Abstract
The optimal sequence of systemic chemotherapy in metastatic breast cancer (MBC) is unknown. We report the case of a woman who was successfully treated with nanoparticle albumin-bound (nab)-paclitaxel for triple negative MBC in our institution. In November 2008, a 48-year-old woman underwent surgical treatment for a triple negative invasive ductal breast cancer and subsequently received adjuvant chemotherapy with fluorouracil/epirubicin/cyclophosphamide and radiotherapy. Sixteen months after surgery, she presented with a left chest wall metastatasis. The patient received combination therapy with conventional paclitaxel (90mg/m² weekly for 3 out of 4 weeks [QW 3/4]) and bevacizumab (10mg/kg every 2 weeks [Q2W]) as first-line treatment for MBC (six cycles; March to September 2010) and achieved a partial response at the metastatic site. Bevacizumab monotherapy was continued until disease progression (April 2011) with the development of a single infraclavicular lymph node metastasis and an increase in the dimensions of the left chest wall lesion. From May to December 2011, the patient received nab-paclitaxel 260mg/m² every 3 weeks (Q3W) as second-line treatment (11 cycles). After three cycles, the left chest wall lesion and the infraclavicular lymph node metastasis were undetectable and the patient was considered to have achieved a complete response. Treatment was well tolerated with no significant toxicity or need for dose reduction. Given our case, here we review the clinical evidence and discuss the potential role of nab-paclitaxel for the treatment of triple negative MBC, a subgroup typically characterized as having aggressive disease and limited treatment options.
Keywords: breast cancer, metastatic, nab-paclitaxel, prognostic factors, triple negative
Introduction
Despite advances in screening and treatment, breast cancer remains a leading cause of death among women worldwide 1. Approximately 15–20% of patients with breast cancer have triple-negative (TN) breast tumors [i.e. no estrogen receptor (ER), progesterone receptor (PgR), or human epidermal growth factor receptor-2 (HER2) expression]. For these patients, no targeted therapy is available and chemotherapy remains the systemic treatment of choice.
TN tumors are usually more aggressive and associated with worse survival compared with non-TN tumors 2–4. Effective and fast-acting chemotherapy is therefore required, although the optimal treatment approach remains controversial. Some suggest that cyclophosphamide is a necessary component of adjuvant chemotherapy, and advocate approaches such as dose-dense cyclophosphamide/methotrexate/fluorouracil or six cycles of fluorouracil/epirubicin/cyclophosphamide (FEC) 5. Others suggest than TN tumors should not be treated with anthracyclines as they are topoisomerase 2-negative and HER2-negative 6. In the neoadjuvant setting, anthracycline/taxane regimens have resulted in a high pathological complete response rate in TN disease 7. Platinum agents are also considered to be particularly effective, especially in TN tumors with BRCA mutations 8. However, the literature is conflicting, and promising results have also been reported for other regimens, such as dose-dense epirubicin plus cyclophosphamide, followed by docetaxel 9.
