Abstract
Background
Abnormal ventricular repolarization is a predictor of cardiovascular mortality. In this study, we tested the hypothesis that glycemic control reverses abnormal ventricular repolarization in patients with type 2 diabetes.
Methods
We analyzed longitudinal changes in repolarization indices of electrocardiograms in retrospectively enrolled 44 patients with type 2 diabetes and 44 age-matched healthy subjects.
Results
In the diabetic group, BMI was greater, levels of HbA1c (10.0 ± 1.6 vs. 5.6 ± 0.3%) and triglyceride were higher and level of HDL cholesterol was lower than those in the control group. Although mean QTc intervals were similar (413.6 ± 18.5 vs. 408.3 ± 22.7 ms), QT dispersion (41.8 ± 15.4 vs. 28.7 ± 7.7 ms) and Tpeak-Tend in lead V5 (83.6 ± 13.6 vs. 71.3 ± 10.3 ms) were significantly longer in the diabetic group than in the control group, indicating increased heterogeneity of ventricular repolarization in type 2 diabetes. During follow-up of 36 patients in the diabetic group for 787 ± 301 days, HbA1c level decreased to 7.3 ± 1.6%, while BMI did not significantly change. In contrast to HbA1c, QT dispersion (45.8 ± 15.0 ms) and Tpeak-Tend in lead V5 (83.6 ± 10.6 ms) were not significantly reduced during the follow-up period. There was no correlation between the change in HbA1c and the change in QT dispersion or Tpeak-Tend.
Conclusions
Increased heterogeneity of ventricular repolarization in type 2 diabetic patients was not reduced during the relatively short follow-up period despite significantly improved glycemic control.
Keywords: Type 2 diabetes, Glycemic control, QT dispersion, Ventricular repolarization
Background
The number of patients with type 2 diabetes has been increasing worldwide in the past two decades, and these patients are predisposed to serious cardiovascular morbidity and mortality [1,2]. Despite recent progress in coronary intervention strategies, diabetes is associated with high mortality after acute myocardial infarction (MI) due to extensive atherosclerotic lesions and also a hypertrophied and dysfunctional left ventricle [3]. It has been reported that post-MI patients with diabetes have higher incidences of heart failure, recurrent myocardial ischemic events and sudden cardiac death (SCD) than do those without diabetes [4]. In the UKPDS, glycemic control significantly reduced the incidence of microvascular disease but had limited effects on cardiovascular events including SCD [5]. Furthermore, recent large clinical trials have shown that no significant reduction of major adverse cardiac events was achieved by 2–5 years of intensive glycemic control [6–10].
An electrocardiogram (ECG) is the most widely used noninvasive diagnostic test for cardiovascular risk stratification. It is well known that repolarization abnormalities, such as prolonged QT interval (or heart rate-corrected QT interval (QTc)) and increased QT dispersion, are associated with increased risk of malignant ventricular arrhythmias and SCD in high-risk populations (i.e., patients with myocardial infarction and cardiomyopathy) [11]. Furthermore, most, but not all, studies have shown that prolonged QTc and increased QT dispersion were predictors of all-cause and cardiovascular mortality in the general population [12,13] and probably in diabetic patients as well [14–17]. The interval from the peak to the end of the T wave (Tpeak-Tend) is known to reflect transmural repolarization heterogeneity and has been associated with increased risk of mortality not only in high-risk patients [18,19] but also in the general population [20,21]. Importantly, Tpeak-Tend has been shown to predict cardiovascular mortality even when the QTc interval is normal [21]. However, its importance in diabetic patients has yet to be determined. In addition, it is unclear whether blood glucose-lowering therapy modifies the spatial heterogeneity in repolarization, if any, in diabetic patients. In the present study, we tested the hypotheses that repolarization heterogeneity is enlarged in patients with type 2 diabetes and that glycemic control alleviates the abnormality in repolarization.
Methods
This study was conducted in strict adherence with the principles of the Declaration of Helsinki and was approved by the Clinical Investigation Ethics Committee of Sapporo Medical University Hospital.
Subjects
We retrospectively analyzed data for 275 consecutive patients who were admitted to our hospital for management of type 2 diabetes from April 2007 to September 2012. Control subjects were chosen from the Tanno-Sobetsu cohort [22], in which residents of two Japanese rural towns, Tanno and Sobetsu, have been prospectively followed up by annual or biannual medical examination, including standard blood tests and ECG. We selected age- and sex-matched healthy subjects who had not been receiving any medications. Exclusion criteria were type 1 diabetes and other specific types of diabetes, atrial fibrillation, past history of cardiac surgery, chronic kidney disease at stage 4 or higher, serum potassium abnormality (<3.5 or >5.5 mEq/l), use of an anti-arrhythmic drug (except a β blocker used for hypertension or coronary artery disease), bundle branch block, overt heart disease, and low T wave amplitude at lead V5 (<0.1 mV). By the exclusion criteria, 231 diabetic patients were excluded, and 44 type 2 diabetic patients and 44 age- and sex-matched non-diabetic controls were enrolled in this study (Figure 1). Autonomic neuropathy was defined as a loss of heart rate variability or postural hypotension with a fall in systolic blood pressure ≥20 mmHg [23,24]. After discharge from our hospital, the patients were followed up at the outpatient clinic. We retrospectively analyzed longitudinal changes in clinical parameters during follow-up for 227–1374 days (i.e., follow-up until April 2014) under glucose-lowering therapy. The medication was adjusted by each physician’s decision to optimize the glycemic control.
Figure 1.
