Abstract
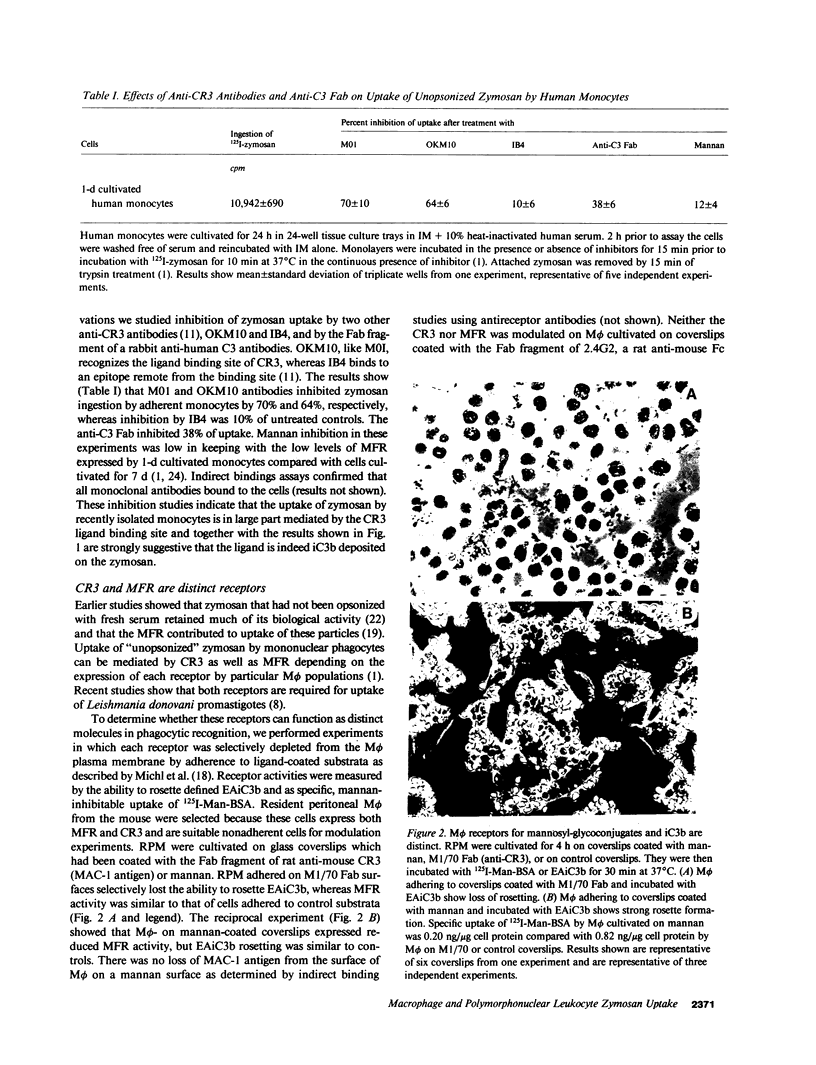
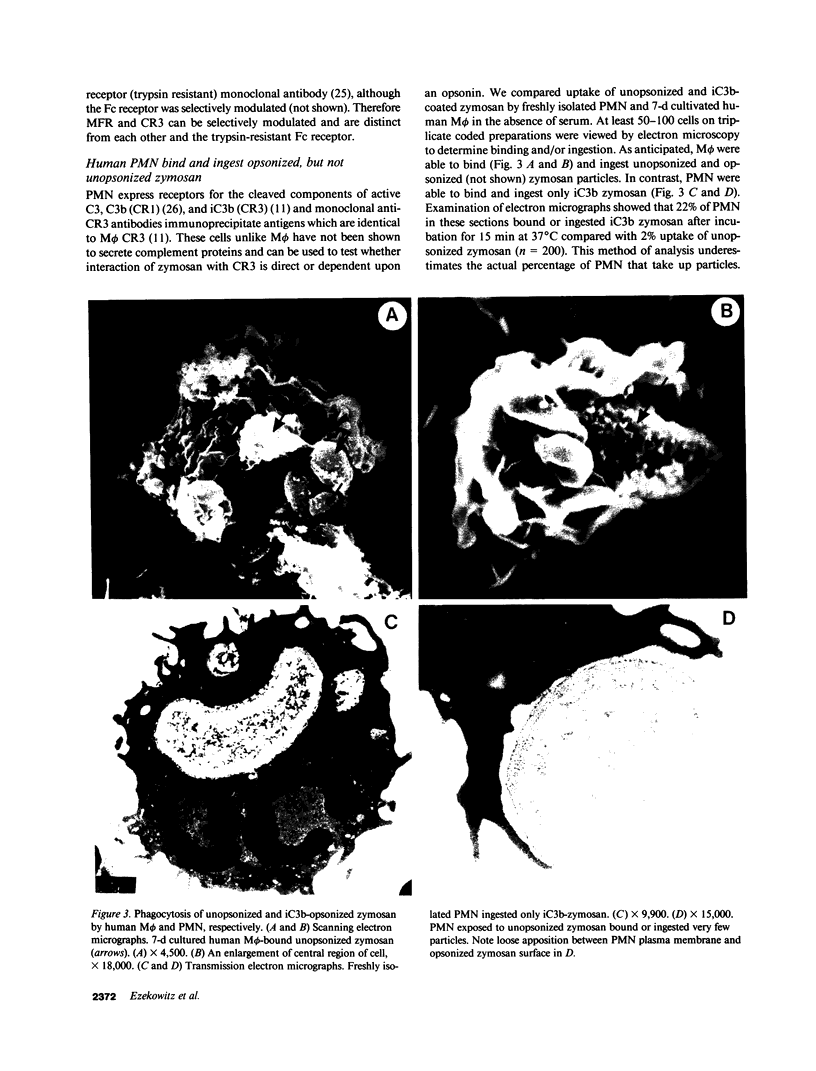
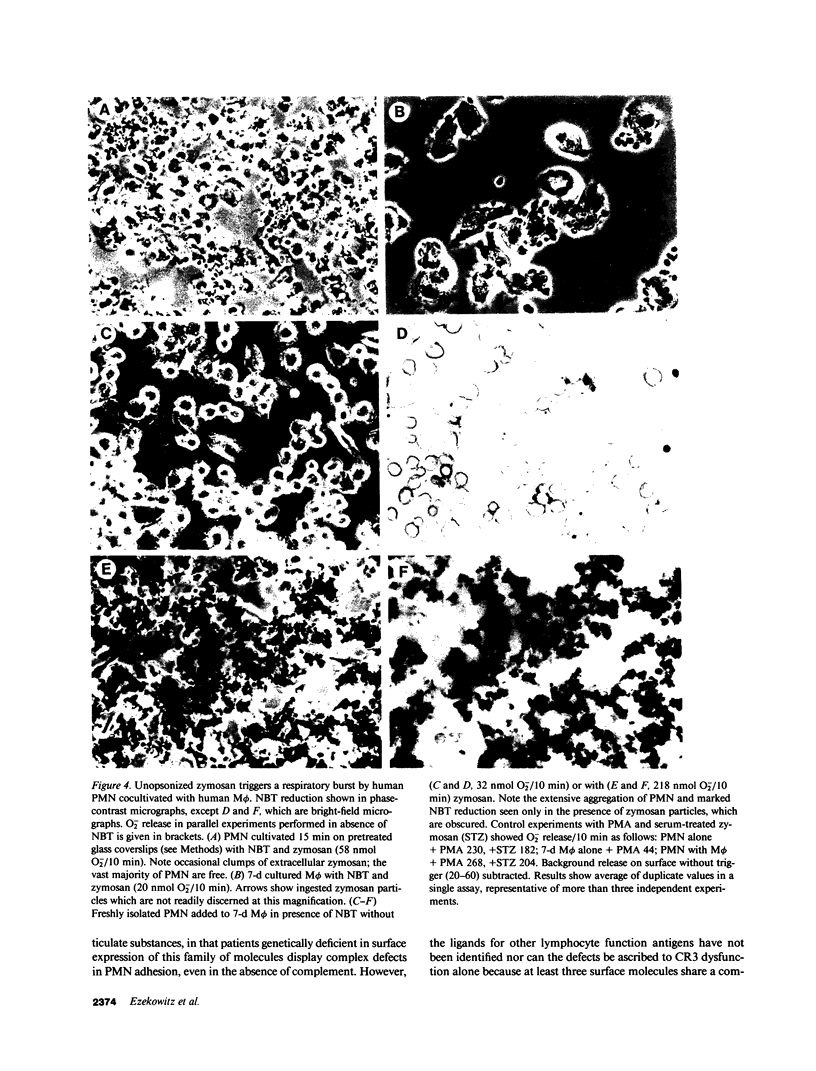
Macrophages take up zymosan in the absence of exogenous complement via receptors for iC3b (type 3 complement receptors) acting with or without lectin-like receptors for mannosyl-fucosyl-terminated glycoconjugates. We previously provided evidence that macrophages themselves secrete complement-alternative pathway components able to opsonize zymosan locally (Ezekowitz et al., J. Exp. Med. 1984. 159:244-260). We show here that covalently bound C3 cleavage products C3b and iC3b can be eluted from zymosan particles cultivated with 36-h adherent human monocytes in the absence of serum. The ligand binding site of type 3 complement receptors is involved in macrophage-zymosan interactions as shown by inhibition studies of zymosan binding and uptake with Fab fragments of anti-C3 antibodies and monoclonal antireceptor antibodies M01 and OKM10. In contrast, antibody IB4, which binds to a receptor epitope distinct from the binding site, does not inhibit zymosan uptake. Selective modulation of macrophage receptors onto anticomplement receptor antibody and mannose-rich yeast mannan, respectively, confirms that the complement and lectin-like receptors are distinct. Human polymorphonuclear leukocytes, which express receptors for complement, but are not known to secrete complement proteins, bind and ingest only exogenously opsonized zymosan. Unopsonized zymosan is a poor trigger of respiratory burst activity in neutrophils or 7-d adherent human macrophages, but induces cell aggregation and secretion of large amounts of superoxide anion when these cells are co-cultivated in serum-free medium and challenged with zymosan. Our studies indicate that complement and/or other products synthesized by macrophages at extravascular sites could play an important role in opsonization and lysis of pathogens able to activate the alternative pathway and mediate macrophage-neutrophil collaboration in first-line host defence.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Hovi T., Vaheri A. Fibronectin is produced by human macrophages. J Exp Med. 1980 Mar 1;151(3):602–613. doi: 10.1084/jem.151.