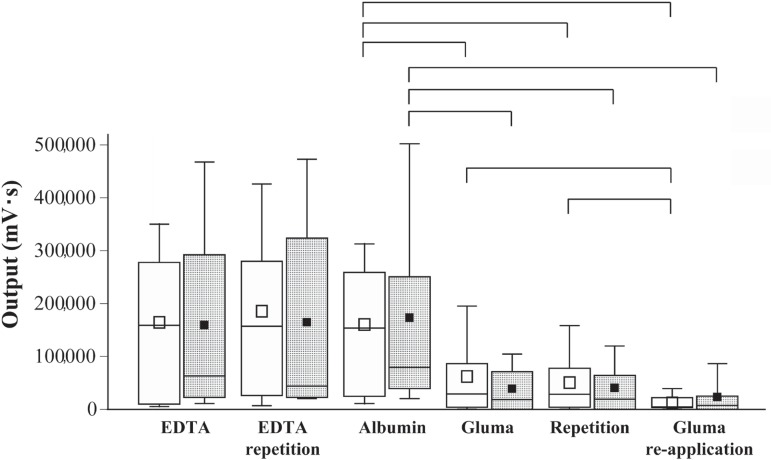
Figures 3 and 4 show the distributions of the integrated outputs of the luminescence signals registered at 2.5 and 13 kPa pressure, respectively, for the EDTA-treated specimens measured in duplicate, the albumin soaked samples, the dentin discs treated with GDL or GDG in duplicate, and finally the dentin specimens following a second application of the desensitizing agents. Kruskal-Wallis ANOVA revealed significantly different permeability (output signals) of the dentin specimens at the different evaluation stages, both at 2.5 and 13 kPa pressure. No significant differences were detected between the EDTA and albumin groups when compared separately at the 2 different pressure levels applied. Multiple comparisons by Mann-Whitney located significant differences in permeability between the albumin-soaked specimens on the one hand, and the GDL or GDG groups on the other. Re-application of GDL resulted in additional significant reduction of permeability when compared with the first GDL application. All significant differences were at p<0.05.
Figure 3.
Permeability of dentin discs (n=10), expressed as integrated output of chemiluminescence (mV•s) under 2.5 kPa pressure at different treatment and evaluation stages. EDTA: samples cleaned for smear on both saw-cut surfaces; Albumin: dentin discs soaked with albumin; Gluma: albumin-soaked specimens topically treated with GDL or GDG; Gluma re-application: specimens of Gluma groups after second application of GDL or GDG. The box-plots show the medians, interquartile distances, the whiskers give the extreme values. The square signatures illustrate the mean values. White and grey boxes refer to groups that received GDL and GDG treatments, respectively. The EDTA and albumin groups’ output signals were not significantly different. The boxes connected with brackets for "Albumin" and the three Gluma groups were significantly different. No significant differences were found between neighboring GDL and GDG boxes. Significant differences by non-parametric tests: (p<0.05)
Figure 4.
As Figure 2, however permeability of dentin discs (n=10) determined under 13 kPa pressure at the different treatment and evaluation stages
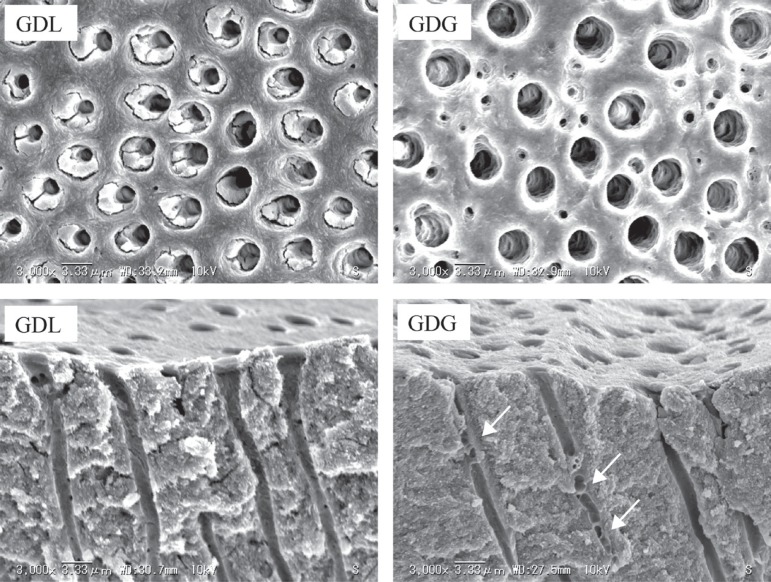
Figure 5 shows representative SEMs of the GDL and GDG treated dentin surfaces and of samples fractured perpendicular through the desensitizer-treated surfaces after rinsing with water. No remnants of the thickening agent of GDG were seen. In two of the longitudinally exposed dentin tubules of GDG transverse septa are displayed (arrows).