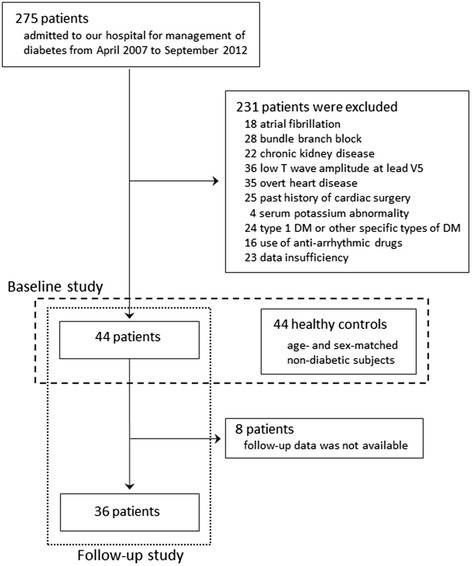
Enrollment and follow-up of study participants.
Clinical characteristics, laboratory data and ECG findings were compared between the type 2 diabetic group and the control group and before and after the glycemic control within the type 2 diabetic group.
ECG recordings and measurements
Standard resting 12-lead ECGs were recorded at 25 mm/s paper speed and 10 mm/mV amplitude. The beginning of the QT interval was defined as first deflection of the QRS complex. The end of the T wave was defined as the intersection of the tangent to the down slope of the T wave and the isoelectric line when not followed by a U wave or if distinct from the following U wave. If a U wave followed by the T wave, T wave offset was measured as the nadir between the T and U waves. In case of a flat T wave or TU merge without nadir, the end of the T wave could not be determined and the lead was excluded from measurements. The QT intervals were measured in all 12 leads and corrected for heart rate (QTc) by Bazett’s formula, together with a sex-specific method described by Rautaharju and Zhang [25] according to AHA/ACCF/HRS guidelines [26]. QT dispersion and QTc dispersion were calculated as the difference between the maximum and minimum QT interval and QTc interval, respectively, among the 12 leads. Tpeak-Tend was defined as the interval from the peak of positive T wave and the end of the T wave and was measured in lead V5.
Statistical analysis
Numeric variables are expressed as means ± SD. Differences between two groups were tested by Student’s t-test. Relationships between parameters were examined by the use of simple linear regression analyses. Multiple regression analyses were performed to determine the relationships between clinical parameters, QT dispersion and Tpeak-Tend. Changes in clinical and electrocardiographic variables and medication during follow-up periods within the type 2 diabetes group (36 patients) were compared by the paired t-test and Fisher’s exact test, respectively. Statistical analyses were carried out using JMP (version11 SAS Institute, Cary, NC, USA). To calculate statistical power for differences in QT dispersion and Tpeak-Tend, we used “Power and Sample Size Calculation version 3.0.43, 2011”. All statistical tests were two-tailed and differences were considered to be statistically significant if p was less than 0.05.
Results
Baseline characteristics
Clinical characteristics in the type 2 diabetes and control groups are shown in Table 1. Body mass index (BMI) was larger and systolic blood pressure (BP) was higher in the type 2 diabetes group than in the control group. However, BP was relatively well-controlled by medications in most of the diabetic patients (124.8 ± 16.8/74.2 ± 9.4 mmHg), with only 8 patients (18%) showing systolic BP ≥140 mmHg. Duration of diabetes was 12.5 ± 12.0 years (median, 6.5 years) and 72% of the patients had one or more complications: 14 with retinopathy, 18 with nephropathy (stage 2 or 3), 7 with autonomic neuropathy, and 9 with coronary artery disease. Autonomic neuropathy in the seven patients was diagnosed from postural hypotension (n = 2), reduced coefficient of variance of RR intervals (CVRR) on ECG (n = 4) or diabetic neurogenic bladder (n = 1). Patients with coronary artery disease had been medically treated with or without prior coronary interventions, and none of them showed myocardial ischemia in exercise ECG tests or stress myocardial scintigraphy at the time of study enrollment. Glycemic control was poor (fasting plasma glucose: 170.3 ± 49.5 mg/dl, HbA1c: 10.0 ± 1.6%) at the time of admission. As expected in poorly controlled diabetes, triglyceride level was significantly higher and high-density lipoprotein cholesterol (HDL-C) level was significantly lower in type 2 diabetic patients than in controls. Low-density lipoprotein cholesterol (LDL-C) levels were comparable in the two groups, most likely as a result of cholesterol-lowering therapy in 36% of the patients, mainly with a statin.
Table 1.
Baseline characteristics
| Type 2 diabetes | Control | P | |
|---|---|---|---|
| (n = 44) | (n = 44) | ||
| Clinical variables | |||
| Age (years) | 60.6 ± 13.8 | 58.4 ± 11.4 | 0.353 |
| Male | 24 (54.5%) | 22 (50.0%) | 0.674 |
| BMI (kg/m2) | 26.7 ± 4.4 | 22.6 ± 2.8 | <0.001 |
| SBP (mmHg) | 124.8 ± 16.8 | 118.3 ± 13.4 | 0.048 |
| DBP (mmHg) | 74.2 ± 9.4 | 71.6 ± 9.7 | 0.204 |
| Smoking | 24 (54.5%) | 20 (45.5%) | 0.400 |
| Duration of DM (years) | 12.5 ± 12.0 | N/A | |
| Retinopathy | 14 (31.8%) | N/A | |
| Nephropathy | 18 (40.9%) | N/A | |
| Autonomic neuropathy | 7 (15.9%) | N/A | |
| CAD | 9 (20.5%) | N/A | |
