Supplemental Digital Content is Available in the Text.
Abstract
Objective:
The association between occupational exposure to lead and amyotrophic lateral sclerosis (ALS) was examined through systematic review and meta-analyses of relevant epidemiological studies and reported according to PRISMA guidelines.
Methods:
Relevant studies were searched in multiple bibliographic databases through September 2013; additional articles were tracked through PubMed until submission. All records were screened in DistillerSR, and the data extracted from included articles were synthesized with meta-analysis.
Results:
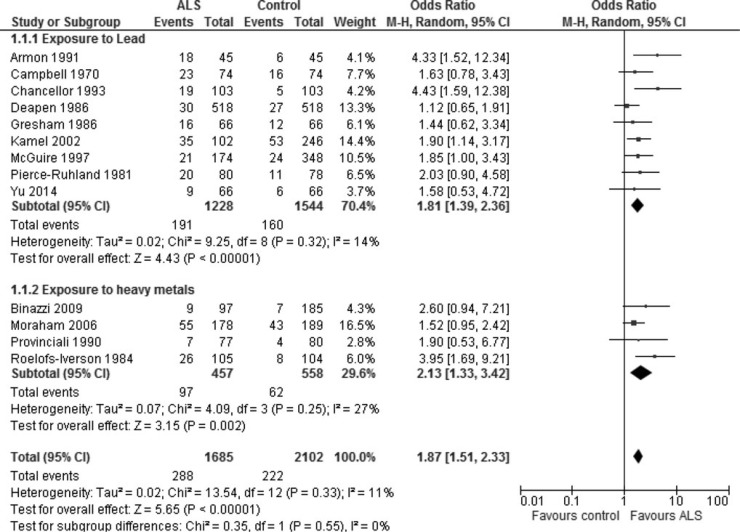
The risk of developing ALS among individuals with a history of exposure to lead was almost doubled (odds ratio, 1.81; 95% confidence interval, 1.39 to 2.36) on the basis of nine included case-control studies with specific lead exposure information, with no apparent heterogeneity across included studies (I2 = 14%). The attributable risk of ALS because of exposure to lead was estimated to be 5%.
Conclusions:
Previous exposure to lead may be a risk factor for ALS.
Learning Objectives
Become familiar with previous research on the association between exposure to lead and heavy metals and the risk of amyotrophic lateral sclerosis (ALS).
Summarize the findings of the new meta-analysis of studies of the association between occupational lead exposure and ALS risk.
Discuss the authors' conclusions on the magnitude of the association, the findings of adjusted analyses, and the fraction of ALS cases attributable to lead.
Amyotrophic lateral sclerosis (ALS), a disease term often used interchangeably with motor neuron disease, is a rare multifactorial degenerative condition of motor neurons, characterized by rapid and irreversible progression. It presents as either a familial (fALS) or a sporadic (sALS) form, accounting for 5% to 10% and 90% to 95% of all ALS cases, respectively.1 Amyotrophic lateral sclerosis begins with either a bulbar or a spinal onset in one third and two thirds of the ALS cases, respectively. In rare instances (about 1% of ALS cases), the initial noticeable clinical symptom is respiratory failure.2 The disease usually occurs in adults, with an average age at onset of 60 years.3 The average age at onset is higher among bulbar than among spinal cases.3 Survival after diagnosis is about 2 to 3 years for bulbar onset cases and 3 to 5 years for spinal onset cases.3
The causes of ALS remain largely unknown, except for a small proportion (about 10%) of cases (including both sALS and fALS cases) that are related to monogenic mutations.4 This type of monogenic mutation has been found in about two dozen genes.5–8 In white patients with ALS, the two most common monogenic mutated genes are C9orf72 and SOD1; mutations in these two genes are responsible for 35% and 25% of fALS cases and 6% and 2% of sALS cases, respectively.3,4,9–11 The mutation in C9orf72, which is rare in nonwhite populations,11 arose in Scandinavia several thousand years ago.12,13 In nonwhite populations, SOD1 is the most common mutated gene in patients with ALS.14 Nevertheless, most (90%) sALS cases are believed to be caused by polygenic variants/polymorphisms, environmental risk factors, and perhaps stochastic factors that exert their influence only in genetically susceptible individuals.15 Environmental and genetic factors are thought to play equally important roles in the development of ALS.16,17 Although many polymorphic gene variants (such as PON1 and VEGF) and environmental factors (such as pesticides, heavy metals, trauma, smoking, and electric shock) have been reported to be associated with ALS, none have been conclusively determined to cause ALS.18–23
Prior exposure to heavy metals, including lead, has long been suspected to be associated with an increased risk of ALS. Case reports have associated the onset of ALS with exposure of several heavy metals, including selenium, mercury, lead, aluminum, and manganese.22 Epidemiological studies investigating the association between prior exposure to lead and ALS began about five decades ago, after a series of ALS cases with antecedent exposure to lead were reported as early as 100 years ago.24–26 Since then, nearly two dozen observational epidemiological studies of ALS, in which assessment of exposure to lead was retrospective, have been conducted.26–46 Most studies showed that reported occupational exposure to lead was associated with a higher risk of developing ALS,34,42,47 although in some studies this was not statistically significant.32,36,42 The major challenge in linking lead and ALS is the retrospective ascertainment of historical lead exposures. Although some studies measured lead levels in blood or other body fluids—these levels may not accurately reflect previous intensity or duration of exposure to lead—it is perhaps not surprising that some previous studies did not observe differences in lead levels between cases and controls.34
A recent study of the spatial distribution of ALS cases showed a gradient in the incidence of ALS in the vicinity of a lead smelting factory in a county of Missouri state.48 In a case-control study in Boston, Kamel and colleagues found a dose–response relationship between ALS and self-reported estimates of exposure to lead33,34,45; this linear association was also observed in measured lead levels in blood and bone.46 These findings have shed new light on the association between previous exposure to lead and ALS. The purpose of this study is to describe the association between prior exposure to lead and ALS by combining multiple relevant observational epidemiological studies using systematic review and meta-analyses.
