Abstract
Fibronectin-binding surface proteins are found in many bacterial species. Most strains of Streptococcus pyogenes, a major human pathogen, express the fibronectin-binding protein F1, which promotes bacterial adherence to and entry into human cells. In this study, the role of fibronectin in S. pyogenes virulence was investigated by introducing the protein F1 gene in an S. pyogenes strain lacking this gene. Furthermore, transgenic mice lacking plasma fibronectin were used to examine the relative contribution of plasma and cellular fibronectin to S. pyogenes virulence. Unexpectedly, protein F1-expressing bacteria were less virulent to normal mice, and virulence was partly restored when these bacteria were used to infect mice lacking plasma fibronectin. Dissemination to the spleen of infected mice was less efficient for fibronectin-binding bacteria. These bacteria also disseminated more efficiently in mice lacking plasma fibronectin, demonstrating that plasma fibronectin bound to the bacterial surface downregulates S. pyogenes virulence by limiting bacterial spread. From an evolutionary point of view, these results suggest that reducing virulence by binding fibronectin adds selective advantages to the bacterium.
Keywords: adherence, fibronectin, internalisation, S. pyogenes, virulence
Introduction
Streptococcus pyogenes (group A streptococcus) is a significant human pathogen. This Gram-positive bacterium causes common and mostly uncomplicated suppurative infections such as pharyngitis, impetigo, and erysipelas, and also nonsuppurative sequelae (acute rheumatic fever, poststreptococcal glomerulonephritis, and reactive arthritis) and severe invasive infections including necrotising fasciitis, sepsis, and a toxic shock-like syndrome (for references, see the review by Cunningham, 2000). S. pyogenes virulence is based on a multitude of secreted and surface-associated components. An important group of virulence factors is the cell wall-attached surface proteins. In this group, M proteins, elongated coiled-coil proteins that bind a variety of human proteins (see the review by Fischetti, 1989), are responsible for the ability of S. pyogenes to survive and multiply in human blood. Several other streptococcal surface proteins have been identified and characterised. For many of these, isogenic mutants are less virulent in animal models of infection. Mutants deficient in the M protein (Ashbaugh et al, 1998), the C5a peptidase that cleaves and inactivates C5a of the complement system, thereby disturbing chemotaxis and neutrophil migration (Wexler et al, 1985), and GRAB that binds the plasma proteinase inhibitor α2-macroglobulin (Rasmussen et al, 1999), are all less virulent in animal challenge studies. Mutants lacking the fibronectin (Fn)-binding surface proteins SOF (Courtney et al, 1999), Fba, or FbaB (Terao et al, 2001, 2002) also show reduced virulence.
Fibronectins are dimeric glycoproteins that exist in human blood plasma (plasma Fn; pFn) at a concentration of 300 μg/ml (Hynes, 1990), and in the extracellular matrix (cellular Fn; cFn). cFn is produced locally in tissues by a variety of cells, whereas hepatocytes are the sole source of pFn (Owens and Cimino, 1982; Mosher, 1989; Hynes, 1990; Schwarzbauer, 1991). Binding of Fn by S. pyogenes mediates bacterial adhesion to human cells (Abraham et al, 1983). Several S. pyogenes surface proteins are implicated in this binding, including M and M-like proteins (Schmidt et al, 1993; Frick et al, 1995; Cue et al, 1998), FBP54 (Courtney et al, 1999), PFBP (Rocha and Fischetti, 1999), glyceraldehyde-3 phosphatase dehydrogenase (Pancholi and Fischetti, 1992), protein F2 (Jaffe et al, 1996), SOF, Fba and FbaB (Terao et al, 2001, 2002), and protein F1/SfbI (Hanski and Caparon, 1992; Talay et al, 1992). The binding of Fn by protein F1 has been studied extensively in vitro. Protein F1 binds Fn via two distinct domains: one repeat-containing domain (RD) in the COOH-terminal region with homology to Fn-binding regions in Staphylococcus aureus and Streptococcus dysgalactiae, and a second more NH2-terminally located domain (upstream Fn-binding domain; UFBD) that binds Fn with higher affinity (Sela et al, 1993). These domains bind to different parts of Fn (Ozeri et al, 1996; Talay et al, 2000). A mutant deficient in protein F1 is defective in adhesion (Hanski and Caparon, 1992), and nonadhesive bacteria devoid of protein F1 became adhesive when complemented with protein F1 (Hanski et al, 1992). Moreover, the protein F1 homologue SfbI blocks S. pyogenes adhesion when added in solution (Talay et al, 1992). Thus, protein F1 is both required and sufficient for effective adhesion in these systems. More recently, it was demonstrated that protein F1 is also important for the entry of S. pyogenes into human cells (Jadoun et al, 1997; Molinari et al, 1997), although Fn-independent internalisation (Molinari et al, 2000) may also occur. Cellular invasion by S. pyogenes is blocked by antibodies against β1-integrins (Cue et al, 1998; Ozeri et al, 1998) and by an α5β1-integrin antagonist (Cue et al, 2000). Streptococcal internalisation occurs in vivo as well as in vitro, and several studies suggest that invasion of host cells by S. pyogenes creates an in vivo reservoir of bacteria that could account for recurrent infection (Österlund and Engstrand, 1995; Kiska et al, 1997; Österlund et al, 1997; Cockerill et al, 1998; Neeman et al, 1998).
Adhesion is an initial and important step in bacterial infections, but so far no animal studies have been published regarding the contribution of protein F1 to the virulence of S. pyogenes. Furthermore, due to the lack of appropriate animal models of infection, the effect of pFn and/or cFn on bacterial virulence has not been studied. Recently, we generated mice lacking pFn using the Cre-IoxP recombinant technology (Sakai et al, 2001). In the present work, such animals and S. pyogenes strains lacking Fn-binding activity or expressing Fn-binding protein F1 were used to investigate the role of Fn in S. pyogenes virulence. Unexpectedly, interactions between protein F1 and Fn were found to downregulate virulence.
Results
Generation and characteristics of AP1 bacteria expressing protein F1
To generate AP1 bacteria with a high binding capacity for Fn, the pLZ12 self-replicating plasmid without (pLZ12) or with (pPTF8) the insert encoding protein F1 were electroporated into AP1 bacteria. The transcomplemented bacteria with or without protein F1 showed the same growth rate at chloramphenicol (Cm) concentrations up to 6 μg/ml (data not shown). Nontransformed, parental AP1 bacteria failed to grow at 1.5 μg Cm/ml. Furthermore, the strains with or without protein F1 had similar growth rates in fresh human blood and bound fibrinogen to the same degree (data not shown). In these strains, the M1 protein is responsible for fibrinogen binding and the binding curves demonstrated that very similar amounts of M1 protein were expressed by the two strains. AP1(pPTF8), but not AP1 or AP1(pLZ12) bacteria, bound significant amounts of radiolabelled human Fn (Figure 1A). Moreover, AP1(pPTF8) bacteria expressing protein F1, but not AP1(pLZ12) bacteria, absorbed Fn from mouse serum (Figure 1B), showing that protein F1-expressing bacteria bind Fn also in a complex mixture of proteins.
Figure 1.