| Laboratory variables | |||
| FPG (mg/dl) | 170.3 ± 49.5 | 89.7 ± 8.0 | <0.001 |
| HbA1c (%) | 10.0 ± 1.6 | 5.6 ± 0.3 | <0.001 |
| Triglyceride (mg/dl) | 228.1 ± 246.0 | 88.2 ± 38.9 | <0.001 |
| HDL-C (mg/dl) | 44.5 ± 12.4 | 54.8 ± 10.2 | <0.001 |
| LDL-C (mg/dl) | 115.9 ± 40.0 | 129.4 ± 27.4 | 0.0069 |
| Creatinine (mg/dl) | 0.66 ± 0.22 | 0.64 ± 0.14 | 0.597 |
| Uric acid (mg/dl) | 5.0 ± 1.4 | 5.1 ± 1.4 | 0.596 |
| Potassium (mEq/l) | 4.1 ± 0.4 | 4.3 ± 0.1 | 0.074 |
| Medications | |||
| ACE-I/ARB | 16 (36.4%) | N/A | |
| CCB | 13 (29.5%) | N/A | |
| β blocker | 5 (11.4%) | N/A | |
| Other antihypertensive drugs | 8 (18.2%) | N/A | |
| Sulphonylurea | 19 (43.2%) | N/A | |
| α-glucosidase inhibitor | 19 (43.2%) | N/A | |
| Biguanide | 16 (36.4%) | N/A | |
| DPP-4 inhibitor | 14 (31.8%) | N/A | |
| Insulin | 8 (18.2%) | N/A | |
| Other antidiabetic drugs | 6 (13.6%) | N/A | |
| Statin | 14 (31.8%) | N/A | |
| Fibrate | 2 (4.5%) | N/A | |
| Electrocardiographic variables | |||
| Heart rate (bpm) | 74.2 ± 15.5 | 61.0 ± 8.6 | <0.001 |
| V1S + V5R (mV) | 2.37 ± 0.58 | 2.14 ± 0.68 | 0.093 |
| QTc mean (ms) | 413.6 ± 18.5 | 408.3 ± 22.7 | 0.229 |
| QT dispersion (ms) | 41.8 ± 15.4 | 28.7 ± 7.7 | <0.001 |
| QTc dispersion (ms) | 45.9 ± 16.3 | 28.8 ± 7.3 | <0.001 |
| Tpeak-Tend in V5 (ms) | 83.6 ± 13.6 | 71.3 ± 10.3 | <0.001 |
BMI = body mass index, SBP = systolic blood pressure, DBP = diastolic blood pressure, CAD = coronary artery disease, FPG = fasting plasma glucose, HbA1c = glycated hemoglobin, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, ACE-I = angiotensin-converting enzyme inhibitor, ARB = angiotensin II receptor blocker, CCB = calcium channel blocker, DPP-4 inhibitor = dipeptidyl peptidase-4 inhibitor. QTc = corrected QT (by Bazett’s formula), N/A= not applicable. Values are means ± SD or absolute numbers (frequency percentages).
Electrocardiographic measurements
Heart rate was significantly higher in type 2 diabetic patients than in controls (Table 1). The sum of S wave depth in lead V1 and R wave height in lead V5 (V1S + V5R, an index for left ventricular mass, with ≥3.5 mV being defined as left ventricular hypertrophy by the Sokolow-Lyon voltage criterion) tended to be larger in diabetic patients, although only two subjects in each group met the ECG criterion for left ventricular hypertrophy. Mean QTc interval (413.6 ± 18.5 vs. 408.3 ± 22.7 ms) was not significantly longer in type 2 diabetic patients than in controls, and a similar trend was observed when QT was adjusted using the method of Rautaharju and Zhang (407.4 ± 15.6 vs. 406.9 ± 21.0 ms). In contrast, QT dispersion (41.8 ± 15.4 vs. 28.7 ± 7.7 ms) and QTc dispersion (45.9 ± 16.3 vs. 28.8 ± 7.3 ms) were significantly increased in type 2 diabetic patients compared with those in controls. Increased QTc dispersion in type 2 diabetic patients was also detected by use of the correction by Rautaharju and Zhang [25] (44.9 ± 15.5 vs. 35.1 ± 12.8 ms, p < 0.01). Tpeak-Tend (83.6 ± 13.6 vs. 71.3 ± 10.3 ms) and Tpeak-Tend/QT ratio (0.220 ± 0.028 vs. 0.175 ± 0.022) were significantly longer in the diabetic group than in the control group, indicating increased heterogeneity of ventricular repolarization in type 2 diabetes.
Multiple regression analysis indicated that HbA1c and systolic BP were independent determinants of both QT dispersion and Tpeak-Tend, indices of heterogeneity in ventricular repolarization (Table 2). On the other hand, neither QT dispersion nor Tpeak-Tend in type 2 diabetes was correlated with duration of diabetes (Figure 2).
Table 2.
Multiple regression analyses for electrical heterogeneity indices
| QT dispersion | Tpeak-Tend | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | t | p | B | SE | β | t | p | |
| Age (years) | 0.043 | 0.120 | 0.039 | 0.353 | 0.720 | −0.001 | 0.110 | −0.001 | −0.008 | 0.990 |
| Sex (male) | −0.148 | 1.400 | −0.011 | −0.109 | 0.910 | −1.520 | 1.300 | −0.113 | −1.180 | 0.240 |
| BMI (kg/m2) | −0.093 | 0.370 | −0.028 | −0.248 | 0.810 | −0.295 | 0.350 | −0.092 | −0.838 | 0.400 |
| SBP (mmHg) | 0.233 | 0.095 | 0.261 | 2.450 | 0.016 | 0.188 | 0.090 | 0.216 | 2.100 | 0.039 |
| HbA1c (%) | 2.050 | 0.660 | 0.374 | 3.130 | 0.002 | 2.710 | 0.620 | 0.504 | 4.380 | <0.001 |
| Triglyceride (mg/dl) | −0.009 | 0.008 | −0.126 | −1.140 | 0.260 | −0.012 | 0.008 | −0.166 | −1.560 | 0.120 |
| n = 88, R2 = 0.224, AIC = 706.0 | n = 88, R2 = 0.281, AIC = 695.5 | |||||||||
BMI = body mass index, SBP = systolic blood pressure, HbA1c = glycated hemoglobin.
Figure 2.
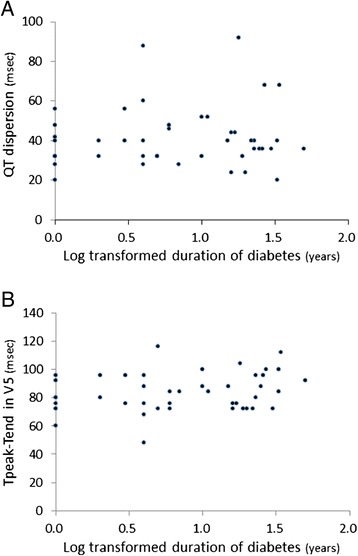
Neither QT dispersion (A) nor Tpeak-Tend (B) was correlated with duration of diabetes (log transformed).