METHODS
Search Strategy
The search strategy was initially developed in Medline using search terms selected to identify relevant scientific publications, including systematic reviews, meta-analyses, and observational (case-control, cohort, and cross-sectional) studies (see Supplemental Digital Content 1, http://links.lww.com/JOM/A175). Once the search strategy was sufficiently well developed, it was used to search other bibliographic databases, with minor modifications to adapt to the requirements of those databases. The search included disease-relevant terms (amyotrophic lateral sclerosis, ALS, motor neuron disease, or Lou Gehrig's disease), and terms of environmental risk factors to identify all environmental risk-related studies. The articles related to exposure to lead were further identified with lead exposure-related terms (see below).
Databases Searched
The following databases were searched through September 2013: Medline, PubMed, EMBASE, Toxiline/toxnet, Ageline, Proquest (including dissertations), PsycInfo, and Google Scholar. Relevant articles were also hand-searched from reference lists and other databases, or through contacting to article authors. Similar articles were tracked with PubMed until the submission of this article.
Screen and Selection of Retrieved Articles
Duplicate articles were identified using Reference Manager by comparing authors and titles in adjacent references, after sorting references by author name; duplicates were then removed. The retained articles were screened in DistillerSR using predesigned screening forms. Level 1 screening was conducted by reading the titles in relation to the inclusion/exclusion criteria (English language, human study, relevant disease terms, environmental risk factor terms, and observational epidemiological study [cohort, case-control, cross-sectional, but not an intervention study or a review or commentary]). Level 2 screening was conducted by examining abstracts with respect to the inclusion/exclusion criteria mentioned above. Level 3 screening was conducted by examining the complete article and applying additional inclusion/exclusion criteria (relating to population characteristics, case ascertainment, environmental risk factors [studies of military personnel were excluded because of the highly selected nature of such populations, and studies with ALS confined to Guam were excluded because the disease on that island and surrounding areas might be associated with specific local risk factors], data analysis methods, and key results and conclusions] using two reviewers. During this step, if we found that an article had only fALS cases, or an article was with only an ecological study design, then the article was excluded. After this step, the retained articles were examined to carefully identify terms related to exposure to lead (lead, heavy metal, solder, soldering, Pb, weld, or welding) by using “find” function in the PDF file or reading through the Methods and Results sections of the article.
Quality Assessment, Data Extraction, and Meta-Analysis
Because ALS is a rare neurological condition, it is important to determine whether information on the same sample of patients with ALS has been reported in more than one article among the set of retained articles. If so, then the earliest article was included in the meta-analysis in the interests of minimizing the potential information fidelity decay with the time when reconstructing historical lead exposure profiles in later studies. The quality of articles selected for meta-analysis was evaluated using a system with a total 20 points, developed by our research team (see Supplemental Digital Content 2, http://links.lww.com/JOM/A176)49,50 as a modification of the quality assessment tool of Downs and Black.51 Information on study participants, study design, data collection, and synthesis was used to evaluate the comparability between cases and controls, risk factor estimation, and the control of the potential biases and confounding. More detail about the items evaluated for each article is presented in Supplemental Digital Content 2, http://links.lww.com/JOM/A176.
In the present meta-analysis, comparisons were based on subjects ever-exposed versus never-exposed to lead through the occupational environment on the basis of the results from included articles. Relevant result information was abstracted from the selected articles, including lead author, country, study type, study period, recruitment method, case ascertainment, control selection, response rates, data analysis methods, risk factor information, study results, and conclusions. A 5% of random sample of the studies from which data had been abstracted was verified by a second reviewer. The odds ratio (OR) for ALS in relation to lead exposure was used as the summary measure of risk in these meta-analyses. The primary meta-analysis was based on random-effects modeling, with a fixed-effects model run as a secondary analysis using Review Manager 5.1.52 Heterogeneity across included studies was estimated by Tau2, χ2, and I2. Forest plots and relevant supporting statistics were examined. Funnel plots were used to evaluate possible publication bias. Meta-analyses for subgroups (on the basis of publication year, exposure to lead vs to heavy metals, adjusted risk estimate) were considered if at least three articles were available for each category. To investigate whether the quality of studies influences the risk estimates from meta-analysis, the articles were divided into two groups, representing articles with higher and lower quality, respectively. If two articles had the identical median quality score, then the more recent article (which would have benefited from the experience of previous investigations and better controlled analyses) was allocated to the higher quality score group to achieve two groups of equal size.
Attributable Risk of ALS Because of Previous Exposure to Lead
The combined prevalence data for meta-analysis were used to estimate the attributable risk (AR) because of previous exposure to lead among ALS cases.53 The assumptions underlying this calculation are the following54,55: (1) the disease prevalence in the general population is low (usually <5%); (2) previous exposure to lead is causally associated with ALS; (3) ALS cases and controls included in the selected observational studies are representative of the total population of patients with ALS and general population; and (4) all excess exposure events among ALS cases as compared with controls are responsible for the development of ALS among those who reported being exposed to lead. Under these assumptions, this attributable risk is given by
where P denotes the prevalence of exposure to lead among control subjects and r denotes the relative risk (RR) for ALS because of exposure to lead. The RR could be replaced by the OR, which is a close approximation to the RR when the OR is not large.