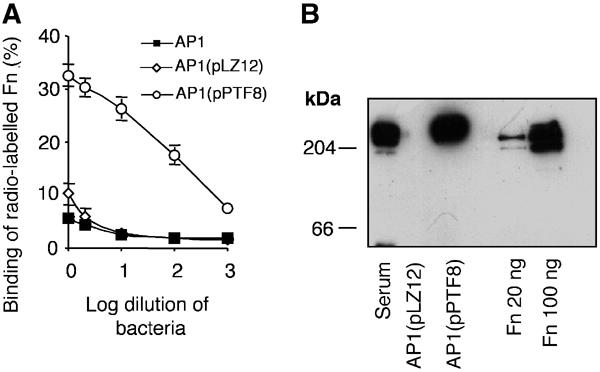
AP1 bacteria expressing protein F1 bind human and mouse Fn. (A) The binding of human 125I-labelled Fn to serial log dilutions (from 2 × 109 cfu's) of AP1, AP1(pLZ12), and AP1(pPTF8) bacteria is shown. The error bars indicate ±s.d. (B) AP1(pLZ12) or AP1(pPTF8) bacteria were incubated with 10% mouse serum, and bound proteins were eluted with 0.1 M glycine-HCl, pH 2.0. The proteins were separated by SDS–PAGE (reducing conditions) and subjected to Western blot analysis using anti-Fn antibodies. Human Fn, 20 or 100 ng, was run as a control.
To determine whether binding of Fn to the bacterial surface would allow secondary binding of other serum proteins, or whether the absence of pFn would result in interactions between protein F1 and other serum proteins, AP1(pPTF8) and AP1(pLZ12) bacteria were incubated with serum from normal (control) and pFn-null mice. Serum proteins bound to the bacteria were eluted, separated by SDS–PAGE under reducing conditions, and silver stained (Figure 2A). A protein with a molecular mass corresponding to pFn was absorbed from normal mouse serum by AP1(pPTF8) bacteria (Figure 2A, STAIN). Western blotting verified that the protein band corresponded to Fn (Figure 2A, BLOT). Furthermore, this protein band was not present in the other eluted samples (Figure 2A, STAIN). Apart from Fn, no qualitative differences were observed between the different lanes. The results demonstrate that AP1(pPTF8), but not AP1(pLZ12) bacteria, bind pFn, and suggest that the binding of Fn does not interfere with binding of other serum proteins. Moreover, no additional protein bands were visible when AP1(pPTF8) was incubated with pFn-null serum, indicating that the absence of pFn does not promote interactions between protein F1 and other serum proteins.
Figure 2.
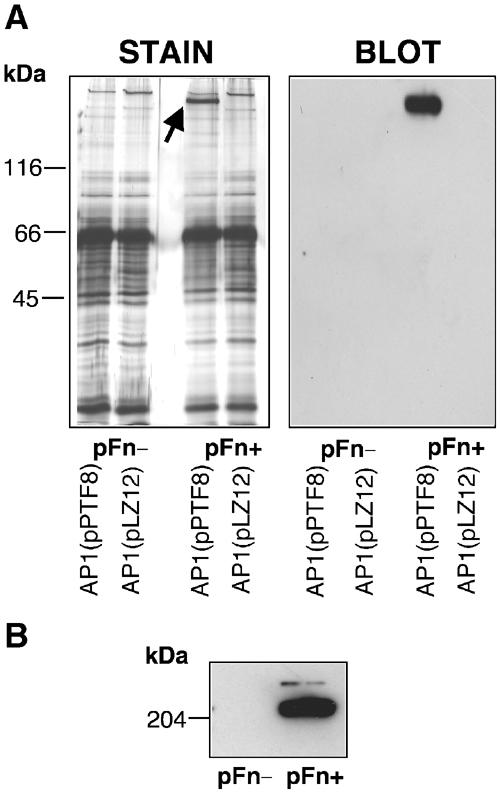
Silver staining of absorbed serum proteins. (A) AP1(pLZ12) or AP1(pPTF8) bacteria were incubated with serum from control (pFn+) or pFn-null (pFn−) mice. Bound proteins were eluted with 0.1 M glycine-HCl, pH 2.0, and separated by SDS–PAGE (reducing conditions) on two identical gels. One gel was silver stained (STAIN), and the other was used for Western blotting (BLOT). A band with the size of Fn (indicated by the arrow) is present in the material eluted from the AP1(pPTF8) bacteria incubated with serum from control mice (pFn+), but not in the other lanes. Western blotting with anti-Fn antibodies confirmed that this band was Fn. (B) Serum proteins from pFn-null mice (pFn-) or control mice (pFn+) were separated by SDS–PAGE and subjected to Western blotting with anti-Fn antibodies.
Effect of fibronectin on S. pyogenes adhesion and internalisation
The effect of pFn on bacterial adhesion and entry into mammalian cells was studied in vitro by performing adhesion and internalisation experiments with the pharyngeal epithelial cell line Detroit 562 or human fibroblasts. First, the density of cFn on the surface of these cells was determined by ELISA (Figure 3). In these experiments, anti-Fn antibodies were applied in different dilutions to pharyngeal epithelial cells or fibroblasts. For comparison, wells were coated with different concentrations of purified Fn, followed by the application of anti-Fn antibodies. The results indicate a four- to eight-fold higher density of Fn on the surface of fibroblasts as compared to Detroit 562 cells. To evaluate the importance of pFn for adhesion and internalisation, experiments were subsequently performed with serum from control mice (control serum) or from pFn-null mice (pFn-null serum).
Figure 3.
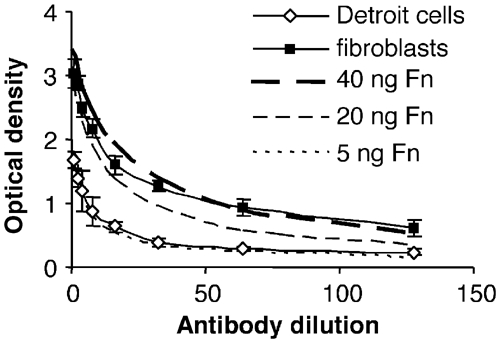
Cellular Fn is more abundant on the surface of fibroblasts than on pharyngeal epithelial cells. Different dilutions of anti-Fn antibodies were applied to wells coated with epithelial cells or fibroblasts, followed by peroxidase-labelled secondary antibodies. The results are from one representative experiment with triplicate samples and the error bars indicate ±s.d. For comparison, the wells were coated with different concentrations of purified Fn, followed by anti-Fn antibodies and secondary antibodies.
When protein F1 was expressed on the surface of AP1 bacteria, the adherence to epithelial cells in the presence of control serum increased by 65% (95% confidence interval 40–90%) (Figure 4A). Importantly, the absence of pFn reduced the adhesion of bacteria expressing protein F1 to epithelial cells (Figure 4A). In the presence of control serum, the entry of AP1(pPTF8) into epithelial cells was increased almost three-fold (95% confidence interval 1.5–4) compared to AP1(pLZ12). When the bacteria and cells were incubated in pFn-null serum, internalisation of the two strains was similar (Figure 4B). The results show that S. pyogenes adhesion to and entry into epithelial cells increase when protein F1 is expressed on the bacterial surface. Furthermore, both effects were only observed in the presence of pFn, demonstrating that protein F1 per se is not sufficient. Finally, the data show that protein F1-independent adhesion and internalisation is not influenced by the presence of pFn.
Figure 4.