Effect of glycemic control on heterogeneity in ventricular repolarization
After discharge from our hospital, 31 patients were followed at the out-patient clinic of our hospital and enrolled in the follow-up study. The remaining 13 patients were followed at affiliated clinics. We confirmed that all of the 13 patients have been free from cardiovascular events, but ECG and laboratory data of 8 patients were not available for the present analyses. Therefore, we analyzed changes in electrocardiographic variables and clinical data for 36 patients at baseline and during follow-up after treatment of diabetes (Table 3, Figure 1). The proportions of patients on each medication did not significantly change during a mean follow-up period of 787.0 ± 300.8 days (median, 754 days), except for increased frequency in use of dipeptidyl peptidase (DPP)-4 inhibitors at the end of the follow-up period. HbA1c level was significantly reduced from 10.0 ± 1.7 to 7.3 ± 1.6%, although BMI and BP were unchanged during the follow-up period. Triglyceride and LDL-C were significantly reduced after treatments, probably due to low calorie/fat intake as diet therapy, improvement of glycemic control, increased use of statins (50.0 vs 33.3%) and/or their combination. Serum creatinine level significantly increased from 0.67 ± 0.23 to 0.99 ± 0.85 mg/dl, and this trend was still observed even after exclusion of two patients who developed stage 5 nephropathy (0.80 ± 0.28 mg/dl).
Table 3.
Changes in parameters in type 2 diabetic patients (n = 36)
| Baseline | Follow-up | P | |
|---|---|---|---|
| Clinical variables | |||
| Duration of treatments (days) | - | 787.0 ± 300.8 | |
| BMI (kg/m2) | 26.5 ± 4.7 | 26.0 ± 4.8 | 0.239 |
| SBP (mmHg) | 126.6 ± 18.0 | 127.5 ± 14.9 | 0.564 |
| DBP (mmHg) | 75.1 ± 9.9 | 71.0 ± 9.4 | 0.051 |
| Laboratory variables | |||
| HbA1c (%) | 10.0 ± 1.7 | 7.3 ± 1.6 | <0.001 |
| Triglyceride (mg/dl) | 184.8 ± 120.2 | 148.5 ± 81.9 | 0.043 |
| HDL-C (mg/dl) | 44.4 ± 11.8 | 46.9 ± 13.1 | 0.289 |
| LDL-C (mg/dl) | 116.0 ± 41.5 | 97.3 ± 28.8 | 0.020 |
| Creatinine (mg/dl) | 0.67 ± 0.23 | 0.99 ± 0.85 | 0.011 |
| Uric acid (mg/dl) | 4.9 ± 1.2 | 5.1 ± 1.4 | 0.504 |
| Potassium (mEq/l) | 4.2 ± 0.4 | 4.1 ± 0.5 | 0.734 |
| Medications | |||
| ACE-I/ARB | 13 (36.1%) | 18 (50.0%) | 0.341 |
| CCB | 11 (30.6%) | 12 (33.3%) | 1.000 |
| β blocker | 5 (13.9%) | 6 (16.7%) | 1.000 |
| Other antihypertensive drugs | 6 (16.7%) | 8 (22.2%) | 0.767 |
| Sulphonylurea | 17 (47.2%) | 13 (36.1%) | 0.474 |
| α-glucosidase inhibitor | 16 (44.4%) | 11 (30.6%) | 0.330 |
| Biguanide | 14 (38.9%) | 18 (50.0%) | 0.477 |
| DPP-4 inhibitor | 10 (27.8%) | 27 (75.0%) | <0.001 |
| Insulin | 7 (19.4%) | 10 (27.8%) | 0.580 |
| Other antidiabetic drugs | 4 (11.1%) | 2 (5.6%) | 0.674 |
| Statin | 12 (33.3%) | 18 (50.0%) | 0.232 |
| Fibrate | 2 (5.6%) | 1 (2.8%) | 1.000 |
| Electrocardiographic variables | |||
| Heart rate (bpm) | 73.6 ± 14.4 | 70.6 ± 11.0 | 0.156 |
| V1S + V5R (mV) | 2.42 ± 0.60 | 2.58 ± 0.74 | 0.097 |
| QTc mean (ms) | 414.1 ± 19.3 | 414.8 ± 23.0 | 0.843 |
| QT dispersion (ms) | 41.4 ± 16.6 | 45.8 ± 15.0 | 0.165 |
| QTc dispersion (ms) | 45.1 ± 16.8 | 46.8 ± 14.4 | 0.564 |
| Tpeak-Tend in V5 (ms) | 85.0 ± 12.9 | 83.6 ± 10.6 | 0.563 |
BMI = body mass index, SBP = systolic blood pressure, DBP = diastolic blood pressure, CAD = coronary artery disease, FPG = fasting plasma glucose, HbA1c = glycated hemoglobin, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, ACE-I = angiotensin-converting enzyme inhibitor, ARB = angiotensin II receptor blocker, CCB = calcium channel blocker, DPP-4 inhibitor = dipeptidyl peptidase-4 inhibitor, QTc = corrected QT (by Bazett’s formula), Values are means ± SD or absolute numbers (frequency percentages).
Heart rate and voltage index in ECG were not different before and after glycemic control. Despite the considerable reduction in HbA1c after treatment of diabetes, mean QTc interval, QT dispersion and Tpeak-Tend in lead V5 did not significantly changed (Table 3). Furthermore, there was no significant correlation between change in HbA1c, creatinine or LDL-C and change in QT dispersion or Tpeak-Tend during the follow-up period (Figure 3).
Figure 3.