RESULTS
Summary of Literature Search
The results of this systematic review and meta-analysis were reported according to the PRISMA guidelines.56 Details of the literature search, article screening, article evaluation, data extraction, and data analyses described in the Methods section are summarized in Fig. 1. All of the selected articles are case-control studies, except for two large cohort studies (see Supplemental Digital Content 3, Supplemental Table 1, http://links.lww.com/JOM/A177).43,44 Of the 21 selected articles, four articles used the same ALS subjects33,34,45,46; the earliest report was selected for inclusion in the meta-analysis,33 leaving 18 articles for further evaluation. Of the 18 retained articles, nine articles used the risk term “lead,” four articles used the terms relating to exposure to “heavy metals,” and five articles did not provide primary prevalence data, but only summary risk estimates. The meta-analysis was therefore conducted with the nine articles, specifically addressing the risk associated with occupational exposure to lead. Sensitivity analyses were conducted to evaluate the effect of various categories of publications, including the four articles focusing on occupational exposure to heavy metals of any type, including lead, and the effect of the study quality.
FIGURE 1.
Literature search, screen, evaluation, data extraction, and data analysis flow chart for the meta-analysis of observational studies of the association between previous exposure and ALS.56
Meta-Analysis of Association Between Previous Exposure to Lead and ALS
The results of meta-analyses on the basis of nine included articles related to occupational exposure to lead revealed that the odds of developing ALS were significantly higher among subjects with a history of occupational exposure to lead than among unexposed subjects (OR, 1.81; 95% confidence interval, 1.39 to 2.36, on the basis of a random-effects model [Fig. 2]; the corresponding estimate on the basis of a fixed-effects model was identical [OR, 1.81; 95% confidence interval, 1.42 to 2.29], with no significant heterogeneity across included studies [P = 0.32, I2 = 14%]), and with no apparent publication bias indicated by the funnel plots (data not shown). A similar increase in the risk of ALS was also found on the basis of the meta-analysis of four articles using heavy metals as the risk factor (OR, 2.13; 95% confidence interval, 1.33 to 2.42) (Fig. 2). Thus, combining the nine articles focusing on lead exposure with the four articles focusing on exposure to all heavy metals yields an OR of 1.87 (1.57 to 2.33). Similar results were obtained using the fixed-effects model (data not shown).
FIGURE 2.
Previous exposure to lead/heavy metals is associated with increased risk of developing ALS. Exposure data were extracted from 13 case-control studies in which exposure to lead (9 studies) or heavy metals (4 studies) and the risk of ALS was assessed. No evidence of heterogeneity across the included studies was observed in a meta-analysis using a random-effects model. Nor was their evidence of significant publication bias (data not shown).
Quality of Articles Seems Not to Significantly Affect the Conclusion
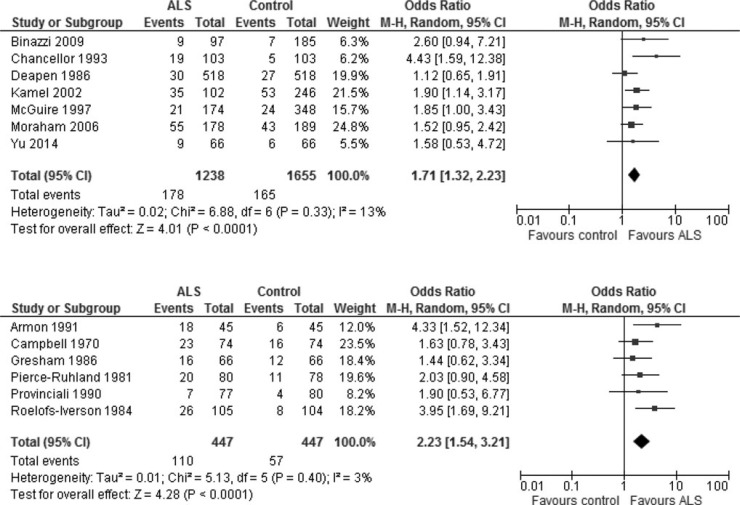
As indicated in Fig. 2, the risk of ALS because of exposure to lead is not materially different from the risk because of exposure to heavy metals. We therefore used all 12 studies to evaluate whether study quality affects the risk estimate of developing ALS. The median quality score among these 12 articles was 7 of a possible total of 20 points, and two articles were assigned this score. We arbitrarily allocated the newer article to the high-quality group to balance the group sizes. A new article became available during the course of the peer review of this article, which is assigned to the high-quality group because its quality score was 14, thus the meta-analysis was updated and included this article.57 This yielded a total of 7 and 6 articles in the higher and lower quality groups, respectively (Fig. 3). The meta-analysis revealed that the publication quality did not significantly change the estimate of risk for ALS, although the risk derived from articles with higher quality (OR, 1.71 [1.32 to 2.23]) is slightly lower than the risk derived from articles with lower quality (OR, 2.23 [1.54 to 3.21]) (Fig. 3).
FIGURE 3.
Article quality among included studies does not affect the risk estimate. Included studies were assessed and divided into two groups (one comprising seven articles and the other six) on the basis of quality scores. If two articles with an identical quality score need to allocate into two groups, then we arbitrarily allocated the newer article to the high-quality group, to balance the group sizes. The meta-analysis of articles (random-effects model) with higher quality scores is shown in the upper panel, and the meta-analysis of articles with lower scores is presented in the lower panel.