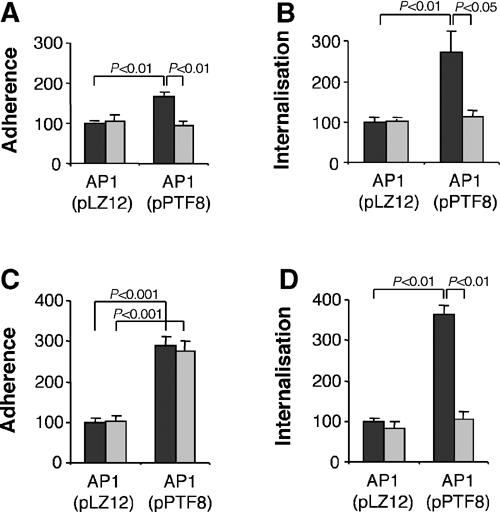
Effect of Fn on S. pyogenes adhesion and internalisation. Adherence and internalisation experiments were performed with AP1(pLZ12) or AP1(pPTF8) bacteria. The cells used were a human pharyngeal cell line (Detroit 562) (A, B) and human foreskin fibroblasts (C, D). Experiments were performed in the presence of serum from control mice (black bars) or serum from pFn-null mice (grey bars). Values are the means of four experiments with triplicate samples, given as a percentage of the mean adhesion or internalisation of AP1(pLZ12). Error bars ±s.e.m. and P-values are indicated.
To examine the relative importance of pFn for bacterial adhesion to and internalisation into cells expressing large amounts of cFn, AP1(pPTF8) or AP1(pLZ12) bacteria were incubated with human foreskin fibroblasts in the presence of either control or pFn-null serum. Adhesion of AP1(pPTF8) bacteria to fibroblasts was increased three-fold (95% confidence interval 2.5–3.4) compared to AP1(pLZ12) (Figure 4C) in control serum. Interestingly, a similar increase was obtained in pFn-null serum, which was in contrast to the results seen with epithelial cells (Figure 4A). Internalisation of AP1 bacteria expressing protein F1 was almost four-fold increased in control serum when compared to AP1 bacteria lacking protein F1 (Figure 4D). In the presence of pFn-null serum, however, internalisation of AP1(pPTF8) was similar to AP1(pLZ12). These findings indicate that efficient internalisation, but not adhesion, depends on pFn bound to the bacterial surface by protein F1.
S. pyogenes bacteria expressing protein F1 are less virulent
Unlike most strains of S. pyogenes, the AP1 strain is virulent to mice (Björck et al, 1989) and it was therefore used in an animal model simulating invasive S. pyogenes disease. A potential problem when introducing the protein F1 gene on a plasmid in the AP1 strain concerns its regulation. However, it has been published that other M1 strains, despite their lack of prtF, still carry RofA, the activator of the gene (Granok et al, 2000). To investigate the virulence of AP1(pPTF8) relative to AP1(pLZ12) bacteria in vivo, 105 or 106 bacteria were injected intraperitoneally (i.p.) into sex- and age-matched groups of control mice. While 81% (13 of 16) of the mice injected with AP1(pLZ12) died within 8 days after injection, only 44% (17 out of 39) mice injected with AP1(pPTF8) died within the same period (Figure 5A). This difference is statistically significant (P=0.016) and suggests that protein F1 attenuates virulence. When 106 bacteria were injected, 50% (three of six) of the AP1(pPTF8)-injected mice died, compared to 100% (six of six) of the mice injected with AP1(pLZ12) (data not shown). Although not statistically significant (P=0.18), the results could indicate that binding of Fn also at this high bacterial load influences virulence.
Figure 5.
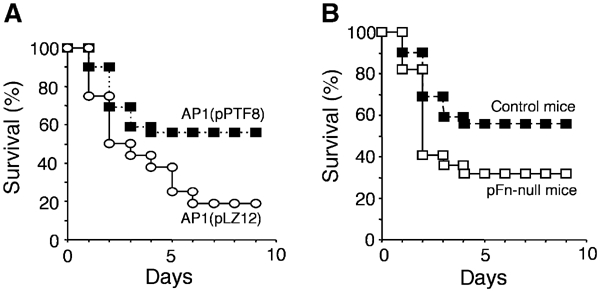
I.p. challenge of mice with S. pyogenes. (A) Control mice were injected i.p. with 105 AP1(pLZ12) or AP1(pPTF8) bacteria. The mice were monitored daily, and the percentage of surviving mice at various days after injection is shown. Significantly more mice infected with AP1(pPTF8) were alive at the end of the experiment (P=0.017). (B) Survival rate in percent when control and pFn-null mice were injected i.p. with 105 AP1(pPTF8) bacteria. At the end of the experiment, 56% of the control mice were alive as compared to 32% of the pFN-null mice (P=0.07).
As Fn-binding bacteria (AP1(pPTF8)) were less virulent to control mice, the effect of pFn on bacterial virulence was investigated using pFn-null mice. In all, 105 AP1(pPTF8) bacteria were injected i.p. into such animals. While 68% (15 of 22) of the pFn-null animals died within 8 days after injection, 44% (17 of 39) of the control mice died during the same time period (Figure 5B). This difference is not statistically significant (P=0.07), but the results could suggest that the reduced virulence of AP1(pPTF8) bacteria in control mice (Figure 5A) is partially restored in pFn-null mice. As pFn-null mice still express cFn in all tissues and protein F1 can interact with cFn (Ozeri et al, 1996; Molinari et al, 1997), the data support the notion that cFn prevents full restoration of virulence in pFn-null mice.
Fn-binding AP1 bacteria disseminate less efficiently to the spleen of infected mice
When AP1 bacteria are injected i.p. into mice, bacteria spread to the spleen within a few hours after administration (data not shown). To investigate whether bacterial Fn binding and pFn could affect the dissemination process, and hence could be responsible for the reduced virulence, a system was designed where different variants of AP1 bacteria were injected into control mice or pFn-null mice. To ensure that the effect of protein F1 on virulence was due to the Fn-binding capacity of the protein, plasmid constructs were made containing the protein F1 gene without the RD and UFBD domains (Figure 6A). These constructs were electroporated into AP1 bacteria, generating AP1(pPTF8ΔBD). AP1(pPTF8ΔBD) bacteria showed the same growth rate as AP1(pLZ12) and AP1(pPTF8) bacteria at Cm concentrations up to 6 μg/ml (data not shown). Also, AP1(pPTF8ΔBD) bacteria only bound background levels of radiolabelled Fn (Figure 6B) and did not absorb Fn from mouse serum (Figure 6C). Finally, AP1(pPTF8ΔBD) bacteria behaved as AP1(pLZ12) bacteria in experiments analysing the adherence to and internalisation into fibroblasts (Figure 6D and E) and epithelial cells (data not shown). These data verify earlier findings that protein F1 interacts with Fn through the RD and the UFBD domains, and demonstrate that these domains are responsible for the contribution of protein F1 to plasma- and cellular Fn-mediated adhesion and internalisation.
Figure 6.