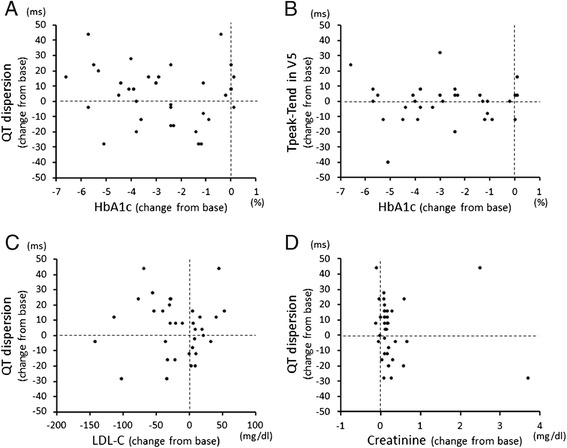
There were no correlations between changes in QT dispersion (A) and Tpeak-Tend (B) and change in HbA1c level. Change in QT dispersion was also not correlated with change in LDL-C level (C) or creatinine level (D).
Discussion
The majority of previous studies have shown that QTc interval was prolonged in diabetic patients compared to that in non-diabetic controls [27,28]. In the present study, however, QTc interval in type 2 diabetic patients (413.6 ± 18.5 ms) was similar to that in control subjects (408.3 ± 22.7 ms). Although Bazett’s formula has been the most frequently used method to adjust QT intervals for heart rate, it tends to underestimate or overestimate the duration of repolarization when heart rate is relatively slow or fast. Since heart rate was faster in diabetic patients than in control subjects, we also corrected QT intervals for heart rate using a method proposed by Rautaharju and Zhang [25]. However, as was the case with Bazett’s formula, the method of Rautaharju and Zhang also did not yield a significant difference in QTc interval between diabetic patients and controls (407.4 ± 15.6 vs. 406.9 ± 21.0 ms). In contrast, QT dispersion and QTc dispersion, indices for global dispersion, and Tpeak-Tend, an index for transmural dispersion, were significantly increased in type 2 diabetic patients compared to those in controls. It has been shown that QT dispersion and/or QTc dispersion are better prognostic markers than QTc interval in diabetes to predict cardiovascular mortality [16,17]. The present results suggest that increases in QT dispersion, QTc dispersion and Tpeak-Tend precede the QTc prolongation, thereby being useful for detection of repolarization abnormality at an earlier stage. Alternatively, QT dispersion, QTc dispersion and Tpeak-Tend may be more sensitive than QTc interval for detecting even slight abnormalities in repolarization.
Electrical repolarization abnormalities have been shown to be associated with increased systolic BP, left ventricular hypertrophy, presence of coronary artery disease, autonomic dysfunction or microalbuminuria in patients with diabetes [24,27,29,30]. Since these complications are increased when the duration of diabetes is prolonged, we presumed that repolarization abnormality would be related to disease duration. However, neither QT dispersion nor Tpeak-Tend was correlated with duration of diabetes (Figure 2). This is consistent with the results of a study by Festa et al. [27] showing that QT interval was already prolonged in newly diagnosed diabetes. We also examined the relationship between presence of coronary artery disease or autonomic dysfunction and repolarization abnormalities. However, the values of both QT dispersion and Tpeak-Tend were similar in diabetic patients regardless of the presence or absence of coronary artery disease or autonomic dysfunction (data not shown). There is a possibility that the small number of patients with these complications made it difficult to detect the difference, but our results suggest that the presence of coronary artery disease and autonomic dysfunction are not major predictors of repolarization abnormalities.
We found that HbA1c was an independent and strong explanatory variable for increased QT dispersion and Tpeak-Tend in this study (Table 2). This result is in accordance with results of studies showing that poor glycemic control was associated with prolonged QT interval [31,32]. However, except for the association with HbA1c and BP, relationships between diabetes-related changes in clinical parameters and repolarization abnormality have not been clarified in previous studies [27,28,31,32]. Therefore, we tested the hypothesis that glycemic control could improve repolarization abnormality in diabetic patients. While HbA1c level significantly decreased from 10.0 ± 1.7 to 7.3 ± 1.6% during the follow-up period, none of the repolarization indices improved after treatment of diabetes. Furthermore, there was no significant correlation between change in HbA1c and change in QT dispersion or Tpeak-Tend during the follow-up period (Figure 3). To the best of our knowledge, this is the first report that glycemic control failed to improve repolarization abnormalities.
Several classes of anti-diabetic drugs were used for glycemic control in the present study subjects. Previous studies have shown that biguanide, DPP-4 inhibitors and sodium glucose cotransporter 2 inhibitors do not modify ventricular repolarization [33–35]. On the other hand, sulfonylurea inhibits ATP-sensitive K+ channels not only in pancreatic β cells but also on the sarcolemma of cardiomyocytes, resulting in prolongation of the QT interval [33]. Although approximately 40% of the patients were treated with sulfonylurea in the present study, those patients were taking glimepiride, which has less effect than glibenclamide on cardiac ATP-sensitive K+ channels [36]. Furthermore, the number of patients treated with sulfonylurea was not increased during the follow-up. Therefore, it is unlikely that the failure of glycemic control to improve repolarization abnormalities is attributable to the medicines used for glycemic control in the present study. Recent large clinical trials have shown that intensive glycemic control failed to reduce cardiovascular and all-cause mortality [6–10] during 2 to 5 years of treatment. A benefit of glycemic control in reducing the risk of cardiovascular disease was observed only when the follow-up period was long (10–20 years) in even newly diagnosed diabetic patients [37]. There is the possibility that the follow-up period (787.0 ± 300.8 days) in the present study was too short to show alleviation of the repolarization abnormality by tight glycemic control. Nevertheless, the present results are consistent with the results of recent clinical trials showing that intensive glycemic control failed to reduce cardiovascular mortality [6–10].