Adjusted Risk Estimates Are Somewhat Attenuated
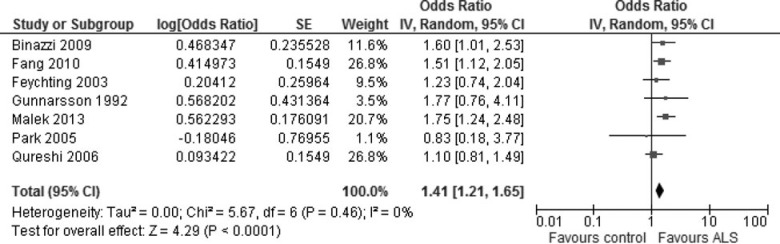
Of the 18 included studies for this article, five articles provided adjusted risk estimates (adjusted for sex and age, for example) only (either RRs or ORs) either for the risk factor “lead” or for the risk factor “heavy metals.”38,39,42–44 Two other articles also provided adjusted risk estimates, along with the prevalence of exposure to lead among cases and controls. One of these two studies has been included in Fig. 2;37 the other46 used the same sample of patients as the article authored with Kamel,33 which was therefore excluded from the analysis in Fig. 2. We conducted an additional meta-analysis with the estimates from these six studies to assess the degree of agreement with the results for the 13 studies in Fig. 2. The results of this analysis indicate that the risk is increased by about 40% (OR, 1.41 [1.21 to 1.65]) (Fig. 4), slightly lower (but not significantly different) than the estimate from Fig. 2 and with no heterogeneity (I2 = 0) among the included studies or evidence of publication bias (Funnel plot, figure not shown).
FIGURE 4.
Meta-analysis using adjusted relative risk estimates provided by included articles. Seven articles were included in this analysis, including 5 case-control studies and 2 cohort studies. Two case-control studies presented risk estimates specifically for exposure to lead,42,45 with the remaining case-control studies focusing on exposure to heavy metals, including lead. The two cohort studies gave estimates for exposure to heavy metals, including lead.43,44 The relative risk (OR or RR) from 7 included articles were first transformed to the log value, then estimated the confidence error on the basis of confidence interval log (95% upper CL − 95% lower CL). The meta-analysis was conducted with RevMan 5.1.
Attributable Risk Because of Previous Exposure to Lead
A total of 191 ALS cases from a population of 1228 individuals with ALS had a history of previous exposure to lead, whereas a total 160 controls from 1544 normal subjects had previous exposure to lead, on the basis of the nine studies using lead rather than heavy metals as the exposure metric in Fig. 2. The AR because of previous exposure to lead is calculated on the basis of these four values using the formula given in the Methods section. The prevalence of exposure is given by P = 160/1544 = 0.1036, and the relative risk is RR = [(191/1228)/(160/1544)] = 1.5009. The AR is then estimated to be 4.9% = [0.1036 × 0.5009/(0.1036 × 1.5009 + 0.8964)] × 100% of total ALS cases. This AR estimate implies that 61 ALS cases of the total 1228 ALS cases from all included studies might have been caused by previous exposure to lead.
DISCUSSION
The present meta-analysis of nine case-control studies with specific lead exposure information indicates that the risk of developing ALS is almost doubled after occupational exposure to lead, compared with unexposed controls. The estimated risk for ALS because of exposure to lead is not materially different from that calculated on the basis of exposure to all heavy metals. In addition, we found that the estimated risk on the basis of the articles of lower quality is not significantly different from the estimated risk on the basis of the articles of higher quality, although the former estimate is slightly higher than the latter (Fig. 3). The available data suggest that about 5% of all sporadic ALS cases may be attributable to occupational exposure to lead, although the actual attributable fraction could be somewhat lower because of the assumptions in this calculation. Nonetheless, our results suggest that previous exposure to lead in the occupational environment is a significant risk factor for ALS. The following observations from the scientific evidence in literature support this association.
Lead Is Toxic to Motor Neurons in Humans
Motor neuron toxicity in humans after exposure to lead was recognized more than a century ago.26 The classic form of lead neuropathy is characterized by weakness that initially involves primarily the wrist and finger extensors, but later spreads to other muscles.58 Sensory involvement is minimal.58 Motor neuropathy is more likely to develop after relatively short-term exposure to high lead concentrations, and evolves in a subacute fashion.58 Many ALS-like or ALS cases with antecedent occupational or cosmetic exposure to lead have been documented.24,59–62 For example, the study by Felmus et al62 documented six ALS cases with antecedent occupational exposure to lead for periods of 8 to 34 years. A number of epidemiological studies on long-term exposure to lead have also shown an association with ALS.34,42,47 These observations suggest that prior exposure to lead may be causally associated with the development of ALS.
Lead Level in Tissues Is Associated With an Increase in the Risk of ALS
Demonstrating a dose–dependent relationship between exposure to lead and ALS is important in detecting a causal relationship. An increasing positive relationship between lead exposure (on the basis of occupational history) and the risk of ALS has been shown in some epidemiological studies.26,35 Nonetheless, the reliability and validity of the exposure ascertainment would be enhanced if supported by biomarkers of lead exposure measured in body tissues or fluids. Higher lead levels have been reported in muscle tissue,63,64 cerebrospinal fluid (CSF), blood, and plasma/serum64–68 in patients with ALS, as compared with controls.26,69–74 Nevertheless, fewer than 30 subjects were included in most of these studies,65–69,74 limiting their power to establishing dose–dependent increased risk of ALS. A report published in 2013 showed that CSF lead concentrations in patients with ALS were higher (even higher than in blood), further supporting the association between ALS and previous lead exposure. The higher CSF lead concentrations in patients with ALS suggest a net influx of lead from blood into the CSF, reflecting a possible mechanism for bioaccumulation of lead in patients with ALS,68 with limited potential for elimination from the body. Such an association has also been observed in a case-control study of 108 ALS cases in the Boston area conducted by Kamel et al,33,46 on the basis of measured blood lead concentrations. Taken together, these studies are compatible with the findings of the present meta-analysis.