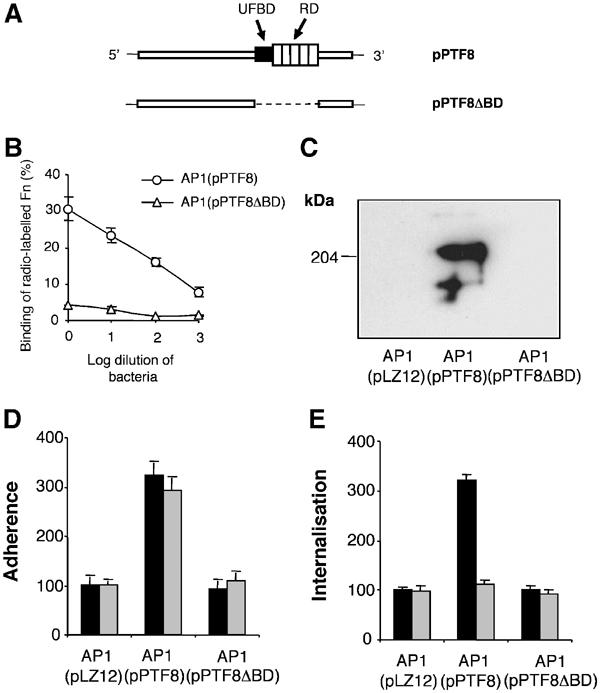
Characterisation of S. pyogenes mutant bacteria expressing protein F1 lacking the Fn-binding domains. (A) A plasmid with a truncated protein F1 gene, lacking the regions coding for the upstream Fn-binding domain (UFBD) and the repeat-containing domain (RD), was generated by performing inverse PCR on the plasmid pPTF8. The resulting plasmid, designated pPTF8ΔBD, was electroporated into AP1 bacteria, generating AP1(pPTF8ΔBD) bacteria. (B) The binding of human 125I-labelled Fn to serial log dilutions (from 2 × 109 cfu's) of AP1(pPTF8) and AP1(pPTF8ΔBD) bacteria is shown. The error bars indicate ±s.d. (C) AP1(pLZ12), AP1(pPTF8), or AP1(pPTF8ΔBD) bacteria were incubated with 10% mouse serum, and bound proteins were eluted with 0.1 M glycine-HCl, pH 2.0. The proteins were separated by SDS–PAGE (reducing conditions) and subjected to Western blot analysis using anti-Fn antibodies. (D, E) The adherence to and internalisation into human fibroblasts was determined for AP1(pLZ12), AP1(pPTF8), and AP1(pPTF8ΔBD) bacteria. Experiments were performed in the presence of serum from control mice (black bars) or serum from pFn-null mice (grey bars). Values are the means of three experiments with triplicate samples, given as a percentage of the mean adhesion or internalisation of AP1(pLZ12). Error bars±s.e.m. are indicated.
For the dissemination experiments, control mice or pFn-null mice were injected ip with AP1(pLZ12), AP1(pPTF8), or AP1(pPTF8ΔBD) bacteria. The mice were killed 24 h later to determine the number of colony-forming units (cfu) in their spleens (Figure 7). Control mice infected with AP1(pPTF8) bacteria expressing protein F1 had significantly less bacteria in the spleens than control mice infected with AP1(pLZ12) or AP1(pPTF8ΔBD) bacteria. Furthermore, a significantly lower number of AP1(pPTF8) bacteria as compared to AP1(pLZ12) or AP1(pPTF8ΔBD) bacteria were isolated from the spleens of pFn-null mice. Finally, the number of AP1(pPTF8) bacteria in the spleens of pFn-null mice was significantly higher than in control mice. Combined, these results suggest that protein F1 reduces the spreading from the peritoneal cavity to the spleen by interacting with both pFn and cFn, and that the reduction is dependent on the presence of the Fn-binding domains of protein F1.
Figure 7.
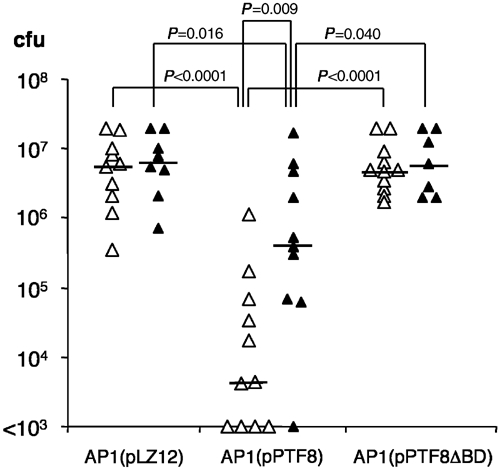
Bacterial dissemination to the spleen. Control mice (open symbols) or pFn-null mice (black symbols) were injected i.p. with AP1(pLZ12), AP1(pPTF8), or AP1(pPTF8ΔBD). The mice were killed 24 h after injection and the total number of cfu's in the spleen was determined for each mouse. The lines represent the median value in each group. P-values were determined with the Mann–Whitney U-test.
S. pyogenes adherence to and entry into mouse fibroblasts lacking cFn
To further test whether cFn can modulate S. pyogenes adherence to and entry into mammalian cells, embryonic fibroblasts were isolated from floxed Fn mice, immortalised with the SV40 large T antigen and subsequently treated with adenovirus transducing Cre to delete both Fn genes (Fn−/−). Western blotting revealed high cFn expression in floxed cells and confirmed the complete absence of cFn expression upon Cre-mediated deletion (data not shown). Initial experiments demonstrated that bacterial adherence and entry of control mouse fibroblasts and human foreskin fibroblasts were similar. In control serum, adhesion of AP1(pPTF8) bacteria to Fn−/− fibroblasts was increased by 78% (95% confidence interval 46–110%) compared to AP1(pLZ12) and by 65% (95% confidence interval 35–90%) as compared to AP1(pPTF8ΔBD). In contrast, there was no difference in adhesion between the three bacterial strains when pFn-null serum was used (Figure 8A). Similarly, in the presence of control serum, internalisation of AP1(pPTF8) was increased over three-fold compared to AP1(pLZ12) (95% confidence interval 2.9–3.5) and AP1(pPTF8ΔBD) (95% confidence interval 3.0–3.5), but there were no significant differences between the bacterial strains in experiments performed in pFn-null serum (Figure 8B). Taken together, these data suggest that both cFn and pFn participate in protein F1-mediated adhesion, whereas protein F1-mediated entry depends on pFn only.
Figure 8.
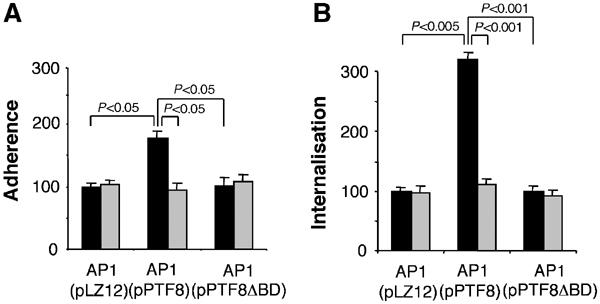
S. pyogenes adhesion and internalisation experiments using murine cells lacking cFn. Adherence and internalisation experiments were performed with mouse fibroblasts lacking the Fn gene and AP1(pLZ12), AP1(pPTF8), or AP1(pPTF8ΔBD) bacteria (A, B). The experiments were performed in the presence of serum from control mice (black bars) or serum from pFn-null mice (grey bars). Values are the means of four experiments with triplicate samples, given as a percentage of the mean adhesion or internalisation of AP1(pLZ12). Error bars±s.e.m. and P-values are indicated.
Discussion
Fn-binding proteins are important for the adhesion and cellular entry of several major bacterial pathogens, suggesting that these molecules could play a role in virulence. However, virulence is a highly polygenic property and it is difficult to design models, especially animal models, by which the significance of a putative virulence factor can be explicitly investigated. The aim of the present study was therefore to establish an experimental system where Fn-dependent adhesion and internalisation could be studied in vitro, and be directly correlated to virulence in vivo. This was achieved by utilising a combination of well-defined S. pyogenes mutant strains, different cell lines, and transgenic mice.