In contrast to the failure of glycemic control to improve repolarization abnormalities in the present study, protective effects of BP and lipid control have been reported in patients with diabetes and/or hypertension [28,38,39]. Treatment with an angiotensin-converting enzyme inhibitor and a calcium channel blocker significantly decreased QT dispersion in patients with hypertension, and this effect was correlated with the degree of left ventricular hypertrophy [38]. In hypertensive patients with diabetes, treatment with aliskiren, a direct renin inhibitor, reduced QT dispersion at 12 weeks after treatment [39]. These results may reflect the outcomes of clinical trials showing that interventions for hypertension and dyslipidemia have improved cardiovascular and all-cause mortality in patients with diabetes [40,41]. Festa et al. [27] showed that systolic BP and LV mass, but not glucose level, were determinants of the QT interval in diabetic patients. Cox et al. [15] reported that systolic BP was higher in a prolonged QTc group (152.8 mmHg) than that in a normal QTc group (139.6 mmHg) of type 2 diabetic patients, though HbA1c levels in the two groups were similar (8.2% vs. 7.7%). In the present study, multiple regression analysis revealed that systolic BP was an independent predictor of QT dispersion and Tpeak-Tend (Table 2), and an ECG marker of left ventricular mass (V1S + V5R) tended to be higher in diabetic patients. These results suggested that high BP and consequent increase in ventricular mass are stronger determinants than HbA1c for increased heterogeneity of ventricular repolarization in diabetic patients. In the present study, BP in diabetic patients was well-controlled by medications both at baseline and during the follow-up periods (Table 3), indicating that significant improvement of glycemic control does not attenuate repolarization abnormality by diabetes even under good BP control.
Treatment with a statin has been shown to improve repolarization heterogeneity in patients with diabetes in a study by Tekin et al. [28]. They reported that treatment of diabetic patients with simvastatin for 12 weeks decreased LDL-C from 142 mg/dl to 80 mg/dl and reduced QT and QTc dispersions by 24% and 27%, respectively. Whether the LDL-C-reducing property of simvastatin or its pleiotropic effect contributed to the improvement of repolarization heterogeneity remains unclear. In the present study, QT dispersion was not reduced during the follow-up period, although LDL-C was reduced by 17% in association with an increase in the proportion of patients on statins (Table 3). A plausible explanation for the discrepancy between the present results and those in the study by Tekin et al. [28] is well-controlled LDL-C level at baseline in the present study: LDL-C level was within the recommendation range (LDL-C <120 mg/dl in diabetic patients, Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2012) at baseline and change in LDL-C level during the follow-up period was within normal ranges.
It has been shown that severe hypoglycemia is associated with increased cardiovascular mortality, and fatal arrhythmias caused by abnormal ventricular repolarization during hypoglycemia could be one of the mechanisms [42,43]. Significant prolongation of QT interval during hypoglycemia has also been observed in previous studies [42–45]. In this study, there was no episode of severe hypoglycemia in patients during hospitalization or the follow-up, but mild hypoglycemia and hypoglycemia unawareness could not be totally excluded in retrospective analysis of medical records. Hence, to examine the possibility that mild hypoglycemic episodes, if any, had an impact on ventricular repolarization in the diabetic patients, we divided the diabetic patients into a subgroup treated with sulfonylurea and/or insulin and a subgroup treated with other agents. Although sulfonylurea and insulin are known to increase the risk of hypoglycemia compared with other agents, QT dispersion and Tpeak-Tend were similar in the subgroups of patients treated with or without sulfonylurea and/or insulin (39.5 ± 15.0 vs. 43.8 ± 15.8 ms for QT dispersion and 84.6 ± 13.0 vs. 82.8 ± 14.2 ms for Tpeak-Tend). We also analyzed blood glucose levels at the outpatient clinic when follow-up ECG was taken in the 36 diabetic patients. Glucose levels ranged from 80 to 280 mg/dl (mean: 156.3 ± 44.3 mg/dl), and thus none of follow-up ECGs were recorded at the time of hypoglycemia. These findings argue against the possibility that improvement of ventricular repolarization by glycemic control was masked by mild ~ modest hypoglycemic episodes in the present study.
There are several limitations in this study. First, the number of patients was small and the follow-up period of diabetic treatment was relatively short. Statistical power for detection of differences in QT dispersion and Tpeak-Tend between type 2 diabetes and control groups (N = 44 each) was 0.998 and 0.987, respectively, suggesting that it was sufficient power (>0.80) in baseline comparison in this study. On the other hand, statistical power for longitudinal changes in the indices of ventricular repolarization is not large due to the relatively small number of patients (i.e., 0.332 for QT dispersion and 0.094 for Tpeak-Tend), and thus the possibility of a type 2 error cannot be excluded. Second, no major adverse cardiac event or death occurred during the follow-up period. Therefore, the observed impact of increased QT dispersion and Tpeak-Tend on the clinical outcome could not be confirmed.
Conclusions
Increased heterogeneity of ventricular repolarization in diabetic patients was not normalized by significant glycemic control within a few years. The results are consistent with the results of recent clinical trials showing that intensive glycemic control failed to reduce cardiovascular mortality. Whether intensive glycemic control for more than a few years ultimately reverses the repolarization heterogeneity remains to be further investigated.
Consent
Informed consent of this retrospective study was obtained from the patient directly or via the study information publicized in the internet.
Acknowledgments
The authors thank Dr. Takeaki Kobayashi, Sapporo Dohkohkai Hospital, Sapporo, Dr. Takayoshi Wada, Wada Internal Medicine Surgery Clinic, Sapporo, Dr. Takuma Hoshino, Japan Community Health Care Organization Hokkaido Hospital, Sapporo, Dr. Shigeo Kakinoki, Otaru Kyokai Hospital, Otaru and Dr. Ayako Hoshiyama, Kashiwazaki Chuo Hospital, Kashiwazaki, for their contributions to the collection of patients’ follow-up data.
Funding
This study was supported by Research and Education Grant 2013 from Sapporo Medical University.