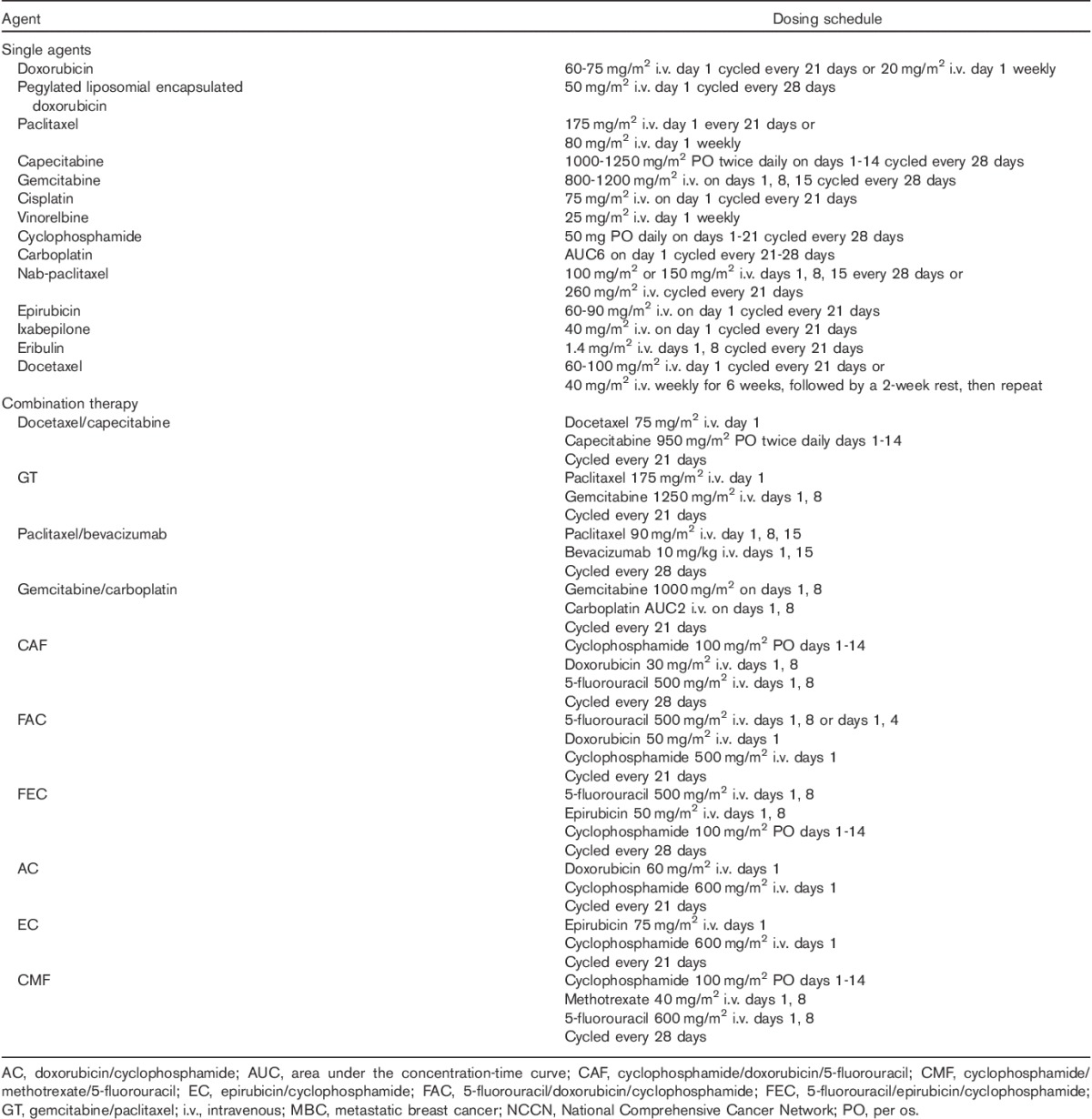
The use of chemotherapy in metastatic breast cancer (MBC) has been studied extensively, and a broad spectrum of agents are now available (Table 1) 10. Current guidelines suggest that as patients with metastatic triple negative breast cancer (mTNBC) frequently have visceral involvement, aggressive disease, and/or a risk of rapid deterioration, combination chemotherapy may be preferred 11. However, whether this offers an advantage over sequential therapy is an unsettled issue as results from clinical trials are conflicting. Currently, taxane-based regimens are the only standard of care first-line therapy for patients who have received anthracycline-based adjuvant therapy 11. However, the considerable toxicity profile of traditional taxanes (i.e. conventional paclitaxel and docetaxel) limits their suitability for some patients, and cumulative toxicity associated with long-term use is a major limitation for many more patients. A significant proportion of patients receiving traditional taxanes require treatment interruptions, delays, and/or discontinuations because of toxicity, and although premedication (corticosteroids, antihistamines, H2 antagonists, and granulocyte colony-stimulating factor) can ameliorate many toxicities, the use of these agents, especially long term, remains challenging 12.
Table 1.