Binding of Fn is crucial for S. pyogenes adhesion and in most S. pyogenes strains protein F1 is the major Fn-binding protein. As shown here, the introduction of protein F1 in a strain with low Fn-binding activity resulted in a 65% increase of adherence to epithelial cells. This effect was dependent on the presence of pFn, suggesting that binding of pFn promotes adherence. Most studies of S. pyogenes adhesion have used epithelial cells (for references, see Courtney et al, 2002), cells that the bacteria will encounter when, for instance, colonising the throat. However, viral infections that often precede S. pyogenes pharyngitis will damage the epithelial cell layer and expose subepithelial cellular and matrix structures. Moreover, in wounded skin or deep tissue infections, S. pyogenes will come across numerous host cell types and matrix components. This study shows that the adhesion of S. pyogenes to fibroblasts does not require serum. The cFn produced by these cells, present both as an intercellular matrix and at the cell surface, is apparently sufficient to promote bacterial adhesion. AP1(pLZ12) bacteria lacking protein F1 and AP1(pPTF8ΔBD) bacteria expressing protein F1 without the Fn-binding domains adhered similarly to fibroblasts and to epithelial cells, whereas the adherence of bacteria expressing intact protein F1 increased about three-fold. The same increase was recorded when normal fibroblasts and pFn-null serum were used, but not with fibroblasts lacking cFn. Thus, a majority of Fn-binding bacteria will adhere to fibroblasts also in the absence of pFn, most likely through binding to cFn via the RD and the UFBD domains of protein F1.
The ability of S. pyogenes to invade human epithelial cells was first described by LaPenta et al (1994), a property that has been correlated with the failure to eradicate the bacteria with penicillin allowing for recurrent infection (Neeman et al, 1998). In the present work, protein F1 was found to enhance internalisation in a pFn-dependent manner. This should be due to interactions between pFn, bound to protein F1 at the bacterial surface, and Fn-binding integrins (Ozeri et al, 1998). Moreover, even though protein F1-mediated adhesion to fibroblasts also occurred in the absence of pFn, the increased entry into fibroblasts by protein F1-expressing bacteria was found to require pFn. At least during inflammatory and exudative phases of S. pyogenes infection, pFn will be available to the bacteria and facilitate entry into human cells.
In the present study, expression of protein F1 was found to reduce the virulence of S. pyogenes. This finding was quite unexpected, as the introduction of protein F1 into the AP1 strain did not affect growth rate or phagocytic killing, measured as the ability of the bacteria to survive in human blood. Moreover, a number of previous investigations have shown that most surface proteins of S. pyogenes enhance virulence (for references, see Navarre and Schneewind, 1999). LaPenta et al (1994) showed a correlation between high internalisation rates and invasive disease, and S. pyogenes mutants that are deficient in the Fn-binding proteins SOF, Fba, and FbaB were all reported to show attenuated virulence (Courtney et al, 1999; Terao et al, 2001, 2002). However, it is possible that functions other than Fn binding could contribute to their role in virulence. Thus, SOF is also a lipoproteinase (Courtney et al, 1999) and Fba limits C3b deposition on the bacterial surface through binding of Factor H and Factor H-like protein-1 (Pandiripally et al, 2002). Moreover, a recent study demonstrated that Fn bound to the protein F1 homologue Sfb1 mediates interactions with collagens (Dinkla et al, 2003). These interactions were found to reduce phagocytosis, but their influence on virulence was not investigated. There are, however, previous studies that support our conclusion that Fn binding and internalisation decrease virulence. Epidemiological data revealed a negative correlation between Fn binding and severity of disease (Natanson et al, 1995), and S. pyogenes isolates from patients with systemic disease were reported to internalise poorly (Jadoun et al, 1998; Molinari and Chhatwal, 1998). Moreover, S. pyogenes expressing a virulence-promoting capsule do not internalise as efficiently as non-capsulated bacteria (Cywes and Wessels, 2001), and a recent study reported that a mutant of Staphylococcus aureus deficient in Fn binding was more virulent than Fn-binding wild-type bacteria (McElroy et al, 2002).
Protein F1 can interact with both pFn and cFn (Okada et al, 1997). As mentioned above, invading S. pyogenes bacteria are likely to encounter cFn present in the extracellular matrix and at the surface of many cell types. As the infection proceeds and an inflammatory response is induced, plasma proteins, including pFn, will extravasate due to increased vascular permeability. In this phase, or if bacteria disseminate to the bloodstream, interactions with pFn will dominate. In order to evaluate the relative contributions of pFn and cFn to S. pyogenes virulence during the course of infection, the Fn-binding AP1(pPTF8) strain was injected into pFn-null mice. These animals have a deletion of the Fn gene only in the hepatocytes where pFn is produced, while the production of cFn is normal in all other tissues (Sakai et al, 2001). Consequently, protein F1-expressing bacteria will interact exclusively with cFn in these mice. Although not statistically significant, there was a tendency that mice without pFn were more susceptible to lethal infections with Fn-binding bacteria. However, in these animals, the bacteria expressing protein F1 are still not as lethal as the bacteria lacking protein F1, when injected into normal mice. This difference could be explained by interactions between protein F1-expressing bacteria and cFn.
To further elucidate the relative importance of protein F1 and its binding to pFn and cFn, mice received a bacterial injection i.p. and were killed 24 h later. For these experiments, AP1(pPTF8ΔBD) bacteria expressing a truncated version of protein F1 lacking the Fn-binding domains (Figure 6A) were also used. Counting the number of bacteria recovered from the spleen revealed a significant difference in dissemination efficiency, depending on the presence of intact protein F1 with retained Fn-binding capacity. The finding that bacterial dissemination is reduced when protein F1 is expressed is in concordance with the challenge experiments (Figure 5A). Furthermore, pFn-null mice infected with protein F1-expressing bacteria had a significantly higher number of bacteria in their spleens than control mice infected with protein F1-expressing bacteria. Thus, pFn bound to S. pyogenes limits bacterial dissemination in this model. Increased internalisation, resulting in a local retention of the infection, could explain this observation. The virulence of protein F1-expressing bacteria was only partially restored in pFn-null mice, and in the absence of pFn S. pyogenes expressing protein F1 still disseminated less efficiently than bacteria lacking protein F1 or the Fn-binding domains of protein F1. The results obtained with control and cFn-null fibroblasts indicate that this is due to the RD and UFBD domains of protein F1 interacting with cFn. However, binding to cFn did not increase internalisation, suggesting that interactions between bacteria and cFn retain bacteria locally and reduce the number of bacteria that can spread, even though they are not internalised. As mentioned above, phagocytic killing was not influenced by protein F1, which further supports the notion that the reduced virulence of Fn-binding S. pyogenes is the result of bacteria being immobilised by host cells and tissues.
