Supplemental digital content is available in the text.
Key Words: axial spondyloarthritis, referral patterns, diagnosis, disease management, survey
Abstract
Background
Recognition, diagnosis, and management of axial spondyloarthritis (axial SpA) continue to advance.
Objectives
The objectives of this study were to compare referrals, diagnosis, and management of axial SpA in Western Europe (WE), North America (US and Canada), and the rest of world (RoW) in academic and community rheumatology practices and to identify areas for further education.
Methods
Rheumatologists responded online to the MAXIMA (Management of Axial SpA International and Multicentric Approaches) survey. Questions pertained to referral, diagnosis, and management of axial SpA.
Results
Rheumatologists (N = 809) from 56 countries completed the survey about patients with chronic back pain (≥3 months) starting before age 45 years. Responses from academic and community practice rheumatologists were generally similar. Most referrals were from primary care providers. Symptom duration of 3 years or more at referral was reported more frequently by WE and RoW than US respondents. More WE and RoW than US rheumatologists referred to the Assessment of SpondyloArthritis International Society criteria for axial SpA in clinical practice. Rheumatologists reported prescribing disease-modifying antirheumatic drugs for the management of axial SpA. Sulfasalazine was frequently prescribed across regions; methotrexate was more commonly prescribed by US rheumatologists compared with other regions.
Conclusions
Referral patterns, diagnosis, and disease management for axial SpA were similar among WE, North America, and RoW rheumatologists and in academic/community practices, although more WE and RoW rheumatologists referred to Assessment of SpondyloArthritis International Society criteria in clinical practice. Disease-modifying antirheumatic drugs were commonly prescribed for axial SpA patients, although it was unclear whether these were prescribed for axial or peripheral symptoms.
Axial spondyloarthritis (SpA) is characterized by inflammation of the spine and sacroiliac (SI) joints.1 Historically, according to modified New York criteria, radiographic evidence of structural damage to the SI joints (radiographic sacroiliitis) was required to identify patients with predominantly axial forms of SpA, diagnosed as ankylosing spondylitis (AS).2 However, there are patients who may have signs and symptoms typical of AS, but who lack radiographic sacroiliitis, and are now classified as having nonradiographic axial SpA (nr-axSpA).3,4
Subsequently, the Amor criteria5 and the European Spondyloarthropathy Study Group (ESSG) criteria6 sought to extend the definition of SpA beyond those with the mandatory radiographic sacroiliitis.
On average, 6 to 8 years elapse between the onset of chronic back pain and a diagnosis of AS based on radiographic evidence.7 In an effort to reduce the delay in diagnosis, the Assessment of SpondyloArthritis International Society (ASAS) expanded its classification criteria to encompass nr-axSpA.3,4 Patients with chronic back pain (≥3 months) that started before 45 years of age can be classified as having axial SpA either by sacroiliitis on imaging (either magnetic resonance imaging [MRI] or radiograph) plus 1 or more feature typical of SpA or the presence of human leukocyte antigen B27 (HLA-B27) plus 2 or more other SpA features.4 Based on these criteria, ASAS more recently proposed a diagnostic algorithm that can be applied in daily clinical practice.8 Despite these advancements, a delay in diagnosis may still occur because of infrequent referrals by other physicians to a rheumatologist. Referral strategies for SpA have been shown to lead to proper diagnosis in a high proportion of patients,9 but referral patterns have not been well studied.
Treatment recommendations for the management of AS have existed for several years.10,11 In 2010, the ASAS and the European League Against Rheumatology updated their recommendations for the management of AS, and ASAS updated the recommendations on treatment with tumor necrosis factor (TNF) inhibitors to include all axial SpA patients (AS and nr-axSpA).12,13 Nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended as first-line treatment for patients with axial SpA.12,13 Prior to initiating anti-TNF therapy, the ASAS recommendations advocate that patients with axial SpA undergo mandatory treatment with 2 or more NSAIDs for a total of 4 weeks or more.13 Disease-modifying antirheumatic drugs (DMARDs) are not recommended for the management of axial disease because of the lack of clinical evidence.
Appropriate identification and referral of patients with symptoms suggestive of axial SpA can facilitate earlier diagnosis and management. The MAXIMA (Management of Axial SpA International and Multicentric Approaches) survey was conducted to understand current referral practices and to identify areas for improvement. The MAXIMA survey also evaluated the use of diagnostic tools and management of axial SpA among rheumatologists. An additional objective was to identify similarities and differences in referral, diagnosis, and disease management among rheumatologists in academic settings compared with those in community practices, as well as among rheumatologists in Western Europe (WE), North America (NA), and regions throughout the rest of the world (RoW).