Figure 5.
Representative scanning electron microscope micrographs (3,000x) of GDL and GDG treated dentin disc surfaces and of samples fractured perpendicular through the desensitizer-treated surfaces after rinsing with water. The morphologies of the treated surfaces are similar; no remnants of the thickening agent of GDG on free surface or inside tubules. In two of the longitudinally exposed tubules of GDG transverse septa are displayed (arrows)
DISCUSSION
This investigation was performed to evaluate quantitatively the permeability of human dentin discs before and after application of Gluma® Desensitizer as original aqueous solution and as experimental gel formulation, a fumed silica dispersion. For determination of permeability a modified split-chamber model was selected using the chemiluminescence method described by Ishihata et al.15,16 (2009,2003). With this method the pressurizing cycles can be freely adjusted within a wide range. The continuously registered photochemical output signal is proportional to the liquid flow through the dentinal tubules. The method does not produce a direct figure for the absolute or the percent hydraulic conductance relative to the baseline permeability of each individual specimen. Thorough removal of the cutting smear with EDTA on both sides of the dentin slices is considered a worst-case scenario, since in clinical dentistry hypersensitive tooth sites are commonly less rigorously treated before application of a desensitizing agent, unless after local anesthesia. Pressurizing the activator liquid with 2.5 kPa is close to the normal pulpal pressure of human teeth (15 cm H2O=1.5 kPa), whereas the non-physiologic exaggerated pressure of 13 kPa was selected to assess the persistence of the tubular obturating effect under extreme conditions7.
The baseline permeability and the comparatively large standard deviations found reflect the differences in tubular density and opening diameter of the donated teeth and the location in coronal dentin from where the slices were cut. The comparatively low permeability registered at the initial pressurizing of specimens under 2.5 kPa indicates that during the first loading cycle the tubules still contain debris that is flushed during subsequent pressurizing cycles. Soaking of the dentin discs prior to the third test procedure with 2 percent albumin solution was necessary to simulate the protein-containing tubular liquid, since the desensitizing effect of Gluma® desensitizer is reportedly based on total or partial closure of the tubules by protein coagulation and precipitation upon reaction with glutaraldehyde and HEMA17,24,25. Irrespective of the application form, aqueous solution or fumed silica dispersion of glutaraldehyde and HEMA, the permeability of the dentin discs was greatly reduced and persistent, as demonstrated with the two loading cycles of the Gluma-treated specimens. A second application of the desensitizing agents reduced permeability further significantly in the GDL group.
It is hypothesized that complete closure of the dentinal tubule diameter is no prerequisite for effective desensitization of dentin with tubules patent at both ends. Under simplifying assumption dentin tubules can be compared with capillaries. For this model assumption Hagen-Poiseuille’s law is applicable. According to this law, the volume of a homogeneous fluid passing per unit time through a capillary tube is directly proportional to the pressure difference between its ends and to the fourth power of its internal radius, and inversely proportional to its length and to the viscosity of the fluid. The dominating factor is the tubular radius influencing on the volume liquid flow in the fourth power, which means that even incomplete obturation of the tubules will result in dramatically reduced fluid flow of the dentinal liquid, reducing the intradental nerve excitability upon stimulus-evoked fluid movements and thus pain perception. Therefore, in agreement with results of laboratory and clinical trials with Gluma, it can be expected that gross reduction of dentin permeability, as found in the present investigation results in elimination or reduction of hypersensitivity.