In cases of severe S. pyogenes infections, including streptococcal toxic shock syndrome (STSS), strains of the M1 serotype are the most frequent (Stevens, 2000). A recent study suggests that the formation of complexes between M1 protein released from the bacterial surface, and fibrinogen, could play a pathogenic role in these severe cases by inducing vascular leakage (Herwald et al, 2004). In relation to the present investigation, it is noteworthy that M1 strains commonly lack the protein F1 gene (Natanson et al, 1995), indicating that this could also contribute to their virulence. Invasive S. pyogenes infections and STSS are connected with high mortality rates, which have led to an increased demand for alternative therapeutic strategies. Specific inhibition of host–bacterial interactions, such as adhesion and internalisation mediated by Fn-binding, has been suggested as a treatment of these serious infections. However, the present finding that expression of protein F1 in a highly virulent M1 strain results in decreased virulence suggests that therapeutic intervention directed toward this protein might actually increase the risk of a fatal outcome. S. pyogenes can be regarded as a relatively unsuccessful parasite that can survive and grow only in humans, where it probably also elicits a too powerful inflammatory response (Kotb et al, 2002). It could be that this pathogen, by the acquisition of Fn-binding activity, is striving to decrease its virulence and establish a more peaceful and evolutionary advantageous co-existence with its human host.
Materials and methods
Bacterial strains, growth conditions, and plasmids
The S. pyogenes AP1 strain is from the AP collection, Institute of Hygiene and Epidemiology, Prague, Czech Republic. Plasmids pLZ12 (carrying Cm and kanamycin (Km) resistance genes) and pPTF8 (pLZ12 also carrying the protein F1 gene) (Hanski and Caparon, 1992) were purified from DH5α strains and electroporated into the AP1 strain, thus generating Cm- and Km-resistant clones AP1(pLZ12) and AP1(pPTF8). For generation of AP1(pPTF8ΔBD), the Fn-binding domains of protein F1 determined previously (Sela et al, 1993) were replaced with an HA tag (influenza A virus haemagglutinin epitope) as follows: pPTF8 was used as template in an ‘inside-out' PCR reaction with primers, prtFΔBD-up (CCGGAATTCAGACTCACCCGCTAGAGGTGATTGG) and prtFΔBD-dn (CCGGAATTCTACCCATACGATGTTCCAGATTACGCTT TCAGTGAAACGGCGACTGTTG). Digestion of the PCR fragment with EcoRI (sites underlined), followed by re-ligation, resulted in an in-frame replacement of the Fn-binding domain of PrtF (G370–G582) with the HA epitope (Y P Y D V P D Y A, the DNA sequence in prtFΔBD-dn is indicated in bold). The fidelity of the construct was verified by sequencing. The resulting plasmid pPTF8ΔBD was used to transform Escherichia coli (DH5α) and S. pyogenes (AP1), and similar levels of prtF1 gene expression from AP1(pPTF8) and AP1(pPTF8ΔBD) bacteria were confirmed with Northern blotting using a prtF1-specific probe (CCTGGATATAATATTTGGACTCGTTATCATGACTTGAGAG TAAATTTAAATGGGAGTCGG), hybridising with the 5′ part of the gene.
S. pyogenes strains were grown in Todd-Hewitt broth (Difco, Detroit, DI, USA) with 0.2% yeast extract (Difco) (THY) in 5% CO2 at 37°C. For growth of the transformed strains, 500 μg/ml of Km and 3 μg/ml of Cm were added. E. coli strains were grown in Luria Bertoni broth (10 g tryptone (Difco), 10 g NaCl, and 5 g yeast extract (Difco)/l), supplemented with 25 μg Km/ml and 15 μg Cm/ml.
Binding assays
Binding assays were performed using human Fn (Sigma) radiolabelled with IODO-BEADS (Pierce). Free 125I was separated from labelled protein on a PD10 column (Amersham Pharmacia Biotech). In the binding assays, bacteria were grown overnight, washed, resuspended in PBS, and bacterial concentrations were determined by measuring optical density at 620 nm. Bacteria were diluted to appropriate concentrations in PBS with 0.05% Tween 20 and 0.02% azide, and incubated with the radiolabelled protein for 30 min. After centrifugation, the radioactivity of the pellets was determined and expressed as a percentage of added radioactivity, deducing binding of the protein to the polyprolene tubes.
Serum absorption experiments were performed by culturing bacteria overnight, washing once, and incubating 2 × 109 bacteria in 100 μl of PBS and 100 μl of serum for 30 min. The bacteria were washed five times with PBS and bound proteins were eluted with 0.1 M glycine-HCl (pH 2.0). The supernatant was collected and neutralised with 1 M Tris. Protein samples were separated by SDS–polyacrylamide gel electrophoresis (PAGE) (Neville, 1971), containing 8 or 12% of acrylamide, and blotted onto an Immobilon-P™ polyvinylidene difluoride filter. The filter was subjected to immunodetection with polyclonal rabbit anti-Fn antibodies diluted 1:5000 (DAKO, Denmark). Horseradish peroxidase-conjugated goat anti-rabbit antibodies (BIO-RAD) were used as secondary antibodies, and immunodetection was achieved by chemiluminescence (Nesbitt and Horton, 1992). Alternatively, the gel was stained using Commassie Brilliant blue or silver staining (Heukeshoven and Dernick, 1988).
Adhesion and internalisation assays
AP1(pLZ12), AP1(pPTF8), or AP1(pPTF8ΔBD) were grown overnight, washed once in PBS, and diluted in minimal essential medium with Earle's salt (MEM) (Life Technologies) with or without supplemented serum, to a final concentration of approximately 5 × 105 bacteria/ml. Foetal bovine serum (FBS), serum from control mice, or serum from pFn-null mice, was used to supplement MEM in the assays (similar results were obtained with FBS supplementation and with serum from normal mice). Human pharyngeal epithelial Detroit 562 cells (ATCC CCL-138) or human foreskin fibroblasts were grown in 24-well tissue plates (Costar) in MEM supplemented with antibiotics, glutamine, and 10% FBS. Near-confluent monolayers of cells were washed three times in PBS and three times in unsupplemented MEM, to remove antibiotics and serum. Subsequently, 1 ml bacterial suspension was added to each well and the plate was incubated for 2 h with 5% CO2 at 37°C, followed by five washes with PBS and release of bacteria as described previously (Berry and Paton, 1996). Alternatively, to determine the number of internalised bacteria, the wells were washed and incubated a further 2 h in MEM supplemented with 100 μg gentamycin and 5 μg of penicillin to kill extracellular bacteria, and then washed three times with PBS to remove antibiotics. Bacteria were released as above and viable counts were performed. The number of adherent and internalised bacteria were related to the size of the inoculum, and expressed as a percentage of the number of adherent or internalised AP1(pLZ12) bacteria, respectively. All calculations for statistical significance were carried out using an unpaired Student's t-test, two-tailed.
ELISA
Human pharyngeal epithelial Detroit 562 cells or fibroblasts were grown to confluence in microtitre plates (NUNCLON™ Surface, NUNC, Denmark). The wells were washed six times in PBST and blocked using PBST with 2% (w/v) bovine serum albumin (BSA) for 30 min at 37°C. As a control, wells were coated with purified Fn in different concentrations. Primary rabbit anti-Fn antibodies (Sigma) were applied to the wells in two-fold dilutions (from 1:500) and the plates were incubated at 37°C for 1 h. The wells were washed and horseradish peroxidase-conjugated goat anti-rabbit antibodies (BIO-RAD) were applied. After a 1 h incubation at 37°C, the wells were washed and substrate solution (0.1% diammonium-2,2-azino-bis (3-ethyl-2,3-dihydrobenzthiazoline)-6-sulphonate (ABTS), 0.012% (v/v) H2O2 in 100 mM citric acid, 100 mM NaH2PO4, pH 4.5) was added. The change in optical density at 415 nm was determined after 10 min.