METHODS
The MAXIMA survey was drafted and conducted by a third-party vendor (excluding the United States: Margaux Orange, Paris, France; United States: Instar, New York) with guidance and approval of the questionnaire by members of a steering committee whose members were selected based on their expertise in the field of SpA: Désirée van der Heijde (the Netherlands), Ruben Burgos-Vargas (Mexico), Eduardo Collantes (Spain), Atul Deodhar (United States), Dirk Elewaut (Belgium), Robert Inman (Canada), Helena Marzo-Ortega (United Kingdom), Philip Mease (United States), Ignazio Olivieri (Italy), Thao Pham (France), and Joachim Sieper (Germany). Questions pertaining to referral, diagnosis, and management of patients with chronic back pain (≥3 months) that started before 45 years of age were developed, reviewed, and approved by the steering committee. The survey questions were not pretested or validated. The MAXIMA survey was funded by AbbVie Inc. Surveys were completed anonymously online by rheumatologists from NA, Latin America, Europe, Asia-Pacific, Africa, and the Middle East. The survey was conducted from November 2011 to December 2011, except in the United States, where the survey was conducted in May 2012. Some questions in the US survey were adapted to match the unique requirements of the US medical system and differed slightly from the surveys used in all other countries. In the United States, respondents were compensated to complete the survey, whereas respondents outside the United States were not compensated for completing the survey.
There was no prespecified goal for the number of surveys to be completed. Results from MAXIMA were summarized descriptively and reported as the percentages of respondents in each survey. The MAXIMA steering committee advisory board reviewed the survey results. Steering committee members specifically reviewed and advised AbbVie on the content of their national survey results and medical educational needs within their respective countries.
RESULTS
Respondents
The MAXIMA survey was completed by 809 rheumatologists from 56 countries (for individual country listing, see Supplementary Table, http://links.lww.com/RHU/A44). In WE, the survey was completed by 258 rheumatologists: 71 rheumatologists (28%) were in academic practice settings, and 187 rheumatologists (72%) were in community practice settings. In NA, 154 surveys were completed by rheumatologists in the United States (academic centers, 34%; community practice, 66%), and 36 surveys were completed by rheumatologists in Canada (academic centers, 25%; community practice, 75%). In the RoW, the survey was completed by 361 rheumatologists: 72 rheumatologists (20%) were in academic practice settings, and 289 rheumatologists (80%) were in community practice settings.
Referral Patterns
For patients with chronic back pain (≥3 months) that started before 45 years of age, the majority of rheumatologists reported that primary care providers were the main source of referral (Table 1). Responses regarding source of referrals of patients with axial SpA were generally similar in both academic and community clinic practice settings.
TABLE 1.
Rates Reported (%) by Rheumatologists Regarding Referral Patterns and Diagnosis
Symptoms triggering referral of patients to rheumatologists were surveyed only in questionnaires conducted outside the United States. Overall, symptoms that most frequently triggered referrals to a rheumatologist were uveitis (range, 78%–89%) and chronic back pain (range, 58%–78%). Rates reported by academic and community rheumatologists were generally similar. The duration of symptoms among referred patients did not differ markedly by rheumatologists’ practice site (Supplementary Figure 1, http://links.lww.com/RHU/A42). Compared with WE, Canada, and the RoW, the reported time to referral to a rheumatologist was shorter in the United States. Few US rheumatologists stated that patients had symptoms of SpA for at least 3 years at the time of referral, and approximately half reported patient referral within 1 year of symptom onset.
Diagnosis
The use of classification criteria for axial SpA is reported in Table 1. In general, NA rheumatologists infrequently referred to classification criteria when making a diagnosis of axial SpA in clinical practice; use of ASAS criteria and modified New York criteria for AS were lower for NA practitioners (27% and 17%, respectively) compared with practitioners from other regions (Table 1). Rates for the use of other classification criteria to guide diagnostic evaluation were also low among NA rheumatologists (ESSG and Amor criteria, 2% each; Table 1). The most common reasons cited for not using ASAS criteria were “don’t use guidelines” (39% of US respondents [response not available in other countries]) and “lack of awareness” (33%).