The null hypothesis, that application of GDL is more effective in reducing dentin permeability than GDG, was rejected. Diffusion of the glutaraldehyde and HEMA, responsible for the obturatory ability of plasma protein should principally be similar, irrespective of being dissolved in aqueous solution or in aqueous gel. Primarily, the network created in the fumed silica dispersion mediates thixotropic behavior. The gel stays in place after application and flows when subjected to stress. When agitated such gels become fluid, and regain their viscosity when left undisturbed. This thixopropic behavior is desirable to limit contact of the desensitizing agent to the hypersensitive tooth surface and to prevent spreading to adjacent tissue. Comparison of the Gluma® Desensitizer effects after application as solution or gel, on liquid flow through dentin showed no significant difference when compared at the same pressure.
It is noteworthy that the gel was completely removed from the dentin surface during the required rinsing procedure; no remnants of the silica thickening agent were seen by SEM inspection of treated dentin discs.
CONCLUSION
This in vitro permeability investigation has proven similarly high efficacy of aqueous glutaraldehyde/HEMA compounds, both when applied as the marketed GDL solution and as the experimental GDG gel formulation. The obvious advantage of the gel formulation is the well-controlled application, limiting the contact of the desensitizing agent to the target area and preventing inadvertent spreading to neighboring gingival tissue, where prolonged contact may result in localized inflammatory response.
ACKNOWLEDGMENTS
The authors thank Heraeus Kulzer (Wehrheim, Germany) for donation of Gluma® Desensitizer and preparation of the experimental gel formulation.
REFERENCES
- 1.Arrais CA, Chan DC, Giannini M. Effects of desensitizing agents on dentinal tubule occlusion. J Appl Oral Sci. 2004;12:144–148. doi: 10.1590/s1678-77572004000200012. [DOI] [PubMed] [Google Scholar]
- 2.Bergenholtz G, Jontell M, Tuttle A, Knutsson G. Inhibition of serum albumin flux across exposed dentine following conditioning with GLUMA primer, glutaraldehyde or potassium oxalates. J Dent. 1993;21:220–227. doi: 10.1016/0300-5712(93)90133-b. [DOI] [PubMed] [Google Scholar]
- 3.Brännström M. Sensitivity of dentine. Oral Surg Oral Med Oral Pathol. 1966;21:517–526. doi: 10.1016/0030-4220(66)90411-7. [DOI] [PubMed] [Google Scholar]
- 4.Brännström M, Lindén LA, Aström A. The hydrodynamics of the dental tubule and of pulp fluid. A discussion of its significance in relation to dentinal sensitivity. Caries Res. 1967;1:310–317. doi: 10.1159/000259530. [DOI] [PubMed] [Google Scholar]
- 5.Brännström M. The transmission and control of dentinal pain. In: Grossman LI, editor. Mechanism and control of pain. New York: Masson Pub. Co.; 1979. pp. 15–35. [Google Scholar]
- 6.Camps J, About I, Van Meerbeek B, Franquin JC. Efficiency and cytotoxicity of resin-based desensitizing agents. Am J Dent. 2002;15:300–304. [PubMed] [Google Scholar]
- 7.Ciucchi B, Bouillaguet S, Holz J, Pashley D. Dentinal fluid dynamics in human teeth, in vivo. J endod. 1995;21:191–194. doi: 10.1016/S0099-2399(06)80564-9. [DOI] [PubMed] [Google Scholar]
- 8.Davidson DF, Suzuki M. The Gluma bonding system: a clinical evaluation of its various components for the treatment of hypersensitive root dentin. J Can Dent Assoc. 1997;63:38–41. [PubMed] [Google Scholar]
- 9.Dondi Dall'Orologio G, Malferrari S. Desensitizing effects of Gluma and Gluma 2000 on hypersensitive dentin. Am J Dent. 1993;6:283–286. [PubMed] [Google Scholar]