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A., Spits H., Terhorst C., Pitt J., Todd R. F., 3rd Deficiency of a leukocyte surface glycoprotein (LFA-1) in two patients with Mo1 deficiency. Effects of cell activation on Mo1/LFA-1 surface expression in normal and deficient leukocytes. J Clin Invest. 1984 Oct;74(4):1291–1300. doi: 10.1172/JCI111539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwey J. A., Curnutte J. T., Robinson J. M., Lazdins J. K., Briggs R. T., Karnovsky M. J., Karnovsky M. L. Comparative aspects of oxidative metabolism of neutrophils from human blood and guinea pig peritonea: magnitude of the respiratory burst, dependence upon stimulating agents, and localization of the oxidases. J Cell Physiol. 1980 Dec;105(3):541–545. doi: 10.1002/jcp.1041050319. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton G., Gordon S. Modulation of macrophage mannosyl-specific receptors by cultivation on immobilized zymosan. Effects on superoxide-anion release and phagocytosis. Immunology. 1983 Aug;49(4):705–715. [PMC free article] [PubMed] [Google Scholar]
- Berton G., Gordon S. Superoxide release by peritoneal and bone marrow-derived mouse macrophages. Modulation by adherence and cell activation. Immunology. 1983 Aug;49(4):693–704. [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade V., Beuscher H. U. Formation of EAC142 and EAC1423 with macrophage culture supernatant containing the secreted complement components C1 to C3. Immunobiology. 1984 Mar;166(2):177–189. doi: 10.1016/s0171-2985(84)80036-4. [DOI] [PubMed] [Google Scholar]
- Colten H. R. Biosynthesis of the MHC-linked complement proteins (C2, C4 and factor B) by mononuclear phagocytes. Mol Immunol. 1982 Oct;19(10):1279–1285. doi: 10.1016/0161-5890(82)90294-2. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Austyn J., Stahl P. D., Gordon S. Surface properties of bacillus Calmette-Guérin-activated mouse macrophages. Reduced expression of mannose-specific endocytosis, Fc receptors, and antigen F4/80 accompanies induction of Ia. J Exp Med. 1981 Jul 1;154(1):60–76. doi: 10.1084/jem.154.