Approximately half of survey respondents in all regions reported systematically requesting HLA-B27 typing when evaluating a patient with chronic back pain in their daily practice (Table 1). North America rheumatologists in community practices reported using HLA-B27 typing at a rate (58%) that was higher than NA rheumatologists in academic settings, WE rheumatologists in academic or community practices, and RoW rheumatologists in community practices (46% each), but similar to that of RoW rheumatologists in academic settings (59%). The use of imaging tests to help guide diagnosis of axial SpA was frequently reported by rheumatologists across regions (Table 1). North America rheumatologists in community practices reported a slightly lower use of pelvic x-ray (74%) compared with NA rheumatologists in academic settings and rheumatologists in WE and RoW. Magnetic resonance imaging of the SI joints was more frequently used than pelvic x-ray across all regions.
Disease Burden and Management
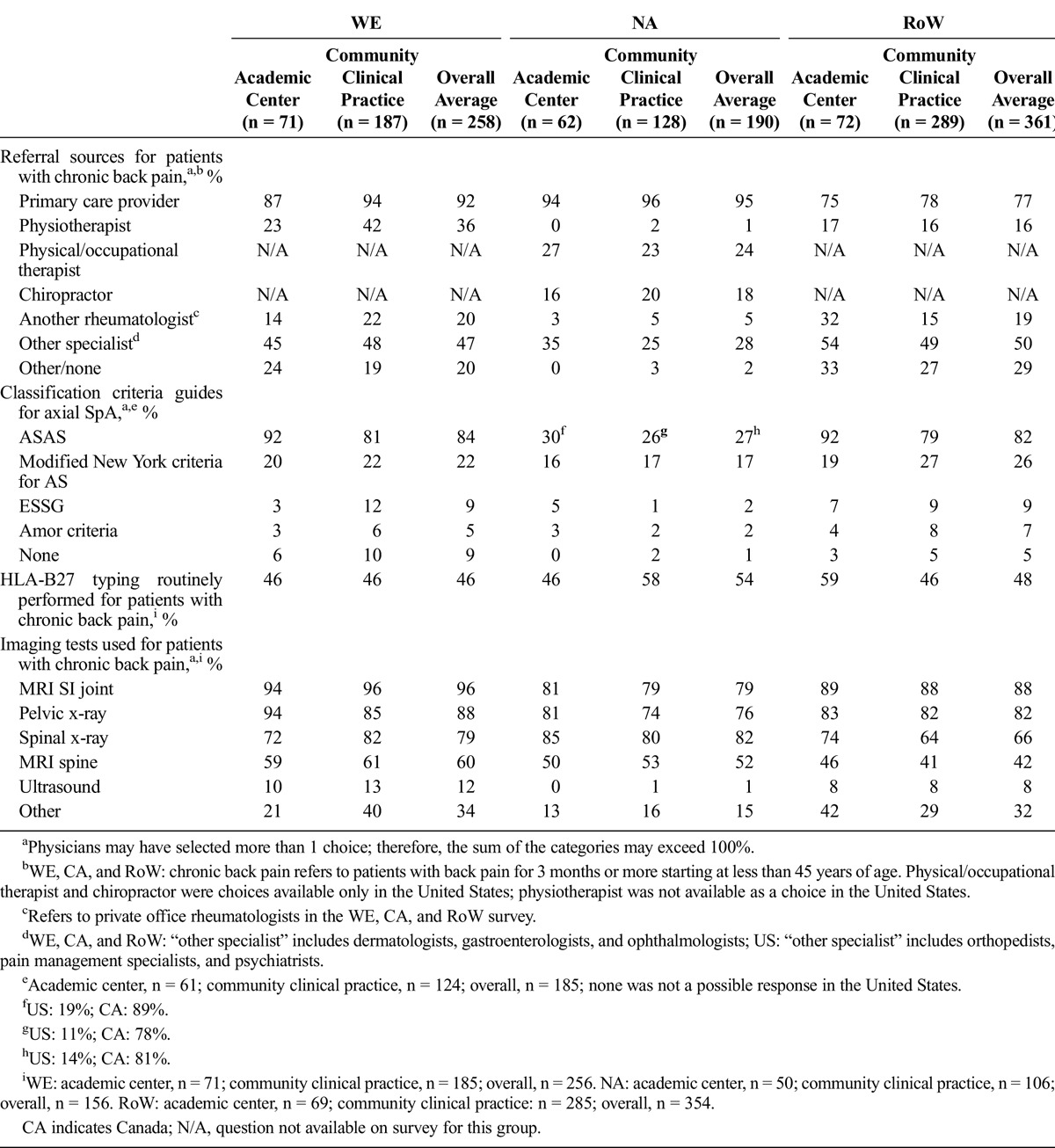
Rheumatologists reported that back pain was the most important disease burden cited by patients with axial SpA across all regions (Fig. 1). Notably, quality of life followed back pain as the second most important burden of disease in WE and RoW, whereas stiffness was more often reported as the second most important burden in the United States. In Canada, stiffness, fatigue, and quality of life were closely matched for the second most important burden of disease, although there was a large difference reported for quality of life between academic centers and community practice.