Generation of fibroblast-like cells lacking fibronectin
To establish fibroblast-like cells, primary cells of E13.5 Fn (flox/flox) mouse embryos were immortalised by the transfection of SV-40 large T antigen, cloned, and several immortalised and clonal fibroblast-like cell lines were generated. Subsequently, two clonal lines were treated with a cre-transducing adenovirus to delete the floxed Fn genes. The deletion of Fn alleles in both cre-treated clones was confirmed by PCR and the lack of Fn protein expression by immunoprecipitation using metabolically labelled conditioned media of these cells.
Animal challenge studies
The construction and characterisation of the pFn-deficient mice was described elsewhere (Sakai et al, 2001). Briefly, mice carrying a floxed Fn gene and expressing Cre under control of the interferon and polyinosinic-polycytidic acid (pI-pC)-inducible Mx promoter (Mx-Cre+) were generated. After i.p. injection with pI-pC, pFn levels were undetectable by Western analysis, whereas production of Fn in other tissues was only marginally affected. The pFn-deficient mice showed no overt phenotype or abnormalities up to 8 months of age. The absence of pFn was confirmed by Western analyses in the animals used in this study, and these mice are referred to as pFn-null mice. Mice carrying a floxed Fn gene (Fn(fl/fl)) were used as controls after injection with pI-pC.
Bacteria were grown to early logarithmic growth phase (OD620∼0.35), harvested, washed in PBS, diluted in PBS to an appropriate concentration, and kept on ice until injection. Colony counts of the inocolum verified that identical numbers of bacteria were injected. The mice were treated twice daily with 300 μg Cm (a nontoxic dose) throughout the experiment in order for the bacteria to retain the transfected plasmids. This corresponds to a peak plasma concentration of approximately 3 μg/ml (Joiner et al, 1981). The first Cm injection took place 2 h before injection of the bacteria. The mice were monitored twice daily for 8 days after injection and the time of death for each mouse was recorded. Post mortem, the spleens were removed and homogenised, in order to recover disseminated bacteria. To verify that the bacteria retained their plasmids throughout the infection, the homogenate was plated on THY plates with or without Km and Cm. This was performed on several mice, and on all occasions the number of bacteria was unaffected by the presence of antibiotics. Colonies from plates without antibiotics were reinoculated with antibiotics. This was repeated with bacterial colonies from several different mice, for a total of 100 colonies, and all colonies grew on antibiotic-containing plates. After 8 days, surviving mice were killed. Statistical analyses for differences in the number of dead animals between groups were carried out using Fisher's exact test.
Dissemination to the spleen
Bacteria were grown to early logarithmic phase (OD620∼0.35), harvested, washed in PBS, diluted in PBS to appropriate concentrations, and kept on ice until injection. After i.p. injection of the bacteria, the mice were killed after 24 h, the spleens were removed, homogenised in 1 ml of PBS, and viable counts were performed. The mice were treated with Cm (300 μg/ml) 2 h prior to injection and 12 h after injection. Statistical analyses for differences between groups were carried out by using the Mann–Whitney U-test.
Acknowledgments
We thank Ingbritt Gustafsson for excellent technical assistance. This work was supported by grants from the Swedish Research Council (projects 7480 and 14379), the Medical Faculty, Lund University, the Foundations of Kock and Österlund, and Hansa Medical AB to LB, and the DFG, Max Planck Society, and the Fonds der Chemischen Industrie to RF.
References
- Abraham SN, Beachey EH, Simpson WA (1983) Adherence of Streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun 41: 1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbaugh CD, Warren HB, Carey VJ, Wessels MR (1998) Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J Clin Invest 102: 550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AM, Paton JC (1996) Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun 64: 5255–5262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björck L, Åkesson P, Bohus M, Trojnar J, Abrahamson M, Olafsson I, Grubb A (1989) Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature 337: 385–386 [DOI] [PubMed] [Google Scholar]
- Cockerill FR III, Thompson RL, Musser JM, Schlievert PM, Talbot J, Holley KE, Harmsen WS, Ilstrup DM, Kohner PC, Kim MH, Frankfort B, Manahan JM, Steckelberg JM, Roberson F, Wilson WR (1998) Molecular, serological, and clinical features of 16 consecutive cases of invasive streptococcal disease. Southeastern Minnesota Streptococcal Working Group. Clin Infect Dis 26: 1448–1458 [DOI] [PubMed] [Google Scholar]
- Courtney HS, Hasty DL, Dale JB (2002) Molecular mechanisms of adhesion, colonization, and invasion of group A streptococci. Ann Med 34: 77–87 [DOI] [PubMed] [Google Scholar]
- Courtney HS, Hasty DL, Li Y, Chiang HC, Thacker JL, Dale JB (1999) Serum opacity factor is a major fibronectin-binding protein and a virulence determinant of M type 2 Streptococcus pyogenes. Mol Microbiol 32: 89–98 [DOI] [PubMed] [Google Scholar]
- Cue D, Dombek PE, Lam H, Cleary PP (1998) Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect Immun 66: 4593–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cue D, Southern SO, Southern PJ, Prabhakar J, Lorelli W, Smallheer JM, Mousa SA, Cleary PP (2000) A nonpeptide integrin antagonist can inhibit epithelial cell ingestion of Streptococcus pyogenes by blocking formation of integrin α5β1–fibronectin–M1 protein complexes. Proc Natl Acad Sci USA 97: 2858–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW (2000) Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13: 470–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cywes C, Wessels MR (2001) Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature 414: 648–652 [DOI] [PubMed] [Google Scholar]
- Dinkla K, Rohde M, Jansen WT, Carapetis JR, Chhatwal GS, Talay SR (2003) Streptococcus pyogenes recruits collagen via surface-bound fibronectin: a novel colonization and immune evasion mechanism. Mol Microbiol 47: 861–869 [DOI] [PubMed] [Google Scholar]
- Fischetti VA (1989) Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev 2: 285–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick IM, Crossin KL, Edelman GM, Björck L (1995) Protein H—a bacterial surface protein with affinity for both immunoglobulin and fibronectin type III domains. EMBO J 14: 1674–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granok AB, Parsonage D, Ross RP, Caparon MG (2000) The RofA binding site in Streptococcus pyogenes is utilized in multiple transcriptional pathways. J Bacteriol 182: 1529–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E, Caparon M (1992) Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci USA 89: 6172–6176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E, Horwitz PA, Caparon MG (1992) Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun 60: 5119–5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwald H, Cramer H, Mörgelin M, Russel W, Sollenberg U, Norrby-Teglund A, Flodgaard H, Lindbom L, Björck L (2004) M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell 116: 367–379 [DOI] [PubMed] [Google Scholar]
- Heukeshoven J, Dernick R (1988) Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis 9: 28–32 [DOI] [PubMed] [Google Scholar]