Abbreviation
- BMI
Body mass index
- BP
Blood pressure
- DPP-4
Dipeptidyl peptidase-4
- ECG
Electrocardiogram
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- MI
Myocardial infarction
- QTc
Heart rate-corrected QT interval
- SCD
Sudden cardiac death
- Tpeak-Tend
The interval from the peak to the end of the T wave
Footnotes
Takayuki Miki and Toshiyuki Tobisawa contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TaM and TeM designed the study. TT, TS, MT, TY, AK and MO collected the patients’ data. TaM, TT, TS, TY, HM and SS analyzed and discussed data, and TT and HA performed statistical analyses. TaM, MT, TY and TeM drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Takayuki Miki, Email: tmiki@sapmed.ac.jp.
Toshiyuki Tobisawa, Email: tobisawadesu@yahoo.co.jp.
Tatsuya Sato, Email: satatsu.bear@gmail.com.
Masaya Tanno, Email: tannom@sapmed.ac.jp.
Toshiyuki Yano, Email: oltomwaits55@gmail.com.
Hiroshi Akasaka, Email: akasaka@sapmed.ac.jp.
Atsushi Kuno, Email: kuno@sapmed.ac.jp.
Makoto Ogasawara, Email: ogasawara13202831@yahoo.co.jp.
Hiromichi Murase, Email: michi_seramu_9754@yahoo.co.jp.
Shigeyuki Saitoh, Email: ssaitoh@sapmed.ac.jp.
Tetsuji Miura, Email: miura@sapmed.ac.jp.
References
- 1.International Diabetes Federation . IDF Diabetes Atlas. 6. Brussels, Belgium: International Diabetes Federation; 2013. [PubMed] [Google Scholar]
- 2.Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB, Sr, Savage PJ, Levy D, Fox CS. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miki T, Yuda S, Kouzu H, Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev. 2013;18:149–166. doi: 10.1007/s10741-012-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeung CY, Lam KS, Li SW, Lam KF, Tse HF, Siu CW. Sudden cardiac death after myocardial infarction in type 2 diabetic patients with no residual myocardial ischemia. Diabetes Care. 2012;35:2564–2569. doi: 10.2337/dc12-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 6.The ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 Diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 7.The Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 Diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 9.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I, SAVOR-TIMI 53 Steering Committee and Investigators Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 10.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F, EXAMINE Investigators Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation. 1978;57:1074–1077. doi: 10.1161/01.CIR.57.6.1074. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Post WS, Blasco-Colmenares E, Dalal D, Tomaselli GF, Guallar E. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology. 2011;22:660–670. doi: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montanez A, Ruskin JN, Hebert PR, Lamas GA, Hennekens CH. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med. 2004;164:943–948. doi: 10.1001/archinte.164.9.943. [DOI] [PubMed] [Google Scholar]
- 14.Okin PM, Devereux RB, Lee ET, Galloway JM, Howard BV. Electrocardiographic repolarization complexity and abnormality predict all-cause and cardiovascular mortality in diabetes: the strong heart study. Diabetes. 2004;53:434–440. doi: 10.2337/diabetes.53.2.434. [DOI] [PubMed] [Google Scholar]
- 15.Cox AJ, Azeem A, Yeboah J, Soliman EZ, Aggarwal SR, Bertoni AG, Carr JJ, Freedman BI, Herrington DM, Bowden DW. Heart rate-corrected QT interval is an independent predictor of all-cause and cardiovascular mortality in individuals with type 2 diabetes: the diabetes heart study. Diabetes Care. 2014;37:1454–1461. doi: 10.2337/dc13-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardoso CR, Salles GF, Deccache W. Prognostic value of QT interval parameters in type 2 diabetes mellitus: results of a long-term follow-up prospective study. J Diabetes Complications. 2003;17:169–178. doi: 10.1016/S1056-8727(02)00206-4. [DOI] [PubMed] [Google Scholar]
- 17.Giunti S, Gruden G, Fornengo P, Barutta F, Amione C, Ghezzo G, Cavallo-Perin P, Bruno G. Increased QT interval dispersion predicts 15-year cardiovascular mortality in type 2 diabetic subjects: the population-based Casale Monferrato Study. Diabetes Care. 2012;35:581–583. doi: 10.2337/dc11-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, Dorantes Sánchez M, Dorticós Balea F, Zayas Molina R, Quiñones Pérez MA, Fayad Rodríguez Y. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828–1834. doi: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbhaiya C, Po JR, Hanon S, Schweitzer P. Tpeak-Tend and Tpeak-Tend/QT ratio as markers of ventricular arrhythmia risk in cardiac resynchronization therapy patients. Pacing Clin Electrophysiol. 2013;36:103–108. doi: 10.1111/pace.12031. [DOI] [PubMed] [Google Scholar]
- 20.Smetana P, Schmidt A, Zabel M, Hnatkova K, Franz M, Huber K, Malik M. Assessment of repolarization heterogeneity for prediction of mortality in cardiovascular disease: peak to the end of the T wave interval and nondipolar repolarization components. J Electrocardiol. 2011;44:301–308. doi: 10.1016/j.jelectrocard.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 2011;4:441–447. doi: 10.1161/CIRCEP.110.960658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsumata K, Saitoh S, Ohnishi H, Akasaka H, Miura T. Effects of parental hypertension on longitudinal trends in blood pressure and plasma metabolic profile: mixed-effects model analysis. Hypertension. 2012;60:1124–1130. doi: 10.1161/HYPERTENSIONAHA.112.201129. [DOI] [PubMed] [Google Scholar]
- 23.Consensus statement Report and recommendations of the San Antonio conference on diabetic neuropathy. American Diabetes Association American Academy of Neurology. Diabetes Care. 1988;11:592–597. doi: 10.2337/diacare.11.7.592. [DOI] [PubMed] [Google Scholar]