Mechanisms Whereby Chronic Exposure to Lead Might Cause ALS
Chronic exposure to lead could cause damage to the renal, nervous, reproductive, endocrinal, and immunological systems,75 possibly obfuscating a diagnosis of ALS in light of similar symptoms associated with other health conditions. Pure motor neuron effects after chronic exposure to lead have been observed in chickens,76 which demonstrated motor neuron disease with characteristics similar to human ALS.76 Lead deposition was identified in both the spinal cord and muscles in chickens exposed to lead. Spinal motor neuron degeneration (in the anterior horn cells), motor axonal loss, and atrophy of muscle tissue were also observed in the chickens exposed to lead. As noted previously, many ALS cases have been diagnosed after chronic exposure to lead.24,59–62 Collectively, these results suggest that chronic exposure to lead could result in ALS in humans, in the absence of symptoms associated with exposure to lead being manifested in nonneuronal or neuronal tissues.
Nearly 200 mutations in metal-binding superoxide dismutase 1 (SOD1) have been linked to ALS.7,77 Animal experiments found that treatment with lead could increase SOD1 expression of mRNA in mice,78 and decrease SOD1 activity in rats,79 suggesting that lead treatment might influence the normal folding process of SOD1 protein, and potentially cause the accumulation of unfolded or misfolded SOD1 protein, a primary mechanism resulting in the apoptosis of motor neurons.80 As a potential link between lead exposure and ALS, we suggest that lead may trigger misfolding of metal-binding proteins,81 such as SOD1, and induce a productive template for propagation of the misfolded protein.82,83
Limitations of This Study
This study is subject to several limitations. First, the observation of an increased risk of ALS in relation to prior exposure to lead on the basis of meta-analysis demonstrates only association, not causation. Nevertheless, the weight of evidence in support of a causal relationship is strengthened when the totality of evidence from clinical case reports, epidemiological studies, and toxicological studies is considered. Second, the accuracy of the risk estimate may be influenced by the quality of included studies, or the quality of the data in meta-analysis, although there is no significant difference among the three estimates (on the basis of articles with low or high quality, and the overall adjusted estimate, compared with the estimated risk on the basis of prevalence data only) in this study. The risk estimate from articles of high quality is attenuated with lower variation. The estimate from adjusted estimates by sex and age is the lowest estimate with lowest variation. To confirm this trend, more observational studies are required. Third, because the present meta-analyses included just two cohort studies, the recall bias in each of the included case-control studies in meta-analysis may influence the risk estimate. Fourth, there remains considerable uncertainty about the fraction of the ALS burden that may be attributed to lead in the general population. The AR estimate in this study represents the first AR estimate for previous occupational lead exposure to ALS; the extent to which this estimate is generalizable to encompass (generally lower) environmental lead exposures as well is unclear. Finally, although it is well known that ALS affects men more frequently than women, sex-specific estimates of the risk for ALS in individuals with previous lead exposure were not derived. Just two studies provided risk information for males and females separately,35,40 and neither study reached a clear conclusion.
CONCLUSIONS
The results of the present meta-analysis of nine case-control studies suggest that previous exposure to lead in the occupational environment is a risk factor for ALS. Lead might not account for a large number of ALS cases at the present time, because lead pollution has been significantly reduced over the last three decades, and lead-containing products have been more stringently regulated. Confirmation of the present findings in future studies would serve both to elucidate the causes of ALS, and to support risk mitigation actions to further reduce the risk of ALS because of exposure to lead from occupational and other sources.
ACKNOWLEDGMENTS
The authors thank Lindsey Sikora, Mona Hersi, and Pauline Quach for helpful advice on the development of the bibliographic search strategy used in this study.
Footnotes
This work was funded in part by a contribution agreement from the Public Health Agency of Canada to conduct systematic review of factors affecting the onset and progression of 14 neurological conditions under the National Population Health Study of Neurological Disease in Canada. Dr Cashman holds a Canada Research Chair in Neurodegeneration and Protein Misfolding Diseases at the University of British Columbia; Dr Little holds a Canada Research Chair in Human Genome Epidemiology at the University of Ottawa; and Dr Krewski holds the McLaughlin Chair in Risk Science at the University of Ottawa.
Authors Wang, Gomes, Cashman, Little, and Krewski have no relationships/conditions/circumstances that present potential conflict of interest.
The JOEM editorial board and planners have no financial interest related to this research.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.joem.org).