FIGURE 1.
Rates reported for the top 3 burden of disease concerns among axial SpA patients in WE (A), NA (B), and RoW (C). Rheumatologists in all countries except the United States responded to the following survey question: What do you consider as the top 3 item(s) of the burden of disease among your axial SpA patients? US rheumatologists responded to the following survey question: What do you consider the top 3 burden of disease concerns among your nr-axSpA patients? QoL indicates quality of life.
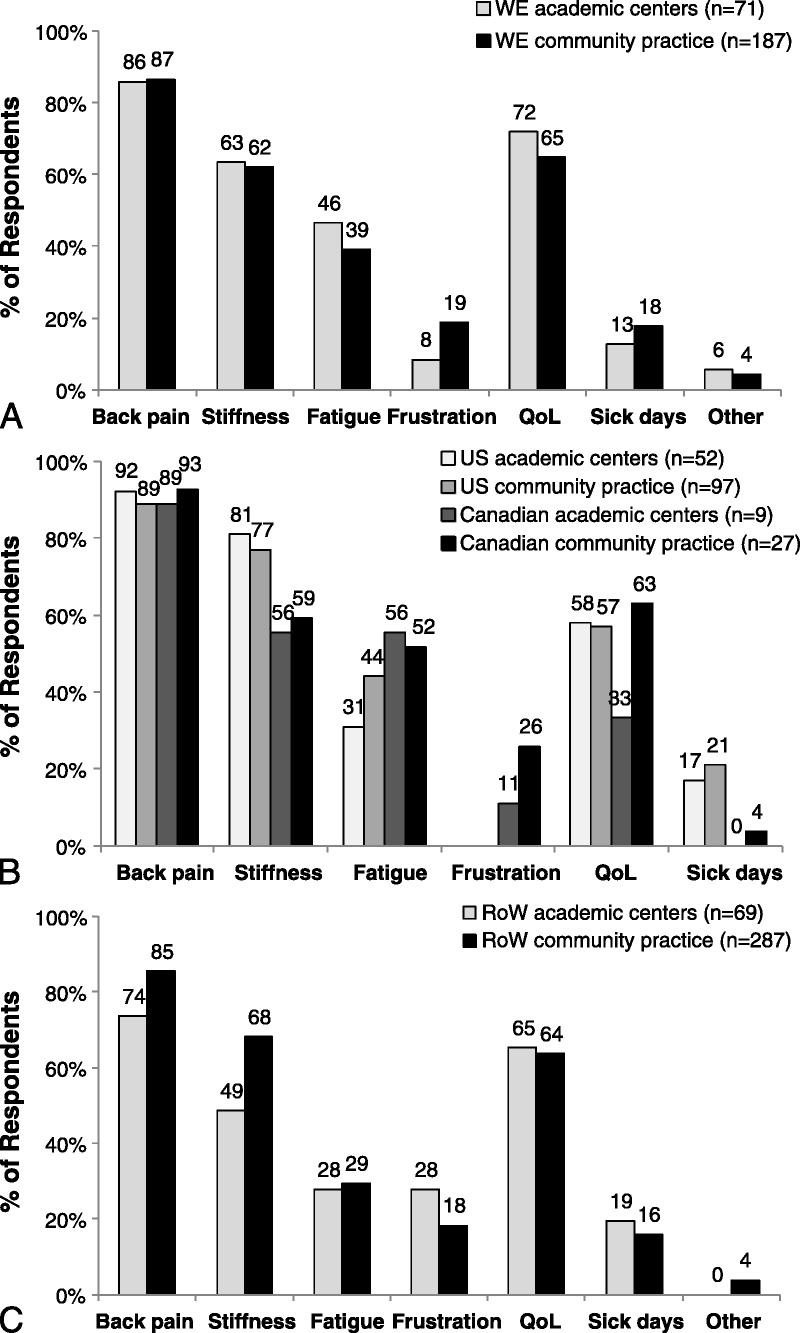
In the MAXIMA survey, most rheumatologists reported prescribing 1 to 2 courses of NSAIDs before considering a treatment class change (Fig. 2). However, Canadian rheumatologists applied a more conservative approach, especially in academic settings, where 63% of rheumatologists prescribed at least 3 courses of NSAIDs. Most rheumatologists reported that they would evaluate the efficacy of NSAIDs after 1 to 3 months of therapy (Supplementary Figure 2, http://links.lww.com/RHU/A43). Trends in prescribing and evaluating the efficacy of NSAIDs were generally similar among academic and community rheumatologists.