Available chemotherapy agents/regimens for metastatic breast cancer recommended by the NCCN 10
Nanoparticle albumin-bound (nab)-paclitaxel (Abraxane; Celgene Corporation, Summit, New Jersey, USA) is a next-generation taxane that does not include a solvent. This reduces the risk of hypersensitivity reactions and eliminates the need for steroids and antihistamine premedication 13. The absence of a solvent and the presence of albumin in nab-paclitaxel allow higher doses of paclitaxel to be delivered compared with traditional taxanes as drug transport to the tumor is considered to be enhanced by albumin receptor (gp60)-mediated transcytosis 14 and by albumin binding to proteins such as SPARC (secreted protein, acidic, and rich in cysteine) at the site of the tumor 15.
The clinical benefits of nab-paclitaxel in MBC were reported in a large Phase III study which showed that, compared with conventional paclitaxel 175 mg/m2 administered every 3 weeks (Q3W), nab-paclitaxel 260 mg/m2 Q3W was associated with a higher objective response rate (33 vs. 19%, P=0.001) and prolonged progression-free survival (22.7 vs. 16.6 weeks, P=0.003) 12,16, and an improvement in median overall survival (OS) in patients who received nab-paclitaxel as greater than first-line therapy (56.4 vs. 46.7 weeks, P=0.024) 16. Nab-paclitaxel was also associated with a rapid and dramatic tumor response (shrinkage), with maximum response to nab-paclitaxel occurring by cycle 3 in 91% of responding patients 16. As expected with a 49% higher paclitaxel dose, treatment with nab-paclitaxel resulted in a higher incidence of grade 3 sensory neuropathy compared with conventional paclitaxel (10 vs. 2%, P<0.001). However, time to improvement of sensory neuropathy from grade 3 to grade ≤2 was more rapid with nab-paclitaxel (22 vs. 79 days) 12,16.
Findings from this Phase III study led to the regulatory approval of nab-paclitaxel 260 mg/m2 Q3W for the treatment of MBC. In Europe, it is licensed for use in adult patients who have failed first-line treatment for metastatic disease and for whom standard, anthracycline-containing therapy is not indicated 13.
The superior therapeutic index of nab-paclitaxel over traditional taxanes could make it a particularly attractive treatment for many patients with MBC, including those with mTNBC. However, there is a dearth of data describing its use in clinical practice. Here, we describe a clinical case of a patient with mTNBC who received nab-paclitaxel as second-line therapy for metastatic disease.
Case presentation
A 48-year-old white woman was diagnosed with early breast cancer in November 2008. She immediately underwent a left quadrantectomy with axillary lymph node dissection for a pT2 (2.1 cm) invasive ductal carcinoma. Histology showed that the disease was pN0 and poorly differentiated. Hormonal receptor status (ER and PgR) was negative, HER2 was not overexpressed, and Ki-67 was 40%. The patients received six cycles of adjuvant FEC (completed June 2009), followed by left breast irradiation.
Sixteen months after surgery, a left chest wall metastasis was detected that was histologically verified as ER negative, PR negative, HER2 1+, and Ki-67 30%. The patient was referred for surgery, but the lesion was considered inoperable as it was infiltrating the pectoral muscle. The patient received conventional paclitaxel [90 mg/m2 weekly for 3 of 4 weeks (QW 3/4)] plus bevacizumab [10 mg/kg every 2 weeks (Q2W)] as a first-line treatment for MBC, with a total of six cycles administered between March and September 2010. Treatment resulted in a partial response at the metastatic site.
In September 2010, conventional paclitaxel was discontinued because of grade 4 neurologic toxicity. Bevacizumab was continued as monotherapy until April 2011, when an increase in the left chest wall lesion dimensions was detected along with a single infraclavicular lymph node metastasis (Fig. 1a). As the patient had received six cycles of epirubicin as part of her adjuvant therapy and developed relapse after 9 months, she was considered anthracycline resistant. Nab-paclitaxel 260 mg/m2 Q3W was therefore selected as the most appropriate second-line treatment. After three cycles, the left chest wall lesion and the infraclavicular lymph node metastasis were undetectable (Fig. 1b), and the patient was considered to have achieved a complete response according to Response Evaluation Criteria for Solid Tumors 17. Nab-paclitaxel was continued at the same dose from May to December 2011 (11 cycles). Importantly, treatment was well tolerated, with no significant toxicity or need for dose reduction. The principal toxicities were mild sensory neuropathy and grade 2 fatigue. No febrile neutropenia was noted.