REFERENCES
- 1.Byrne S, Walsh C, Lynch C, et al. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:623–627. [DOI] [PubMed] [Google Scholar]
- 2.Sato K, Morimoto N, Deguchi K, Ikeda Y, Matsuura T, Abe K. Seven amyotrophic lateral sclerosis patients diagnosed only after development of respiratory failure. J Clin Neurosci. 2014;21:1341–1343. [DOI] [PubMed] [Google Scholar]
- 3.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chio A, Calvo A, Mazzini L, et al. Extensive genetics of ALS: a population-based study in Italy. Neurology. 2012;79:1983–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S, Sayana P, Zhang X, Le W. Genetics of amyotrophic lateral sclerosis: an update. Molecular Neurodegeneration. 2013;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Chalabi A, Jones A, Troakes C, King A, Al-Sarraj S, Van Den Berg LH. The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol. 2012;124:339–352. [DOI] [PubMed] [Google Scholar]
- 7.Abel O, Powell JF, Andersen PM, Al-Chalabi A. ALSoD: a user-friendly online bioinformatics tool for amyotrophic lateral sclerosis genetics. Hum Mutat. 2012;33:1345–1351. [DOI] [PubMed] [Google Scholar]
- 8.ALS Online Genetics Database. World federation of neurology and European network to cure ALS. 2014. .
- 9.Pfister T, Sekhon R, White M, et al. Familial amyotrophic lateral sclerosis in Alberta, Canada. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:273–277. [DOI] [PubMed] [Google Scholar]
- 10.Robberecht W. Genetics of amyotrophic lateral sclerosis. J Neurol. 2000;247(suppl 6):VI/2–6. [DOI] [PubMed] [Google Scholar]
- 11.van Blitterswijk M, DeJesus-Hernandez M, Rademakers R. How do C9ORF72 repeat expansions cause amyotrophic lateral sclerosis and frontotemporal dementia: can we learn from other noncoding repeat expansion disorders? Curr Opin Neurol. 2012;25:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith BN, Newhouse S, Shatunov A, et al. The C9ORF72 expansion mutation is a common cause of ALS+/-FTD in Europe and has a single founder. Eur J Hum Genet. 2013;21:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratti A, Corrado L, Castellotti B, et al. C9ORF72 repeat expansion in a large Italian ALS cohort: evidence of a founder effect. Neurobiol Aging. 2012;33:2528.e7–14. [DOI] [PubMed] [Google Scholar]
- 14.Soong BW, Lin KP, Guo YC, et al. Extensive molecular genetic survey of Taiwanese patients with amyotrophic lateral sclerosis. Neurobiol Aging. 2014;35:2423.e1–6. [DOI] [PubMed] [Google Scholar]
- 15.Vinceti M, Fiore M, Signorelli C, et al. Environmental risk factors for amyotrophic lateral sclerosis: methodological issues in epidemiologic studies. Ann Ig. 2012;24:407–415. [PubMed] [Google Scholar]
- 16.Al-Chalabi A, Fang F, Hanby MF, et al. An estimate of amyotrophic lateral sclerosis heritability using twin data. J Neurol Neurosurg Psychiatry. 2010;PMC2988617:1324–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wingo TS, Cutler DJ, Yarab N, Kelly CM, Glass JD. The heritability of amyotrophic lateral sclerosis in a clinically ascertained united states research registry. PLoS One. 2011;6:e27985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meireles A, Al-Chalabi A. Genetic studies of amyotrophic lateral sclerosis: controversies and perspectives. Amyotrophic Lat Scler. 2009;10:1–14. [DOI] [PubMed] [Google Scholar]
- 19.Johnson F, Atchison W. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology. 2009;30:761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutedja NA, Veldink JH, Fischer K, et al. Exposure to chemicals and metals and risk of amyotrophic lateral sclerosis: a systematic review. Amyotroph Lateral Scler. 2009;10:302–309. [DOI] [PubMed] [Google Scholar]
- 21.Sutedja NA, Fischer K, Veldink JH, et al. What we truly know about occupation as a risk factor for ALS: a critical and systematic review. Amyotroph Lateral Scler. 2009;10:295–301. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed A, Wicklund MP. Amyotrophic lateral sclerosis: what role does environment play? Neurol Clin. 2011;29:689–711. [DOI] [PubMed] [Google Scholar]
- 23.Migliore L, Coppede F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res. 2009;667:82–97. [DOI] [PubMed] [Google Scholar]
- 24.Oh SS, Kim EA, Lee SW, Kim MK, Kang SK. A case of amyotrophic lateral sclerosis in electronic parts manufacturing worker exposed to lead. Neurotoxicology. 2007;28:324–327. [DOI] [PubMed] [Google Scholar]
- 25.Livesley B, Sissons CE. Chronic lead intoxication mimicking motor neurone disease. Br Med J. 1968;4:387–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell AM, Williams ER, Barltrop D. Motor neurone disease and exposure to lead. J Neurol Neurosurg Psychiatry. 1970;33:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chancellor AM, Slattery JM, Fraser H, Warlow CP. Risk factors for motor neuron disease: a case-control study based on patients from the Scottish motor neuron disease register. J Neurol Neurosurg Psychiatry. 1993;56:1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deapen DM, Henderson BE. A case-control study of amyotrophic lateral sclerosis. Am J Epidemiol. 1986;123:790–799. [DOI] [PubMed] [Google Scholar]
- 29.Deapen DM. A case-control study of amyotrophic lateral sclerosis. ProQuest Dissertations and Theses. 1982.