FIGURE 2.
Nonsteroidal anti-inflammatory drugs in the management of AS in WE (A), NA (B), and RoW (C). Proportions of rheumatologists prescribing different courses of NSAIDs for AS before considering a treatment class change. Rheumatologists in all countries except the United States responded to the following question: Considering those patients diagnosed with AS, how many courses of NSAIDs do you prescribe before considering a treatment class change? US rheumatologists responded to the following survey question: When thinking about your AS patient, how many different NSAIDs do you prescribe before considering a treatment class change? *1% selected “other.”
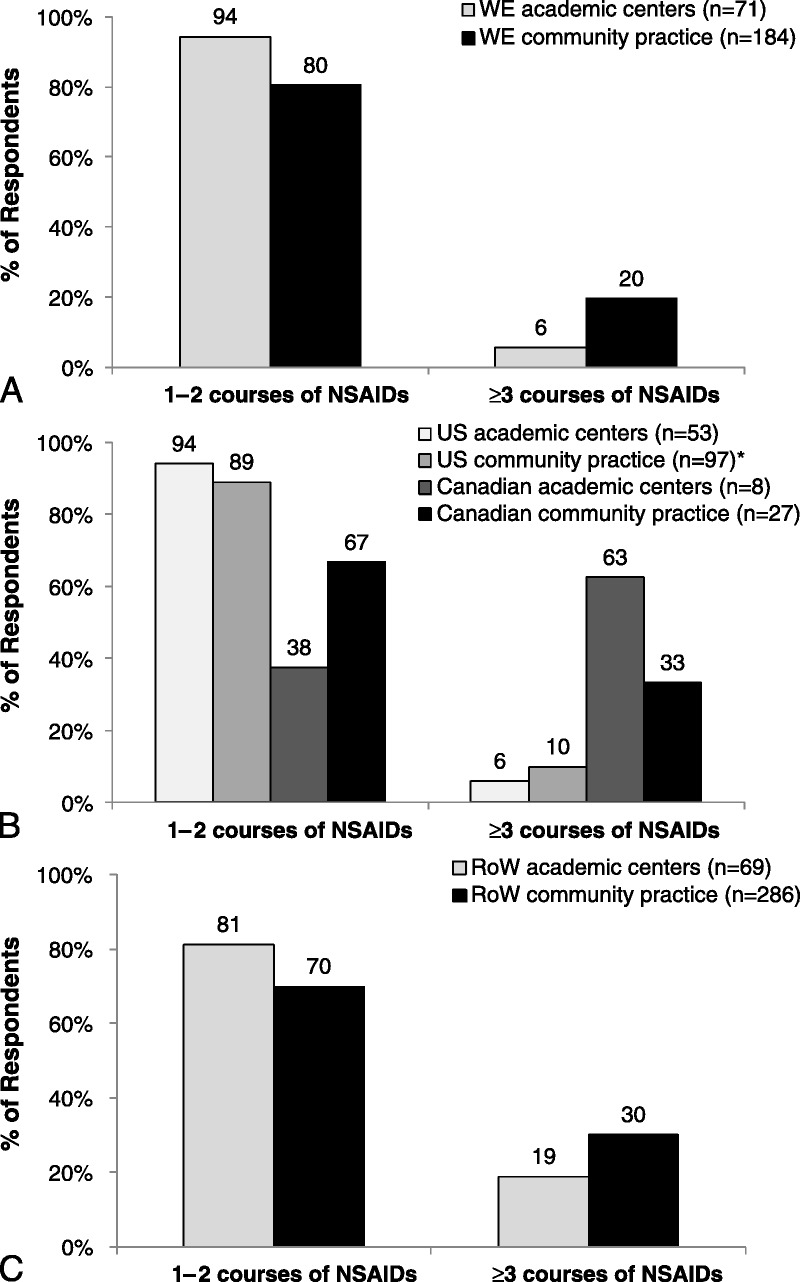
Most respondents in academic or community practice settings, independent of location, reported prescribing DMARDs for the management of axial SpA. Sulfasalazine was the most commonly prescribed DMARD for AS and nr-axSpA across most regions (Fig. 3). Methotrexate was more frequently prescribed by rheumatologists in the United States (AS, 76%–81%; nr-axSpA, 69%–81%) compared with WE (AS, 51%–68%; nr-axSpA, 51%–70%); rates of prescribing methotrexate were particularly low among RoW rheumatologists (AS, 35%–56%; nr-axSpA, 47%–50%). “Other DMARDs” (which included leflunomide, hydroxychloroquine, and antimalarials) were prescribed more frequently by US and Canadian rheumatologists compared with WE and RoW.