Fig. 1.

Computed tomography imaging of a 48-year-old patient with metastatic breast cancer at progression following first-line treatment with six cycles of conventional paclitaxel 90 mg/m2 QW 3/4 plus bevacizumab 10 mg/kg Q2W for 12 months (a) and then after the first three cycles of second-line treatment with nab-paclitaxel 260 mg/m2 Q3W (b). Arrows show the extent of the left chest wall lesion before and after nab-paclitaxel therapy. Q2W, every 2 weeks; Q3W, every 3 weeks; QW 3/4, weekly for 3 weeks of a 4 week cycle.
In July 2012, the patient experienced disease progression at the previously described metastatic sites. Subsequent treatments, including gemcitabine (1000 mg/m2 QW 2/3) plus carboplatin (AUC5 on day 1 Q3W), pegylated liposomal doxorubicin (50 mg/m2 every 4 weeks), and vinorelbine (25 mg/m2 QW), all yielded poor results. In May 2013, further disease progression resulted in general deterioration and the patient received best supportive care.
Discussion
MBC remains an incurable disease with a poor prognosis and a median 5-year survival of only 23–26% 18,19. As such, effective long-term management of MBC poses significant clinical challenges, and more effective and better tolerated agents are urgently needed 11. For patients with mTNBC, the lack of biomarkers to predict chemotherapy responsiveness or a consensus on the best agents to use mean that clinical judgment of the potential risk and benefit is the principal factor driving the physician’s choice of which chemotherapy to administer.
The patient presented in this clinical case had very aggressive early breast cancer, relapsing after less than 1 year of completing adjuvant chemotherapy. She experienced an excellent tumor response to first-line taxane chemotherapy, but had to discontinue conventional paclitaxel after six cycles because of unbearable neurotoxicity that significantly limited her quality of life. However, on the basis of her apparent sensitivity to taxane therapy, nab-paclitaxel was chosen as her second-line treatment, and despite having aggressive disease, our patient achieved a prolonged period of response with minimal and acceptable toxicities.
The decision to treat this patient with nab-paclitaxel is supported by the superior therapeutic index of this agent shown in the large Phase III trial 16. Emerging data from Phase II studies and retrospective analyses also suggest that nab-paclitaxel may be effective as part of combination therapy in patients with mTNBC 20,21 and in those who have received taxane previously 22,23 as it does not show cross-resistance with conventional paclitaxel or docetaxel, even in heavily pretreated patients 22. In addition, findings from a recent post-hoc analysis of data from the Phase III study indicate that nab-paclitaxel is associated with improved efficacy in subgroups of patients with characteristics typical of aggressive disease, such as those with at least three metastatic lesions, visceral-dominant metastases, and patients with a short disease-free interval 24–26. For patients receiving study treatment as greater than first-line therapy, i.e. the patient subgroup for whom nab-paclitaxel is currently licensed in MBC, nab-paclitaxel was associated with a significant improvement in OS in patients with at least three metastatic lesions (hazard ratio 0.71, P=0.037; Fig. 2a) and a trend toward improved OS in those with visceral-dominant disease (P=0.145; Fig. 2b) 24. As patients with mTNBC frequently have visceral involvement, aggressive disease, and/or a risk of rapid deterioration, these data also support the decision to treat our patient with nab-paclitaxel. Moreover, if confirmed in prospectively designed clinical studies, these findings would suggest that nab-paclitaxel shows considerable clinical activity in virulent MBC and may be preferentially selected to treat patients with more aggressive tumor characteristics.
Fig. 2.