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., Hill M., Gordon S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J Exp Med. 1984 Jan 1;159(1):244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Griffin J. A., Griffin F. M., Jr Augmentation of macrophage complement receptor function in vitro. I. Characterization of the cellular interactions required for the generation of a T-lymphocyte product that enhances macrophage complement receptor function. J Exp Med. 1979 Sep 19;150(3):653–675. doi: 10.1084/jem.150.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Hsiung L., Barclay A. N., Brandon M. R., Sim E., Porter R. R. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem J. 1982 Apr 1;203(1):293–298. doi: 10.1042/bj2030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Silverstein S. C. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979 Sep 19;150(3):607–621. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micklem K. J., Sim R. B., Sim E. Analysis of C3-receptor activity on human B-lymphocytes and isolation of the complement receptor type 2 (CR2). Biochem J. 1984 Nov 15;224(1):75–86. doi: 10.1042/bj2240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokoena T., Gordon S. Human macrophage activation. Modulation of mannosyl, fucosyl receptor activity in vitro by lymphokines, gamma and alpha interferons, and dexamethasone. J Clin Invest. 1985 Feb;75(2):624–631. doi: 10.1172/JCI111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier C. G., O'Shea J., Chused T., Takahashi T., Ochoa M., Nutman T. B., Bianco C., Brown E. J. Differentiation stimuli induce receptors for plasma fibronectin on the human myelomonocytic cell line HL-60. Blood. 1984 Oct;64(4):858–866. [PubMed] [Google Scholar]
- Roos D., Bot A. A., van Schaik M. L., de Boer M., Daha M. R. Interaction between human neutrophils and zymosan particles: the role of opsonins and divalent cations. J Immunol. 1981 Feb;126(2):433–440. [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Hamill A. L., Cohn Z. A. Synthesis of leukotriene C and other arachidonic acid metabolites by mouse pulmonary macrophages. J Exp Med. 1982 Mar 1;155(3):720–733. doi: 10.1084/jem.155.