FIGURE 3.
Rates of DMARD use among rheumatologists prescribing DMARDs for the management of AS and nr-axSpA in WE (A), NA (B), and RoW (C). “Other DMARDs” include leflunomide, hydroxychloroquine, and antimalarials. Rheumatologists in all countries except the United States responded to the following questions: (1) Considering those patients diagnosed with AS, do you use DMARDs in AS? If yes, please specify which one(s). (2) If you do differentially treat nr-axSpA patients, do you use DMARDs in nr-axSpA treatment? US rheumatologists responded to the following survey questions: (1) When thinking about your AS patient, which DMARDs do you prescribe? (2) Please specify which DMARD(s) you use for nr-axSpA treatment. *In responses where the percentage was greater than 100%, more respondents answered the question regarding which type of DMARD was used (numerator for calculation) than respondents who answered the question regarding if they used DMARDs (denominator for calculation), resulting in a percentage greater than 100%.
DISCUSSION
Results of the MAXIMA survey demonstrated general agreement in referral patterns, diagnosis, and management of axial SpA among rheumatologists in WE, NA, and RoW, with some differences noted for the time to referral to a rheumatologist, use of classification criteria, NSAID courses before treatment change, and DMARD use. Furthermore, practice patterns for axial SpA were generally similar in academic and community clinic settings.
Referral to a rheumatologist is one of the first steps toward an accurate and timely diagnosis. Although time to referral was not based on a precise measurement and may have been influenced by recall bias, survey results showed that the reported time to referral was longer in WE and RoW, with approximately half of patients referred to rheumatologists for evaluation several years after the onset of symptoms. In contrast, the majority of patients in the United States were referred to rheumatologists within 2 years. This finding is unexpected, as it is frequently stated that rheumatologists in the United States do not see patients with back pain early. Differences in national health care systems, the presence of additional medical specialties in the United States (eg, physical medicine and rehabilitation, chiropractors), and the low number of rheumatologists per capita in countries such as Germany may have contributed to the regional differences in the time to referral.
Although these results suggest a need for improvement, time to referral has decreased over the past several years. In 1988, Kidd and Cawley14 reported that patients with SpA in the United Kingdom had experienced multiple referrals and delayed diagnosis. The median time from first symptom to diagnosis for patients referred to nonrheumatologists and rheumatologists was 6 years and 3 years, respectively.14 In Germany, the average delay from first AS symptoms to diagnosis decreased from 15 years in patients with disease onset in the 1950s, to approximately 7.5 years for patients with SpA onset between 1975 and 1979.15
Survey results showed that rheumatologists prefer imaging tests, especially MRI, over HLA-B27 testing for the diagnosis of axial SpA. In our survey, only approximately half of the rheumatologists stated that they used HLA-B27 assessments in making the diagnosis. The slow uptake of the HLA-B27 test might be explained by the lack of understanding of its positive predictive value for making the diagnosis of axial SpA in a suitable patient. Moreover, it shows that there is a stronger belief in imaging in the diagnostic process.