- Hynes RO (1990) Fibronectins. New York: Springer-Verlag [Google Scholar]
- Jadoun J, Burstein E, Hanski E, Sela S (1997) Proteins M6 and F1 are required for efficient invasion of group A streptococci into cultured epithelial cells. Adv Exp Med Biol 418: 511–515 [DOI] [PubMed] [Google Scholar]
- Jadoun J, Ozeri V, Burstein E, Skutelsky E, Hanski E, Sela S (1998) Protein F1 is required for efficient entry of Streptococcus pyogenes into epithelial cells. J Infect Dis 178: 147–158 [DOI] [PubMed] [Google Scholar]
- Jaffe J, Natanson-Yaron S, Caparon MG, Hanski E (1996) Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two binding domains. Mol Microbiol 21: 373–384 [DOI] [PubMed] [Google Scholar]
- Joiner KA, Lowe BR, Dzink JL, Bartlett JG (1981) Antibiotic levels in infected and sterile subcutaneous abscesses in mice. J Infect Dis 143: 487–494 [DOI] [PubMed] [Google Scholar]
- Kiska DL, Thiede B, Caracciolo J, Jordan M, Johnson D, Kaplan EL, Gruninger RP, Lohr JA, Gilligan PH, Denny FW Jr (1997) Invasive group A streptococcal infections in North Carolina: epidemiology, clinical features, and genetic and serotype analysis of causative organisms. J Infect Dis 176: 992–1000 [DOI] [PubMed] [Google Scholar]
- Kotb M, Norrby-Teglund A, McGeer A, El-Sherbini H, Dorak MT, Khurshid A, Green K, Peeples J, Wade J, Thomson G, Schwartz B, Low DE (2002) An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat Med 8: 1349–1350 [DOI] [PubMed] [Google Scholar]
- LaPenta D, Rubens C, Chi E, Cleary PP (1994) Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Sci USA 91: 12115–12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy MC, Cain DJ, Tyrrell C, Foster TJ, Haslett C (2002) Increased virulence of a fibronectin-binding protein mutant of Staphylococcus aureus in a rat model of pneumonia. Infect Immun 70: 3865–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari G, Chhatwal GS (1998) Invasion and survival of Streptococcus pyogenes in eukaryotic cells correlates with the source of the clinical isolates. J Infect Dis 177: 1600–1607 [DOI] [PubMed] [Google Scholar]
- Molinari G, Rohde M, Guzman CA, Chhatwal GS (2000) Two distinct pathways for the invasion of Streptococcus pyogenes in non-phagocytic cells. Cell Microbiol 2: 145–154 [DOI] [PubMed] [Google Scholar]
- Molinari G, Talay SR, Valentin-Weigand P, Rohde M, Chhatwal GS (1997) The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun 65: 1357–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF (1989) Fibronectin. San Diego: Academic Press Inc [Google Scholar]
- Natanson S, Sela S, Moses AE, Musser JM, Caparon MG, Hanski E (1995) Distribution of fibronectin-binding proteins among group A streptococci of different M types. J Infect Dis 171: 871–878 [DOI] [PubMed] [Google Scholar]
- Navarre WW, Schneewind O (1999) Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev 63: 174–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman R, Keller N, Barzilai A, Korenman Z, Sela S (1998) Prevalence of internalisation-associated gene, prtF1, among persisting group-A streptococcus strains isolated from asymptomatic carriers. Lancet 352: 1974–1977 [DOI] [PubMed] [Google Scholar]
- Nesbitt SA, Horton MA (1992) A nonradioactive biochemical characterization of membrane proteins using enhanced chemiluminescence. Anal Biochem 206: 267–272 [DOI] [PubMed] [Google Scholar]
- Neville DM Jr (1971) Molecular weight determination of protein–dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem 246: 6328–6334 [PubMed] [Google Scholar]
- Okada N, Watarai M, Ozeri V, Hanski E, Caparon M, Sasakawa C (1997) A matrix form of fibronectin mediates enhanced binding of Streptococcus pyogenes to host tissue. J Biol Chem 272: 26978–26984 [DOI] [PubMed] [Google Scholar]
- Österlund A, Engstrand L (1995) Intracellular penetration and survival of Streptococcus pyogenes in respiratory epithelial cells in vitro. Acta Otolaryngol 115: 685–688 [DOI] [PubMed] [Google Scholar]
- Österlund A, Popa R, Nikkila T, Scheynius A, Engstrand L (1997) Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope 107: 640–647 [DOI] [PubMed] [Google Scholar]
- Owens MR, Cimino CD (1982) Synthesis of fibronectin by the isolated perfused rat liver. Blood 59: 1305–1309 [PubMed] [Google Scholar]
- Ozeri V, Rosenshine I, Mosher DF, Fassler R, Hanski E (1998) Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol Microbiol 30: 625–637 [DOI] [PubMed] [Google Scholar]
- Ozeri V, Tovi A, Burstein I, Natanson-Yaron S, Caparon MG, Yamada KM, Akiyama SK, Vlodavsky I, Hanski E (1996) A two-domain mechanism for group A streptococcal adherence through protein F to the extracellular matrix. EMBO J 15: 989–998 [PMC free article] [PubMed] [Google Scholar]
- Pancholi V, Fischetti VA (1992) A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med 176: 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiripally V, Gregory E, Cue D (2002) Acquisition of regulators of complement activation by Streptococcus pyogenes serotype M1. Infect Immun 70: 6206–6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M, Müller HP, Björck L (1999) Protein GRAB of Streptococcus pyogenes regulates proteolysis at the bacterial surface by binding α2-macroglobulin. J Biol Chem 274: 15336–15344 [DOI] [PubMed] [Google Scholar]
- Rocha CL, Fischetti VA (1999) Identification and characterization of a novel fibronectin-binding protein on the surface of group A streptococci. Infect Immun 67: 2720–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Johnson KJ, Murozono M, Sakai K, Magnuson MA, Wieloch T, Cronberg T, Isshiki A, Erickson HP, Fässler R (2001) Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med 7: 324–330 [DOI] [PubMed] [Google Scholar]
- Schmidt KH, Mann K, Cooney J, Köhler W (1993) Multiple binding of type 3 streptococcal M protein to human fibrinogen, albumin and fibronectin. FEMS Immunol Med Microbiol 7: 135–143 [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE (1991) Fibronectin: from gene to protein. Curr Opin Cell Biol 3: 786–791 [DOI] [PubMed] [Google Scholar]
- Sela S, Aviv A, Tovi A, Burstein I, Caparon MG, Hanski E (1993) Protein F: an adhesin of Streptococcus pyogenes binds fibronectin via two distinct domains. Mol Microbiol 10: 1049–1055 [DOI] [PubMed] [Google Scholar]
- Stevens DL (2000) Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu Rev Med 51: 271–288 [DOI] [PubMed] [Google Scholar]
- Talay SR, Valentin-Weigand P, Jerlström PG, Timmis KN, Chhatwal GS (1992) Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun 60: 3837–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talay SR, Zock A, Rohde M, Molinari G, Oggion M, Pozzi G, Guzman CA, Chhatwal GS (2000) Co-operative binding of human fibronectin to Sfbl protein triggers streptococcal invasion into respiratory epithelial cells. Cell Microbiol 2: 521–535 [DOI] [PubMed] [Google Scholar]
- Terao Y, Kawabata S, Kunitomo E, Murakami J, Nakagawa I, Hamada S (2001) Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol Microbiol 42: 75–86 [DOI] [PubMed] [Google Scholar]
- Terao Y, Kawabata S, Nakata M, Nakagawa I, Hamada S (2002) Molecular characterization of a novel fibronectin-binding protein of Streptococcus pyogenes strains isolated from toxic shock-like syndrome patients. J Biol Chem 277: 47428–47435 [DOI] [PubMed] [Google Scholar]
- Wexler DE, Chenoweth DE, Cleary PP (1985) Mechanism of action of the group A streptococcal C5a inactivator. Proc Natl Acad Sci USA 82: 8144–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]