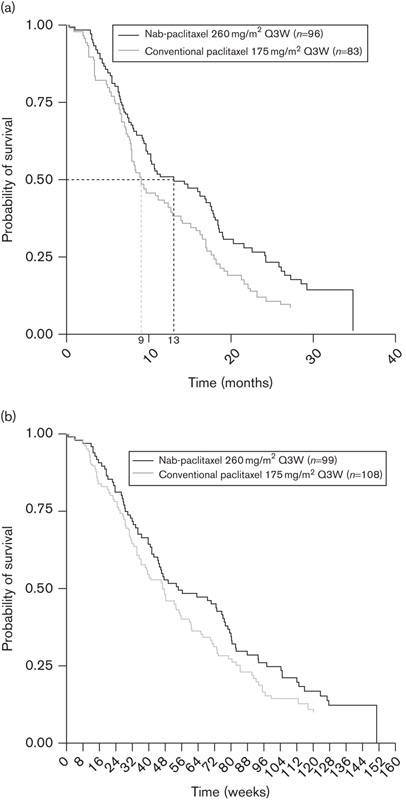
Kaplan–Meier curve of overall survival in patients with metastatic breast cancer who received treatment with nab-paclitaxel or conventional paclitaxel as greater than first-line therapy in the randomized Phase III study. (a) Patients with at least three metastases (HR=0.71; P=0.037). (b) Patients with visceral-dominant disease (P=0.145) 16,24. HR, hazard ratio; Q3W, every 3 weeks.
Nab-paclitaxel therefore appears to be an effective treatment for many patients with MBC, and may be preferred for patients with characteristics of aggressive disease, including those with mTNBC. Further trials to explore this theory are ongoing. For example, the Phase II/III tnAcity trial is evaluating nab-paclitaxel in combination with carboplatin or gemcitabine versus carboplatin plus gemcitabine in patients with mTNBC 27. SNAP (Schedules of Nab-Paclitaxel in Metastatic Breast Cancer) 28 is evaluating induction therapy with three cycles of high-dose weekly nab-paclitaxel, followed by different maintenance therapy doses in women with HER2 negative and ER negative or ER positive/refractory MBC. Findings from SNAP could also lead to the introduction of alternative nab-paclitaxel dosing schedules, which could allow physicians to tailor the use of this agent according to individual patient needs since balancing efficacy and safety is a key goal for delivering a positive risk–benefit profile for each patient.
Conclusion
Nab-paclitaxel can safely be offered to many women with MBC, with reasonable expectations of clinical benefit and without concern of significant toxicity. Moreover, nab-paclitaxel may be particularly beneficial for patients with aggressive disease, including those with mTNBC. Thus, nab-paclitaxel 260 mg/m2 Q3W is an effective treatment for MBC and a welcome addition to the treatment armamentarium. Ongoing research to evaluate different dosing schedules and combination regimens of nab-paclitaxel could broaden the clinical utility of this agent in the future.
Acknowledgements
The authors received medical writing support in the preparation of this manuscript from Angela Corstorphine of Kstorfin Medical Communications Ltd, funded by Celgene International Sàrl.
Conflicts of interest
G. Arpino has received honorarium from Celgene International Sàrl. For the remaining authors there are no conflicts of interest.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687–1717. [DOI] [PubMed] [Google Scholar]
- 2.Malorni L, Shetty PB, De Angelis C, Hilsenbeck S, Rimawi MF, Elledge R, et al. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res Treat 2012; 136:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertucci F, Finetti P, Birnbaum D. Basal breast cancer: a complex and deadly molecular subtype. Curr Mol Med 2012; 12:96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007; 13:4429–4434. [DOI] [PubMed] [Google Scholar]
- 5.Colleoni M, Cole BF, Viale G, Regan MM, Price KN, Maiorano E, et al. Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Clin Oncol 2010; 28:2966–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weigelt B, Kreike B, Reis-Filho JS. Metaplastic breast carcinomas are basal-like breast cancers: a genomic profiling analysis. Breast Cancer Res Treat 2009; 117:273–280. [DOI] [PubMed] [Google Scholar]
- 7.von Minckwitz G, Untch M, Nüesch E, Loibl S, Kaufmann M, Kümmel S, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat 2011; 125:145–156. [DOI] [PubMed] [Google Scholar]
- 8.Chang HR, Glaspy J, Allison MA, Kass FC, Elashoff R, Chung DU, Gornbein J. Differential response of triple-negative breast cancer to a docetaxel and carboplatin-based neoadjuvant treatment. Cancer 2010; 116:4227–4237. [DOI] [PubMed] [Google Scholar]