The ability to detect sacroiliitis on MRI has been shown to substantially contribute to transitioning patients from an undifferentiated status to a diagnosis of axial SpA.3 Magnetic resonance imaging of the SI joints was widely used by WE, NA, and RoW rheumatologists, regardless of practice setting. A high level of awareness and utilization of the ASAS axial SpA classification criteria was identified among WE and RoW rheumatologists, whereas use of the ASAS criteria was lower among NA rheumatologists. Reasons for not using ASAS classification criteria cited by NA rheumatologists included lack of awareness and, in the United States, not using guidelines in general. Another possible explanation for lower use of ASAS criteria in NA may be that in Europe and other parts of the world, the ESSG and Amor criteria have been used since 1991 to identify nr-axSpA patients, whereas this was generally not the case in the United States. In the United States, radiographic changes have remained a more crucial component for classification. In addition, more than a third of US rheumatologists reported that they “don’t use guidelines.” This response by US rheumatologists compared with the other survey respondents may be a result of interpretation of the wording. Classification criteria are meant to be a guide, not a strict application for diagnosis. The wording of the questionnaire may have been such that US rheumatologists were reluctant to answer a question that contradicts good clinical practice or may have had difficulty with the word “guidelines” rather than “criteria.”
Disease management approaches used for AS and nr-axSpA were generally similar across geographic regions. Consistent with recent recommendations,12,13 most rheumatologists prescribed 1 to 2 courses of NSAIDs and evaluated efficacy after 1 to 3 months before considering a treatment class change. The differences noted in Canada are a reflection of the Canadian Rheumatologists Association/Spondyloarthritis Research Consortium of Canada guidelines that require the use of at least 3 NSAIDs, each administered over a minimum 2-week period.16 The use of DMARDs for axial symptoms prior to anti-TNF therapy is not recommended by ASAS/European League Against Rheumatology recommendations because of the lack of evidence that these medications can improve the signs and symptoms of axial disease.12,13 Despite these recommendations, the majority of rheumatologists participating in this survey prescribed DMARDs for patients with AS and nr-axSpA, suggesting the need for additional effective therapies for axial SpA and further education. Furthermore, it is unclear if DMARDs were prescribed for patients with pure axial disease or for peripheral symptoms in patients with axial disease.
One notable disparity in disease management was the more frequent use of methotrexate among US rheumatologists compared with WE and RoW rheumatologists. Furthermore, WE and RoW rheumatologists prescribed sulfasalazine more frequently than methotrexate for the treatment of AS and nr-axSpA, whereas rates of the use of sulfasalazine and methotrexate were similar among US rheumatologists. Similar to rheumatoid arthritis, historically, the use of methotrexate has been higher than sulfasalazine in the United States,17 whereas the converse has been true in other countries. Methotrexate could also have been used in the United States as a combination therapy with TNF inhibitors to reduce anti–drug antibody production, although this information was not collected.
The MAXIMA survey had more than 800 participants from 56 countries across the world, allowing for a robust analysis of referral, disease, and axial SpA patient management. In addition, both academic and community clinic rheumatologists were represented. The data were not derived from a clinical study with follow-up and data verification. Limitations of the current survey include the selection of respondents and survey questions by the study sponsor and the slightly different phrasing of some survey questions in the US questionnaire compared with the questionnaire used in all of the other countries, allowing only limited comparisons for some of the questions. Furthermore, US respondents, but not respondents from other countries, were compensated for completing the survey, potentially affecting responses.
In summary, there is increasing awareness of axial SpA, but delays in referral and diagnosis persist. The delay from symptom onset to referral and diagnosis of axial SpA highlights the need for continuing education of both referring health care providers and rheumatologists to improve axial SpA recognition in appropriate patients with chronic back pain. Although some differences exist across geographic regions, rheumatologists in academic and community clinical practice settings used similar disease management approaches for axial SpA. Despite the lack of evidence of the efficacy of DMARDs in treating axial symptoms, DMARDs were commonly prescribed for patients with axial SpA. Although it was unclear whether the DMARDs were intended to treat axial or peripheral symptoms, or whether patients presented with peripheral symptoms, this observation suggests the need for more effective treatment options for this disease and further education regarding management. Overall, the results highlight the need for further education to facilitate early diagnosis and referral of patients with axial SpA, as well as to increase awareness of therapies recommended for the management of axial SpA.
Figure.
No caption available.
ACKNOWLEDGMENT
The authors thank Dianne Nguyen and Benjamin Wolfe (AbbVie Inc) for support in developing this manuscript and thank all members of the steering committee of axial SpA experts (MAXIMA Steering Committee) for guidance and approval of the questionnaire.