- 30.Gresham LS, Molgaard CA, Golbeck AL, Smith R. Amyotrophic lateral sclerosis and occupational heavy metal exposure: a case-control study. Neuroepidemiology. 1986;5:29–38. [DOI] [PubMed] [Google Scholar]
- 31.Gresham LS, Molgaard CA, Golbeck AL, Smith R. Amyotrophic lateral sclerosis and history of skeletal fracture: a case-control study. Neurology. 1987;37:717–719. [DOI] [PubMed] [Google Scholar]
- 32.Gresham LS, Molgaard CA, Golbeck AL, Smith R. Lead exposure and ALS. Neurology. 1992;42:2228–2229. [DOI] [PubMed] [Google Scholar]
- 33.Kamel F, Umbach D, Munsat T, Shefner J, Hu H, Sandler D. Lead exposure and amyotrophic lateral sclerosis. Epidemiology. 2002;13:311–319. [DOI] [PubMed] [Google Scholar]
- 34.Kamel F, Umbach DM, Hu H, et al. Lead exposure as a risk factor for amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:195–201. [DOI] [PubMed] [Google Scholar]
- 35.McGuire V, Longstreth WT, Jr, Nelson LM, et al. Occupational exposures and amyotrophic lateral sclerosis. A population-based case-control study. Am J Epidemiol. 1997;145:1076–1088. [DOI] [PubMed] [Google Scholar]
- 36.Pierce-Ruhland R, Patten BM. Repeat study of antecedent events in motor neuron disease. Ann Clin Res. 1981;13:102–107. [PubMed] [Google Scholar]
- 37.Binazzi A, Belli S, Uccelli R, et al. An exploratory case-control study on spinal and bulbar forms of amyotrophic lateral sclerosis in the province of Rome. Amyotroph Lateral Scler. 2009;10:361–369. [DOI] [PubMed] [Google Scholar]
- 38.Gunnarsson LG, Bodin L, Soderfeldt B, Axelson O. A case-control study of motor neurone disease: Its relation to heritability, and occupational exposures, particularly to solvents. Br J Ind Med. 1992;49:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malek AM, Barchowsky A, Bowser R, et al. Environmental and occupational risk factors for amyotrophic lateral sclerosis: a case-control study. Neurodegener Dis. 2014;14:31–38. [DOI] [PubMed] [Google Scholar]
- 40.Morahan JM, Pamphlett R. Amyotrophic lateral sclerosis and exposure to environmental toxins: An Australian case-control study. Neuroepidemiology. 2006;27:130–135. [DOI] [PubMed] [Google Scholar]
- 41.Provinciali L, Giovagnoli AR. Antecedent events in amyotrophic lateral sclerosis: Do they influence clinical onset and progression? Neuroepidemiology. 1990;9:255–262. [DOI] [PubMed] [Google Scholar]
- 42.Qureshi MM, Hayden D, Urbinelli L, et al. Analysis of factors that modify susceptibility and rate of progression in amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler. 2006;7:173–182. [DOI] [PubMed] [Google Scholar]
- 43.Feychting M, Jonsson F, Pedersen NL, Ahlbom A. Occupational magnetic field exposure and neurodegenerative disease. Epidemiology. 2003;14:413–419; discussion 427–428. [DOI] [PubMed] [Google Scholar]
- 44.Park R, Schulte P, Bowman J, et al. Potential occupational risks for neurodegenerative diseases. Am J Ind Med. 2005;48:63–77. [DOI] [PubMed] [Google Scholar]
- 45.Fang F, Quinlan P, Ye W, et al. Workplace exposures and the risk of amyotrophic lateral sclerosis. Environ Health Perspect. 2009;117:1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang F, Kwee LC, Allen KD, et al. Association between blood lead and the risk of amyotrophic lateral sclerosis. Am J Epidemiol. 2010;171:1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scarpa M, Colombo A, Panzetti P, Sorgato P. Epidemiology of amyotrophic lateral sclerosis in the province of Modena, Italy. influence of environmental exposure to lead. Acta Neurol Scand. 1988;77:456–460. [DOI] [PubMed] [Google Scholar]
- 48.Turabelidze G, Zhu BP, Schootman M, et al. An epidemiologic investigation of amyotrophic lateral sclerosis in Jefferson county, Missouri, 1998–2002. Neurotoxicology. 2008;29:81–86. [DOI] [PubMed] [Google Scholar]
- 49.Turner MC, Wigle DT, Krewski D. Residential pesticides and childhood leukemia: a systematic review and meta-analysis. Cien Saude Colet. 2011;16:1915–1931. [DOI] [PubMed] [Google Scholar]
- 50.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 51.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu L, Fu DL, Li HQ, Liu AJ, Li JH, Zheng GQ. Diabetes and risk of Parkinson's disease: an updated meta-analysis of case-control studies. PLoS One. 2014;9:e85781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breslow N, Day N. Satatistical methods in cancer research. IARC Sci Publ. 1980;32:5–338. [PubMed] [Google Scholar]
- 54.Wartenberg D. Residential EMF exposure and childhood leukemia: meta-analysis and population attributable risk. Bioelectromagnetics. 2001;5(suppl):S86–104. [DOI] [PubMed] [Google Scholar]
- 55.Sahai H, Khurshid A. Statistics in Epidemiology—-Methods, Techniques, and Applications. Boca Raton, FL: CRC Press; 1996. [Google Scholar]
- 56.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- 57.Yu Y, Su FC, Callaghan BC, Goutman SA, Batterman SA, Feldman EL. Environmental risk factors and amyotrophic lateral sclerosis (ALS): a case-control study of ALS in Michigan. PLoS One. 2014;9:e101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomson RM, Parry GJ. Neuropathies associated with excessive exposure to lead. Muscle Nerve. 2006;33:732–741. [DOI] [PubMed] [Google Scholar]