- 9.Warm M, Kates R, Grosse-Onnebrink E, Stoff-Khalili M, Hoopmann M, Mallmann P, et al. Impact of tumor biology, particularly triple-negative status, on response to pre-operative sequential, dose-dense epirubicin, cyclophosphamide followed by docetaxel in breast cancer. Anticancer Res 2010; 30:4251–4259. [PubMed] [Google Scholar]
- 10.NCCN. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Version 2 2013NCCN; Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Google Scholar]
- 11.Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E. ESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23Suppl 7vii11–vii19. [DOI] [PubMed] [Google Scholar]
- 12.von Minckwitz G, Martin M, Wilson G, Alba E, Schmidt M, Biganzoli L, Awada A. Optimizing taxane use in MBC in the emerging era of targeted chemotherapy. Crit Rev Oncol Hematol 2013; 85:315–331. [DOI] [PubMed] [Google Scholar]
- 13.EMA. Abraxane summary of product characteristics 2008EMA; Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000778/WC500020435.pdf. [Google Scholar]
- 14.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 2006; 12:1317–1324. [DOI] [PubMed] [Google Scholar]
- 15.Desai NP, Trieu V, Hwang LY, Wu R, Soon-Shiong P, Gradishar WJ. Improved effectiveness of nanoparticle albumin-bound (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of HER2 and SPARC status. Anticancer Drugs 2008; 19:899–909. [DOI] [PubMed] [Google Scholar]
- 16.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005; 23:7794–7803. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228–247. [DOI] [PubMed] [Google Scholar]
- 18.Telli ML, Carlson RW. First-line chemotherapy for metastatic breast cancer. Clin Breast Cancer 2009; 9Suppl 2S66–S72. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. SEER Stat Fact Sheets: Breast Cancer 2013National Cancer Institute; Available at: http://seer.cancer.gov/statfacts/html/breast.html. [Google Scholar]
- 20.Hamilton E, Kimmick G, Hopkins J, Marcom PK, Rocha G, Welch R, et al. Nab-paclitaxel/bevacizumab/carboplatin chemotherapy in first-line triple negative metastatic breast cancer. Clin Breast Cancer 2013; 13:416–420. [DOI] [PubMed] [Google Scholar]
- 21.Lobo C, Lopes G, Baez O, Castrellon A, Ferrell A, Higgins C, et al. Final results of a phase II study of nab-paclitaxel, bevacizumab, and gemcitabine as first-line therapy for patients with HER2-negative metastatic breast cancer. Breast Cancer Res Treat 2010; 123:427–435. [DOI] [PubMed] [Google Scholar]
- 22.Blum JL, Savin MA, Edelman G, Pippen JE, Robert NJ, Geister BV, et al. Phase II study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer 2007; 7:850–856. [DOI] [PubMed] [Google Scholar]
- 23.Lohmann AE, Speers CH, Chia SK. Evaluation of the clinical benefits of nanoparticle albumin-bound paclitaxel in women with metastatic breast cancer in British Columbia. Curr Oncol 2013; 20:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celgene. Data on file. 2014.
- 25.Ciruelos E, Jackisch C. Evaluating the role of nab-paclitaxel (Abraxane) in women with aggressive metastatic breast cancer. Expert Rev Anticancer Ther 2014; 14:511–521. [DOI] [PubMed] [Google Scholar]
- 26.O’Shaughnessy J, Gradishar WJ, Bhar P, Iglesias J. Nab-paclitaxel for first-line treatment of patients with metastatic breast cancer and poor prognostic factors: a retrospective analysis. Breast Cancer Res Treat 2013; 138:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NCT01881230. 2013Evaluate Risk/Benefit of Nab paclitaxel in combination with gemcitabine or carboplatin compared to gemcitabine and carboplatin in triple negative metastatic breast cancer (tnAcity).
- 28.NCT01746225. 2013Schedules of Nab-Paclitaxel in metastatic breast cancer (SNAP).