- 24.Takebayashi K, Sugita R, Tayama K, Aso Y, Takemura Y, Inukai T. The connection between QT dispersion and autonomic neuropathy in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003;111:351–357. doi: 10.1055/s-2003-42726. [DOI] [PubMed] [Google Scholar]
- 25.Rautaharju PM, Zhang ZM. Linearly scaled, rate-invariant normal limits for QT interval: eight decades of incorrect application of power functions. J Cardiovasc Electrophysiol. 2002;13:1211–1218. doi: 10.1046/j.1540-8167.2002.01211.x. [DOI] [PubMed] [Google Scholar]
- 26.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H, American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:982–991. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Festa A, D’Agostino R, Jr, Rautaharju P, Mykkänen L, Haffner SM. Relation of systemic blood pressure, left ventricular mass, insulin sensitivity, and coronary artery disease to QT interval duration in nondiabetic and type 2 diabetic subjects. Am J Cardiol. 2000;86:1117–1122. doi: 10.1016/S0002-9149(00)01170-X. [DOI] [PubMed] [Google Scholar]
- 28.Tekin A, Tekin G, Sezgin AT, Müderrisoğlu H. Short- and long-term effect of simvastatin therapy on the heterogeneity of cardiac repolarization in diabetic patients. Pharmacol Res. 2008;57:393–397. doi: 10.1016/j.phrs.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Veglio M, Chinaglia A, Cavallo-Perin P. QT interval, cardiovascular risk factors and risk of death in diabetes. J Endocrinol Invest. 2004;27:175–181. doi: 10.1007/BF03346265. [DOI] [PubMed] [Google Scholar]
- 30.Psallas M, Tentolouris N, Papadogiannis D, Doulgerakis D, Kokkinos A, Cokkinos DV, Katsilambros N. QT dispersion: comparison between participants with Type 1 and 2 diabetes and association with microalbuminuria in diabetes. J Diabetes Complications. 2006;20:88–97. doi: 10.1016/j.jdiacomp.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Giunti S, Bruno G, Lillaz E, Gruden G, Lolli V, Chaturvedi N, Fuller JH, Veglio M, Cavallo-Perin P, EURODIAB IDDM Complications Study Group Incidence and risk factors of prolonged QTc interval in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care. 2007;30:2057–2063. doi: 10.2337/dc07-0063. [DOI] [PubMed] [Google Scholar]
- 32.Suys B, Heuten S, De Wolf D, Verherstraeten M, de Beeck LO, Matthys D, Vrints C, Rooman R. Glycemia and corrected QT interval prolongation in young type 1 diabetic patients: what is the relation? Diabetes Care. 2006;29:427–429. doi: 10.2337/diacare.29.02.06.dc05-1450. [DOI] [PubMed] [Google Scholar]
- 33.Najeed SA, Khan IA, Molnar J, Somberg JC. Differential effect of glyburide (glibenclamide) and metformin on QT dispersion: a potential adenosine triphosphate sensitive K + channel effect. Am J Cardiol. 2002;90:1103–1106. doi: 10.1016/S0002-9149(02)02776-5. [DOI] [PubMed] [Google Scholar]
- 34.Bloomfield DM, Krishna R, Hreniuk D, Hickey L, Ghosh K, Bergman AJ, Miller J, Gutierrez MJ, Stoltz R, Gottesdiener KM, Herman GA, Wagner JA. A thorough QTc study to assess the effect of sitagliptin, a DPP4 inhibitor, on ventricular repolarization in healthy subjects. J Clin Pharmacol. 2009;49:937–946. doi: 10.1177/0091270009337511. [DOI] [PubMed] [Google Scholar]
- 35.Ring A, Brand T, Macha S, Breithaupt-Groegler K, Simons G, Walter B, Woerle HJ, Broedl UC. The sodium glucose cotransporter 2 inhibitor empagliflozin does not prolong QT interval in a thorough QT (TQT) study. Cardiovasc Diabetol. 2013;12:70. doi: 10.1186/1475-2840-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geisen K, Végh A, Krause E, Papp JG. Cardiovascular effects of conventional sulfonylureas and glimepiride. Horm Metab Res. 1996;28:496–507. doi: 10.1055/s-2007-979841. [DOI] [PubMed] [Google Scholar]
- 37.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 38.Karpanou EA, Vyssoulis GP, Psichogios A, Malakou C, Kyrozi EA, Cokkinos DV, Toutouzas PK. Regression of left ventricular hypertrophy results in improvement of QT dispersion in patients with hypertension. Am Heart J. 1998;136:765–768. doi: 10.1016/S0002-8703(98)70119-X. [DOI] [PubMed] [Google Scholar]
- 39.Fogari R, Zoppi A, Maffioli P, Monti C, Lazzari P, Mugellini A, Derosa G. Effects of aliskiren on QT duration and dispersion in hypertensive patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:341–347. doi: 10.1111/j.1463-1326.2011.01535.x. [DOI] [PubMed] [Google Scholar]
- 40.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 41.Cholesterol Treatment Trialists’ (CTT) Collaborators. Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60761-8. [DOI] [PubMed] [Google Scholar]
- 42.Hanefeld M, Duetting E, Bramlage P. Cardiac implications of hypoglycaemia in patients with diabetes - a systematic review. Cardiovasc Diabetol. 2013;12:135. doi: 10.1186/1475-2840-12-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chow E, Bernjak A, Williams S, Fawdry RA, Hibbert S, Freeman J, Sheridan PJ, Heller SR. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63:1738–1747. doi: 10.2337/db13-0468. [DOI] [PubMed] [Google Scholar]
- 44.Beom JW, Kim JM, Chung EJ, Kim JY, Ko SY, Na SD, Kim CH, Park G, Kang MY. Corrected QT interval prolongation during severe hypoglycemia without hypokalemia in patients with type 2 diabetes. Diabetes Metab J. 2013;37:190–195. doi: 10.4093/dmj.2013.37.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ling S, Nguyen HT. Ventricular repolarization variability for hypoglycemia detection. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:7961–7964. doi: 10.1109/IEMBS.2011.6091963. [DOI] [PubMed] [Google Scholar]