Footnotes
Medical writing support was provided by Amanda Sheldon of Complete Publication Solutions, LLC; this support was funded by AbbVie Inc. AbbVie participated in selecting the survey respondents; survey question content; interpretation of data; and writing, reviewing, and approving of the manuscript.
Dr Heijde has received consulting fees from AbbVie, Amgen, AstraZeneca, BMS, Celgene, Centocor, Chugai, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Otsuka, Pfizer, Roche, Sanofi Aventis, Schering-Plough, UCB, and Wyeth and is the director of Imaging Rheumatology. Dr Sieper has received grant/research support, consulting fees, and/or speaker’s fees from AbbVie, Merck, Pfizer, and UCB. Dr Elewaut has received grant/research support, consulting fees, and/or speaker’s fees from AbbVie. Dr Deodhar has received grant/research support from AbbVie, Amgen, Janssen, Novartis, Pfizer, and UCB and has received consulting fees and/or speaker’s fees from AbbVie, Pfizer, Novartis, MSD, and UCB. Drs Pangan and Dorr are AbbVie employees and may hold AbbVie stock or stock options.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jclinrheum.com).
REFERENCES
- 1. van Tubergen A, Weber U. Diagnosis and classification in spondyloarthritis: identifying a chameleon. Nat Rev Rheumatol. 2012; 8: 253– 261. [DOI] [PubMed] [Google Scholar]
- 2. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984; 27: 361– 368. [DOI] [PubMed] [Google Scholar]
- 3. Rudwaleit M, Landewe R, van der Heijde D, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009; 68: 770– 776. [DOI] [PubMed] [Google Scholar]
- 4. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009; 68: 777– 783. [DOI] [PubMed] [Google Scholar]
- 5. Amor B, Dougados M, Mijiyawa M. Criteria of the classification of spondylarthropathies [in French]. Rev Rhum Mal Osteoartic. 1990; 57: 85– 89. [PubMed] [Google Scholar]
- 6. Dougados M, van der Linden S, Juhlin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991; 34: 1218– 1227. [DOI] [PubMed] [Google Scholar]
- 7. Rudwaleit M. New approaches to diagnosis and classification of axial and peripheral spondyloarthritis. Curr Opin Rheumatol. 2010; 22: 375– 380. [DOI] [PubMed] [Google Scholar]
- 8. van den Berg R, de Hooge M, Rudwaleit M, et al. ASAS modification of the Berlin algorithm for diagnosing axial spondyloarthritis: results from the SPondyloArthritis Caught Early (SPACE) cohort and from the Assessment of SpondyloArthritis International Society (ASAS) cohort. Ann Rheum Dis. 2013; 72: 1646– 1653. [DOI] [PubMed] [Google Scholar]
- 9. Rudwaleit M, Sieper J. Referral strategies for early diagnosis of axial spondyloarthritis. Nat Rev Rheumatol. 2012; 8: 262– 268. [DOI] [PubMed] [Google Scholar]
- 10. Braun J, Pham T, Sieper J, et al. International ASAS consensus statement for the use of anti–tumour necrosis factor agents in patients with ankylosing spondylitis. Ann Rheum Dis. 2003; 62: 817– 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zochling J, van der Heijde D, Burgos-Vargas R, et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2006; 65: 442– 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011; 70: 896– 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Heijde D, Sieper J, Maksymowych WP, et al. 2010 Update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis. 2011; 70: 905– 908. [DOI] [PubMed] [Google Scholar]
- 14. Kidd BL, Cawley MI. Delay in diagnosis of spondarthritis. Br J Rheumatol. 1988; 27: 230– 232. [DOI] [PubMed] [Google Scholar]
- 15. Feldtkeller E, Bruckel J, Khan MA. Scientific contributions of ankylosing spondylitis patient advocacy groups. Curr Opin Rheumatol. 2000; 12: 239– 247. [DOI] [PubMed] [Google Scholar]
- 16. Maksymowych WP, Gladman D, Rahman P, et al. The Canadian Rheumatology Association/ Spondyloarthritis Research Consortium of Canada treatment recommendations for the management of spondyloarthritis: a national multidisciplinary stakeholder project. J Rheumatol. 2007; 34: 2273– 2284. [PubMed] [Google Scholar]
- 17. Griffiths RI, Bar-Din M, MacLean CH, et al. Medical resource use and costs among rheumatoid arthritis patients receiving disease-modifying antirheumatic drug therapy. Arthritis Care Res. 2000; 13: 213– 226. [DOI] [PubMed] [Google Scholar]