- 10.Duran I, Sengun A. The long-term effectiveness of five current desensitizing products on cervical dentine sensitivity. J Oral Rehabil. 2004;31:351–356. doi: 10.1046/j.1365-2842.2003.01241.x. [DOI] [PubMed] [Google Scholar]
- 11.Duran I, Sengun A, Yildirim T, Ozturk B. In vitro dentine permeability evaluation of HeMA-based (desensitizing) products using split-chamber model following in vivo application in the dog. J Oral Rehabil. 2005;32:34–38. doi: 10.1111/j.1365-2842.2004.01132.x. [DOI] [PubMed] [Google Scholar]
- 12.Felton DA, Bergenholtz G, Kanoy BE. Evaluation of the desensitizing effect of Gluma Dentin Bond on teeth prepared for complete-coverage restorations. Int J Prosthodont. 1991;4:292–298. [PubMed] [Google Scholar]
- 13.Gillam DG, Mordan NJ, Newman HN. The Dentine Disc surface: a plausible model for dentine physiology and dentine sensitivity evaluation. Adv Dent Res. 1997;11:487–501. doi: 10.1177/08959374970110041701. [DOI] [PubMed] [Google Scholar]
- 14.Greenhill JD, Pashley DH. The effects of desensitizing agents on the hydraulic conductance of human dentin in vitro. J Dent Res. 1981;60:686–698. doi: 10.1177/00220345810600030401. [DOI] [PubMed] [Google Scholar]
- 15.Ishihata H, Kanehira M, Nagai T, Finger WJ, Shimauchi H, Komatsu M. Effect of desensitizing agents on dentin permeability. Am J Dent. 2009;22:143–146. [PubMed] [Google Scholar]
- 16.Ishihata H, Shoji S, Shimauchi H, Matthews B. A photochemical method for the investigation of dentin permeability. J Dent Res. 2003;82(Spec Issue):B-47. [Google Scholar]
- 17.Kolker JL, Vargas MA, Armstrong SR, Dawson DV. Effect of desensitizing agents on dentin permeability and dentin tubule occlusion. J Adhes Dent. 2002;4:211–221. [PubMed] [Google Scholar]
- 18.Mordan NJ, Barber PM, Gillam DG. The dentine disc. A review of its applicability as a model for the in vitro testing of dentine hypersensitivity. J Oral Rehabil. 1997;24:148–156. doi: 10.1046/j.1365-2842.1997.d01-260.x. [DOI] [PubMed] [Google Scholar]
- 19.Närhi MV, Hirvonen TJ, Hakumäki MO. Responses of intradental nerve fibers to stimulation of dentin and pulp. Acta Physiol Scand. 1982;115:173–178. doi: 10.1111/j.1748-1716.1982.tb07062.x. [DOI] [PubMed] [Google Scholar]
- 20.Närhi MV. Dentin sensitivity: a review. J Biol Buccale. 1985;13:75–96. [PubMed] [Google Scholar]
- 21.Pashley DH, O'Meara JA, Kepler EE, Galloway SE, Thompson SM, Stewart FP. Dentin Permeability. effects of desensitizing dentifrices in vitro. J Periodontol. 1984;55:522–525. doi: 10.1902/jop.1984.55.9.522. [DOI] [PubMed] [Google Scholar]
- 22.Pereira JC, Martinelli AC, Tung MS. Replica of human dentin treated with different desensitizing agents: a methodological SeM study in vitro. Braz Dent J. 2002;13:75–85. doi: 10.1590/s0103-64402002000200001. [DOI] [PubMed] [Google Scholar]
- 23.Pereira JC, Segala AD, Gillam DG. Effect of desensitizing agents on the hydraulic conductance of human dentin subjected to different surface pre- treatments: a in vitro study. Dent Mater. 2005;21:129–138. doi: 10.1016/j.dental.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Qin C, Xu J, Zhang Y. Spectroscopic investigation of the function of aqueous 2-hydroxyethylmethacrylate/glutaraldehyde solution as a dentin desensitizer. Eur J Oral Sci. 2006;114:354–359. doi: 10.1111/j.1600-0722.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 25.Schüpbach P, Lutz F, Finger WJ. Closing of dentinal tubules by Gluma desensitizer. Eur J Oral Sci. 1997;105:414–421. doi: 10.1111/j.1600-0722.1997.tb02138.x. [DOI] [PubMed] [Google Scholar]