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., DiScipio R. G. Purification and structural studies on the complement-system control protein beta 1H (Factor H). Biochem J. 1982 Aug 1;205(2):285–293. doi: 10.1042/bj2050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Twose T. M., Paterson D. S., Sim E. The covalent-binding reaction of complement component C3. Biochem J. 1981 Jan 1;193(1):115–127. doi: 10.1042/bj1930115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Thompson W. S., Miller L. J., Schmalstieg F. C., Anderson D. C. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984 Dec 1;160(6):1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Gordon S. Expression of a mannosyl-fucosyl receptor for endocytosis on cultured primary macrophages and their hybrids. J Cell Biol. 1982 Apr;93(1):49–56. doi: 10.1083/jcb.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J Cell Biol. 1983 Jan;96(1):160–166. doi: 10.1083/jcb.96.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartio T., Hovi T., Vaheri A. Human macrophages synthesize and secrete a major 95,000-dalton gelatin-binding protein distinct from fibronectin. J Biol Chem. 1982 Aug 10;257(15):8862–8866. [PubMed] [Google Scholar]
- Whaley K. Biosynthesis of the complement components and the regulatory proteins of the alternative complement pathway by human peripheral blood monocytes. J Exp Med. 1980 Mar 1;151(3):501–516. doi: 10.1084/jem.151.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Licht M. R., Craigmyle L. S., Silverstein S. C. Communication between receptors for different ligands on a single cell: ligation of fibronectin receptors induces a reversible alteration in the function of complement receptors on cultured human monocytes. J Cell Biol. 1984 Jul;99(1 Pt 1):336–339. doi: 10.1083/jcb.99.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Johnston R. B., Jr Dissociation of phagocytosis from stimulation of the oxidative metabolic burst in macrophages. J Exp Med. 1984 Feb 1;159(2):405–416. doi: 10.1084/jem.159.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]