- 59.Bachmeyer C, Bagur E, Lenglet T, Maier-Redelsperger M, Lecomte I. Lead poisoning mimicking amyotrophic lateral sclerosis: an adverse effect of rituals. Am J Med. 2012;125:e5–e6. [DOI] [PubMed] [Google Scholar]
- 60.Fluri F, Lyrer P, Gratwohl A, Raetz-Bravo AE, Steck AJ. Lead poisoning from the beauty case: neurologic manifestations in an elderly woman. Neurology. 2007;69:929–930. [DOI] [PubMed] [Google Scholar]
- 61.Boothby JA, DeJesus PV, Rowland LP. Reversible forms of motor neuron disease lead “neuritis.” Arch Neurol. 1974;31:18–23. [DOI] [PubMed] [Google Scholar]
- 62.Felmus MT, Patten BM, Swanke L. Antecedent events in amyotrophic lateral sclerosis. Neurology. 1976;26:167–172. [DOI] [PubMed] [Google Scholar]
- 63.Mandybur TI, Cooper GP. Increased spinal cord lead content in amyotrophic lateral sclerosis—possibly a secondary phenomenon. Med Hypotheses. 1979;5:1313–1315. [DOI] [PubMed] [Google Scholar]
- 64.Petkau A, Sawatzky A, Hillier CR, Hoogstraten J. Lead content of neuromuscular tissue in amyotrophic lateral sclerosis: Case report and other considerations. Br J Ind Med. 1974;31:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Conradi S, Ronnevi LO, Vesterberg O. Abnormal tissue distribution of lead in amyotrophic lateral sclerosis. J Neurol Sci. 1976;29:259–265. [DOI] [PubMed] [Google Scholar]
- 66.Conradi S, Ronnevi LO, Vesterberg O. Increased plasma levels of lead in patients with amyotrophic lateral sclerosis compared with control subjects as determined by flameless atomic absorption spectrophotometry. J Neurol Neurosurg Psychiatry. 1978;41:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kurlander HM, Patten BM. Metals in spinal cord tissue of patients dying of motor neuron disease. Ann Neurol. 1979;6:21–24. [DOI] [PubMed] [Google Scholar]
- 68.Roos PM, Vesterberg O, Syversen T, Flaten TP, Nordberg M. Metal concentrations in cerebrospinal fluid and blood plasma from patients with amyotrophic lateral sclerosis. Biol Trace Elem Res. 2013;151:159–170. [DOI] [PubMed] [Google Scholar]
- 69.Conradi S, Ronnevi LO, Vesterberg O. Lead concentration in skeletal muscle in amyotrophic lateral sclerosis patients and control subjects. J Neurol Neurosurg Psychiatry. 1978;41:1001–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vinceti M, Guidetti D, Bergomi M, et al. Lead, cadmium, and selenium in the blood of patients with sporadic amyotrophic lateral sclerosis. Ital J Neurol Sci. 1997;18:87–92. [DOI] [PubMed] [Google Scholar]
- 71.Kapaki E, Segditsa J, Zournas C, Xenos D, Papageorgiou C. Determination of cerebrospinal fluid and serum lead levels in patients with amyotrophic lateral sclerosis and other neurological diseases. Experientia. 1989;45:1108–1110. [DOI] [PubMed] [Google Scholar]
- 72.Cavalleri A, Minoia C, Ceroni M, Poloni M. Lead in cerebrospinal fluid and its relationship to plasma lead in humans. J Appl Toxicol. 1984;4:63–65. [DOI] [PubMed] [Google Scholar]
- 73.Stober T, Stelte W, Kunze K. Lead concentrations in blood, plasma, erythrocytes, and cerebrospinal fluid in amyotrophic lateral sclerosis. J Neurol Sci. 1983;61:21–26. [DOI] [PubMed] [Google Scholar]
- 74.Conradi S, Ronnevi LO, Nise G, Vesterberg O. Abnormal distribution of lead in amyotrophic lateral sclerosis–reestimation of lead in the cerebrospinal fluid. J Neurol Sci. 1980;48:413–418. [DOI] [PubMed] [Google Scholar]
- 75.Damstra T. Toxicological properties of lead. Environ Health Perspect. 1977;19:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mazliah J, Barron S, Bental E, Rogowski Z, Coleman R, Silbermann M. The effects of long-term lead intoxication on the nervous system of the chicken. Neurosci Lett. 1989;101:253–257. [DOI] [PubMed] [Google Scholar]
- 77.Mulligan VK, Chakrabartty A. Protein misfolding in the late-onset neurodegenerative diseases: common themes and the unique case of amyotrophic lateral sclerosis. Proteins. 2013;81:1285–1303. [DOI] [PubMed] [Google Scholar]
- 78.Kim S, Hyun J, Kim H, et al. Effects of lead exposure on nitric oxide-associated gene expression in the olfactory bulb of mice. Biol Trace Elem Res. 2011;142:683–692. [DOI] [PubMed] [Google Scholar]
- 79.Baranowska-Bosiacka I, Gutowska I, Marchlewicz M, et al. Disrupted pro- and antioxidative balance as a mechanism of neurotoxicity induced by perinatal exposure to lead. Brain Res. 2012;1435:56–71. [DOI] [PubMed] [Google Scholar]
- 80.Rotunno MS, Bosco DA. An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. Front Cell Neurosci. 2013;7:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goering PL. Lead-protein interactions as a basis for lead toxicity. Neurotoxicology. 1993;14:45–60. [PubMed] [Google Scholar]
- 82.Grad LI, Cashman NR. Prion-like activity of Cu/Zn superoxide dismutase: Implications for amyotrophic lateral sclerosis. Prion. 2014;8:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grad LI, Guest WC, Yanai A, et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc Natl Acad Sci U S A. 2011;108:16398–16403. [DOI] [PMC free article] [PubMed] [Google Scholar]